Abstract
The present study extends our previous finding that the endothelium-independent relaxation in porcine coronary artery rings is enhanced after short-term (20 min) exposure to a physiological concentration (1 nM) of 17β-estradiol and demonstrates that this effect may be attributable to activation of the cyclic AMP pathway.
Isometric tension was recorded in isolated rings of porcine coronary arteries.
Relaxation by levcromakalim and sodium nitroprusside, but not bradykinin and calcium ionophore A23187, were significantly potentiated following 20 min treatment with 1 nM 17β-estradiol. This enhancing effect was insensitive to the transcriptional and translational inhibitors, actinomycin D and cycloheximide respectively and absent following repeated washing of the rings prior to construction of relaxation-response curves.
The potentiating actions of 1 nM 17β-estradiol on endothelium-independent relaxation were mimicked by the cyclic AMP analogue 8-Bromo-cyclic AMP and the protein kinase A activator Sp-cyclic AMPS but not by the cyclic GMP analogue 8-Bromo-cyclic GMP. The modulatory effect of 17β-estradiol was increased in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine.
The cyclic AMP-dependent protein kinase A inhibitor Rp-cyclic AMPS, but not the cyclic GMP antagonist Rp-8-Bromo-cyclic GMPS, effectively inhibited the enhancing effects 1 M 17β-estradiol had on the relaxation responses of levcromakalim and sodium nitroprusside.
These data support our earlier findings that physiologically relevant concentrations of 17β-estradiol can acutely modify vasorelaxation in vitro. Furthermore, we report that this short-term effect of 17β-estradiol on vasorelaxation appears to be mediated via non-genomic pathways and involves the cyclic AMP cascade.
Keywords: 17β-estradiol, cyclic AMP, cyclic GMP, cyclic AMP-dependent protein kinase, cyclic GMP-dependent protein kinase, porcine coronary artery, levcromakalim, sodium nitroprusside
Introduction
The observation that premenopausal women are less susceptible to coronary artery disease (CAD) than age-matched men and post-menopausal women has led to the postulation that the circulating levels of estrogens in premenopausal women curtail some of the established CAD risk factors (Barrett-Connor, 1997). Indeed, post-menopausal women who undergo estrogen replacement therapy not only demonstrate decreases in CAD morbidity (Dallongeville et al., 1995; Stampfer et al., 1991) but also reduction in CAD mortality (Ettinger et al., 1996; Stampfer et al., 1991).
Although the exact mechanism(s) behind the cardioprotective nature of estrogen is unresolved, the current consensus is that part of these benefits stem from its favourable modulation of serum lipid profiles (Ettinger et al., 1996; Dallongeville et al., 1995) and its antioxidant activities (Shwaery et al., 1997; Keaney et al., 1994). However, the modulation of lipoprotein levels can only account for 25–50% of the advantageous estrogenic effects (Bush et al., 1987; Lobo, 1991) while the contributory extent of the antioxidant events remains unknown.
Besides being able to influence the body's biochemical status, estrogen also acts directly on the physiology of the vasculature and this latter characteristic is now believed to also contribute significantly to the favourable influences of estrogen. Clinical studies indicate that acute arterial infusion of estrogen augments endothelium-dependent vasodilatation (Gilligan et al., 1994a,1994b). In vitro (Teoh et al., 1999; le Tran et al., 1997; Jiang et al., 1991) and in vivo (Sudhir et al., 1997; Williams et al., 1992) experiments have confirmed that such rapid vasoactive responses to 17β-estradiol are also apparent in the laboratory setting.
The promptness with which these vasodilatory responses were enhanced following exposure to estrogen suggests that the mechanisms responsible for de novo protein synthesis may not be involved in these events. This conflicts with the traditional concept that gene transcription and translation are mandatory for facilitating estrogen-induced activities. Recently, however, increasing numbers of studies have provided evidence for the existence of a neuronal membrane-bound estrogen receptor that swiftly mediates estrogen-evoked events. Some investigators have further implicated a role for the cyclic AMP cascade in these rapid estrogen effects (e.g. Gu & Moss, 1996; Minami et al., 1990). Previously, we reported that short-term exposure to physiological levels of 17β-estradiol enhanced endothelium-independent relaxation in isolated porcine coronary artery rings (Teoh et al., 1999). In the present study, we tried to determine if the acute phenomenon we recorded then involves gene-based mechanisms and if like the putative membrane-bound estrogen receptor, it is caused by activation of the cyclic AMP cascade.
