Abstract
The effect of single administrations of aerosolized heparin, low molecular weight heparin (LMWH) and the linear polyanionic molecule, polyglutamic acid (PGA) were examined on antigen-induced airway hyperresponsiveness and leukocyte accumulation in neonatally immunized rabbits.
Adult litter-matched NZW rabbits immunized within 24 h of birth with Alternaria tenuis antigen were treated with heparin, LMWH or PGA prior to or following antigen challenge (Alternaria tenuis). For each drug-treated group, a parallel group of rabbits were treated with the appropriate vehicle. In all groups, airway responsiveness to inhaled histamine and bronchoalveolar lavage (BAL) was performed 24 h prior to and following antigen challenge.
Basal lung function in terms of resistance (RL) and dynamic compliance (Cdyn) and acute bronchoconstriction was unaltered by pre-treatment with heparin, LMWH or PGA compared to their respective vehicles 24 h prior to or following antigen challenge.
In vehicle-treated animals, airway hyperresponsiveness to inhaled histamine was indicated by an increase in the maximal responses of the cumulative concentration-effect curves to histamine and reductions in RLPC50 and CdynPC35 values 24 h following antigen challenge.
Heparin and LMWH given prior to antigen challenge significantly inhibited the development of airway hyperresponsiveness, whereas PGA did not. When given following antigen challenge, all three drugs failed to inhibit the development of airway hyperresponsiveness.
Eosinophil and neutrophil cell numbers in BAL fluid increased significantly 24 h following antigen challenge. Heparin, LMWH and PGA failed to inhibit the increase in cell numbers following antigen challenge whether given prior to or following antigen challenge.
Keywords: Heparin, glycosaminoglycans, antigen, inflammation, airway hyperresponsivenss
Introduction
Asthma is now widely accepted as an inflammatory disease and is characterized by hyperresponsiveness of the pulmonary airways to stimuli such as histamine, as well as by an increased population of inflammatory cells in both the submucosal area and lumen exudate, comprised predominantly of eosinophils. We have previously shown that rabbits immunized with Alternaria tenuis antigen at birth and repeatedly exposed to this antigen during the first 3 months of life develop persistent airway hyperresponsiveness (Minshall et al., 1993) and an increased presence of inflammatory leukocytes in the bronchoalveolar lavage fluid (Herd et al., 1994). Studies have demonstrated that immunization of neonatal rabbits induces the production of predominantly IgE antibodies (Pinckard et al., 1972; Shampain et al., 1982). As adults, these rabbits display both early- and late-onset airways obstruction associated with airway inflammation following antigen challenge (Shampain et al., 1982; Larsen et al., 1987; Coyle et al., 1989; 1990).
One of the predominant inflammatory cells present in the lung in asthma is the mast cell which is activated by cross-linking of IgE and pharmacological stimuli such as calcium ionophores to cause degranulation with the subsequent release into the surrounding tissue of histamine and other mediators (reviewed in Page & Minshall, 1993). Degranulation of mast cells results in the release of the glycosaminoglycan heparin (Lasser et al., 1987; Green et al., 1993) which is synthesized and stored in these cells (Selye, 1965; Bloom, 1974). It is released along with histamine upon activation of the mast cells. Heparin is also known to have an inhibitory effect on mast cell degranulation (Ahmed et al., 1994) which may act as a regulatory mechanism. Natural heparin-like compounds exist as heparan sulphate and occur primarily as a component of proteoglycans when the structurally heterogeneous glycosaminoglycan polymers are covalently linked to a core protein. Heparan sulphate proteoglycans are widely present on cell surfaces (Hook et al., 1984) bound to components of the extracellular matrix and in basal lamina (reviewed in Conrad, 1989).
Although its anticoagulant activity is well documented, heparin is also known to possess a range of other activities (Tyrrell et al., 1995) including activation of macrophages to become scavengers (Dolowitz & Dougherty, 1965), inhibition of vascular leakage induced by histamine, bradykinin and PGE1 (Carr, 1979) and to induce lymphocytosis (Sasaki, 1967). It is also known to decrease myointimal thickening and vascular smooth muscle cell proliferation (Clowes & Karnovsky, 1977; Hirsch & Karnovsky, 1991). In addition to these varied activities it has long been known that heparin inhibits the allergic anaphylactic response (Van der Carr & Williams, 1928).
Previous studies in our laboratory have demonstrated that heparin and related heparinoids inhibit airway hyperresponsiveness and pulmonary cell infiltration induced by PAF in the rabbit (Sasaki et al., 1993) and guinea-pig (Seeds et al., 1993) and by allergen in the guinea-pig (Seeds et al., 1995). Therefore, in the present study, we have investigated the effect of heparin, low molecular weight heparin (LMWH) and the linear polyanionic molecule, polyglutamic acid (PGA), given both prior to and immediately following antigen challenge on antigen-induced airway hyperresponsiveness and inflammatory leukocyte infiltration in rabbits.
Methods
Animals
New Zealand White (NZW) rabbits (Froxfield Farms, Petersfield, Hampshire, U.K.) of either sex were used in this study. The immunization protocol is that described previously (Minshall et al., 1993) whereby rabbits were injected intraperitoneally within 24 h of birth with 0.5 ml of Alternaria tenuis extract in aluminium hydroxide and saline in the ratio 2 : 1 : 1. Further administration followed on a weekly basis for the next month and then on a biweekly basis for a further 2 months.
Pulmonary function measurements
At 3 months of age, immunized rabbits were premedicated with diazepam (2.5 mg.kg−1, i.p.) and subsequently administered Hypnorm (0.4 ml.kg−1, i.m.). This régime produces neuroleptanalgesia and is recommended for recovery procedures in laboratory animals (Flecknall, 1987). Throughout the course of the experiments, neuoleptanalgesia was maintained by further administration of 0.5 ml Hypnorm intramuscularly at 25–30 min intervals. Animals were intubated with a cuffed endotracheal tube (3.0 mm internal diameter; Mallinckrodt Laboratories, Athlone, Ireland) which was then inflated and attached to a heated (37°C) Fleisch pneumotachograph (size 00). Respiratory flow, pleural pressure, transpulmonary pressure and tidal volume were measured according to the methods previously described (Minshall et al., 1993). Total lung resistance (RL) and dynamic compliance (Cdyn) were calculated by an online respiratory analyser (PMS Version 5.2; Mumed Ltd, London, U.K.).
