Abstract
The pharmacology of the orexin-like peptides, hypocretin-1 and hypocretin-2, was studied in Chinese hamster ovary (CHO) cells stably expressing orexin-1 (OX1) or orexin-2 (OX2) receptors by measuring intracellular calcium ([Ca2+]i) using Fluo-3AM. Orexin-A and orexin-B increased [Ca2+]i in CHO-OX1 (pEC50=7.99±0.05 and 7.00±0.10 respectively, n=8) and CHO-OX2 (pEC50=8.30±0.05 and 8.21±0.07 respectively, n=5). However, hypocretin-1 and hypocretin-2 were markedly less potent, with pEC50 values of 5.31±0.04 and 5.41±0.04 respectively in CHO-OX2 cells (n=5). In CHO-OX1 cells 10 μM hypocretin-1 only elicited a 37.5±3.4% response whilst 10 μM hypocretin-2 elicited a 18.0±2.1% response (n=8). Desensitisation of OX1 or OX2 with orexin-A (100 nM) abolished the response to orexin-A (10 nM) and the hypocretins (10 μM), but not to UTP (3 μM). In conclusion, the hypocretins are only weak agonists at the orexin receptors.
Keywords: Orexin, hypocretin, calcium, FLIPR
Introduction
Orexin-A and orexin-B are 33 and 28 residue peptides respectively, which were recently isolated from the rat hypothalamus and are derived from a 130 amino acid precursor, prepro-orexin (Sakurai et al., 1998). Both peptides bind to two receptors, orexin-1 (OX1) and orexin-2 (OX2), although orexin-B apparently has a low affinity for OX1 (Sakurai et al., 1998). The binding of these ligands is associated with an increase in intracellular calcium concentrations ([Ca2+]i) (Smart et al., 1999).
The orexins are located predominantly in the hypothalamus and locus coeruleus (Sakurai et al., 1998; Peyron et al., 1998), but are also found elsewhere in the CNS (Smart, 1999; Van den Pol, 1999). The orexins have a range of physiological functions including the control of feeding and energy metabolism (Sakurai et al., 1998), modulation of neuroendocrine function (Van den Pol et al., 1998; Smart, 1999), and regulation of the sleep-wake cycle (Smart, 1999).
Independently, DeLecea and colleagues (1998) identified a hypothalamic-specific mRNA encoding a precursor protein which they called prepro-hypocretin, and predicted that processing of this prepro-peptide would yield two peptides, hypocretin-1 (residues 28–66) and hypocretin-2 (residues 69–97). Furthermore, they showed synthetic hypocretin-2 was excitatory when applied to hypothalamic neurons (DeLecea et al., 1998). Subsequent comparisons revealed that prepro-orexin and prepro-hypocretin were the same peptide (Flier & Maratos-Flier 1998; Sakurai et al., 1998), and that the sequences of the orexins and the hypocretins overlapped (Sakurai et al., 1998; DeLecea et al., 1998). This has led to some authors erroneously referring to the orexins as hypocretins in some published studies (Van den Pol, 1999; Samson et al., 1999). However, the pharmacology of the hypocretins at the orexin receptors has not been examined. Therefore, the present study examined the pharmacology of the hypocretins at the recombinant human receptors using the calcium-sensitive dye, Fluo-3AM, in a fluorometric imaging plate reader (FLIPR), and demonstrated that hypocretin-1 and hypocretin-2 are weak agonists at OX1 and OX2.
Methods
Cloning and expression of OX1 and OX2 receptors in CHO cells
OX1 and OX2 were produced by PCR from in-house foetal and adult brain cDNA libraries respectively, using primers located across the start and stop codons. The receptors were sub-cloned into the pCDN vector (with neomycin resistance) and transfected into CHO cells using lipofectamine (Life Technologies). Clones were selected using 400 μg ml−1 G418 (Life Technologies) and single cell clones were produced by limiting dilution cloning.
Cell culture
CHO-OX1 and CHO-OX2 cells were routinely grown as monolayers in MEM-Alpha medium supplemented with 10% foetal calf serum and 400 μg ml−1 G418, and maintained under 95%/5% O2/CO2 at 37°C. Cells were passaged every 3–4 days and the highest passage number used was 18.
Measurement of [Ca2+]i using the FLIPR
CHO-OX1 or CHO-OX2 cells were seeded into black walled clear-base 96 well plates (Costar U.K.) at a density of 20,000 cells per well in MEM-Alpha medium, supplemented as above and cultured overnight. The cells were then incubated with MEM-Alpha medium containing the cytoplasmic calcium indicator, Fluo-3AM (4 μM; Teflabs, Austin, Texas) and 2.5 mM probenecid at 37°C for 60 min. The cells were washed four times with, and finally resuspended in, Tyrode's medium containing 2.5 mM probenecid and 1% gelatine, before being incubated for 30 min at 37°C with either buffer alone (control) or buffer containing orexin-A or orexin-B. The plates were then placed into a FLIPR (Molecular Devices, U.K.) to monitor cell fluorescence (λex=488 nM, λEM=540 nM) (Sullivan et al., 1999) before and after the addition of orexin-A, orexin-B, hypocretin-1 or hypocretin-2 (10 pM–10 μM).