Methods
Tissue samples
Pigs were culled according to the regulations laid down by the Urban Services Department, The Government of the Hong Kong SAR. Hearts from pigs of either sex were collected from the local abattoir and rinsed in cold, oxygenated (95% O2: 5% CO2) Krebs-Henseleit solution (KHS; composition in mM: NaCl 120, KCl 4.76, NaHCO3 25, NaH2PO4.H2O 1.18, CaCl2 1.25, MgSO4.7H2O 1.18, glucose 5.5) before the left anterior descending and right coronary arteries were isolated. After removal of the surrounding connective tissues, artery rings (3 mm) were suspended between stainless steel stirrups and stationary support rods positioned in 5 ml jacketed organ baths filled with oxygenated KHS maintained at 37°C. Rings were placed under a 2.0 g tension for at least 100 min during which bath KHS was changed periodically. Isometric tension was measured by force transducers (FT03, Grass Instrument Co., Quincy, U.S.A.) coupled to an amplifier and a personal computer for data collection (PICO Data Logger, Pico Technology Ltd., Cambridge, U.K.).
Functional Studies
The viability of each porcine coronary artery ring was determined by contracting with 30 nM U46619 in the presence of 10 μM indomethacin before relaxing with 1 μM bradykinin. Rings that failed to produce ⩾4.0 g contraction to U46619 and ⩾80% relaxation to bradykinin were excluded from the study. Indomethacin, U46619 and bradykinin were then removed by repeated changes of bath KHS. After baseline tensions were re-established, rings were incubated again with KHS containing 10 μM indomethacin for 20 min and then for 20 min with 17β-estradiol (final bath concentration 1 nM) or estradiol vehicle before contraction to 30 nM U46619 was elicited. The use of a 20 min incubation time was based on initial studies on the acute effect of 17β-estradiol where 20–30 min incubation produced similar enhancement of relaxation when compared to 4 h incubation. Upon achievement of a stable contraction, cumulative relaxation-response curves were constructed to bradykinin (0.1 nM–1 μM), calcium ionophore A23187 (0.1 nM–1 μM), levcromakalim (1 nM–100 μM) or sodium nitroprusside (SNP; 1 nM–100 μM). Relaxation-response curves of U46619-contracted rings to 8-Bromo-cyclic AMP, Sp-cyclic AMPS, 8-Bromo-cyclic GMP and 3-isobutyl-1-methylxanthine (all in the range of 1 nM–100 μM) were also determined.
In experiments requiring endothelium-disrupted rings, porcine coronary arteries were perfused at a rate of 1 ml min−1 for 30 s with either 0.5% Triton X-100 or KHS before being cut into 3 mm ring segments. Porcine coronary artery rings were considered to have disrupted endothelium after perfusion with Triton X-100 if they exhibited less than 20% relaxation in response to 1 μM bradykinin.
In some experiments, half the number of rings were ‘washed' every 15 min for 45 min after the 20 min vehicle/17β-estradiol incubation period ended. Rings that did not undergo this ‘washing' procedure were termed ‘unwashed' while those that did were referred to as ‘washed'. At the end of the 45 min ‘washing' session, rings were once again exposed to 10 μM indomethacin for 20 min before being contracted with 30 nM U46619. Relaxation-response curves to levcromakalim or SNP were then recorded.
The participation of genomic events in the acute enhancing effect of 1 nM 17β-estradiol was investigated by introducing actinomycin D or cycloheximide (both at final concentrations of 10 μM) into the baths together with indomethacin (20 min incubation before testing). To examine the involvement of cyclic nucleotides in this response, 8-Bromo-cyclic AMP, Sp-cyclic AMPs, 8-Bromo-cyclic GMP or 3-isobutyl-1-methylxanthine were added after rings had been incubated with 10 μM indomethacin for 20 min. In parallel experiments, Rp-cyclic AMPS or Rp-8-Bromo-cyclic GMPS were concomitantly administered with indomethacin. With the exception of the ‘wash-out' studies, all drugs remained in the baths for the duration of the experiment.
Data and statistical analyses
Data are reported as means±s.e.mean with n indicating the number of porcine hearts from which the arteries were obtained. Relaxation responses were expressed as a percentage of U46619-induced contraction. pD2 values were determined using a curve-fitting program (SigmaPlot, Jandel Scientific Software, CA, U.S.A.). Analysis of variance (ANOVA) followed by Bonferroni's correction were applied where appropriate to determine individual differences between multiple groups of data using a computer statistical package (SPSS, SPSS Inc., Chicago, IL, U.S.A.), A P value of <0.05 was considered to be significant.
