Abstract
This study investigated the cannabinoid receptor, known to inhibit neuronally-evoked contractions of the mouse isolated urinary bladder, in bladder sections isolated from mouse, rat, dog, pig non-human primate or human.
The CB1-like pharmacology of the cannabinoid receptor in mouse isolated bladder observed previously was confirmed in this study by the rank order of agonist potencies: CP 55940⩾WIN 55212-2>HU 210>JWH 015>anandamide, the high affinity of the CB1 selective antagonist, SR 141716A (apparent pKB 8.7), and the low affinity of the CB2 antagonist, SR 144528 (apparent pKB<6.5). In these studies, SR 141716A (10–100 nM) significantly potentiated electrically-evoked contractions in this tissue by an undetermined mechanism.
A similar rank order of agonist potencies was determined in rat isolated bladder sections (CP 55, 940⩾WIN 55212-2>JWH 015). In this tissue, the maximal inhibitory effect of all agonists was lower than in the mouse bladder. Indeed, the effects of both HU 210 and anandamide were too modest to quantify potency accurately.
In the rat isolated bladder, SR 141716A (30 nM) or SR 144528 (100 nM), reversed the inhibitory effect of WIN 55212-2 (apparent pKB=8.4 and 8.0, respectively) or JWH 015 (apparent pKB=8.2 and 7.4, respectively). These findings may demonstrate pharmacological differences between the rat and mouse orthologues of the CB1 receptor. Alternatively, they may be attributed to a mixed population of CB1 and CB2 receptors that jointly influence neurogenic contraction of the rat bladder, but cannot be differentiated without more selective ligands.
WIN 55212-2 had no effect on electrically-evoked contractions of bladder sections isolated from dog, pig, cynomolgus monkey and human. These findings suggest that the effect of cannabinoid agonists to inhibit neurogenic contraction of the mouse and rat bladder is not conserved across all mammalian species.
Keywords: Cannabinoid receptor, CB1, CB2, neurogenic contraction, urinary bladder, receptor orthologue, WIN 55212-2
Introduction
Cannabinoid receptor stimulation inhibits electrically-evoked contractions in certain isolated mammalian tissues (vas deferens, myenteric plexus, small intestine, urinary bladder, Pacheco et al., 1991; Pertwee et al., 1992; Pertwee & Fernando, 1996). In mouse isolated bladder, several cannabinoid receptor agonists, including WIN 55212-2, Δ9-tetrahydrocannabinol and anandamide, inhibit electrically-evoked bladder contractions in a concentration-dependent fashion. Furthermore, this inhibitory effect appears not to be post-junctionally-mediated since contractile responses to muscarinic or purinergic receptor agonists, acetylcholine or β,γ-methylene ATP respectively, are unaffected by pre-treatment with Δ9-tetrahydrocannabinol (Pertwee et al., 1996).
Sparse evidence for cannabinoid-mediated effects in urinary bladder has been reported to date. Indeed, autoradiographic studies fail to identify specific binding of the radiolabelled cannabinoid agonist, [3H]-CP-55940 (Lynn & Herkenham, 1994), in rat bladder. However, this observation may reflect the low sensitivity of the autoradiographic assay, since no specific binding of [3H]-CP-55940 was observed in either the vas deferens or the small intestine; tissues in which cannabinoid receptor-mediated effects were demonstrated previously (see above).
Some evidence exists to suggest a role for cannabinoid receptor agonists in the control of urinary bladder function in humans. In a self-dosing regimen, nabilone, a non-selective cannabinoid agonist, has been found to alleviate the nocturia associated with multiple sclerosis (Martyn et al., 1995). However, the cannabinoid receptor subtype(s), or their anatomical distribution, through which the nabilone effect is mediated remain unknown. Indeed, such relief from nocturia might be attributed to the effects of nabilone at any point in the peripheral (afferent or efferent) nervous system, and/or in the micturition centres of the central nervous system.
This study attempted to investigate whether there are cannabinoid receptors in the efferent peripheral nervous system that control urinary bladder tone. In order to establish whether any such peripheral localization is conserved across mammalian species, this study also investigated the effect of cannabinoid receptor activation on neurogenically-mediated contractions of bladder sections isolated from mouse, rat, pig, dog, non-human primate and human.
Firstly, the effect of cannabinoid receptor ligands on electrically-evoked contractions was examined both to establish and confirm a role of cannabinoid receptors in rat and mouse isolated bladders. The effects on mouse or rat urinary bladders were characterized using the cannabinoid ligands previously used to characterize the CB1 receptor in the mouse isolated bladder (Pertwee et al., 1996). In addition, the affinities at these receptors of the more selective CB2 ligands, SR 144528 (Rinaldi-Carmona et al., 1998), AM 630 (Ross et al., 1999) and JWH 015 (Griffin et al., 1997), which hitherto have not been reported for mouse or rat cannabinoid receptors, were determined in these assays.
The second aim of this study was to determine the effects of the non-selective cannabinoid receptor agonist, WIN 55212-2, on isolated bladder sections from mouse, rat, dog, pig, cynomolgus monkey and human were investigated. Since excitatory neurotransmission in mammalian urinary bladders is predominantly mediated by acetylcholine and ATP (see Anderson, 1993 for review; Burnstock et al., 1972), we examined the response to WIN 55212-2 on its own, or under conditions of muscarinic or purinergic receptor blockade using atropine or α,β-methylene ATP, respectively.
