Abstract
Andrographolide, an active component found in leaves of Andrographis paniculata, has been reported to exhibit nitric oxide (NO) inhibitory property in endotoxin-stimulated macrophages, however, the detailed mechanisms remain unclear. In the present study we investigated the effect of andrographolide on the expression of inducible NO synthase (iNOS) mRNA, protein, and enzyme activity in RAW 264.7 macrophages stimulated with lipopolysaccharide (LPS) plus interferon-γ (IFN-γ).
RAW 264.7 cells stimulated with LPS/IFN-γ activated NO production; in this condition andrographolide (1–100 μM) inhibited NO production in a dose-dependent manner with an IC50 value of 17.4±1.1 μM. Andrographolide also reduces the expression of iNOS protein level but without a significant effect on iNOS mRNA. The reduction of iNOS activity is thought to be caused by decreased expression of iNOS protein.
In a protein stability assay, andrographolide moderately but significantly reduced the amount of iNOS protein as suggested by accelerating degradation. Furthermore, andrographolide also inhibited total protein de novo synthesis as demonstrated by [35S]-methionine incorporation.
As a whole, these data suggest that andrographolide inhibits NO synthesis in RAW 264.7 cells by reducing the expression of iNOS protein and the reduction could occur through two additional mechanisms: prevention of the de novo protein synthesis and decreasing the protein stability via a post-transcriptional mechanism. It is also possible that inhibition of iNOS protein expression and NO production under immune stimulation and/or bacteria infection may explain, in part, the beneficial effects of andrographolide as an anti-inflammatory agent.
Keywords: Andrographolide, nitric oxide, inducible NO synthase (iNOS), macrophage, de novo protein synthesis
Introduction
Bacterial infection or immunological stimuli including lipopolysaccharide (LPS), interferon-γ (IFN-γ) or interleukin-1 cause the expression of an inducible isoform of nitric oxide synthase (iNOS) which, once expressed, produces large amounts of nitric oxide (NO) (Kilbourn & Griffith, 1992). In pathological conditions, macrophages can greatly increase their production of both NO and superoxide anion simultaneously, resulting in the formation of ONOO−, which, through further reaction, can exert even stronger oxidant effects (Ischiropoulos et al., 1992). Therefore, high amounts of NO are potentially cytotoxic, capable of injuring the surrounding cells and tissues indiscriminately either by itself or by formation of ONOO−. Indeed, it has been reported that excess production of NO by macrophages and other cells exposed to endotoxin may contribute to septic shock (Rees et al., 1990), cerebral injury (Dawson et al., 1993), myocardial ischemia (Matheis et al., 1992), local (e.g., polyarthritis, osteoarthritis) or systemic inflammatory disorders, diabetes, arteriosclerosis, and other diseases (Stefanovic-Racic et al., 1993; Connor et al., 1995; Wu & Thiemermann, 1996). Thus, the inhibition of iNOS expression and/or activity represents an important therapeutic goal.
Andrographis paniculata (Chuan-Chin-Lian) has been used medically in mainland China for more than 30 years and has been effective in the treatment of disorders related to bacteria infection (e.g., pneumonia bacteria) and inflammation (e.g., rheumatoid arthritis) (Chang & But, 1987). Extract of Andrographis paniculata and its chemical constituents have been shown to display a number of biological activities such as antiviral (Yao et al., 1992), anti-infective (George & Pandalai, 1949), anti-inflammation (Chang & But, 1987) and immunostimulative (Puri et al., 1993). Diterpenoid chemicals are the primary constituents found in leaves of Andrographis paniculata, with andrographolide as the major constituent (Lu et al., 1981). Although the mechanism(s) by which Andrographis paniculata produces its antiinflammatory biological effects remain unknown, it was reported that andrographolide can protect against lipid peroxidation (Choudhury & Poddar, 1984).
NO synthesized by iNOS has also been implicated as a mediator of inflammation (Stichtenoth & Frölich, 1998) and sepsis (Thimermann & Vane, 1990). In a previous study we found that andrographolide displayed beneficial haemodynamic effect in rats with endotoxaemia, which it was suggested could be due to its NO inhibitory action (Chiou et al., 1998). Since NO production by iNOS is the final step after immune stimulation, it can be regulated at many sites including transcription, translation and post-translational modification (Nathan & Xie, 1994). Thus, we became interested in how andrographolide may affect the NO activation pathway leading to inflammation. In this report we investigate the detail mechanisms of action of andrographolide on LPS/IFN-γ-stimulated murine macrophage-like cell line, RAW 264.7. The present study suggested that the post-transcriptional mechanisms, notably the total protein synthesis and the degradation of iNOS protein are involved in the suppression of iNOS expression even when iNOS is already induced.
Methods
Cell culture
RAW 264.7 cells (American Type Culture Collection ATCC, TIB 71, Rockville, MD, U.S.A.) were cultured in 75 cm2 plastic flasks (Corning-Costar) with Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% heat-inactivated foetal calf serum (FCS), penicillin (100 U ml−1) and streptomycin (100 μg ml−1) as previously described (Chiou et al., 1997). For experiments, macrophages were detached by vigorous pipetting and, after centrifugation, plated in fresh medium. These cells were activated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1), and cultured for 24 h at 37°C in an atmosphere of 5% CO2 plus air.
Nitrite measurement
Nitrite production, an indicator of NO synthesis, was measured in the supernatant of RAW264.7 macrophages as described previously (Chiou et al., 1997). Briefly, the cells were cultured in 96-well plates with 200 μl of culture medium until cells reached confluence (approximately 200,000 cells per well). In order to induce iNOS, fresh culture medium containing LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) was added. Nitrite accumulation in the medium was measured at 24 h after the application of LPS/IFN-γ. To assay the effect of drugs on nitrite production, andrographolide (final concentration, 1 to 100 μM) was added together with LPS/IFN-γ. Viability was assessed by the MTT assay.