Drugs
U46619 (9,11-dideoxy-9α, 11α-methanoepoxy prostaglandin F2α) was obtained from Biomol, PA, U.S.A. Levcromakalim was a gift from SmithKline Beecham, Harlow, Essex, U.K. 8-Bromo-cyclic AMP, Sp-cyclic AMPS, 8-Bromo-cyclic GMP, Rp-cyclic AMPS and Rp-8-Bromo-cyclic GMPS were purchased from BioLog Life Science Institute, Breman, Germany. 3-isobutyl-1-methylxanthine and the remaining chemicals were from Sigma, St. Louis, MO, U.S.A. Stocks of 17β-estradiol, U46619 and levcromakalim were made up in ethanol. The final concentration of ethanol in each bath was always ⩽0.2%. Calcium ionophore A23187 was dissolved in dimethyl sulphoxide (final bath concentration was 0.1%) and indomethacin was made up in a 1 mM Na2CO3 solution. Stock solutions of the remaining drugs were dissolved in deionized water. All working solutions were obtained by dilutions in KHS.
Results
Effects of actinomycin D and cycloheximide on the acute enhancing effects of 1 nM 17β-estradiol
Under control conditions (addition of vehicle), rings contracted 6.33±0.18 g to 30 nM U46619 (n=28). Baseline tensions as well as the contractile efforts of the thromboxane analogue were not appreciably affected by 20 min incubation with 1 nM 17β-estradiol. In agreement with Teoh et al. (1999), the presence of 1 nM 17β-estradiol in the bathing medium did not alter bradykinin- and calcium ionophore A23187-mediated endothelium-dependent relaxation (data not shown). However, this 20 min treatment with 17β-estradiol did potentiate endothelium-independent relaxation elicited by levcromakalim and SNP (Figure 1).
Figure 1.

Effects of actinomycin D and cycloheximide on the acute (20 min) enhancing effect 1 nM 17β-estradiol had on the concentration-response curves for (a) levcromakalim and (b) SNP. Data are means±s.e.mean with n=8 in each case. The response curves of all three treatment groups were significantly different to that of the vehicle-treated control group (*P<0.05).
Actinomycin D (10 μM) and cycloheximide (10 μM), on their own, had no effect on the resting tension and the U46619-induced contractions. Similarly, the enhancing effects that 1 nM 17β-estradiol had on drug-induced endothelium-independent relaxation were still evident in the presence of the transcription and translation inhibitors (Figure 1).
Effect of ‘washing out' on the acute enhancing effects of 1 nM 17β-estradiol
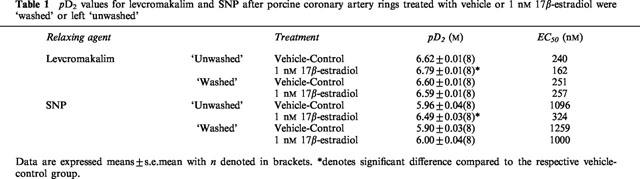
Contractions elicited by 30 nM U46619 in ‘washed' and ‘unwashed' rings were 6.54±0.20 g (n=14) and 6.63±0.24 g (n=14) respectively. There was no significant difference between the contraction responses attained by both groups of coronary artery rings (P>0.05). As shown in Figure 2, the enhancing effect of 1 nM 17β-estradiol was no longer apparent following the ‘washing out' procedure. The pD2 values for levcromakalim and SNP for this series of experiments are tabulated in Table 1.
Figure 2.
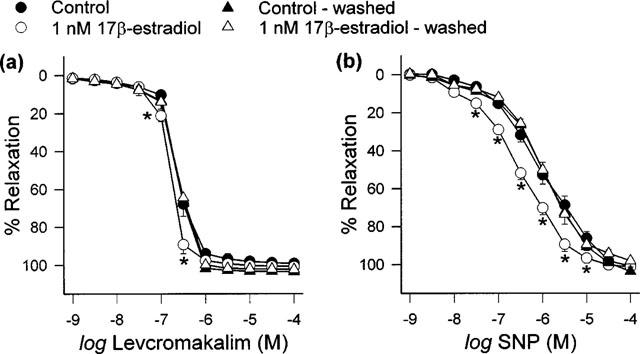
Effect of ‘washing out' on the acute (20 min) enhancing effect 1 nM 17β-estradiol had on (a) levcromakalim- and (b) SNP-mediated relaxation responses. Data are means±s.e.mean with n=8 in each case. *P<0.05 when compared to respective ‘unwashed' group.
Table 1.
pD2 values for levcromakalim and SNP after porcine coronary artery rings treated with vehicle or 1 nM 17β-estradiol were ‘washed' or left ‘unwashed'
Involvement of cyclic nucleotides in the acute enhancing effects of 1 nM 17β-estradiol
Relaxation-response curves to the cyclic nucleotide analogues and IBMX were recorded in U46619-contracted coronary artery rings. Within the concentration range studied, the rank order vasodilatory effectiveness was IBMX>8-Bromo-cyclic GMP>Sp-cyclic AMPS>8-Bromo-cyclic AMP (Figure 3). For each of the above drugs, subsequent experiments were performed with a concentration that did not itself have any significant effect on U46619-induced contractions. The concentrations chosen for 8-Bromo-cyclic AMP, Sp-cyclic AMPs, 8-Bromo-cyclic GMP and IBMX were 10, 3, 3 μM and 300 nM respectively. This definition was necessitated by our attempt to carry out comparisons between the potentiating effect of 1 nM 17β-estradiol which on its own did not produce any significant vasorelaxation and those of cyclic nucleotides that should also have no significant vasorelaxing effect.