Methods
Urinary bladders from adult male CD-1 mice (Charles River, CA, U.S.A.), adult male Sprague Dawley rats (Charles River, CA, U.S.A.), adult Yucatan micro pigs (STS Farms, CA, U.S.A.), adult beagles (Marshal Farms, CA, U.S.A.), and adult cynomolgus monkeys (Charles River, TX, U.S.A.) were rapidly removed following euthanasia, and immersed in Krebs solution (of the following composition (mM): NaCl, 118.41; NaHCO3, 25.0; CaCl2, 2.5; KCl 4.75; KH2PO4, 1.19; MgSO4, 1.19; glucose, 11.10) for immediate use or overnight storage at 4°C. Sections of human bladder were obtained following surgical resection from seven patients with differing pathologies (four adult male or female patients who underwent cystectomy or prostatectomy, a 77-year-old male with transitional cell carcinoma, a 4-year-old female, and post-mortem tissue from a 50-year-old female).
Tissue sections (2×4 mm2 for rat, pig, dog, monkey and human; upper and lower halves of mouse bladder) were mounted using stainless steel hooks between two parallel plate electrodes in thermostatically-controlled organ baths containing 20 ml Krebs buffer, gassed continuously with 95% O2, 5% CO2 mixture at 37°C. The mucosal layer of the pig bladder was removed in order to reduce spontaneous contractions. Tissues were subjected to tensile forces by vertical displacement of the supporting hooks until they attained a resting tension of 0.5 g for mouse bladders, 0.75–1 g for rat bladders, and 3–4 g for dog, pig, monkey and human tissues. Changes in isometric force were measured from Grass FT03c (MA, U.S.A.) transducers and recorded using MacLab data acquisition software (version 3.5, AD Instruments, MA, U.S.A.). Stimuli were generated using a Grass S88 (MA, U.S.A.) stimulator, and divided across eight organ baths using a MedLab StimuSplitter (CO, U.S.A.). Stimulus timing was controlled using a GraLab 451 Intervalometer (OH, U.S.A.). For all electrically-evoked contractions, the size of contraction was determined using MacLab software as the difference between maximum and minimum tensile forces (g) across the tissue over a given stimulation interval.
In order to remove lipophilic drugs from apparatus, organ baths, electrodes and hooks were washed in ethanol at the end of every experiment. In addition, organ baths were coated with Sigmacote (Sigma, MO, U.S.A.) and allowed to dry before commencement of every experiment.
Concentration-response data: effect on electrically-evoked contraction
The pharmacology of the inhibitory effect of cannabinoid receptor agonists on electrically-evoked contractions was investigated in mouse and rat isolated bladder sections. For these studies, concentration-response curves were generated using ascending semi-logarithmic increments in agonist concentration, each administered 30 min prior to a 2 min period of electrical stimulation (0.5 ms pulse width, 0.5 s train duration, 0.1 trains s−1, with pulse frequency of 12 and 8 Hz for mouse and rat, respectively, voltage as for frequency-response curves, below). After the stimulation period, tissues were washed immediately with two exchanges of the surrounding Krebs medium, and agonist was re-administered at the appropriate concentration.
Agonist-mediated responses were expressed as the per cent inhibition of electrically-evoked contraction prior to agonist administration. Agonist potency (EC50) and relative intrinsic activities (α) were determined by fitting individual concentration-response curve data to the Hill equation (1):
 |
where [A] and nH are the agonist concentration and slope parameter, respectively. The equation was adapted for non-linear regression to estimate −log EC50 (i.e. pEC50). For each agonist, responses are summarised as the mean and standard errors of pEC50 and α values determined in bladder tissue from 4–6 animals.
For antagonist affinity estimation, tissues were incubated, without electrical stimulation, with antagonists or equivalent solvent vehicle for 1 h prior to the commencement of agonist concentration-response curves. Agonist-mediated effects were expressed relative to the electrically-evoked contractile responses elicited at the end of the antagonist incubation, prior to agonist administration. If antagonists produced parallel, surmountable displacement of agonist concentration-response curves, their affinities were determined by Schild analysis (Arunlakshana & Schild, 1959) using a range of competing antagonist concentrations, and simultaneously fitting agonist pEC50 values estimated for control and antagonist-treated tissues to equation (2) (Trist & Leff, 1985).
where  are the mid-points of the agonist concentration-response curves generated in the presence and absence of a competing concentration of antagonist, respectively and KB and n are the antagonist affinity and Schild slope, respectively. Schild slope, was tested for deviation from unity by a t-test comparison of observed log (r−1) vs estimated log (r−1) (using SAS software, SAS Institute Inc., Cary, NC, U.S.A., release 6.12 for Windows). If the Schild slope was not significantly different from unity, it was constrained to 1 for determination of antagonist KB.
are the mid-points of the agonist concentration-response curves generated in the presence and absence of a competing concentration of antagonist, respectively and KB and n are the antagonist affinity and Schild slope, respectively. Schild slope, was tested for deviation from unity by a t-test comparison of observed log (r−1) vs estimated log (r−1) (using SAS software, SAS Institute Inc., Cary, NC, U.S.A., release 6.12 for Windows). If the Schild slope was not significantly different from unity, it was constrained to 1 for determination of antagonist KB.