Aerosols of drugs and antigen were generated by an ultrasonic nebulizer (Ultra-Neb 99; DeVilbiss Health Care Ltd, Heston, Middlesex, U.K.) and delivered to the animal via the tracheal cannula.
Experimental protocol
Experiments were conducted over a period of three consecutive days with recovery of animals following days 1 and 2. On day 1, following measurements of lung function for baseline and aerosolized saline, airway hyperresponsiveness to histamine was determined. Aerosolized histamine was given in doubling, cumulative concentrations of 1.25–160 mg.ml−1 for a period of 2 min per concentration with lung function measurements taken between each dose. Cumulative concentration-effect curves were thus established and the provocation concentration (PC) of histamine which cumulatively produced a 50% increase in RL (PC50) or a 35% fall in Cdyn (PC35) was determined for each animal. These values were used as indices of airway responsiveness.
On day 2, animals were administered either heparin (Multiparin; 1000 U.ml−1 or 5000 U.ml−1), low molecular weight heparin (LMWH; 1000 U.ml−1), polyglutamic acid (5 mg.ml−1) or vehicle (chlorocresol or saline) in aerosolized form for 2 min, 20 min prior to antigen challenge or immediately following the last administration of antigen. Antigen challenge consisted of an initial administration of aerosolized saline for 2 min, followed by aerosolized Alternaria tenuis (20,000 PNU.ml−1) for 2×2 min periods with a further 4×4 min periods, resulting in a total of 20 min of antigen challenge. Lung function measurements were made following each aerosolization and then again 15 and 30 min following the final antigen aerosol challenge. In animals administered with the treatment drugs following the final antigen challenge, a further lung function measurement was made following the administration of the drug. On day 3, histamine challenge was repeated as for day 1.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) was performed immediately following histamine concentration-effect curves on days 1 and 3. This involved the administration of 5 ml saline via the endotracheal tube with immediate aspiration and collection into a sterile tube on ice. Total cell counts were determined under light microscopy using an improved Neubauer haemocytometer. Differential cell counts of neutrophils, eosinophils and mononuclear cells were also determined from cytospin preparations as previously described (Minshall et al., 1993).
Data analysis
Acute changes in airway responsiveness following drug administration or antigen challenge are expressed as the maximum percentage change in RL and Cdyn from baseline. Unpaired Student's t-tests were used to compare differences between treatment groups.
Cumulative concentration-effect curves to histamine were constructed for all animals on days 1 and 3. Curves were compared using 2-way analysis of variance (ANOVA). Histamine PC50 and PC35 values were obtained from each curve from each animal 24 h prior to and 24 h following antigen challenge. Mean values were calculated from each group of animals and data presented in the form of concentrations (mg.ml−1). For statistical analysis the data was log10-transformed and the geometric means±standard error of the mean of PC50 and PC35 values measured before and after antigen challenge were compared using paired Student's t-tests. Unpaired Student's t-tests were used to compare data between groups of animals. For all statistical comparisons, results were considered statistically significant when P<0.05.
Bronchoalveolar lavage cell counts were differentiated as either eosinophils, neutrophils or mononuclear cells based on morphological criteria and expressed as absolute cell counts per ml of lavage fluid. Mean values were calculated for each group of animals and results from different groups of animals were compared using paired Student's t-tests. For all statistical comparisons, results were considered statistically significant when P<0.05.
Drugs and chemicals
Drugs and chemicals used in this study included: Heparin (Multiparin, average mol. wt 12,000 Da; C.P. Pharmaceuticals, Wrexham, Clwyd, U.K.), low molecular weight heparin (LMWH (Fragmin®), average mol. wt 4000–6000 Da; gift of Dr Max Pauling, Pfizer ltd, Sandwich, Kent, U.K.), polyglutamic acid (50,000–100,000 mol. wt); histamine diphosphate (Sigma Chem. Co.), chlorocresol (heparin vehicle; Sigma Chem. Co.); Alternaria tenuis extract (40,000 PNU.ml−1; Greer Laboratories Inc.); haematoxylin (BDH Chemicals); diazepam (Valium 5 mg.ml−1; Roche Products Ltd); Hypnorm (a mixture of fentanyl citrate 0.315 mg.ml−1 and fluanisone 10 mg.ml−1; Janssen Pharmaceutical Ltd); sterile pyrogen-free 0.9% sodium chloride solution (saline; Baxter Healthcare Ltd). All reagents were of analytical grade and all dilute solutions were prepared in sterile saline.
Results
Airway responses
Basal lung function
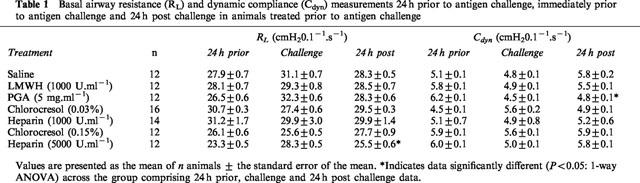
In animals given drug treatment prior to antigen challenge, basal airway resistance (RL) and dynamic compliance (Cdyn) were similar between the different groups of animals 24 h prior to antigen challenge, immediately prior to challenge, or 24 h post challenge. The only exceptions were that observed in the RL group for heparin (5000 U.ml−1) which demonstrated a small but significant variation in baseline values from that obtained 24 h prior to antigen and a significant decrease in values observed for the Cdyn group for PGA (Table 1).
Table 1.
Basal airway resistance (RL) and dynamic compliance (Cdyn) measurements 24 h prior to antigen challenge, immediately prior to antigen challenge and 24 h post challenge in animals treated prior to antigen challenge
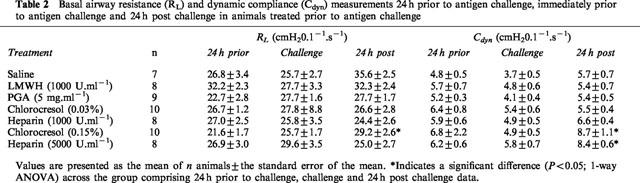
In animals treated following antigen challenge, basal airway resistance (RL) and dynamic compliance (Cdyn) values were not significantly different (P>0.05; 1-way ANOVA) across the treatment groups for any of the days (24 h prior, challenge or 24 h post challenge) examined (Table 2). In addition, within the groups, basal resistance and compliance remained similar over the 3 days of the experimental protocol except in the case of Cdyn for heparin (5000 U.ml−1) and both RL and Cdyn for chlorocresol (0.15%) (Table 2).