Figure 3.
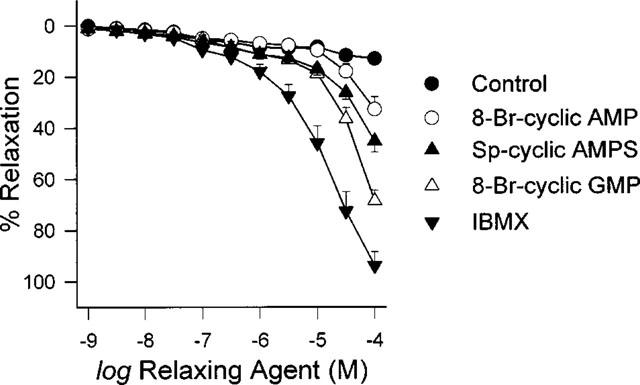
Relaxing effects of cyclic nucleotide analogues and IBMX. Rings were contracted with 30 nM U46619 before 8-Bromo-cyclic AMP, Sp-cyclic AMPs, 8-Bromo-cyclic GMP and IBMX were cumulatively added. Control data indicates curve obtained in the absence of any drug addition. Data are means±s.e.mean with n=6–7 in each case.
In our hands, 1 nM 17β-estradiol, 10 μM 8-Bromo-cyclic AMP, 3 μM Sp-cyclic AMPS and 3 μM 8-Bromo-cyclic GMP had no effect on relaxation responses elicited by bradykinin and calcium ionophore A23187 (data not shown). The effects on pD2 were summarized in Table 2. As illustrated in Figure 4, both levcromakalim- and SNP-evoked relaxation were enhanced following 20 min exposure to 1 nM 17β-estradiol, 10 μM 8-Bromo-cyclic AMP and 3 μM Sp-cyclic AMPS. Further comparisons indicated that these three agents also diminished the pD2 values for levcromakalim and SNP (Table 2). In contrast, the relaxation curves recorded for levcromakalim and SNP demonstrated little difference under control conditions and in the presence of 3 μM 8-bromo-cyclic GMP (Figure 4) as did their pD2 values (Table 2).
Table 2.
pD2 values for different relaxing agents after treatment with vehicle, 17β-estradiol, 8-Bromo-cyclic AMP, Sp-cyclic AMPS or 8-Bromo-cyclic GMP
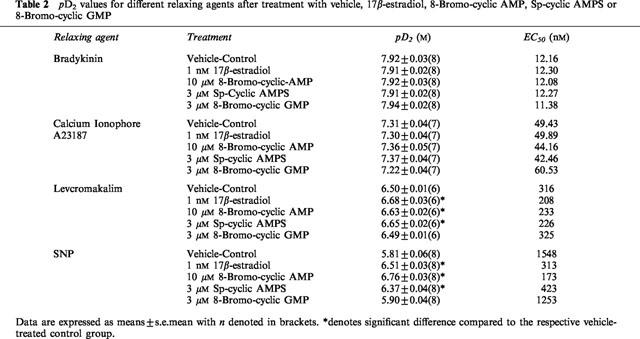
Figure 4.
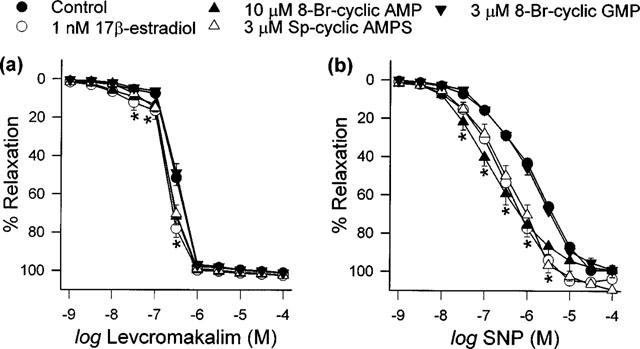
Effects of 20 min exposure to vehicle, 17β-estradiol, 8-Bromo-cyclic AMP, Sp-cyclic AMPS and 8-Bromo-cyclic GMP on relaxation responses to (a) levcromakalim and (b) SNP. Data are means±s.e.mean with n=6–8 in each case. Concentration-response curves obtained in the presence of 17β-estradiol, 8-Bromo-cyclic AMP and Sp-cyclic AMPS were significantly different to the vehicle-control curve (*P<0.05).