Alternatively, an apparent pKB was calculated using the Schild equation (3) following incubation with a single concentration of antagonist, [B]:
where r is the ratio of EC50s for agonists in the absence and presence of antagonist. This calculation was only attempted when the antagonist caused significant (determined by ANOVA, P<0.05) rightward-displacement of the agonist E/[A] curve, relative to control. The Schild slope parameter, n, is constrained to unity, as the assumption is made that antagonist interacts competitively with receptor.
All non-linear regression was performed in SAS software (SAS Institute Inc., Cary, NC, U.S.A., release 6.12 for Windows). In addition in antagonist studies, pEC50, α, slope parameters derived from logistic curve fitting to agonist concentration-effect data in the absence or presence of antagonist were routinely subjected to ANOVA analysis to determine statistically significant difference between control and antagonist-treated tissues.
Concentration-response curves: effect of drugs on direct smooth muscle contraction
The effects of pre-incubating either WIN 55212-2 (3 μM) or SR 141716A (30 nM) on concentration-effect data to carbachol or α,β-methylene ATP were investigated in order to determine whether the effects of these drugs can be attributed to interactions with post-junctional receptors in the bladder. In these studies, a paired curve design was employed. Cumulative concentration-effect curves to carbachol or single-exposure concentration-effect curves to α,β-methylene ATP were constructed. When these drugs had been removed by exchanging the surrounding Krebs solution, WIN 55212-2, or SR 141716A or equivalent solvent vehicle was administered to the surrounding Krebs media and incubated for 1 h prior to the construction of a second concentration-effect curve to the agonist. Contractile responses to carbachol or α,β-methylene ATP were scaled to the within-tissue response to KCl (80 mM). Concentration-effect data were fitted to the logistic equation (1) above, and an analysis of variance was performed to determine whether treatment with WIN 55212-2 or SR 141617A gave rise to differences in intrinsic activity (α), potency (EC50) or slope parameter (n) between first and second curves.
Frequency response-curves
For the construction of frequency-response curves, a train of electrical pulses was applied for 0.5 s once per minute, with pulse frequency increasing in 2 fold increments (0.5 ms pulse width, 1–128 Hz). For each species the minimum voltage to give reliable contractile responses at 4 Hz was chosen (8, 7, 4, 8, 12 and 10 V for mouse, rat, dog, pig, monkey and human bladders respectively). Before commencement of electrical stimulation, the contractile response to 0.3 mM carbachol was determined in all tissues. All electrically-evoked responses were scaled to this carbachol response. Electrically-induced contractile responses that were sensitive to 0.3 μM tetrodotoxin were considered to be neurogenically mediated.
In tissues where multiple frequency-response curves could be constructed reproducibly within one tissue (mouse, primate) paired Student t-tests (using SAS software, SAS Institute Inc., Cary, NC, U.S.A., release 6.12 for Windows) were used to compare within-tissue control and drug-treated contractile responses at every frequency from 4–6 different animals. When multiple curves could not be generated reproducibly (rat, dog, human), an unpaired Student's t-test was performed (using SAS software, SAS Institute Inc., Cary, NC, U.S.A., release 6.12 for Windows) to compare contractile responses in control and treated tissues at every stimulation frequency. Under either experimental protocol, tissues were incubated for 1 h with drug or corresponding vehicle prior to construction of frequency-response curves.
Finally, in order to determine whether cannabinoid receptor(s) selectively interfere with the muscarinic or purinergic components of neuronal transmission, the effects of WIN 55212-2 were investigated in mouse and rat isolated bladder under conditions of muscarinic and/or purinergic receptor blockade. In these studies, the non-selective muscarinic receptor antagonist, atropine (0.3 μM), and/or α,β-methylene ATP (3 μM) which causes rapid densensitization of P2X purinoceptors were used to inhibit the respective receptor population(s).
Materials
The following drugs were purchased: WIN 55212-2 mesylate ((R)(+)-[2,3-dihydro -5 -methyl -3 -[(morpholinyl)methyl]pyrolo[1,2,3-de] -1,4 - benzoxazin - yl] - (1 - naphthalenyl)methanone mesylate), anandamide from Research Biochemicals Inc. (MA, U.S.A.); tetrodotoxin, atropine, α,β-methylene ATP, carbachol from Sigma Chemicals (MO, U.S.A.); HU 210 ((6aR)-trans-3-(1,1-dimethylheptyl) - 6a,7,10,10a - tetrahydro-1-hydroxy-6,6-dimethyl-6H-dibenzo[b,d]pyran-9-methanol) and SR 141716A (N-(piperidin-lyl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) from Tocris Cookson Inc. (U.K.). SR 144528 (N-[(1S)-endo-1,3,3-trimethyl bicyclo [2,2,1] heptan-2-yl]-5-(4-chloro-3-methylphenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide) was a generous gift from Dr Francis Barth, Sanofi Research, Montpellier, France. CP 55940 ((−)cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cyclohexanol) and JWH 015 (1-propyl-2-methyl-3-(1-naphthoyl)indole) were obtained from the Medicinal Chemistry Department at Roche Bioscience.