Nitrite was measured by adding 100 μl of Griess reagent (1% sulphanilamide and 0.1% naphthylenediamine in 5% phosphoric acid) to 100 μl samples of medium. The optical density at 550 nm (OD550) was measured with a microplate reader. Concentrations were calculated by comparison with OD550 of standard solutions of sodium nitrite prepared in culture medium.
Cell viability
Cell viability was assessed by the mitochondria-dependent reduction of MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide] to formazan (Mosmann, 1983). Cells in 96-well plates were incubated (37°C) with MTT (5 mg ml−1 for 4 h). Culture medium was removed by aspiration and cells were dissolved in acid-SDS (100 μl) (Chiou et al., 1997). The extent of reduction of MTT to formazan within cells was quantitated by measurement of OD570 against OD630.
RT–PCR
Total cellular RNA was extracted from control and treated macrophages by acid guanidinium thiocyanate-phenol-chloroform extraction (Chomczynski & Sacchi, 1987). RT was performed using an Access RT–PCR System (Promega Corporation, U.S.A.). One microgram of total RNA was reverse transcribed to cDNA following the manufacturer's recommended procedures. RT-generated cDNA encoding iNOS and β-actin (as a positive control and an internal standard) genes were amplified using PCR (35 cycles). Oligonucleotide primers which correspond to the mouse macrophages iNOS (5′-CCCTTCCGAAGTTTCTGGCAGCAGC-3′ and 5′-GGCTGTCAGAGAGCCTCGTGGCTTTGG-3′) and mouse β-actin (5′-ATGCCATCCTGCGTCTGGACCTGG-C-3′ and 5′-AGCATTTGCGGTGCACGATGGAGGG-3′) cDNA were purchased from Cashmere Scientific Company (Taiwan) and used following the manufacturer's procedures. PCR was also performed using an Access RT–PCR System. The reaction volume was 50 μl containing (final concentration): PCR buffer (1×), deoxynucleotide (0.2 mM each), MgSO4 (1 mM), Tfl DNA polymerase (5 U), oligonucleotide primer (1 μM each), and RT products. After an initial denaturation for 2 min at 94°C, 35 cycles of amplification (94°C for 45 s, 65°C for 45 s, and 72°C for 2 min) were performed followed by a 10-min extension at 72°C. PCR products were analysed on 2% agarose gel.
Western blots
Confluent monolayers of RAW264.7 cells in 75 cm2 culture flasks (∼10×106 cells) were incubated for 24 h in DMEM with either LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) or in combination with andrographolide (50 μM). Incubations were terminated by rapid aspiration of the cell supernatant followed by washing with ice-cold phosphate buffer saline (PBS, Sigma Chemical Company, U.S.A.). Cells were lysed in PBS containing EDTA (10 mM), 1% Triton X-100, phenylmethylsulphonyl fluoride (1 mM), and 0.1% leupeptin. Lysates (40 μg protein per lane) were separated by SDS–PAGE on 7.5% polyacrylamide gel and transferred onto nitrocellulose membrane. Membranes were blocked at 4°C for overnight in NaCl (100 mM), Tris (10 mM), 0.1% (v v−1) Tween-20, pH 7.4 (STT) containing 5% milk. Membranes were subsequently probed with mouse monoclonal anti-iNOS antibody (1 : 2500 dilution in STT, Transduction Lab., U.S.A.) for 2 h at room temperature. Blots were washed with STT (2×15 min) and incubated with horseradish peroxidase-conjugated rabbit anti-mouse IgG (1 : 5000, Amersham) for 2 h at room temperature. Following further washing in STT (3×15 min), immunoreactive bands were visualized using ECL detection system (Amersham, U.K.). Thereafter, the same membrane was stripped and reprobed with mouse anti-α-tubulin antibody.
Inducible NOS protein stability analysis
Macrophage monolayers were stimulated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) for 18 h. Cycloheximide (added at time 0 h) was thereafter applied to the medium to interrupt further protein synthesis. Andrographolide (50 μM) or vehicle control was added immediately following cycloheximide for extra 2, 6 and 12 h incubation respectively. Total protein was prepared at the indicated time points and processed for Western blotting as described above.
[35S]-Methionine incorporation into RAW 264.7 macrophages
2×106 macrophages were cultured in 10 ml DMEM for 24 h. After the cultures reached about 70–80% of confluence, the medium was aspirated and replaced with fresh DMEM without L-methionine. The macrophages were further incubated at 37°C, 5% CO2 for 1 h in order to deplete cellular stores of L-methionine. Thereafter, [35S]-methionine (300 μCi dish−1) was added back to the culture medium. After a 24 h culture period, the medium was removed and the dishes were washed three time with PBS to remove unincorporated radiolabel. The cells were scraped into cold PBS. After centrifugation the cell pellet was lysed in NaCl (150 mM), EDTA (10 mM), 1% Triton X-100, 0.1% leupeptin and phenylmethylsulphonyl fluoride (1 mM). Then, 200 μl of ice-cold trichloric acid (TCA, 30%, v v−1) was added to precipitate protein. The protein was sequentially washed and pelleted three times with the ice-cold TCA (10%, v v−1) solution. The 35S content of the pellet was then determined by liquid scintillation counting (Beckman).