Neither Rp-cyclic AMPS (50 μM) nor Rp-8-Bromo-cyclic GMPS (10 μM) affected the baseline tensions or contraction responses to 30 nM U46619. Figure 5 shows the effects the cyclic AMP-dependent protein kinase A inhibitor and cyclic GMP antagonists had on the short-term enhancing actions of 1 nM 17β-estradiol in this model. Whilst the potentiating effects of 17β-estradiol on endothelium-independent relaxation were no longer evident in the presence of Rp-cyclic AMPS, they were retained in rings exposed to Rp-8-Bromo-cyclic GMPS. The pD2 values for levcromakalim and SNP under this set of conditions are tabulated in Table 3.
Figure 5.
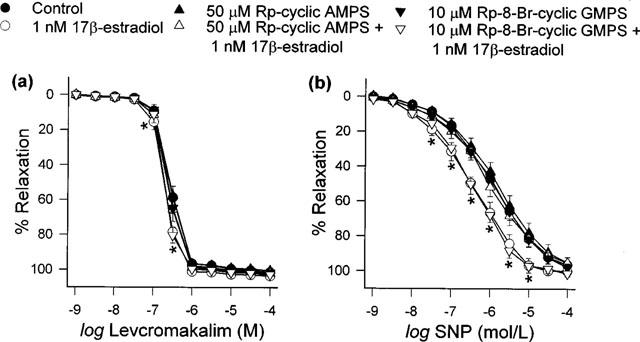
Effects of Rp-cyclic AMPS and Rp-8-Bromo-cyclic GMPS, alone and together with 1 nM 17β-estradiol, on relaxation responses produced by (a) levcromakalim and (b) SNP. Data are means±s.e.mean with n=6–8 in each case. Concentration-response curves obtained in the presence of 17β-estradiol (with and without Rp-8-Bromo-cyclic GMPS) were significantly different to the vehicle-control curve (*P<0.05).
Table 3.
pD2 values for levcromakalim and SNP after treatment of porcine coronary artery rings with 17β-estradiol under different conditions
IBMX (300 nM), on its own or in the presence of 1 nM 17β-estradiol, did not affect endothelium-dependent relaxation by bradykinin and calcium ionophore A23187 (data not shown). On the other hand, whilst this concentration of IBMX did not itself affect endothelium-independent relaxation by levcromakalim and SNP, there were evident leftward shifts in the relaxation-response curves of levcromakalim and SNP after artery rings had been co-incubated with 1 nM 17β-estradiol and 300 nM IBMX (Figure 6). Interestingly, these shifts were greater than those observed in the presence of 1 nM 17β-estradiol alone and thus resulted in significant reduction of pD2 values (Table 4).
Figure 6.
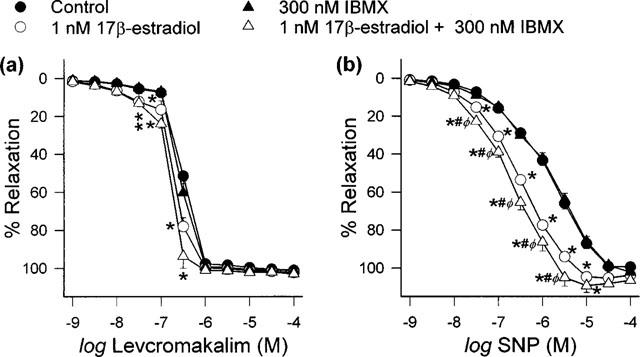
Effect of 300 nM IBMX, alone and with 1 nM 17β-estradiol, on (a) levcromakalim- and (b) SNP-evoked relaxation. Data are means±s.e.mean with n=7–8 in each case. *P<0.05 when compared to respective vehicle-treated control group; #P<0.05 when compared to respective 17β-estradiol-treated group; φP<0.05 when compared to respective IBMX-treated group.
Table 4.
pD2 values for different relaxing agents after treatment of porcine coronary artery rings with 1 nM 17β-estradiol and/or 300 nM IBMX
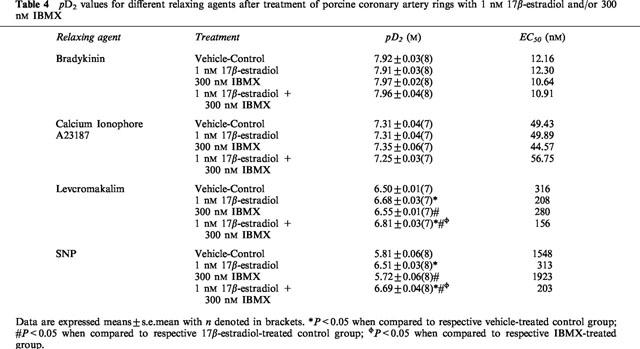
In order to further examine if in this model, the actions of 17β-estradiol were mediated via the cyclic AMP cascade, 17β-estradiol was concomitantly added to the baths with either 8-Bromo-cyclic AMP or Sp-cyclic AMPS. As illustrated in Figures 7 and 8, the responses observed when 17β-estradiol was incubated together with either 8-Bromo-cyclic AMP or Sp-cyclic AMPS were similar to those in the presence of only one of these agents.