Table 2.
Basal airway resistance (RL) and dynamic compliance (Cdyn) measurements 24 h prior to antigen challenge, immediately prior to antigen challenge and 24 h post challenge in animals treated prior to antigen challenge
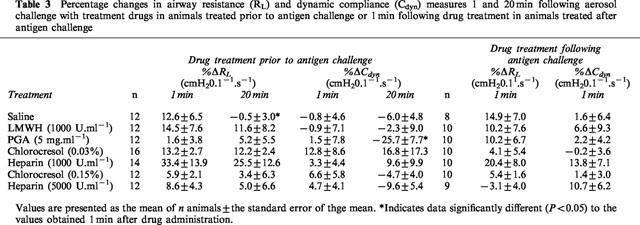
The acute effects of drug administration on lung responses were determined as RL and Cdyn measured 1 and 20 min following aerosol administration of saline, heparin vehicle, heparin (1000 U.ml−1 and 5000 U.ml−1), LMWH (1000 U.ml−1) or PGA (5 mg.ml−1) prior to antigen challenge. Values measured 20 min following aerosol administration of drugs were largely unaltered compared to the values obtained 1 min following drug administration. The obvious exception to this was the baseline %ΔCdyn results obtained for PGA which were significantly lower after 20 min compared to after 1 min (Table 3).
Table 3.
Percentage changes in airway resistance (RL) and dynamic compliance (Cdyn) measures 1 and 20 min following aerosol challenge with treatment drugs in animals treated prior to antigen challenge or 1 min following drug treatment in animals treated after antigen challenge
In animals treated with drugs following antigen challenge, lung function responses were determined 1 min following aerosol administration of the drugs. There was no significant difference between any of the treatment drugs and their corresponding vehicles.
Acute bronchoconstriction
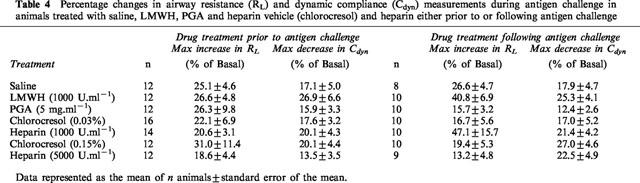
In animals administered with the drugs prior to antigen challenge, acute antigen-induced bronchoconstriction, determined as a percentage increase in total lung resistance and decrease in dynamic compliance and measured during the antigen challenge and for the following 30 min, was not significantly different in vehicle-treated or drug-treated animals (Table 4). In animals administered with the drugs following antigen challenge, acute antigen-induced bronchoconstriction was similarly unchanged in drug-treated animals compared to the corresponding vehicle-treated animals (Table 4).
Table 4.
Percentage changes in airway resistance (RL) and dynamic compliance (Cdyn) measurements during antigen challenge in animals treated with saline, LMWH, PGA and heparin vehicle (chlorocresol) and heparin either prior to or following antigen challenge
Airway responsiveness to histamine
Prior to antigen challenge, responsiveness to histamine was similar in terms of RLPC50 and CdynPC35 in all groups (Tables 5 and 6). In animals treated with saline prior to antigen challenge, airway hyperresponsiveness was demonstrated by a significant decrease in RLPC50 in addition to a significant increase in maximum airway resistance (Figure 1a). Similarly, the cumulative concentration-effect curves for histamine Cdyn had a significantly lower CdynPC35 with a decreased minimum dynamic compliance (Figure 1b). These changes in airway responsiveness to histamine were quantitatively observed as a 3.3 fold decrease in RLPC50 and a 3.4 fold decrease in CdynPC35 (Table 5). Similar changes in the responsiveness of the rabbit airways to histamine in terms of both RL and Cdyn were observed in animals treated with the heparin vehicle at both concentrations examined (0.03 and 0.15%) and were highly significant with P<0.0001 in all cases (Figures 2 and 3). Animals treated with the heparin vehicles (chlorocresol 0.03 and 0.15%) also demonstrated significant changes in RLPC50 and CdynPC35 (Table 5).
Table 5.
Airway resistance (RL PC50) and dynamic compliance (Cdyn PC35) measurements 24 h prior to and 24 h post antigen challenge in animals administered with the drugs prior to antigen challenge
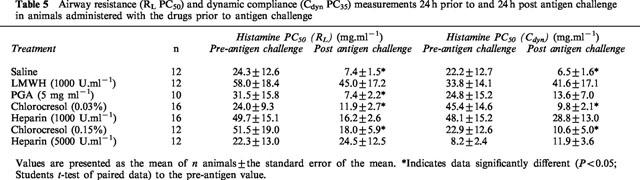
Table 6.
Airway resistance (RL PC50) and dynamic compliance (Cdyn PC35) measurements 24 h prior to and 24 h post antigen challenge in animals administered with the drugs following antigen challenge
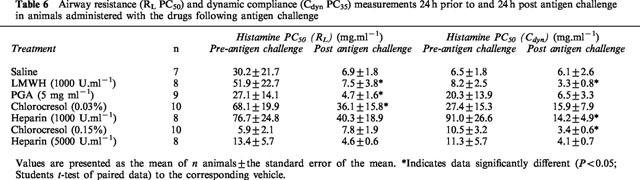
Figure 1.