All compounds were dissolved in water with the exception of anandamide, CP 55940, HU 210, JWH 015, SR 141716A, SR 144528 and WIN 55212-2, which were dissolved (10 mM) in dimethylsulphoxide (DMSO).
Krebs solution was purchased from Gibco Life Technologies, NY, U.S.A.
Results
Inhibitory effect of cannabinoid receptor agonists on electrically-evoked bladder contractions in rat and mouse
Cannabinoid receptor agonists inhibited electrically-evoked contractions in a concentration-dependent manner in both mouse and rat isolated bladder sections (see Table 1, Figure 1). The maximal inhibitory effect of cannabinoid receptor stimulation in the mouse bladder was higher than that for the rat (55–80% and 5–40% inhibition of electrically-evoked response in the absence of agonist, respectively).
Table 1.
Estimates of agonist potency (pEC50) and intrinsic activity (α) in mouse or rat isolated bladder

Figure 1.

Inhibitory effect of WIN 55212, CP 55940, HU 210, anandamide and JWH 015 on electrically-evoked contractions in mouse (A) and rat (B) urinary bladder. Data are the means±s.e.mean of replicate determinations from tissues isolated from 3–6 animals.
The rank order of potencies of cannabinoid agonists were similar in both species, CP 55940⩾WIN 55212-2>HU 210>JWH 015>anandamide, although the very low intrinsic activities of both anandamide and HU 210 at the cannabinoid receptor in the rat isolated bladder precluded reliable estimation of potency.
Antagonist studies in mouse and rat isolated bladder
In the mouse isolated bladder, the CB1 selective antagonist, SR 141716A (10–100 nM), caused parallel rightward shifts of the concentration-response curves to WIN 55212-2 or JWH 015, with no significant effect on slope (pKB=8.66±0.13 and 8.22±0.17, respectively: Data for WIN 55212-2 are presented in Table 2). However, an increase in electrically-evoked contractions was observed 1 h after treatment with SR 141716A but not in vehicle controls (Figure 2, increases over pre-dose contractions due to 10, 30, or 100 nM SR 141716A=108±68, 187±66, 154±47%, respectively, n=4, P<0.05 for 10 nM SR 141716A). This increase in contractile response was not dose-dependent over the concentration range of SR 141716A investigated. Mean data for a single concentration of SR 141716A (30 nM), in competition with WIN 55212-2 or JWH 015 are presented in Figures 3 and 4.
Table 2.
Antagonist affinity estimates determined in competition with WIN 55212-2 in the mouse and rat isolated urinary bladder
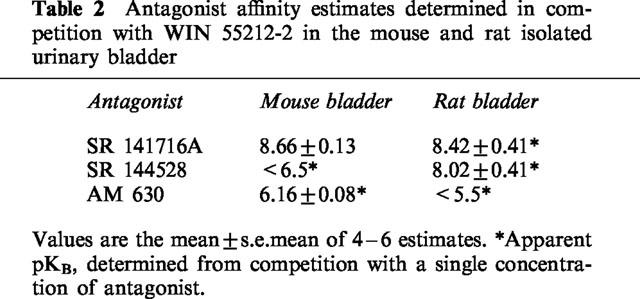
Figure 2.

Electrically-evoked contractions of mouse isolated urinary bladder. Contractions were evoked by electrical stimulation over 2 min intervals (8 V, 12 pulses s−1, 0.5 ms pulse width, 0.1 trains s−1) in sections of mouse isolated bladder before and after 1 h incubation with (A) SR 141716A (30 nM) or (B) DMSO (0.005% v v−1).
Figure 3.

Antagonism of WIN 55212-2 -mediated effect by SR 141716A (30 nM), SR 144528 (100 or 300 nM) and AM 630 (3 μM) in mouse (A) and rat (B) urinary bladder. Data are the mean±s.e.mean of replicate determinations from tissues isolated from 4–6 animals.
Figure 4.

Antagonism of JWH 015-mediated effect by SR 141716A (100 nM) and SR 144528 (100 nM) in mouse (A) and rat (B) isolated bladder. Data are the means±s.e.mean of replicate determinations in tissues from 4–6 animals.
As with the mouse bladder, SR 141716A caused parallel rightward shifts of the concentration-response curve to WIN 55212-2, JWH 015 and CP 55940 in the rat isolated bladder, with no significant effect on E/[A] curve slope (Figures 3 and 4). In these studies, the affinity of SR 141716A in the rat isolated bladder was determined using a single concentration of antagonist (30 nM, apparent pKB=8.42±0.41, 8.19±0.27 and 8.26±0.18 determined in competition with WIN 55212-2, JWH 015 and CP 55940, respectively). Unlike in the mouse bladder, no increase in electrically-evoked contractions following treatment with SR 141716A was observed in rat isolated bladder (P>0.05 following 30 nM SR 141716A).
The CB2 selective antagonist, SR 144528, attenuated the effects of WIN 55212-2 or JWH 015 in the rat (100 nM, apparent pKB=8.02±0.41 and 7.74±0.21, respectively, Table 2 and Figures 3 and 4) but not the mouse isolated urinary bladder (100 or 300 nM, pKB<6.5). At the concentrations investigated, SR 144528 on its own had no significant effect on electrically-evoked contractions in either mouse or rat isolated bladder.