Figure 7.
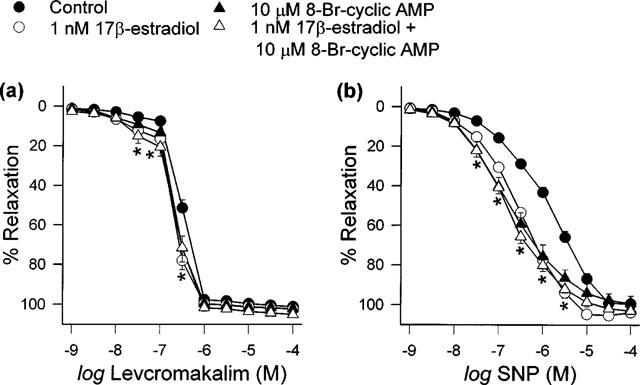
Effects of 8-Bromo-cyclic AMP, alone and together with 1 nM 17β-estradiol, on (a) levcromakalim- and (b) SNP-elicited relaxation. Data are means±s.e.mean with n=6–8 in each case. The response curves of all three treatment groups were significantly different to that of the vehicle-treated control group (*P<0.05).
Figure 8.
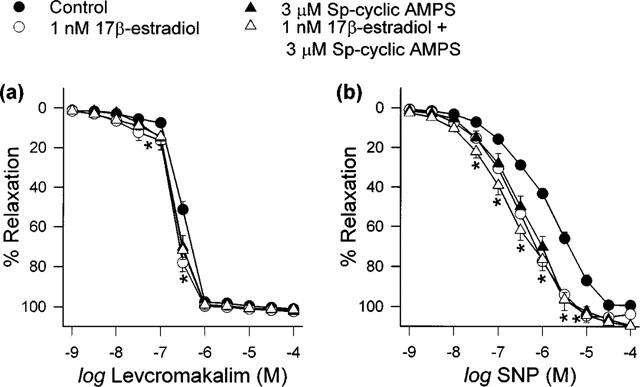
Effects of Sp-cyclic AMPS, alone and together with 1 nM 17β-estradiol, on (a) levcromakalim- and (b) SNP-elicited relaxation. Data are means±s.e.mean with n=6–8 in each case. The response curves of all three treatment groups were significantly different to that of the vehicle-treated control group (*P<0.05).
Discussion
In our earlier study, we found that endothelium-independent relaxation in isolated porcine coronary artery rings was potentiated following 20 min incubation with a physiologically relevant concentration of 17β-estradiol (Teoh et al., 1999). Here, using the same model, we have demonstrated that this rapid steroid-mediated effect is independent of genome-based pathways and the mechanism of this action involves activation of the cyclic AMP cascade.
A striking feature of this study is the concentration of the steroid required to present a prominent and quick response. Earlier work indicated that only micromolar concentrations of 17β-estradiol could produce rapid vasorelaxing effects in vitro (Teoh et al., 1999; Han et al., 1995; Jiang et al., 1991). In contrast, we have also noted that 20 min exposure to circulating concentrations (low nanomolar) of 17β-estradiol is sufficient to augment levcromakalim- and SNP-mediated relaxation in isolated coronary artery rings and that this modulation occurs in an all-or-nothing manner (Teoh et al., 1999). Interestingly, the potentiating effect was specific to 17β-estradiol as the same concentration of 17α-estradiol (Teoh et al., 1999), testosterone (Quan et al., 1999) and progresterone (Teoh & Man, 1999) had either no or opposite effects. This indicates that the response we recorded with 1 nM 17β-estradiol was not due to non-specific steroid-mediated activities.
That the transcription and translation inhibitors, actinomycin D and cycloheximide respectively could not limit the enhancing actions of 17β-estradiol implied that the nuclear estrogen receptor is not involved in this phenomenon. Furthermore, this effect was reproducible in the presence of the estrogen receptor antagonists tamoxifen and ICI 182,780 (Teoh et al., 1999). To the best of our knowledge, there is not yet any evidence for a vascular estrogen membrane binding site. Intriguingly, estrogen membrane receptors are speculated to exist in non-vascular tissues (see references in Farhat et al., 1996) with the strongest evidence thus far originating from electrophysiological studies on neuronal cells. The amplitude of kainate-induced currents was increased in hypothalamic neurons by 3 min application of 100 nM 17β-estradiol Gu & Moss (1996) whilst L-type currents in neostriatal neurons were diminished seconds after administration of 100 pM 17β-estradiol (Mermelstein et al., 1996). In addition, alterations in membrane potential shortly (⩽20 min) after application of low nanomolar concentrations of 17β-estradiol have been demonstrated in medial amygdala (Nabekura et al., 1986) and ventromedial hypothalamic neurons (Minami et al., 1990). The efficient low levels of 17β-estradiol together with the rate and stereospecific manner at which the membrane electrical properties were altered have hence led many to postulate the existence of a membrane-bound estrogen receptor on neuronal cells.