Determination of iNOS activity
The enzyme preparation was obtained from RAW 264.7 cells activated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) in the absence or presence of andrographolide (30 and 50 μM) for 6–24 h, respectively. The cells were collected, washed in DMEM, and disrupted in 50 mM HEPES, pH 7.5, containing MgCl2 (1 mM), dithiothreitol (0.5 mM), phenylmethulsulphonyl fluoride (1 mM) and 2 μM leupeptin. The lysate was centrifuged at 15,000×g for 30 min at 4°C, and the supernatant was collected for assay of NOS enzyme activity. The NOS specific activity was determined by the conversion of [3H]-arginine to [3H]-citrulline as described by Weinberg et al. (1995) and Kobuchi et al. (1997) with minor modifications. Briefly, the reaction mixture (100 μl) contained NADPH (1 mM), CaCl2 (1.25 mM), 1 μg of calmodulin, 5 μM FAD, 5 μM FMN, tetrahydrobiopterin (0.1 mM), dithiothreitol (1 mM), 0.24 μM [3H]-arginine and 60 μl of supernatant in 50 mM HEPES (pH 7.5). After 30 min of incubation at 37°C, the reaction was terminated by addition of 1 ml of cold stop buffer (20 mM HEPES, pH 5.5, containing 10 mM EDTA). The reaction mixture was then applied to a 1 ml column of Dowex-50 W cation exchange resin (Na+ form) pre-equilibrated with stop buffer, which was eluted with 1 ml of cold distilled water. Radiolabelled citrulline was quantified using a liquid scintillation counter. In another set of experiment, iNOS activity was determined by adding various concentrations of andrographolide (10, 30, and 50 μM) or aminoguanidine (0.3 mM) to a cell-free cytosolic preparation activated by LPS/IFN-γ for 24 h.
Andrographolide and other chemicals
Andrographolide was purchased from Aldrich (Milwaukee, WI, U.S.A). Prior to use, the drug was dissolved in dimethylsulphoxide at 100 mM and then serially diluted in PBS to the appropriate concentration. All other chemicals were dissolved in saline or distilled water. LPS (Escherichia coli serotype 0111 : B4), penicillin, streptomycin, sulphanilamide, naphthylenediamine, EDTA, Triton-X 100, phenylmethylsulphonyl fluoride, Tween-20, leupeptin, dithiothreitol, HEPES, NADPH, FAD, and FMN were obtained from Sigma Chemical Company (St. Louis, U.S.A.). IFN-γ was obtained from Genzyme (Cambridge, MA, U.S.A.). DMEM was purchased from GIBCO (Grand Island, NY, U.S.A.). L-[3H]-arginine and L-[35S]-methionine were obtained from Amersham (Buckinghamshire, U.K.).
Statistical evaluation
The results are expressed as mean±s.e.mean, and NO production is indicated as absolute concentrations in μM. Computation of 50% inhibitory concentration (IC50) and the slope of regression line were computer-assisted (PHARM/PCS v.4.2, Tallarida & Murray, 1987). [35S]-Methionine incorporation and [3H]-L-citrulline formation were indicated as c.p.m./2×106 cells and pmol min−1mg−1 protein, respectively. Statistical comparisons were made between groups using a one-way analysis of variance (ANOVA) followed by Scheffé F test post hoc analysis. A P-value less than 0.05 was considered to be statistically significant.
Results
Andrographolide inhibits nitrite production
The efficacy of andrographolide on nitrite production in macrophages is shown in Figure 1. Nitrite production was dependent on the activating state of the cells. Unstimulated macrophages, after 24 h of culture, produced the lowest levels of nitrite (4.7±0.8 μM) (Figure 1A). Andrographolide did not significantly affect basal nitrite production. Even at a concentration of 100 μM, amounts of nitrite in the medium were maintained at a level similar to that of the unstimulated sample (Figure 1A). However, stimulating the cells with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) for 24 h induced a 16 fold increase in nitrite production from the basal level to 74.4±1.2 μM (Figure 1B). In these experiments, andrographolide (1, 10, 30, 50 and 100 μM) evoked a concentration-dependent inhibition of nitrite accumulation to 70.9±0.4, 62.2±1.1, 43.0±0.8, 7.8±0.7 and 4.5±0.6 μM (n=12), respectively (Figure 1B). The IC50 of andrographolide for inhibition of nitrite production was 17.4±1.1 μM. Drug's effects were significantly distinguished from vehicle. Significant inhibition by andrographolide was observed at 10 μM and greater than 80% inhibition was noted at concentrations ⩾50 μM. Based on both trypan blue uptake experiments (data not shown) and the MTT test (cell viability after 50 μM andrographolide treatment>95% when compared with control groups), this level of andrographolide was not toxic to macrophages during a 24 h incubation. This result indicates that the inhibition of nitrite production by andrographolide is not due to cell death. However, 100 μM andrographolide was associated with a small degree of cytotoxicity (cell viability less than 80% as compared with control group), so in subsequent experiments the maximum concentration of andrographolide used was 50 μM.
Figure 1.
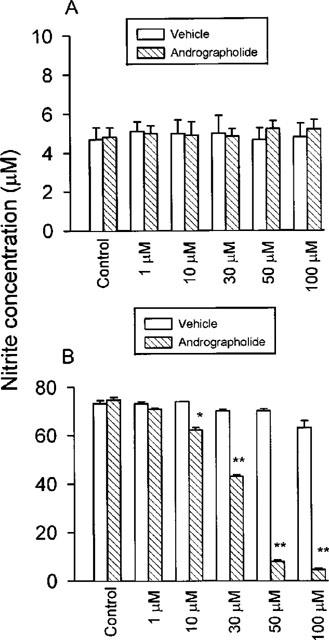
Effects of andrographolide on LPS/IFN-γ-induced nitrite formation by RAW 264.7 macrophages. RAW 264.7 macrophages were cultured with (A) medium alone or (B) with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) at 37°C for 24 h in 96-well plate in the absence and presence of indicated concentrations of andrographolide or vehicle control. Data are expressed as mean±s.e.mean of six individual experiments (triplicate in each experiment) for andrographolide and for vehicle control. Statistically significant inhibition of nitrite formation (*P<0.05, **P<0.01), as compared with the control group.