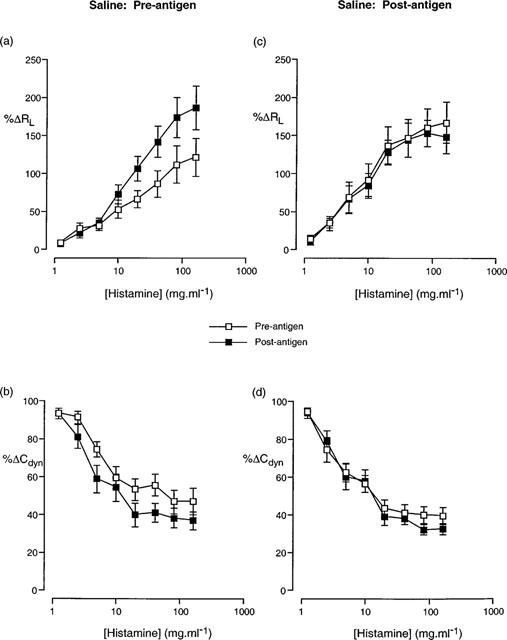
Cumulative concentration-effect curves described in terms of (a,c) increase in airway resistance (%RL) and (b,d) decrease in dynamic compliance (%Cdyn) from basal levels in response to aerosolized histamine prior to and following antigen challenge in animals that were treated with saline prior to (a,b) and following (c,d) antigen challenge. Data presented as the mean±standard error of the mean from 12 and 8 animals respectively.
Figure 2.
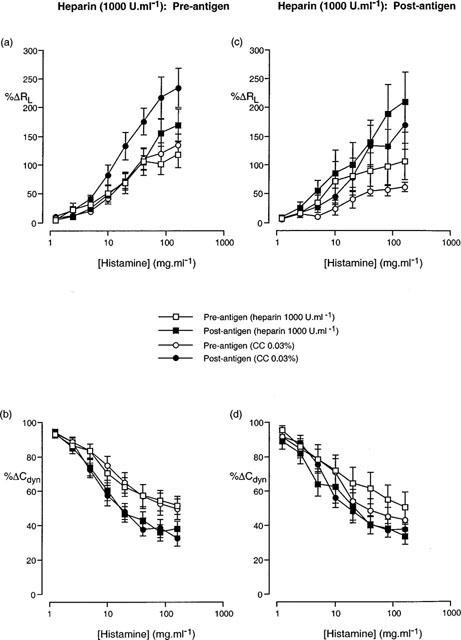
Cumulative concentration-effect curves described in terms (a,c) increase in airway resistance (%RL) and (b,d) decrease in dynamic compliance (%Cdyn) from basal levels in response to aerosolized histamine prior to antigen challenge and following antigen challenge in animals treated with either heparin (1000 U.ml−1) or heparin vehicle (chlorocresol, 0.03%), prior to (a,b) and following (c,d) antigen challenge. Data presented as the mean±standard error of the mean from 9–14 animals.
Figure 3.
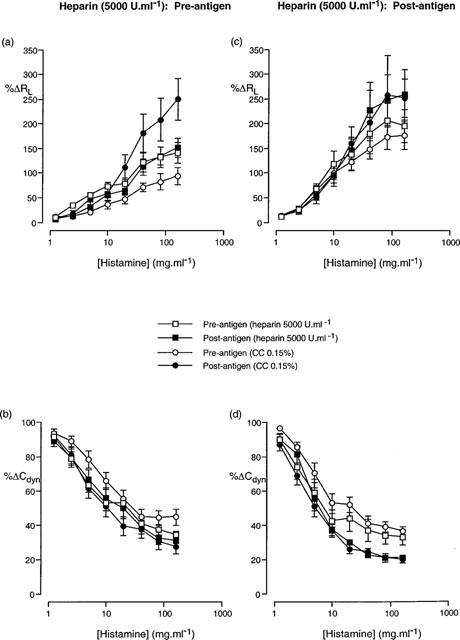
Cumulative concentration-effect curves described in terms of (a,c) increase in airway resistance (%RL) and (b,d) decrease in dynamic compliance (%Cdyn) from basal levels in response to aerosolized histamine prior to antigen challenge and following antigen challenge in animals treated with either heparin (5000 U.ml−1) or heparin vehicle (chlorocresol, 0.15%), prior to (a,b) and following (c,d) antigen challenge. Data presented as the mean±standard error of the mean from 12 and 10 animals respectively.
In animals treated with saline following antigen challenge, there was no significant difference in the cumulative concentration effect curve to histamine following antigen challenge compared to that prior to antigen challenge (Figure 1c,d). Conversely, in animals treated with the heparin vehicles (chlorocresol 0.03 and 0.15%) following antigen challenge, there was a significant (P<0.05; 2-way ANOVA) alteration in the concentration-effect curves to histamine following antigen challenge compared to that prior to antigen challenge (Figures 2 and 3). Despite these changes in the histamine curves, only the RLPC50 for chlorocresol (0.03%)-treated animals and the CdynPC35 for chlorocresol (0.15%)-treated animals reached statistical significance (Table 6).
In animals treated with heparin (1000 U.ml−1) prior to antigen challenge, the antigen-induced change in the cumulative concentration-effect curves to histamine observed in saline- or vehicle-treated animals was abolished (Figure 2). The RLPC50 and CdynPC35 values determined from the curves following antigen challenge were not significantly different from those prior to antigen challenge (Table 5). Animals treated with heparin (5000 U.ml−1) demonstrated a similar inhibition of the development of airway hyperresponsiveness (Figure 3 and Table 5) to that observed with 1000 U.ml−1 heparin.
Treatment of animals with heparin (1000 U.ml−1) following antigen challenge did not alter the significant (P<0.05; 2-way ANOVA) change in the histamine concentration-effect curve that was observed in vehicle-treated animals, although only the PC35Cdyn reached statistical significance (Table 6). Conversely, animals administered heparin (5000 U.ml−1) following antigen challenge demonstrated a statistically significant alteration in the histamine concentration-effect curve for Cdyn, but not for RL (Figure 3) and neither the RLPC50 nor the CdynPC35 reached statistical significance (P>0.05; Student's paired t-test).
Aerosol administration of LMWH (1000 U.ml−1) prior to antigen challenge also resulted in a significant difference in the cumulative concentration-effect curves for histamine RL but not for Cdyn (Figure 4) although RLPC50 and CdynPC35 values were not significantly different prior to and following antigen challenge (Table 5). The disparity of these results is due to the greater change in the curves at the higher concentrations of histamine than at the lower dose range, resulting in a flatter shape to the concentration-effect curve (Figure 4). Similar changes in the cumulative concentration-effect curves for histamine RL but not for Cdyn (Figure 5) were observed in animals treated with PGA, (5 mg.ml−1) and in this case, the RLPC50 and CdynPC35 values reflected these changes (Table 5).