Reverse phase HPLC
The purity of the commercial hypocretins was confirmed by reverse phase HPLC using a Waters Symmetry C18 (5 μM, 300A, 2.1×150 mm) column and a Hewlett-Packard HP1090 chromatograph at 40°C, with UV detection (210 nm). Eluent A was 0.1% TFA and Eluent B was 80 : 20 acetonitrile : water (+0.085% TFA), were run as a linear gradient (1%/min) from 5–95% Eluent B, with a flow rate of 200 μl min−1. Synthetic orexin-A and orexin-B were run as standards.
Data analysis
Responses were measured as peak fluorescence intensity (FI) minus basal FI, and where appropriate were expressed as a percentage of a maximum orexin-A-induced response. Data are expressed as mean±s.e.mean unless otherwise stated. Curve-fitting and parameter estimation were carried out using Graph Pad Prism 3.00 (GraphPad Software Inc., CA, U.S.A.).
Materials
Orexin-A and orexin-B were synthesized for SmithKline Beecham at California Peptides (CA, U.S.A.). Orexin-A, orexin-B, hypocretin-1 and hypocretin-2 were purchased from Phoenix Pharmaceuticals (CA, U.S.A.). All cell culture media were obtained from Life Technologies, Paisley, U.K.
Results
Orexin-A and -B caused a concentration-dependent increase in [Ca2+]i in CHO-OX1 cells (Figure 1), with pEC50 values of 7.99±0.05 and 7.00±0.10 respectively (n=8). Similarly, both peptides increased [Ca2+]i in CHO-OX2 cells (Figure 1), with pEC50 values of 8.30±0.05 and 8.21±0.07, respectively (n=5). Commercial orexin-A and orexin-B produced similar responses (data not shown).
Figure 1.
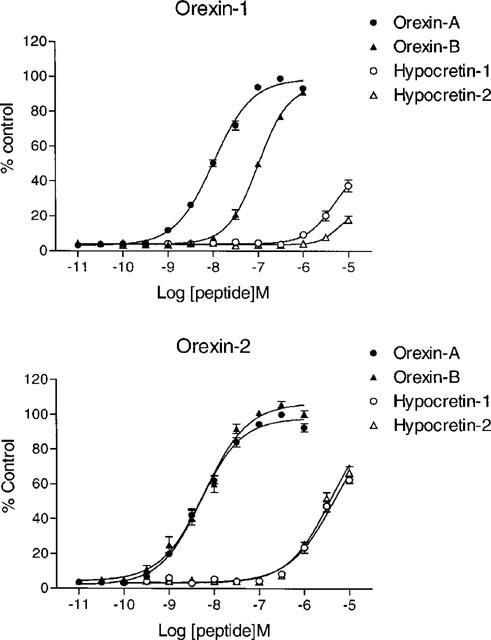
Orexins and hypocretins cause a concentration-dependent increase in [Ca2+]i. [Ca2+]i was monitored using Fluo-3AM in CHO cells stably expressing OX1 (upper panel) or OX2 (lower panel) before and after addition of orexin-A (10 pM–1 μM), orexin-B (10 pM–1 μM), hypocretin-1 (100 pM–10 μM) or hypocretin-2 (100 pM–10 μM). Responses were measured as peak increase in fluorescence minus basal and are given as mean±s.e.mean, where n=5–8.
Hypocretin-1 and hypocretin-2 also elicited concentration-related Ca2+ responses in CHO-OX1 and CHO-OX2 cells (Figure 1), but were markedly less potent than the orexins. Indeed, in CHO-OX1 cells the concentration-response relationship could not be fully defined as the highest concentration tested (10 μM) only elicited a 37.5±3.4% response for hypocretin-1 and a 18.0±2.1% response for hypocretin-2 (n=8). However, in the CHO-OX2 cells the concentration-response curves were better defined, allowing pEC50 values of 5.31±0.04 and 5.41±0.04 to be estimated for hypocretin-1 and hypocretin-2 respectively (n=5). The hypocretins had no effect on [Ca2+]i in non-transfected CHO cells (data not shown).
The hypocretin-induced response had a similar kinetic profile to that of the orexin-induced response (Figure 2), with a rapid initial peak (maximal 6–10 s after addition) followed by a gradual decline towards baseline values over a period of ∼150 s. Moreover, analysis by HPLC showed that there was no trace of contaminating orexins in either the hypocretin-1 or hypocretin-2 (data not shown).