To date, several lines of evidence have further implicated cyclic AMP as a mediator of the fast-acting actions of 17β-estradiol in neuronal tissues. Minami et al. (1990) and Gu & Moss (1996) showed that the influence 17β-estradiol (nM concentrations) had on neuronal membrane electrical activity was mimicked by the cyclic AMP analogue 8-Bromo-cyclic AMP, enhanced by the phosphodiesterase inhibitor IBMX and not reproduced by the cyclic GMP analogue 8-Bromo-cyclic GMP. Similarly, in guinea-pig hypothalamic neurones, the potency of the μ agonist DAMGO was reduced by the protein kinase A activator Sp-cyclic AMPS in the same manner as by 20 nM 17β-estradiol while the cyclic AMP-dependent protein kinase A inhibitor Rp-cyclic AMPS reversed the steroid-induced effect (Lagrange et al., 1997). In vascular tissues, Rodriguez et al., 1996 also showed that the effect of high concentrations of estrogens involved cyclic AMP. Furthermore, a role for the cyclic AMP cascade in the regulation of gene transcription was demonstrated with physiological levels of 17β-estradiol (Aronica et al., 1994).
In our hands, both 8-Bromo-cyclic AMP and Sp-cyclic AMPS caused the levcromakalim and SNP concentration-response curves to be displaced to the left. This shift was similar in terms of both magnitude and latency to that produced by 1 nM 17β-estradiol. Interestingly, when 17β-estradiol was co-administered with either 8-Bromo-cyclic AMP or Sp-cyclic AMPS, the outcome was not significantly different from those obtained with each of these drugs alone. We postulate that the potentiating effect was not further increased because all three compounds act through the same mechanistic pathway. Conversely, the concentration of 8-Bromo-cyclic GMP we chose did not alter contraction to U46619 or affect relaxation by levcromakalim and SNP. These observations suggest that cyclic AMP but not cyclic GMP might be involved in the rapid response to 17β-estradiol seen in our preparation. We subsequently found that the phosphodiesterase inhibitor IBMX could potentiate the 17β-estradiol enhancing action. Finally, with Rp-cyclic AMPS and the cyclic GMP antagonist Rp-8-Bromo-cyclic GMPS, we demonstrated that only the former was capable of reversing the modulatory effects 17β-estradiol had on endothelium-independent relaxation. Taken together, these data indicate that the cyclic AMP cascade mediates the acute potentiating activity of 17β-estradiol.
Inasmuch as the enhancing response recorded in our model has similar pharmacodynamic and pharmacological properties as the putative fast-acting estrogen receptor reported in brain cells, it seems possible that the same or a similar receptor might be responsible for the phenomenon we observed. However, as to whether these membrane estrogen receptors exist in vascular tissues and are responsible for the rapid responses we describe with low nanomolar concentrations of 17β-estradiol remain unknown. Evidently, further investigations are warranted to clarify this question.
To conclude, we report that the enhancement of endothelium-independent relaxation in isolated porcine coronary artery rings following short-term exposure to a low concentration of 17β-estradiol (1 nM) appears to not require the activation of genomic signalling pathways and may involve an as yet unidentified vascular membrane estrogen receptor. In addition, our current findings suggest that part of the potentiating effect that is caused by 17β-estradiol may be attributed to activation of the cyclic AMP cascade.
Acknowledgments
A Committee on Research and Conference Grant, a University of Hong Kong Block grant and a post-doctoral fellowship (H. Teoh) from The University of Hong Kong supported this study. We thank Adrian Quan for his assistance in some of these experiments and Godfrey S.K. Man for his indispensable technical support. Both authors are members of the Institute of Cardiovascular Science and Medicine at the University of Hong Kong.