Figure 4.
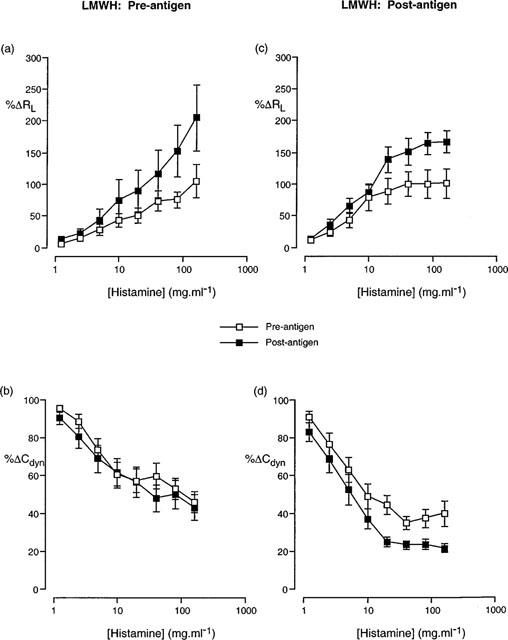
Cumulative concentration-effect curves described in terms of (a,c) increase in airway resistance (%RL) and (b,d) decrease in dynamic compliance (%Cdyn) from basal levels in response to aerosolized histamine prior to and following antigen challenge in animals treated with LMWH 1000 U.ml−1 prior to (a,b) and following (c,d) antigen challenge. Data presented as the mean±standard error of the mean from 12 and 10 animals respectively.
Figure 5.
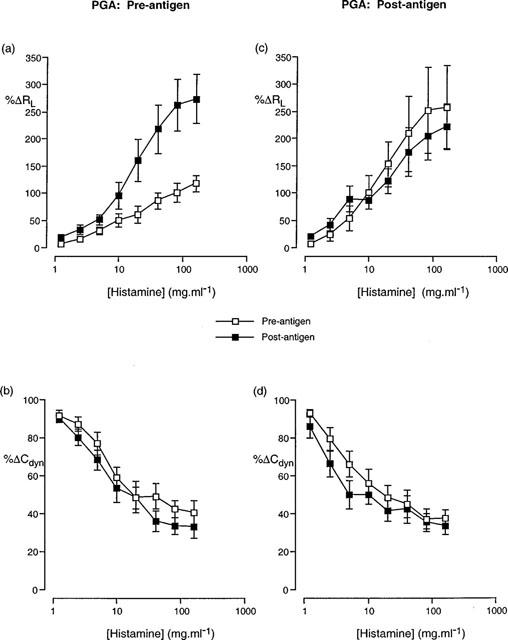
Cumulative concentration-effect curves described in terms of (a,c) increase in airway resistance (%RL) and (b,d) decrease in dynamic compliance (%Cdyn) from basal levels in response to aerosolized histamine prior to and following antigen challenge in animals treated with PGA 5 mg.ml−1 prior to (a,b) and following (c,d) antigen challenge. Data presented as the mean±standard error of the mean from 12 and 10 animals respectively.
PC50 values for airway resistance (RL) prior to and following antigen challenge were significantly different for LMWH and PGA-treated animals in animals treated with the drugs following antigen challenge (Table 6). Analysis of the cumulative concentration-effect curves to histamine indicated a significant (P<0.05; 2-way ANOVA) change in the RL curve following antigen challenge in those animals treated with LMWH (Figure 4c). PC35 values for dynamic compliance (Cdyn) prior to and following antigen challenge were also significantly different in animals treated with LMWH and a clear difference (P<0.05; 2-way ANOVA) in the cumulative concentration-effect curves was observed (Figure 4d). Whilst not reaching statistical significance, a clear trend towards an alteration in the Cdyn cumulative concentration effect curve could be seen after antigen challenge in animals treated with PGA (Figure 5).
Bronchoalveolar lavage
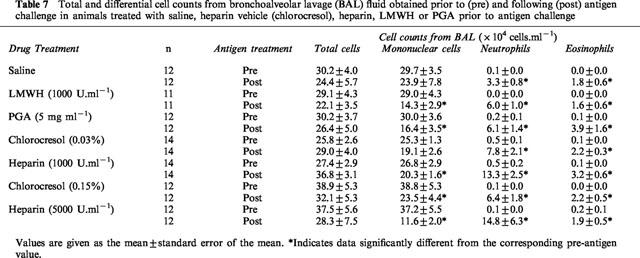
The volume of fluid recovered from BAL was not significantly different between drug and vehicle-treated rabbits before or after antigen challenge (2.0–4.0 ml, 40–80% recovery). Total and differential cell counts prior to antigen challenge were similar in all groups of animals. Following antigen challenge in rabbits treated with saline prior to antigen challenge, there was no change in total or mononuclear cell numbers but neutrophil and eosinophil numbers were increased 41 fold and 37 fold respectively above that obtained 24 h prior to antigen challenge (Table 7). Drug-treated groups of animals did not have significantly different neutrophil or eosinophil numbers following antigen challenge from that of the corresponding saline or vehicle control groups.
Table 7.
Total and differential cell counts from bronchoalveolar lavage (BAL) fluid obtained prior to (pre) and following (post) antigen challenge in animals treated with saline, heparin vehicle (chlorocresol), heparin, LMWH or PGA prior to antigen challenge
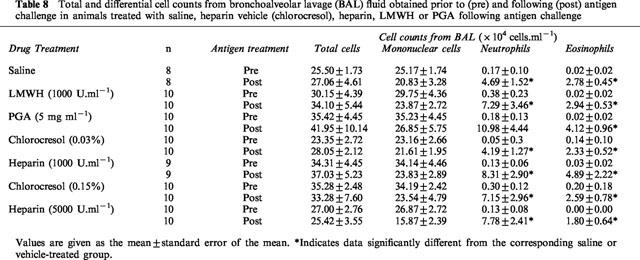
In animals treated with saline following antigen challenge, there was no change in total or mononuclear cell numbers but neutrophil and eosinophil numbers were increased 28 fold and 139 fold respectively (Table 8). Drug-treated groups of animals did not have significantly different neutrophil or eosinophil numbers following antigen challenge from that of the corresponding saline or vehicle control groups.