AM 630 (3 μM), previously reported to be an antagonist in mouse isolated vas deferens and inactive in the mouse isolated bladder, caused parallel rightward shifts in concentration-response curves to WIN 55212-2, in mouse but not rat isolated bladder sections (apparent pKB 6.16±0.08 and <5.5, Table 2 and Figure 3). At the concentration investigated, AM 630 had no significant effect on electrically-evoked contractions in either mouse or rat isolated bladder sections.
Effect of WIN 55212-2 and SR 141716A on post-junctional responses to carbachol and α,β-methylene ATP
In mammalian bladders, the major component of neurogenic contraction is mediated by activation of post-junctional muscarinic and purinergic receptors on bladder smooth muscle. Carbachol or α,β-methylene ATP evoked concentration-dependent increases in muscle contractility in the mouse and rat isolated bladders, although the relative magnitude of these responses varied profoundly between the two species. Relative to the responses to potassium chloride (KCl, 80 mM), different sensitivities to carbachol (respective maximal responses=105.5±14% and 63±5%) or α,β-methylene ATP (respective maximal responses=23±3% and 88±10%) were detected in mouse and rat isolated bladder sections, respectively. In spite of the differential sensitivity to muscarinic or purinergic receptor activation, neither WIN 55212-2 (3 μM) nor SR 141716A (30 nM) had any effect on the contractile responses mediated by carbachol or α,β-methylene ATP in the mouse or rat isolated bladders (Figure 5).
Figure 5.

Effect of WIN 55212-2 (3 μM) or SR 141716A (30 nM) on contractile responses to α,β-methylene ATP or carbachol (control responses) in isolated bladder sections from the mouse (A,B) and rat (C,D). Data are the mean±s.e.mean from 4–6 replicates.
Effect of WIN 55212-2 on frequency-response curves in isolated bladder tissue
Electrically-evoked responses in the mouse isolated bladder were attenuated by tetrodotoxin (0.3 μM) by greater than 90% (data not shown) over the full frequency range investigated (1–128 Hz). This is consistent with a neuronally-mediated contraction.
An inhibition of electrically-evoked contractile responses by WIN 55212-2 (3 μM) was observed over the full range of stimulation frequencies in the mouse isolated bladder (1–128 Hz, P<0.05, Figure 6A). In the rat isolated bladder, a decrease in electrically-evoked contractions by WIN 55212-2 (3 μM) was observed only at higher frequencies (32 and 64 Hz, P<0.05, Figure 6B). Conversely, WIN 55212-2 (3 μM) had no effect on electrically-evoked contractile responses (1–128 Hz) in isolated sections of dog, pig, monkey and human bladder tissues, relative to time-matched vehicle controls (Figure 6C–F).
Figure 6.

Effect of WIN 55212-2 (3 μM) on frequency response curves in isolated bladder tissue from mouse (A), rat (B), dog (C), pig (D), non-human primate (E), human (F). Data are the mean±s.e.mean from 4–7 replicates, significant difference between control and WIN 55212-2 (3 μM)-treated tissues indicated by *(P<0.05) and **(P<0.01).
The combined effects of WIN 55212-2 and muscarinic and/or purinergic receptor blockade on frequency-response curves were investigated in isolated bladder sections. In the mouse isolated bladder, treatment with atropine (0.3 μM) attenuated electrically evoked contractions across all the frequencies investigated (1–128 Hz, P<0.05). Conversely, α,β-methylene ATP (3 μM) had no effect on electrically-evoked contractions at any of the frequencies investigated (1–128 Hz). Co-treatment of atropine or α,β-methylene ATP with WIN 55212-2 (3μM) resulted in attenuation (P<0.05) of electrically-evoked contraction when stimulated at 4, 64 and 128 Hz frequencies, or at all frequencies, relative to atropine or α,β-methylene ATP -treated tissues in the absence of WIN 55212-2 (Figure 7A,B).
Figure 7.

Effect of co-administration of atropine (0.3 μM; A,B) or α,β-methylene ATP (3 μM; B,D) with WIN 55212-2 (3 μM) on frequency-response curves in mouse (A,B) or rat (C,D) isolated urinary bladder. Significant difference between treatment with atropine or α,β-methylene ATP alone or in combination with WIN 55212-2, determined by Student's t-test, is indicated by *(P<0.05) and **(P<0.01). Data are the mean±s.e.mean of 4–6 replicates.
In similar experiments in the rat isolated bladder. WIN 55212-2 attenuated electrically-evoked contractile responses to high frequency stimulation (32 and 64 Hz, P<0.05). As with the mouse isolated bladder, treatment with atropine (0.3 μM) attenuated electrically-evoked contractions in the rat isolated bladder at all frequencies investigated (1–128 Hz, P<0.05). Treatment with α,β-methylene ATP (3 μM) inhibited contractions evoked by stimulation at 4, 8 and 16 Hz (P<0.05). However, the combined inhibitory effect of WIN 55212-2 (3 μM) and atropine or α,β-methylene ATP was no greater than that for either atropine or α,β-methylene ATP alone (Figure 7C,D). Finally, co-treatment with WIN 55212-2, atropine and α,β-methylene ATP was not significantly different from treatment with atropine and α,β-methylene ATP (data not shown).