Effect of andrographolide on iNOS mRNA expression
To determine whether andrographolide inhibits NO production at the level of transcription, we used RT–PCR to examine the expression of the iNOS gene in activated macrophages. The housekeeping gene β-actin was also amplified from each RNA preparation to enable comparisons of the PCR products in different samples. Size of PCR products were estimated by comparison with a size marker and found to correspond to 497 bp for iNOS and 607 bp for β-actin. As shown in Figure 2A, RAW 264.7 macrophages did not express detectable levels of iNOS mRNA after 6 h of incubation with medium alone (indicated as Blank). However, LPS/IFN-γ caused a time-dependent increase in iNOS mRNA expression at 3 and 6 h. Figure 2B shows quantification of iNOS mRNA expression by densitometry, normalized by calculating the ratio of iNOS to β-actin. In the presence of 50 μM andrographolide, iNOS mRNA level showed no significant difference before and after treatment. These experiments were performed in three separate experiments with similar results.
Figure 2.
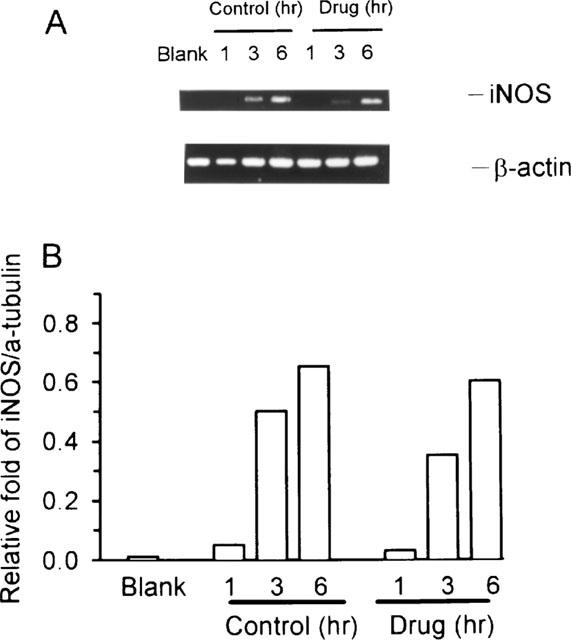
RT–PCR analysis of iNOS mRNA expression. (A) RAW 264.7 macrophages were incubated in the presence LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) with or without andrographolide (50 μM) for 1, 3, and 6 h, respectively. (B) Band intensities were quantified by densitometer. This experiment was repeated three times with similar results.
Andrographolide inhibits the expression of iNOS protein in macrophages stimulated with LPS/IFN-γ
To determine whether the NO inhibitory effect of andrographolide was due to inhibition of iNOS protein expression, Western blot analysis was carried out on whole cell lysates using a monoclonal antibody for mouse macrophage iNOS. The dose responses for inhibition of 130-kDa iNOS protein and nitrite generation by andrographolide are shown in Figure 3. At 10, 30, and 50 μM, andrographolide inhibited the levels of iNOS protein by 0.1±0.1, 35.2±2.7 and 99.3±1.4% (n=5), respectively (Figure 3C), and inhibited NO generation by 12.0±1.7, 84.3±0.9, and 92.1±1.3% (n=5), respectively (Figure 3A). Significant inhibition of iNOS protein by andrographolide was observed at 30 μM, and 50 μM almost completely abolished iNOS expression. Cell viability, however, seems to be intact in the presence of 50 μM andrographolide because α-tubulin protein is minimally affected (Figure 3B, lower traces). Figure 3C shows quantification of iNOS protein expression by densitometry.
Figure 3.
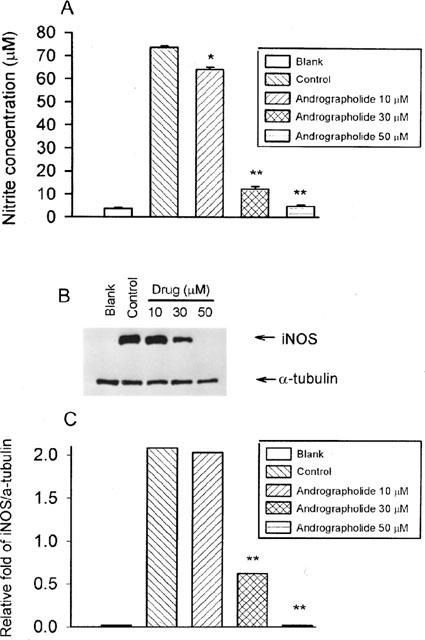
Inhibition of iNOS protein expression in activated macrophages by andrographolide. RAW 264.7 cells were treated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) in the co-existed of various concentrations (10, 30, and 50 μM) of andrographolide for 24 h. At the end of the incubation time, the culture medium was collected for nitrite assay (A) and extraction of total protein for iNOS protein and α-tubulin analysis (B). Band intensities were quantified by densitometer and indicated in (C). This experiment was repeated three times with similar results. Statistically significant inhibition of nitrite formation (*P<0.05, **P<0.01), as compared with the control group.
We also determined the time course of changes in synthesized iNOS protein expression and released nitrite at 6, 12, and 18 h. Upon stimulation with LPS/IFN-γ, both nitrite (Figure 4A) and iNOS protein (Figure 4B) were induced in increasing amounts from 6 to 18 h. Andrographolide (50 μM), in conjunction with the stimuli, blocked this induction. The normal expression of α-tubulin suggested that inhibition by andrographolide was not due to nonspecific or cytotoxic effects. Figure 4C shows quantification of iNOS protein expression by densitometry, normalized by calculating the ratio of iNOS to α-tubulin.
Figure 4.