Table 8.
Total and differential cell counts from bronchoalveolar lavage (BAL) fluid obtained prior to (pre) and following (post) antigen challenge in animals treated with saline, heparin vehicle (chlorocresol), heparin, LMWH or PGA following antigen challenge
Discussion
The present study has demonstrated that aerosolized heparin differentially inhibits the development of airway hyperresponsiveness but not the associated inflammatory leukocyte influx into the airways, depending on whether it is administered prior to or following antigen challenge of actively sensitized rabbits.
There is conflicting evidence concerning the relationship between the presence of inflammatory cells in the airways and the development of airway hyperresponsiveness (Brusasco et al., 1998). Whilst the exact mechanism of action responsible for the development of airway hyperresponsiveness is unknown (Lötvall et al., 1998), mast cells are known to play a role through the release of secondary mediators such as histamine, prostaglandin D2, leukotrienes, cytokines and platelet-activating factor (PAF) (Harvima & Schwartz, 1993; Page & Minshall, 1993). Heparin, whilst synthesized, stored and released from mast cells (Selye, 1965; Bloom, 1974; Lasser et al., 1987; Green et al., 1993), is also known to have an inhibitory effect on mast cell degranulation (Ahmed et al., 1994). It is plausible therefore that administration of heparin prior to allergen challenge may prevent the degranulation of the mast cells and thus inhibit the secondary release of mediators which may be responsible, at least in part, for the development of airway hyperresponsiveness. Indeed it has long been known that heparin inhibits the allergic anaphylactic response (Van der Carr & Williams, 1928).
More recent studies have demonstrated that heparin can modulate the activities of a number of inflammatory cells (Tyrrell et al., 1995). It has previously been shown that heparin inhibits the adhesion of leukocytes to the endothelium (Rogers et al., 1993) most likely by displacing pro-adhesive cytokines (which are responsible for the formation of cell adhesion complexes) from the luminal surface of the endothelium (Tanaka et al., 1993a,1993b).
Preliminary studies in our laboratory have demonstrated that intravenously administered heparin and related heparinoids inhibit airway hyperresponsiveness and pulmonary cell infiltration induced by PAF in the rabbit (Sasaki et al., 1993) and guinea-pig (Seeds et al., 1993). Clinical studies examining the effectiveness of inhaled heparin have produced mixed results with some studies demonstrating a significant inhibition of exercise-induced bronchoconstriction (Ahmed et al., 1993a), the early response to house dust mite (Bowler et al., 1993) and the late asthmatic response to allergens (Weersink et al., 1994; Diamant et al., 1996), whilst others have been unable to demonstrate any significant attenuation of allergen-induced acute bronchoconstriction following heparin (O'Donnell et al., 1992) or the responses to sodium metabisulphite and methacholine challenge (Pavord et al., 1996).
The present study has examined the effect of pretreatment with aerosolized heparin, low molecular weight heparin (LMWH) and the polyanionic molecule, polyglutamic acid (PGA) on the development of airway hyperresponsiveness in an established animal model. The results demonstrate that following antigen challenge of actively sensitized animals treated with either saline or vehicle, the histamine concentration-effect curves for RL and Cdyn are significantly altered, as evidenced by a significant increase in the RLPC50 and a significant decrease in the CdynPC35 values, concurrent with an increase in the maximal responses obtained. These data demonstrate the development of airway hyperresponsiveness to histamine following antigen challenge in these animals. Following pretreatment with heparin or LMWH prior to antigen challenge however, the airway hyperresponsiveness to histamine is significantly inhibited. These results are in agreement with previous studies in the sheep (Ahmed et al., 1992; 1993b) where aerosol administration of heparin inhibited the development of airway hyperresponsiveness.
In those animals treated with heparin immediately following antigen challenge, the hyperresponsiveness to histamine in terms of changes in RL and Cdyn was still evident in animals treated with the lower dose (1000 U.ml−1) of heparin. At the higher dose (5000 U.ml−1) of heparin, hyperresponsiveness was still observed in Cdyn, but was not evident in RL. This latter result is most likely due to the large variation surrounding the data, as there is a clear trend towards a change in the curve following antigen challenge.
Administration of LMWH to the animals immediately following antigen challenge did not inhibit the development of hyperresponsiveness to histamine. This is in contrast to the results obtained when LMWH was administered prior to antigen challenge and suggests that the timing of the administration of LMWH is important in inhibiting the development of hyperresponsiveness to histamine in actively sensitized rabbits.
The linear polyanionic molecule, PGA, was used to determine whether the large negative charge of the heparin molecule may be responsible for the results obtained. When given prior to antigen challenge, PGA inhibited the change in RL following antigen challenge, but did not significantly inhibit the change in Cdyn. This latter result may be due to the significantly decreased baseline values for Cdyn observed 20 min after administration of PGA, the reasons for which are unknown. The results obtained from animals treated with PGA following antigen challenge were similar to that obtained when PGA was administered prior to antigen challenge, except that the RL curves were not significantly different in animals treated following antigen challenge. These results therefore, suggest that the large negative charge associated with heparin is unlikely to be responsible for the inhibition of hyperresponsiveness observed following treatment with heparin.
Previous studies (Sasaki et al., 1993; Seeds et al., 1993) have demonstrated a PAF-induced increase in eosinophil and neutrophil numbers in the BAL. Similarly, the present study has demonstrated a significant increase in antigen-induced neutrophil and eosinophil numbers in saline or vehicle-treated rabbits. In contrast to previous studies whereby heparin was administered intravenously (Sasaki et al., 1993; Seeds et al., 1993), the aerosol administration of heparin, LMWH and PGA in the present study failed to inhibit eosinophil and neutrophil infiltration following antigen challenge. In a study using a guinea-pig model of airway hyperresponsiveness, inhaled heparin inhibited eosinophil infiltration only at the highest administered dose and had no significant effect on neutrophil infiltration (Seeds et al., 1995). The failure of inhaled heparin to inhibit inflammatory cell infiltration into the airways is most likely due to difficulties in the penetration of these drugs into the circulation in concentrations sufficient to inhibit the migration of inflammatory cells. The ability of heparin and LMWH to inhibit the development of airway hyperresponsiveness whilst failing to inhibit the influx of inflammatory cells into the airways is in line with other studies (Herd et al., 1995) which have suggested that the presence of inflammatory cells in the airways is not necessarily a pre-requisite to the development of airway hyperresponsiveness.