In similar experiments with pig or dog isolated bladder, occlusion of either or both the muscarinic or purinergic components of the electrically-evoked response with atropine or α,β,-methylene ATP did not unmask an inhibitory effect of WIN 55212-2 (data not shown).
Discussion
Previous data suggest the presence of a prejunctional CB1 receptor in mouse isolated bladder that mediates inhibition of electrically-evoked contractions (Pertwee & Fernando, 1996). The data in this study confirm and extend the previous findings in the mouse bladder using a selection of synthetic cannabinoid receptor agonists and antagonists. Definitive characterization of the cannabinoid receptor subtype associated with this effect is confounded by the poor selectivity of several cannabinoid ligands for the two known receptor subtypes, and an ignorance of the affinity of several of these drugs at mouse and rat cannabinoid receptor homologues. Nonetheless, clear similarities in the agonist potencies and antagonist affinity estimates were identified between the human recombinant CB1 receptor and the cannabinoid receptor in the mouse isolated urinary bladder. In this study, the potencies estimated for all agonists and antagonists were consistent with those reported for human CB1 receptors (Showalter et al., 1996; Rinaldi-Carmona et al., 1998). In particular, the observed rank order of agonist potencies: CP 55940⩾WIN 55212-2>HU 210>JWH 015>anandamide, the high affinity of the CB1 selective antagonist, SR 141716A (apparent pKB=8.7), and the low affinity of the CB2 antagonist, SR 144528 (apparent pKB<6.5) are consistent with a CB1-like cannabinoid receptor in the mouse isolated bladder.
Neither WIN 55212-2 (3 μM) nor SR 141716A (30 nM) had any effect on the post-junctional effects of carbachol or α,β-methylene ATP in mouse or rat isolated bladder. As such, the effects of these cannabinoid ligands respectively to inhibit or potentiate electrically-evoked contractions cannot be ascribed to a post-junctional interaction with muscarinic or purinergic receptors on bladder smooth muscle, consistent with the findings in mouse bladder of Pertwee & Fernando (1996). Although the mechanism by which SR 141716A potentiates electrically-evoked contractions in this tissue was not determined, it is likely to be independent of the CB1 receptor as it was concentration-independent over the concentration range (10–100 nM) which, from its affinity for the CB1 receptor, can be predicted to occupy 80–99% of the CB1 receptors investigated. However, given the poor signal-to-noise of the mouse and rat isolated bladder assays, the possibility remains that the increases in electrically-evoked contraction due to effects of 10, 30 or 100 nM SR 141716A are not different from each other, and are a consequence of receptor-mediated inverse agonism or antagonism of endocannabinoid. Notwithstanding the unknown mechanism of action, the affinity of SR 141716A estimated in the mouse bladder appears not to have been confounded by its observed potentiation of electrically-evoked contractions as the values determined in competition with WIN 55212-2 or JWH 015 (8.66 and 8.22, respectively) are similar to other affinities estimated at the human recombinant receptor or rat brain membranes (pKi=8.3, Rinaldi-Carmona et al., 1996; pKD=9.4–9.9, Breivogel et al., 1997).
This study also demonstrated an inhibitory effect of cannabinoid receptor agonists on electrically-evoked contractions in the rat isolated bladder. In this tissue, the rank order of agonist potencies determined was similar to that in mouse isolated bladder sections and at human recombinant CB1 receptors (Showalter et al., 1996). In this assay however, the maximal inhibitory effects of all agonists were lower when compared to responses in mouse bladder. Indeed, the effects of both HU 210 and anandamide were too modest to allow accurate quantification of potency. The lower maximal effect of agonists in rat bladder may result from differences between mouse and rat in the nerves and neurotransmitters that control neurogenic contraction, or the expression level or G-protein coupling efficiency of cannabinoid receptors along these pathways.
In contrast to the CB1-like pharmacology of the mouse isolated bladder, parallel rightward shifts of concentration-effects curves to WIN 55212-2 or JWH 015 were observed in the presence of either SR 141716A (30 nM) or SR 144528 (100 nM) in the rat bladder. These antagonists are known to bind selectively to the human recombinant CB1 and CB2 receptors, respectively (pKi at CB1=7.91 and 6.4; pKi at CB2=6.2 and 9.2 for SR 141716A and SR 144528, respectively, Showalter et al., 1996; Rinaldi-Carmona et al., 1998). Based on these data, the high affinity with which both antagonists inhibit the effects of WIN 55212-2 or JWH 015 in the rat bladder (apparent pKB 8.4 and 8.0, respectively) is consistent with interactions at CB1 and CB2 receptors. The CB2-like component of the responses in the rat bladder cannot be attributed to pharmacological differences between human and rat orthologues of the CB1 receptors as the selectivity of SR 144528 for rat CB2 receptors over rat CB1 previously has been demonstrated in its higher affinity at membranes derived from rat spleen microsomes (CB2 receptor pKi=9.4) over rat brain (CB1 receptor pKi=6.5, Rinaldi-Carmona et al., 1998). It remains possible however that a mixed population of CB1 and CB2 receptors jointly influence neurogenic contraction of the rat bladder, although no evidence for the CB2 receptor subtype has been demonstrated in the peripheral nervous system to date. This suggestion is not consistent with the observed failure to demonstrate CB2-like pharmacology using the CB2-selective antagonist, AM 630 (pKi at human recombinant CB1 and CB2 receptors of 5.29 and 7.51, respectively, Ross et al., 1999; mouse brain apparent pKB=5.5, Hosohata et al., 1997). Definitive characterization of the cannabinoid receptor(s) that inhibit neurogenic contractions in the rat bladder is confounded by the poor signal-to-noise ratio of the assay.