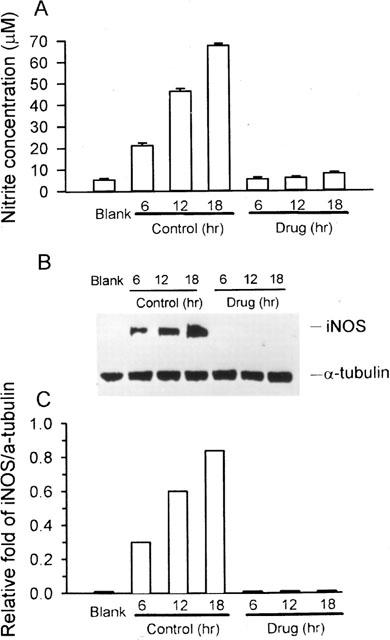
Western blot analysis of time-course protein expression of iNOS. RAW 264.7 macrophages were incubated in the presence of LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) with or without 50 μM andrographolide for 6, 12, and 18 h, respectively. At the end of the incubation time, the culture medium was collected for nitrite assay (A) and extraction of total protein for iNOS protein and α-tubulin analysis (B). (C) Band intensities were quantified by densitometer. This experiment was repeated four times with similar results.
Andrographolide treatment reduces iNOS protein stability
To examine whether andrographolide suppresses iNOS protein expression by reducing its stability, experiments were performed using the protein synthesis inhibitor cycloheximide (added at 18 h after LPS/IFN-γ, and indicated as time 0). In the continuous presence of cycloheximide, iNOS protein expression is stable and peaks within 6 h (total incubation interval for 24 h after LPS/IFN-γ), and decreases progressively after a 12-h (a total 30-h period after LPS/IFN-γ) exposure to medium alone (control). In the presence of 50 μM andrographolide (lanes 6, 7, 8), iNOS protein expression decreased faster than in the control group (lanes 3, 4, 5) (Figure 5A). The slope of the regression line for control and drug groups were −1.29 and −5.88, respectively (P<0.01). Figure 5B shows quantification of iNOS protein expression by densitometery, normalized by calculating the ratio of iNOS over α-tubulin.
Figure 5.
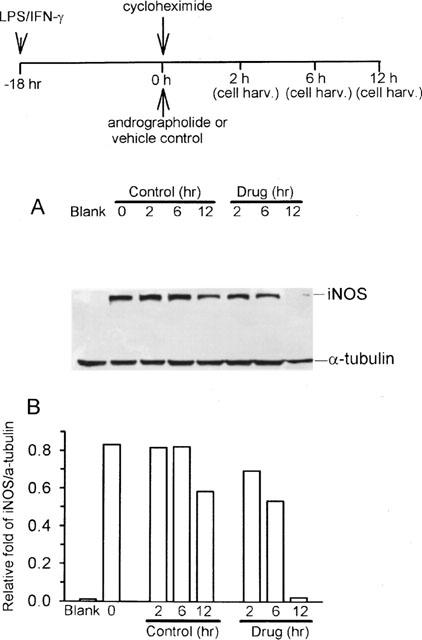
Andrographolide treatment reduces iNOS protein stability. After macrophages were stimulated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) for 18 h, cycloheximide (added at time 0 h) was added to the medium to interrupt further protein synthesis. Andrographolide (50 μM) or vehicle control was added closed behind cycloheximide for 2, 6, and 12 h incubation respectively, and iNOS protein stability was analysed in the continuous presence of cycloheximide (A). Protein was prepared for Western blot with anti-iNOS antibody. As a control, the blot was reacted simultaneously with an antibody for α-tubulin as an internal control. (B) Band intensities were quantified by densitometer and indicated as relative fold of iNOS/α-tubulin. This experiment was repeated four times with similar results.
Effect of andrographolide on [35S]-methionine incorporation into macrophages
Incubation of RAW 264.7 cells for 24 h in the presence of 10–50 μM andrographolide did not affect cell viability significantly based on trypan blue dye exclusion experiment and MTT assay (data not shown). These concentrations of andrographolide, however, significantly inhibited [35S]-methionine incorporation to a percentage of 65.1±5.8, 23.3±4.2 and 12.5±5.0 (n=6) in quiescent cells, respectively (Figure 6). Total de novo protein synthesis suppressed by 50 μM andrographolide was comparable to 3 μM cycloheximide.
Figure 6.
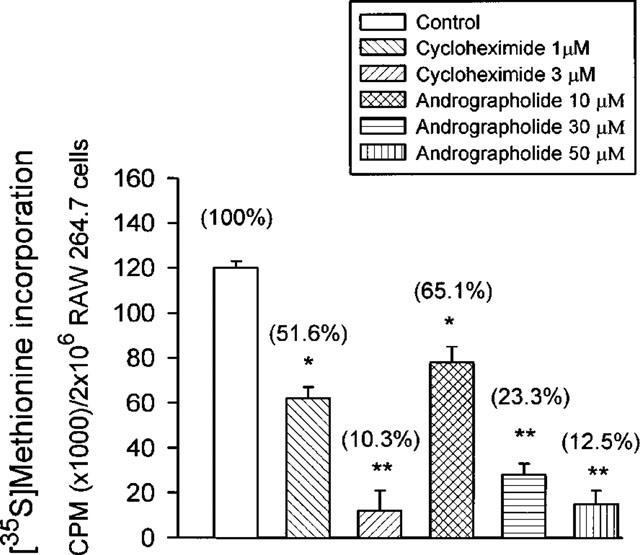
Effect of andrographolide on [35S]-methionine incorporation into RAW 264.7 macrophages. Cells were cultured at 37°C for 24 h then were aspirated and replaced with fresh DMEM without L-methionine. The macrophages were further incubated at 37°C, 5% CO2 for 1 h in order to deplete cellular stores of L-methionine. Thereafter, [35S]-methionine was included in all samples. [35S]-methionine incorporation was measured as described in Methods. Data are expressed as mean±s.e.mean of five individual experiments for control, cycloheximide (1 and 3 μM) and for andrographolide (10, 30, and 50 μM). Statistically significant inhibition of [35S]-methionine incorporation (*P<0.05, **P<0.01), as compared with the control groups.