In conclusion, the present study has demonstrated that heparin and LMWH can inhibit the development of airway hyperresponsiveness to histamine in a rabbit model. The linear polyanionic molecule, PGA was not as effective in inhibiting the airway hyperresponsiveness to inhaled histamine, suggesting that the inhibitory effects of heparin and LMWH are not due entirely to their anionic nature. In addition, the failure of these drugs to inhibit the inflammatory cell influx into the airway tissue suggests that the presence of inflammatory cells into the airways is not a pre-requisite to the development of airway hyperresponsiveness.
Abbreviations
- ANOVA
analysis of variance
- BAL
bronchoalveolar lavage
- Cdyn
dynamic compliance
- LMWH
low molecular weight heparin
- PAF
platelet activating factor
- PC
provocation concentration
- PGA
polyglutamic acid
- RL
total lung resistance
References
- AHMED T., ABRAHAM W.M., D'BROT J. Effects of inhaled heparin on immunologic and nonimmunologic bronchoconstrictor responses in sheep. Am. Rev. Respir. Dis. 1992;145:566–570. doi: 10.1164/ajrccm/145.3.566. [DOI] [PubMed] [Google Scholar]
- AHMED T., GARRIGO J., DANTA I. Preventing bronchoconstriction in exercise-induced asthma with inhaled heparin. N. Engl. J. Med. 1993a;329:90–95. doi: 10.1056/NEJM199307083290204. [DOI] [PubMed] [Google Scholar]
- AHMED T., SYRISTE T., LUCIO J., ABRAHAM W., ROBINSON M., D'BROT J. Inhibition of antigen-induced airway and cutaneous responses by heparin: a pharmacodynamic study. J. Appl. Physiol. 1993b;74:1492–1498. doi: 10.1152/jappl.1993.74.4.1492. [DOI] [PubMed] [Google Scholar]
- AHMED T., SYRISTE T., MENDELSSOHN R., SORACE D., MANSOUR E., LANSING M., ABRAHAM W.M., ROBINSON M.J. Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation. J. Appl. Physiol. 1994;76:893–901. doi: 10.1152/jappl.1994.76.2.893. [DOI] [PubMed] [Google Scholar]
- BLOOM G.D.Structural and biochemical characteristics of mast cells The inflammatory process 1974Volume 1Academic Press, Inc.: New York; 545–599.In: Zweifach, B.W., Grant, L. and McCluskey, R.T. (eds)2nd Ed [Google Scholar]
- BOWLER S.D., SMITH S.M., LAVERCOMBE P.S. Heparin inhibits the immediate response to antigen in the skin and lungs of allergic subjects. Am. Rev. Respir. Dis. 1993;147:160–163. doi: 10.1164/ajrccm/147.1.160. [DOI] [PubMed] [Google Scholar]
- BRUSASCO V., CRIMI E., PELLEGRINO R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53:992–998. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR J. The anti-inflammatory action of heparin: heparin as an antagonist to histamine, bradykinin and prostaglandin E1. Thromb. Res. 1979;16:507–516. doi: 10.1016/0049-3848(79)90097-5. [DOI] [PubMed] [Google Scholar]
- CLOWES A.W., KARNOVSKY M.J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;256:625–626. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- CONRAD H.E. Structure of heparan sulfate and dermatan sulfate. Ann. N.Y. Acad. Sci. 1989;556:18–28. doi: 10.1111/j.1749-6632.1989.tb22486.x. [DOI] [PubMed] [Google Scholar]
- COYLE A.J., PAGE C.P., ATKINSON L., FLANAGAN R., METZGER W.J. The requirement for platelets in allergen-induced late asthmatic airway obstruction. Am. Rev. Respir. Dis. 1990;142:587–593. doi: 10.1164/ajrccm/142.3.587. [DOI] [PubMed] [Google Scholar]
- COYLE A.J., PAGE C.P., ATKINSON L., SJOERDSMA K., TOUVAY C., METZGER W.J. Modification of allergen-induced airway obstruction and airway hyperresponsiveness in an allergic rabbit model by the selective platelet-activating factor antagonist, BN 52021. J. Allergy Clin. Immunol. 1989;84:960–967. doi: 10.1016/0091-6749(89)90395-3. [DOI] [PubMed] [Google Scholar]
- DIAMANT Z., TIMMERS M.C, , VAN DER VEEN H., PAGE C.P., VAN DER MEER F.J., STERK P.J. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. Am. J. Respir. Crit. Care Med. 1996;153:1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- DOLOWITZ D.A., DOUGHERTY T.F. The use of heparin in the control of allergies. Ann. Allergy. 1965;23:309–313. [PubMed] [Google Scholar]
- FLECKNALL P.A.Anaesthesia of common laboratory species: special considerations Laboratory animal anaesthesia: an introduction for research workers and technicians 1987Academic Press: London, U.K.98–100.In: Flecknall, P.A. (ed.) [Google Scholar]
- GREEN W.F., KONNARIS K., WOOLCOCK A.J. Effect of salbutamol, fenoterol, and sodium cromoglycate on the release of heparin from sensitized human lung fragments challenged with Dermatophagoides pteronyssinus allergen. Am. J. Respir. Cell Mol. Biol. 1993;8:518–521. doi: 10.1165/ajrcmb/8.5.518. [DOI] [PubMed] [Google Scholar]
- HARVIMA R.J., SCHWARTZ L.B.Mast cell-derived mediators The handbook of immunopharmacology: immunopharmacology of mast cells and basophils 1993Academic Press Ltd: London; 115–138.In: Foreman, J.C. (ed.) [Google Scholar]