Figure 2.
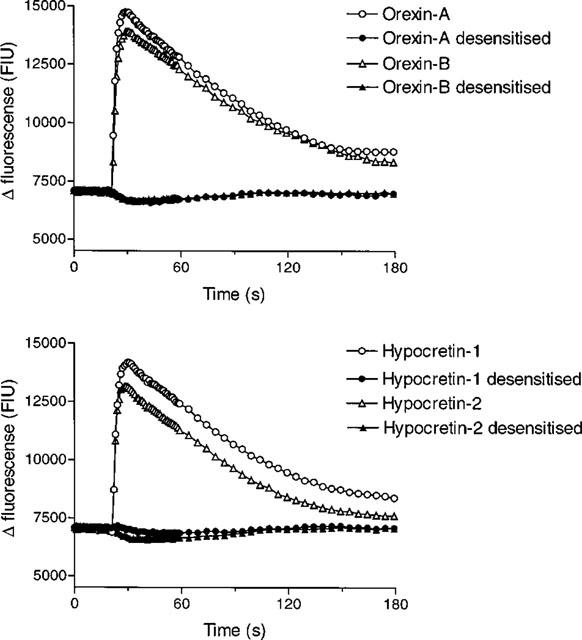
The hypocretin-induced Ca2+ responses are desensitized by orexin-A. [Ca2+]i was monitored using Fluo-3AM in CHO-OX2 cells which had been incubated with buffer or orexin-A (100 nM) for 30 min prior to the addition of orexin-A (10 nM), orexin-B (10 nM), hypocretin-1 (10 μM) or hypocretin-2 (10 μM). Data are representative traces, typical of n=3–8.
In the absence of an available orexin receptor antagonist desensitization studies were carried out to confirm that the orexins and hypocretins were acting via the same receptors. Pre-exposure to orexin-A (100 nM) or orexin-B (100 nM) for 30 min abolished the Ca2+ response to a subsequent orexin-A (10 nM), orexin-B (10 nM), hypocretin-1 (10 μM) or hypocretin-2 (10 μM) challenge in CHO-OX2 cells (Figure 2). Similarly, pretreatment with orexin-A (100 nM) or orexin-B (1 μM) abolished the Ca2+ response to all four ligands in CHO-OX1 cells (n=3). However, the Ca2+ response to UTP (3 μM), which activates an endogenous purinergic receptor, was unaffected in these cells (data not shown).
Discussion
The orexins are a recently discovered family of neuropeptides (Sakurai et al., 1998) with a wide range of physiological functions (Smart, 1999). These two peptides were originally isolated from rat hypothalamic extracts and shown to interact with two receptors, OX1 and OX2 (Sakurai et al., 1998). Another group independently identified a hypothalamic precursor protein, prepro-hypocretin, which sequence analysis indicated would yield two peptides, hypocretin-1 (residues 28–66) and hypocretin-2 (residues 69–97) although these were not isolated (DeLecea et al., 1998). Subsequent comparisons revealed considerable homology between the hypocretins and the orexins (Flier & Maratos-Flier, 1998; Sakurai et al., 1998). Hypocretin-1 has the same sequence as orexin-A, but with five additional N-terminal amino acids and a C-terminal glycine, whilst hypocretin-2 shares the same sequence as orexin-B, but with a C-terminal glycine (DeLecea et al., 1998; Sakurai et al., 1998). Thus, it was proposed that the hypocretins act at the orexin receptors, but no evidence has been offered to support this hypothesis (Van den Pol et al., 1998; 1999). Moreover, this has even resulted in some authors erroneously referring to having used hypocretins when they have actually used orexins (Samson et al., 1999). The present study has demonstrated that hypocretin-1 and hypocretin-2 act as weak agonists at OX1 and OX2, with ∼1000 fold lower potency than orexin-A and orexin-B respectively.
In the present study orexin-A and orexin-B caused a concentration-dependent increase in [Ca2+]i in CHO cells expressing either OX1 or OX2, with potencies similar to those reported previously (Smart et al., 1999). The potency of orexin-A was also consistent with the published radioligand binding data (Sakurai et al., 1998). Furthermore, orexin-A was equipotent at OX1 and OX2, whilst orexin-B displayed moderate selectivity for OX2, again consistent with the literature (Sakurai et al., 1998; Smart et al., 1999).