Effect of andrographolide on iNOS activity
Inducible NOS activity in soluble extracts of activated macrophages in the absence of andrographolide was increased in a time-dependent manner within 6–24 h (Figure 7A, control). Andrographolide was added to the medium together with the LPS/IFN-γ for 6, 12, 18, and 24 h, respectively. In the presence of andrographolide (30 and 50 μM), the specific activity of iNOS was significantly inhibited in a dose-dependent manner (Figure 7A). At 30 and 50 μM, andrographolide inhibited induction of iNOS activity by 52.3±5.4 and 82.7±6.5% (n=7) at 24 h, respectively.
Figure 7.
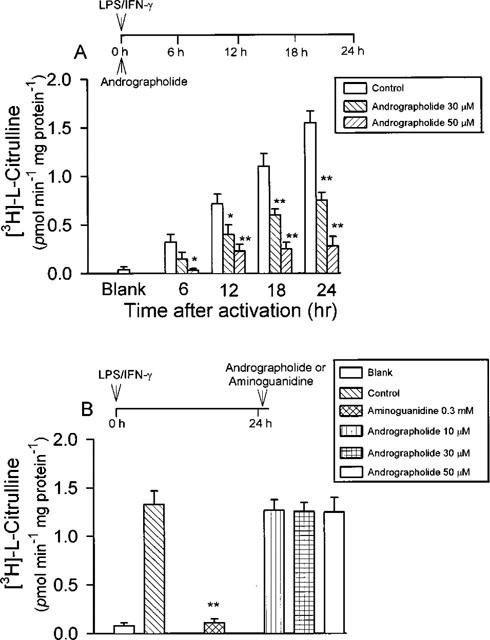
Effect of andrographolide on iNOS activity. (A) The enzyme preparation was obtained from RAW 264.7 cells activated with LPS (1 μg ml−1) plus IFN-γ (50 U ml−1) in the absence or simultaneous presence of andrographolide (30 and 50 μM) for 6–24 h, respectively. (B) iNOS activity was determined by adding various concentrations of andrographolide (10, 30, and 50 μM) or aminoguanidine (0.3 mM) back to a cell-free cytosolic enzyme fraction well-activated by LPS/IFN-γ for 24 h. Data are expressed as mean±s.e.mean of five individual experiments for andrographolide and for aminoguanidine. Statistically significant inhibition of [3H]-arginine conversion (*P<0.05, **P<0.01), as compared with the control groups.
The followed experiment was to examine whether andrographolide could directly affect the enzyme activity of iNOS. Cells stimulated with LPS/IFN-γ for 24 h were lysed for enzyme extraction. Then, iNOS activity was determined by adding andrographolide (10, 30, and 50 μM) or aminoguanidine (0.3 mM) to the cell-free cytosolic reparation. Under these conditions citrulline formation was greatly suppressed by aminoguanidine (from 1.27±0.12 to 0.18±0.06 pmol min−1mg−1 protein, n=5, P<0.01). In contrast, iNOS activities were not significantly affected by andrographolide treatment.
Discussion
NO is a short-lived bioactive molecule that participates in the physiology and pathophysiology of many system (Moncada et al., 1991). In recent years it has become evidence that high output production of nitric oxide by iNOS is responsible for the development of a variety of diverse pathological events such as atherosclerosis, circulatory shock, diabetes, chronic inflammation and cancer, although the detailed molecular mechanisms involved are not clear. Recent research has focused on the development of selective inhibitors of iNOS catalytic activity. The goal is to inhibit excessive formation of NO without interfering with the production of small quantities of NO generated by the endothelial and neuronal NOS isoforms (Moncada & Higgs, 1995; Pfeilschifter et al., 1996). Understanding the mechanisms involved in the induction and modulation of iNOS gene expression is important for the development of pharmacological strategies aimed to selectively prevent excessive NO formation.
Extracts of Andrographis paniculata have been utilized in traditional Chinese medicine for the treatment of bacterial infection and inflammatory diseases for many years. However, how this herb carries out these functions is not clear. In a previous study we reported that andrographolide, the major diterpenoid isolated from Andrographis paniculata, can suppress LPS-induced NO formation in vitro and ameliorate the haemodynamic parameters in endotoxic shock in vivo (Chiou et al., 1998). This might account for the therapeutic effectiveness of Andrographis paniculata against a variety of related diseases.
In the present study we report that andrographolide acts at a post-transcriptional level to inhibit the formation of NO. The suppression of iNOS expression is probably as important to its regulation as its induction. The complex biochemistry of NO production affords many potential sites for regulatory action. NO production by macrophages is regulated by various factors (LPS, IFN-γ, transforming growth factor-β), and the induction of iNOS requires gene transcription and new protein synthesis, since actinomycin-D and cycloheximide inhibited LPS- or IFN-γ-induced NO generation (Hughes et al., 1990; Welsh et al., 1991; Corbett et al., 1992; Xie et al., 1994). Thus, NO production by iNOS may be regulated at many sites, including transcription, post-transcription, translation, and post-translational modification (Nathan & Xie, 1994). The present data obtained from RT–PCR demonstrated that interference with the expression of iNOS mRNA is not the factor contributing to the inhibitory effect of andrographolide on NO production in macrophages and this was consistent with our previous work (Chiou et al., 1998). In the previous study, we reported that the kinetic inhibition of nitrite synthesis by andrographolide was obviously different from actinomycin D, an agent which disrupts gene transcription. Additionally, it is worth noting that the inhibitory effect of andrographolide was long lasting for 18 h after LPS stimulation. These data suggest that andrographolide acts more like a protein synthesis inhibitor. In this study we confirmed that andrographolide reduces NO production at the level of iNOS protein expression without affecting iNOS mRNA level.