- HERD C.M., DONIGI-GALE D., SHOUPE T.S., BURROUGHS D.A., YEADON M., PAGE C.P. Effect of a 5-lipoxygenase inhibitor and leukotriene antagonist (PF 5901) on antigen-induced airway responses in neonatally immunized rabbits. Br. J. Pharmacol. 1994;112:292–298. doi: 10.1111/j.1476-5381.1994.tb13067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERD C.M., GOZZARD N., PAGE C.P. Capsaicin pre-treatment prevents the development of antigen-induced airway hyperresponsiveness in neonatally immunised rabbits. Eur. J. Pharmacol. 1995;282:111–119. doi: 10.1016/0014-2999(95)00291-r. [DOI] [PubMed] [Google Scholar]
- HIRSCH G.M., KARNOVSKY M.J. Inhibition of vein graft intimal proliferative lesions in the rat by heparin. Am. J. Pathol. 1991;139:581–587. [PMC free article] [PubMed] [Google Scholar]
- HOOK M., KJELLEN L., JOHANSSON S., ROBINSON J. Cell surface glycosaminoglycans. Ann. Rev. Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- LARSEN G.L., WILSON M.C., CLARK R.A.F., BEHRENS B.L. The inflammatory reaction in the airways in an animal model of the late asthmatic response. Fed. Proc. 1987;46:105–112. [PubMed] [Google Scholar]
- LASSER E.C., SIMON R.A., LYON S.G., HAMBLIN A.E., STEIN R. Heparin-like anticoagulants and asthma. Allergy. 1987;42:619–625. doi: 10.1111/j.1398-9995.1987.tb00393.x. [DOI] [PubMed] [Google Scholar]
- LÖTVALL J., INMAN M., O'BYRNE P. Measurement of airway hyperresponsiveness: new considerations. Thorax. 1998;53:419–424. doi: 10.1136/thx.53.5.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSHALL E.M., RICCIO M.M., HERD C.M., DOUGLAS G.J., SEEDS E.A.M., MCKENNIFF M.G., SASAKI M., SPINA D., PAGE C.P. A novel animal model for investigating persistent airway hyperresponsiveness. J. Pharmacol. Toxicol. Meth. 1993;30:177–188. doi: 10.1016/1056-8719(93)90015-7. [DOI] [PubMed] [Google Scholar]
- O'DONNELL W.J., ROSENBERG M.A., KELLEHER K., DRAZEN J.M., ISRAEL E. Inhaled heparin does not protect asthmatics against antigen-induced bronchoconstriction. Am. Rev. Respir. Dis. 1992;145:A422. [Google Scholar]
- PAGE C.P., MINSHALL E.Mast cells and the lung The handbook of immunopharmacology: immunopharmacology of mast cells and basophils 1993Academic Press Ltd: London; 181–195.In: Foreman, J.C. (ed.) [Google Scholar]
- PAVORD I., MUDASSAR T., BENNETT J., WILDING P., KNOX A. The effect of inhaled heparin on bronchial reactivity to sodium metabisulphite and methacholine in patients with asthma. Eur. Respir. J. 1996;9:217–219. doi: 10.1183/09031936.96.09020217. [DOI] [PubMed] [Google Scholar]
- PINCKARD R.N., HALONEN M., MENG A.L. Preferential expression of anti-bovine serum albumin IgE homocytotropic antibody synthesis and anaphylactic sensitivity in the neonatal rabbit. J. Allergy Clin. Immunol. 1972;49:301–310. [Google Scholar]
- ROGERS C., KARNOVSKY M.J., EDELMAN E.R. Inhibition of experimental neointimal hyperplasia and thrombosis depends on the type of vascular injury and the site of drug administration. Circulation. 1993;88:1215–1221. doi: 10.1161/01.cir.88.3.1215. [DOI] [PubMed] [Google Scholar]
- SASAKI S. Production of lymphocytosis by polysaccharide polyphosphates (heparinoids) Nature. 1967;214:1041–1042. doi: 10.1038/2141041a0. [DOI] [PubMed] [Google Scholar]
- SASAKI M., HERD C.M., PAGE C.P. Effect of heparin and a low-molecular weight heparinoid on PAF-induced airway responses in neonatally immunized rabbits. Br. J. Pharmacol. 1993;110:107–112. doi: 10.1111/j.1476-5381.1993.tb13778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEDS E.A.M., HANSS J., PAGE C.P. The effect of heparin and related proteoglycans on allergen and PAF-induced eosinophil infiltration. J. Lipid. Med. 1993;7:269–278. [PubMed] [Google Scholar]
- SEEDS E.A.M., HORNE A.P., TYRELL D.J., PAGE C.P. The effect of inhaled heparin and related glycosaminoglycans on allergen-induced eosinophil infiltration in guinea-pigs. Pulmon. Pharmacol. 1995;8:97–105. doi: 10.1006/pulp.1995.1012. [DOI] [PubMed] [Google Scholar]
- SELYE H. The mast cells. Butterworths Inc., Washington D.C.; 1965. [Google Scholar]
- SHAMPAIN M.P., BEHRENS B.L., LARSEN G.L., HENSON P.M. An animal model of late pulmonary responses to Alternaria challenge. Am. Rev. Respir. Dis. 1982;126:493–498. doi: 10.1164/arrd.1982.126.3.493. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., ADAMS D.H., HUBSCHER S., HIRANO H., SIEBENLIST U., SHAW S. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993a;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- TANAKA Y., ADAMS D.H., SHAW S. Proteoglycans on endothelial cells present adhesion-inducing cytokines to leukocytes. Immunol. Today. 1993b;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- TYRRELL D.J., KILFEATHER S., PAGE C.P. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Tr. Pharmacol. Sci. 1995;16:198–204. doi: 10.1016/s0165-6147(00)89022-7. [DOI] [PubMed] [Google Scholar]
- VAN DER CARR F.R., WILLIAMS O.B. Further studies on the influence of heparin on anaphylactic shock in the guinea pig. J. Immunol. 1928;15:13–20. [Google Scholar]
- WEERSINK E.J.M., POSTMA D.S., AALBERS R., DEMONCHY J.G.R. Early and late asthmatic reaction after allergen challenge. Respir. Med. 1994;88:103–114. doi: 10.1016/0954-6111(94)90021-3. [DOI] [PubMed] [Google Scholar]