Previous investigations in the mouse isolated urinary bladder have shown that pre-junctional cannabinoid receptors mediate inhibition of electrically-induced contractions by reducing release of contractile transmitters (Pertwee & Fernando, 1996). Our findings extend these observations to suggest that while this holds true for mouse and rat isolated bladders, there are profound differences between mammalian bladders since electrically-evoked contractions of bladder sections from dog, pig, primate or human are completely resistant to cannabinoid receptor activation by WIN 55212-2. This may be the consequence of inter-species differences in: (i) cannabinoid receptor expression or distribution, (ii) the effect of these receptors on the release of contractile transmitters, and (iii) anatomical variations in bladder innervation.
Relative to the responses to potassium chloride, different sensitivities to carbachol or α,β-methylene ATP were detected in mouse and rat isolated bladders. These are likely to relate to the relative importance of muscarinic or purinergic neurotransmission in the mouse or rat bladders, as demonstrated by the differences in the effects of α,β-methylene ATP or carbachol on frequency-response curves in these tissues. Such inter-species differences in neuronal control of bladder contraction may account, in part, for the variability in effect of WIN 55212-2. Profound inter-species differences in the neuroanatomy of the mammalian bladders are known to exist. For example, while no parasympathetic ganglia are located within the urinary bladders of mice and rats, several have been demonstrated in isolated bladder tissue from guinea-pigs and human (Gabella, 1990; Gilpin et al., 1983). Our study suggests that in mouse and rat isolated bladders, which are devoid of parasympathetic ganglia, cannabinoid receptors are located at the neuro-effector junction. Therefore, the known differences in the location of ganglia cannot account for the observed inter-species differences in response to WIN 55212-2.
Data in this study demonstrate species differences in the relative contributions of muscarinic and purinergic receptors in the control of bladder contractility between the mouse and rat. In particular, the low responsiveness of isolated sections of mouse bladder to α,β-methylene ATP compared with carbachol suggests a dominant control of murine urinary bladder tone by post-junctional muscarinic receptors over purinoceptors. Conversely, very similar responses to α,β-methylene ATP and carbachol were determined in rat isolated bladder sections. These data are consistent with the inter-species differences in transmitter release reported previously (see Anderson, 1993 for review; Sibley, 1984).
With specific regard to the neuronal pathways affected by cannabinoid receptor activation, our studies demonstrate that WIN 55212-2 inhibits one or both of the muscarinic and purinergic components of neuronal transmission in the mouse isolated bladder. In particular, the effect of WIN 55212-2 to reduce electrically-evoked contractions in the mouse bladder is attenuated in the presence of atropine or α,β-methylene ATP, and is completely removed by co-incubation with these two agents in combination. Conversely, in rat isolated bladder, the inhibitory effect of WIN 55212-2 is likely to be mediated by nerves that utilize both muscarinic and purinergic transmission as the effect of WIN 55212-2 is removed by treatment with either atropine or α,β-methylene ATP.
Taken together, the data in this study demonstrate differences in the role of cannabinoid receptors in the control of peripheral neurogenic contraction in mouse, rat, dog, pig, monkey and human bladders. Based on these data, the therapeutic success of nabilone in modulating bladder emptying in humans (Martyn et al., 1995) is unlikely to be mediated by interaction with pre-synaptic receptors in the bladder. However, it remains possible that cannabinoid agonists may attenuate bladder contractility at other points along the neuronal pathway including the spinal cord, the periaqueductal gray or the pons micturition centre. Indeed, the cannabinoid receptors known to be expressed in the periaqueductal gray (Lichtman et al., 1995) may be the mechanism through which nabilone exerts its effects over the micturition reflex (Vanderhorst et al., 1996). Furthermore, it is possible that cannabinoid receptor expression levels, release of endocannabinoids, and the role of different neurotransmitters may differ between normal and disease states such that a role for cannabinoids in control of bladder contractility, although absent in healthy bladder tissue, may become apparent in pathologically altered tissue. Analogous changes in purinoceptor expression under disease conditions are known to occur (Palea et al., 1993).
The lack of response of dog, pig, monkey and human bladder sections to WIN 55212-2, and the non-CB1-like pharmacology of the rat bladder challenge both the suitability of peripheral cannabinoid CB1 receptors as a potential therapeutic target for urinary incontinence, and the reliability with which mammalian organs represent human physiology. Clearly, investigations into the role of cannabinoid receptor agonists in neurogenic contractions of diseased human bladder sections will need to be investigated before any conclusions can be made about utility of the peripheral CB1 receptor as a drug target.