Protein expression can be regulated both by up-stream protein synthetic process and by down-stream protein degradation. We therefore studied the effects of andrographolide on protein biosynthesis by determining [35S]-methionine incorporation. Results indicated that andrographolide is able to inhibit iNOS expression by impairing protein de novo synthesis. The first report suggesting that post-transcriptional mechanisms are involved in the regulation of iNOS expression were provided by Vodovotz et al. (1993). These authors demonstrated that transforming growth factor β1 inhibits IFN-γ-induced iNOS expression in mouse peritoneal macrophages at multiple levels, including translation of the iNOS mRNA and stability of the iNOS protein. Also, aspirin and dexamethasone have been reported to exert their anti-inflammatory effects by inhibiting total protein synthesis and/or by accelerating protein degradation (Kwon et al., 1997; Walker et al., 1996). Interestingly, andrographolide seems to activate the same post-transcriptional mechanisms. We showed that andrographolide reduces the de novo protein biosynthetic process and, in addition, increases the degradation of iNOS protein even after iNOS has already been expressed. If, indeed, our observations are confirmed by independent investigators, they will suggest that dissociation between iNOS mRNA content and NO production after treatment with different drugs is not an uncommon event (Cetkovic-Cvrlje et al., 1993). However, whether andrographolide impairs the specific translational machinery and which degrading enzyme is involved in accelerating iNOS protein degradation need further investigation.
Finally, andrographolide when added together with LPS/IFN-γ inhibits iNOS enzyme activity in intact cells but is without effect in the cell-free enzyme fraction, as measured by the conversion of 3H-labeled L-arginine to citrulline. The reduction of iNOS activity is caused by decreased iNOS protein level. These results indicate that andrographolide can not regulate the iNOS enzyme activity directly.
Taken together, the present data shows that andrographolide significantly inhibits LPS/IFN-γ-induced NO production. The primary mechanism responsible for this effect is inhibition of iNOS protein expression that is probably mediated at a post-transcriptional level by andrographolide since iNOS mRNA expression was not significantly altered. More importantly, andrographolide reduces the amount of iNOS protein in RAW 264.7 cells through potentially synergistic action by two additional mechanisms: reduction of de novo protein synthesis and increased degradation of the iNOS protein. This may have important implications for the clinical treatment of diseases associated with overproduction of NO and strongly for the effectiveness of andrographolide in the anti-inflammatory therapy.
Acknowledgments
The authors thank Dr Huang RL for technical assistance of [35S]-methionine incorporation study. This investigation was supported in part by grants NSC88-2314-B-077-011 to Prof Chen and NSC88-2314-B-077-002 to Dr Chiou from the National Science Council and funds from the National Research Institute of Chinese Medicine, Taipei, Taiwan, the Republic of China.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- FCS
foetal calf serum
- IFN-γ
interferon-γ
- LPS
lipopolysaccharide
- MTT
3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NO
nitric oxide
- iNOS
inducible NO synthase
- STT
sodium chloride/Tris/Tween-20
- TCA
trichloric acid
References
- CETKOVIC-CVRLJE M., SANDLER S., EIZIRIK D. Nicotinamide and dexamethasone inhibit interleukin-1-induced nitric oxide production by RINm5F cells without decreasing messenger ribonucleic acid expression for nitric oxide synthase. Endocrinology. 1993;133:1739–1743. doi: 10.1210/endo.133.4.7691579. [DOI] [PubMed] [Google Scholar]
- CHANG H.M., BUT P.P.H. Pharmacology and Applications of Chinese Materia Medica. Vol. 1. World Scientific: Singapore; 1987. pp. 918–928. [Google Scholar]
- CHIOU W.F., LIN J.J., CHEN C.F. Andrographolide suppresses the expression of inducible nitric oxide synthase in macrophage and restores the vasoconstriction in rat aorta treated with lipopolysaccharide. Br. J. Pharmacol. 1998;125:327–334. doi: 10.1038/sj.bjp.0702073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIOU W.F., SUNG Y.J., LIAO J.F., SHUM A.Y.C., CHEN C.F. Inhibitory effect of dehydroevodiamine and evodiamine on nitric oxide production in cultured murine macrophages. J. Natl. Proc. 1997;60:707–711. doi: 10.1021/np960495z. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- CHOUDHURY B.R., PODDAR M.K. Andrographolide and kalmegh (Andrographis paniculata) extract: in vivo and in vitro effect on hepatic lipid peroxidation. Methods Findings Exp. Clin. Pharmacol. 1984;6:481–485. [PubMed] [Google Scholar]
- CONNOR J.R., MANNING P.T., SETTLE S.L., MOORE W.M., JEROMA G.M., WEBBER R.K., TJOENG F.S., CURRIE M.G. Suppression of adjuvant-induced arthritis by selective inhibition of inducible nitric oxide synthase. Eur. J. Pharmacol. 1995;273:15–24. doi: 10.1016/0014-2999(94)00672-t. [DOI] [PubMed] [Google Scholar]
- CORBETT J.A., WANG J.L., SWEETLAND M.A., LANCASTER J.R., MCDANIEL M.L. Interleukin 1β induces the formation of nitric oxide by β-cells purified from roden islets of Langerhans. Evidence for the β-cell as source and site of action of nitric oxide. J. Clin. Invest. 1992;90:2384–2391. doi: 10.1172/JCI116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON V., DAWSON T., BARTLEY D., UHL G., SNYDER S.H. Mechanisms of nitric oxide mediated neurotoxicity in primary brain cultures. J. Neurosci. 1993;13:2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., PANDALAI K.M. Investigations on plant antibiotics, Part IV. Further search for antibiotics substances in Indian medicinal plants. Indian J. Med. Res. 1949;37:169–181. [Google Scholar]