Hypocretin-1 and hypocretin-2 also elicited concentration-related Ca2+ responses in CHO-OX1 and CHO-OX2 cells, but were ∼1000 fold less potent than the orexins. Indeed, in CHO-OX1 cells the concentration-response relationship could not be defined as the response to the highest concentration (10 μM) of either hypocretin tested was <40%. However, the response to hypocretin-1 was greater than that to hypocretin-2, suggesting a similar rank order of potency to that displayed by the orexins at OX1 (Sakurai et al., 1998; Smart et al., 1999). Neither hypocretin-1 nor hypocretin-2 affected [Ca2+]i in parental CHO cells, indicating the hypocretin-induced Ca2+ response was mediated by the orexin receptor. Furthermore, the hypocretin-induced Ca2+ response displayed an identical kinetic profile to that of the orexin-induced Ca2+ response. This biphasic response is consistent with previous reports (Van den Pol, 1999; Smart et al., 1999) and indicative of an initial mobilization of intracellular Ca2+ and subsequent influx of extracellular Ca2+ (Smart et al., 1999).
Pre-exposure of the cells to EC80 concentrations of orexin-A or orexin-B for 30 min fully desensitized OX1 and OX2, as the Ca2+ response to a subsequent orexin-A or orexin-B challenge were abolished. Similarly, the responses to hypocretin-1 or hypocretin-2 were also abolished in orexin-desensitized CHO-OX1 or CHO-OX2 cells. However, the Ca2+ response elicited by UTP (3 μM), which activates an endogenous purinergic receptor, was not affected by orexin-pretreatment in these cells. Therefore, the desensitization of OX1 and OX2 was homologous, and thus receptor-specific (Lohse, 1993), confirming that the hypocretins and orexins were acting at the same receptors. Furthermore, the hypocretin-induced responses were not due to contaminating orexins as HPLC showed no trace of these peptides in either the hypocretin-1 or hypocretin-2.
In conclusion, the present study has shown that hypocretin-1 and hypocretin-2 act as weak agonists at OX1 or OX2, but are ∼1000 fold less potent than the orexins at these receptors.
Acknowledgments
The authors would like to thank Shabina Nasir for additional tissue culture support.
Abbreviations
- [Ca2+]i
intracellular calcium concentration
- CHO
Chinese hamster ovary
- FIU
fluorescence intensity units
- FLIPR
flurometric imaging plate reader
- OX1
orexin-1 receptor
- OX2
orexin-2 receptor
References
- DELECEA L., KILDUFF T.S., PEYRON C., GAO X.B., FOYE P.E., DANIELSON P.E., FUKUHARA C., BATTENBERG E.L.F., GAUTVIK V.T., BARTLETT F.S., FRANKEL W.N., VAN DEN POL A.N., BLOOM F.E., GAUTVIK K.M., SUTCLIFFE J.G. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLIER J.S., MARATOS-FLIER E. Obesity and the hypothalamus: novel peptides for new pathways. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J. Molecular mechanisms of membrane receptor desensitization. Biochim. Biophys. Acta. 1993;1179:171–188. doi: 10.1016/0167-4889(93)90139-g. [DOI] [PubMed] [Google Scholar]
- PEYRON C., TIGHE D.K., VAN DEN POL A.N., DELECEA L., HELLER H.C., SUTCLIFFE J.G., KILDUFF T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI T., AMEMIYA A., ISHII M., MATSUZAKI I., CHEMELLI R.M., TANAKA H., WILLIAMS S.C., RICHARDSON J.A., KOZLOWSKI G.P., WILSON S., ARCH J.R.S., BUCKINHAM R.C., HAYNES A.C., CARR S.A., ANNAN R.S., MCNULTY D.E., LIU W.S., TERRETT J.A., ELSHOURBAGY N.A., BERGSMA D.J., YANGISAWA M. Orexins and orexin receptors: a family of hypothelamic neuropeptides and G-protein coupled receptors that regulate feeding behaviour. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- SAMSON W.K., GOSNELL B., CHANG J.-K., RESCH Z.T., MURPHY T.C. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;31:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- SMART D. Orexins: a new family of neuropeptides. Br. J. Anaesthes. 1999;83:695–697. doi: 10.1093/bja/83.5.695. [DOI] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., BROUGH S.J., RUSHTON S.L., MURDOCK P.R., JEWITT F., ELSHOURBAGY N.A., ELLIS C.E., MIDDLEMISS D.N., BROWN F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999;128:1–4. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULLIVAN E., TUCKER E.M., DALE I.L.Measurement of [Ca2+]i using the fluometric imaging plate reader (FLIPR) Calcium Signaling Protocols 1999New Jersey: Humana Press; 125–136.In: Lambert, D.G. (ed.) [DOI] [PubMed] [Google Scholar]
- VAN DEN POL A.N. Hypocretin (orexin): robust innervation of the rat spinal cord. J. Neurosci. 1999;19:3171–3182. doi: 10.1523/JNEUROSCI.19-08-03171.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DEN POL A.N., GAO X.B., OBRIETAN K., KILDUFF T.S., BELOUSOV A.B. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