Abbreviations
- DMSO
dimethylsulphoxide
- α,β-methylene ATP
α,β-methylene adenosine 5′-triphosphate
- β,γ-methylene ATP
β,γ-methylene adenosine 5′-triphosphate
References
- ANDERSON K.E. Pharmacology of the lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREIVOGEL C.S., SIM L.J., CHILDERS S.R. Regional differences in cannabinoid receptor/G-protein coupling in rat brain. J. Pharmacol. Exp. Ther. 1997;282:1632–1642. [PubMed] [Google Scholar]
- BURNSTOCK G., DUMSDAY B., SMYTHE A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br. J. Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GABELLA G. Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res. 1990;261:231–237. doi: 10.1007/BF00318664. [DOI] [PubMed] [Google Scholar]
- GILPIN C.J., DIXON J.S., GILPIN S.A., GOSLING G.A. The fine structure of autonomic neurons in the wall of the human urinary bladder. J. Anatomy. 1983;137:705–713. [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN G., FERNANDO S.R., ROSS R.A., MCKAY N.G., ASHFORD M.L.J., SHIRE D., HUFFMAN J.W., YU S., LAINTON J.A.H., PERTWEE R.G. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur. J. Pharmacol. 1997;339:53–61. doi: 10.1016/s0014-2999(97)01336-8. [DOI] [PubMed] [Google Scholar]
- HOSOHATA Y., QUOCK R.M., HOSOHATA K., MAKRIYANNIS A., CONSROE P., ROESKE W.R., YAMAMURA H.I. AM 630 antagonism of cannabinoid-stimulated [35S]GTPγS binding in the mouse brain. Eur. J. Pharmacol. 1997;321:R1–R3. doi: 10.1016/s0014-2999(97)00047-2. [DOI] [PubMed] [Google Scholar]
- LICHTMAN A.H., COOK S.A., MARTIN B.R. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J. Pharmacol. Exp. Ther. 1995;276:585–593. [PubMed] [Google Scholar]
- LYNN A.B., HERKENHAM M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding sites in peripheral tissues of the rat: Implications for receptor-mediated immune modulation by cannabinoids. J. Pharmacol. Exp. Ther. 1994;268:1612–1623. [PubMed] [Google Scholar]
- MARTYN C.N., ILLIS L.S., THOM J. Nabilone in the treatment of multiple sclerosis. Lancet. 1995;345:579. doi: 10.1016/s0140-6736(95)90485-9. [DOI] [PubMed] [Google Scholar]
- PACHECO M., CHILDERS S.R., ARNOLD R., CASIANO F., WARD S.J. Aminoalkylindoles: actions on specific G-protein-linked receptors. J. Pharmacol. Exp. Ther. 1991;257:170–183. [PubMed] [Google Scholar]
- PALEA S., ARTIBANI W., OSTARDO E., TRIST D.G., PIETRA C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J. Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G., FERNANDO S.R. Evidence for the presence of cannabinoid CB1 receptors in mouse urinary bladder. Br. J. Pharmacol. 1996;118:2053–2058. doi: 10.1111/j.1476-5381.1996.tb15643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERTWEE R.G., STEVENSON L.A., ELRICK D.B., MECHOULAM R., CORBETT A.D. Inhibitory effects of certain enantiomeric cannabinoids in the mouse vas deferens and myenteric plexus preparation of guinea-pig small intestine. Br. J. Pharmacol. 1992;105:980–984. doi: 10.1111/j.1476-5381.1992.tb09088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., BARTH F., MILLAN J., DEROCQ J., CASELLAS P., CONGY C., OUSTRIC D., SARRAN M., BOUBOULA M., CALANDRA B., PORTIER M., SHIRE D., BRELIÈRE J.-C., LEFUR G. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- RINALDI-CARMONA M., CALANDRA B. , SHIRE D., BOUABOULA M., OUSTRIC D., BARTH F., CASELLAS P., FERRARA P., LE FUR G. Characterization of two cloned human CB1 cannabinoid isoforms. J. Pharmacol. Exp. Ther. 1996;278:871–878. [PubMed] [Google Scholar]
- ROSS R.A., BROCKIE H.C., STEVENSON L.A., MURPHY V.L., TEMPLETON F., MAKRIYANNIS A., PERTWEE R.G. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656 and AM630. Br. J. Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOWALTER V.M., COMPTON D.R., MARTIN B.R., ABOOD M.E. Evaluation of binding in a transfected cell line expressing peripheral cannabinoid receptor (CB2): Identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 1996;278:989–999. [PubMed] [Google Scholar]
- SIBLEY G.N.A. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–443. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIST D.G., LEFF P. Quantification of H2-antagonism by clonidine and dimiprit in an adenylate cyclase assay. Agents Actions. 1985;16:222–226. doi: 10.1007/BF01983145. [DOI] [PubMed] [Google Scholar]
- VANDERHORST V.G., MOUTON L.J., BLOK B.F., HOLSTEGE G. Distinct cell groups in the lumbosacral cord of the cat project to different areas in the periaqueductal gray. J. Comp. Neurol. 1996;376:361–385. doi: 10.1002/(SICI)1096-9861(19961216)376:3<361::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]