- HUGHES J.H., COLCA J.R., EASOM R.A., TURK J., MCDANIEL M.L. Interleukin 1 inhibits insulin secretion from isolated rat pancreatic islets by a process that requires gene transcription and mRNA translation. J. Clin. Invest. 1990;86:856–863. doi: 10.1172/JCI114785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISCHIROPOULOS H., ZHU L., BECKMAN J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Arch. Biochem. Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- KILBOURN R.G., GRIFFITH O.W. Overproduction of nitric oxide in cytokine-mediated and septic shock. J. Natl. Cancer Institute. 1992;84:827–831. doi: 10.1093/jnci/84.11.827. [DOI] [PubMed] [Google Scholar]
- KOBUCHI H., DROY-LEFAIX M.T., CHRISTEN Y., PACKER L. Ginkgo biloba extract (EGb 761): Inhibitory effect on nitric oxide production in the macrophage cell line RAW 264.7. Biochem. Pharmacol. 1997;53:897–903. doi: 10.1016/s0006-2952(96)00873-8. [DOI] [PubMed] [Google Scholar]
- KWON G., HILL J.R., CORBETT J.A., MCDANIEL M.L. Effects of Aspirin on nitric oxide formation and de novo protein synthesis by RINm5F cells and rat islets. Mol. Pharmacol. 1997;52:398–405. doi: 10.1124/mol.52.3.398. [DOI] [PubMed] [Google Scholar]
- LU K.J., ZHANG S.L., WANG Z.S. Analysis of andrographolie compounds. I. Ion pair high performance liquid chromatographic analysis of andrographolide derivatives. Acta Pharmaceuticaa Sinica. 1981;16:182–189. [PubMed] [Google Scholar]
- MATHEIS G., SHEMAN M.P., BUCKBERG G.D., HAYBRON D.M., YOUNG H.H, IGNARRO L.J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am. J. Physiol. 1992;262:H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- MONCADA S., HIGGS E.A. Molecular mechanisms and therapeutic strategies related to nitric oxide. FASEB J. 1995;9:1319–1330. [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–141. [PubMed] [Google Scholar]
- MOSMANN T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- NATHAN C., XIE Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- PFEILSCHIFTER J., EBERHARDT W., HUMMEL R., KUNZ D., MUHL H., NITSCH D., PLUSS C., EALKER G. Therapeutic strategies for the inhibition of inducible nitric oxide synthase–potential for a novel class of anti-inflammatory agents. Cell. Biol. Int. 1996;20:51–58. doi: 10.1006/cbir.1996.0008. [DOI] [PubMed] [Google Scholar]
- PURI A., SAXENA R., SAXENA R.P., SAXENA K.C., SRIVASTAVA V., TANDON J.S. Immunostimulant agents from Andrographis paniculata. J. Natl. Prod. 1993;56:995–999. doi: 10.1021/np50097a002. [DOI] [PubMed] [Google Scholar]
- REES D.D., CELLEK S., PALMER R.M., MONCADA S. Dexamethasone prevents the induction by endotoxin of a nitric oxide synthase and the associated effects on vascular tone: An insight into endotoxin shock. Biochem. Biophys. Res. Commun. 1990;173:541–547. doi: 10.1016/s0006-291x(05)80068-3. [DOI] [PubMed] [Google Scholar]
- STEFANOVIC-RACIC S., STADLER J., EVANS C.H. Nitric oxide and arthritis. Arthritis Rheum. 1993;36:1036–1044. doi: 10.1002/art.1780360803. [DOI] [PubMed] [Google Scholar]
- STICHTENOTH D.O., FRÖLICH J.C. Nitric oxide and inflammatory joint diseases. Br. J. Rheum. 1998;37:246–257. doi: 10.1093/rheumatology/37.3.246. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B.Pharmacological Calculation System (PHARM/PCS v.4.2) Manual of pharmacologic calculations with computer programs 1987New York: Springer-Verlag; 2nd edn [Google Scholar]
- THIEMERMANN C., VANE J.R. Inhibition of nitric oxide synthesis reduces the hypotension induced by bacterial lipopolysaccharide in the rat in vivo. Eur. J. Pharmacol. 1990;182:591–595. doi: 10.1016/0014-2999(90)90062-b. [DOI] [PubMed] [Google Scholar]
- VODOVOTZ Y., BOGDAN C., PAIK J., XIE Q.W., NATHAN C. Mechanisms of supression of macrophages nitric oxide release by transforming growth factor β. J. Exp. Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER G., PFEILSCHIFTER J., KUNZ D. Mechanisms of suppression of inducible nitric oxide synthase (iNOS) expression in interferon (IFN)-γ-stimulated RAW 264.7 cells by dexamethasone. J. Biol. Chem. 1996;271:16679–16687. doi: 10.1074/jbc.272.26.16679. [DOI] [PubMed] [Google Scholar]
- WEINBERG J.B., MISUKONIS M.A., SHAMI P.J., MASON S.N., SAULS D.L., DITTMAN W.A., WOOD E.R., SMITH G.K., MCDONALD B., BACHUS K.E., HANEY A.F., GRANGER D.L. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood. 1995;86:1184–1195. [PubMed] [Google Scholar]
- WELSH N., EIZIRIK D.L., BENDTZEN K., SANDLER S. Interleukin-1 beta-induced nitric oxide production in isolated rat pancreatic islets requires gene transcription and may lead to inhibition of the Krebs cycle enzyme aconitase. Endocrinology. 1991;129:3167–3173. doi: 10.1210/endo-129-6-3167. [DOI] [PubMed] [Google Scholar]
- WU C.C., THIEMERMANN C. Biological control and inhibition of induction of nitric oxide synthase. Method Enzymol. 1996;268:408–420. doi: 10.1016/s0076-6879(96)68043-4. [DOI] [PubMed] [Google Scholar]
- XIE Q.W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- YAO X.J., WAINBERG M.A., PARNIAK M.A. Mechanism of inhibition of HIV-1 infection in vitro by purified extract of Prunella vulgaris. Virology. 1992;187:56–62. doi: 10.1016/0042-6822(92)90294-y. [DOI] [PubMed] [Google Scholar]