Abbreviations
- CAD
coronary artery disease
- cyclic AMP
adenosine 3′, 5′-cyclic monophosphate
- cyclic GMP
guanosine 3′,5′-cyclic monophosphate
- IBMX
3-isobutyl-1-methylxanthine
- KHS
Krebs-Henseleit solution
- SNP
sodium nitroprusside
References
- ARONICA S.M., KRAUSE W.L., KATZENELLENBOGEN B.S. Estrogen action via the cAMP signaling pathway: Stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT-CONNOR E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- BUSH T.L., BARRETT-CONNOR E., COWAN L.D., CRIQUI M.H., WALLACE R.B., SUCHINDRAN C.M., TYROLER H.A., RIFKIND B.M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- DALLONGEVILLE J., MARECAUX N., ISOREZ D., ZYLBERBERG G., FRUCHART J.-C., AMOUYEL P. Multiple coronary heart disease risk factors are associated with menopause and influenced by substitutive hormonal therapy in a cohort of French women. Atherosclerosis. 1995;118:2123–2133. doi: 10.1016/0021-9150(95)05599-r. [DOI] [PubMed] [Google Scholar]
- ETTINGER N., FRIEDMAN G.D., BUSH T., QUESENBERRY C.P., Jr Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet. Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- FARHAT M.Y., ABI-YOUNES S., RAMWELL P.W. Non-genomic effects of estrogen and the vessel wall. Biochem. Pharmacol. 1996;51:571–576. doi: 10.1016/s0006-2952(95)02159-0. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., BADAR D.M., PANZA J.A., QUYYUMI A.A., CANNON R.O. , 3RD Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994a;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., QUYYUMI A.A., CANNON R.O. , 3RD Effects of physiological levels of estrogen on coronary vasomotor function in postmenopausal women. Circulation. 1994b;89:2545–2551. doi: 10.1161/01.cir.89.6.2545. [DOI] [PubMed] [Google Scholar]
- GU Q., MOSS R.L. 17β-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J. Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN S.-Z., KARAKI H., OUCHI Y., AKISHITA M., ORIMO H. 17β-Estradiol inhibits Ca2+ influx and Ca2+ release induced by thromboxane A2 in porcine coronary artery. Circulation. 1995;91:2619–2626. doi: 10.1161/01.cir.91.10.2619. [DOI] [PubMed] [Google Scholar]
- JIANG C., SARREL P.M., LINDSAY D.C., POOLE-WILSON P.A., COLLINS P. Endothelium-independent relaxation of rabbit coronary artery by 17β-estradiol in vitro. Brit. J. Pharmacol. 1991;104:1033–1037. doi: 10.1111/j.1476-5381.1991.tb12545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEANEY J.F., SHWAERY G.T., XU A.-M. 17β-estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994;89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- LAGRANGE A.H., RØNNEKLEIV O.K., KELLY M.J. Modulation of G-protein-coupled receptors by an estrogen receptor that activates protein kinase A. Mol. Pharmacol. 1997;51:605–612. doi: 10.1124/mol.51.4.605. [DOI] [PubMed] [Google Scholar]
- LE TRAN Y., FUNG A., FORSTER C. Role of gender and vascular endothelium in rat aorta response to 17β-estradiol. Can. J. Physiol. Pharmacol. 1997;75:1393–1397. [PubMed] [Google Scholar]
- LOBO R.A. Effects of hormonal replacement on lipids and lipoproteins in postmenopausal women. J. Clin. Endocrinol. Metab. 1991;73:925–930. doi: 10.1210/jcem-73-5-925. [DOI] [PubMed] [Google Scholar]
- MERMELSTEIN P.G., BECKER J.B., SURMEIER D.J. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J. Neurosci. 1996;16:585–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINAMI T., OOMURA Y., NABEKURA J., FUKUDA A. 17β-Estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res. 1990;519:301–307. doi: 10.1016/0006-8993(90)90092-p. [DOI] [PubMed] [Google Scholar]
- NABEKURA J., OOMURA Y., MINAMI T., MIZUNO Y., FUKUDA A. Mechanism of the rapid effect of 17β-estradiol on medial amygdala neurons. Science. 1986;233:226–229. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- QUAN A., TEOH H., MAN R.Y.K. Acute exposure to a low level of testosterone impairs relaxation in porcine coronary arteries. Clin. Exp. Physiol. Pharmacol. 1999;26:830–832. doi: 10.1046/j.1440-1681.1999.03138.x. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ J., GARCIA DE BOTO M.J., HIDALGO A. Mechanisms involved in the relaxant effect of estrogens on rat aorta strips. Life Sciences. 1996;58:607–615. doi: 10.1016/0024-3205(95)02330-5. [DOI] [PubMed] [Google Scholar]
- SHWAERY G.T., VITA J.A., KEANEY J.F. Antioxidant protection of LDL by physiological concentrations of 17β-estradiol. Requirement for estradiol modification. Circulation. 1997;95:1378–1385. doi: 10.1161/01.cir.95.6.1378. [DOI] [PubMed] [Google Scholar]
- STAMPFER M.J., COLDITZ G.A., WILLETT W.C., MANSON J.E., ROSNER B., SPEIZER F.E., HENNEKENS C.H. Postmenopausal estrogen therapy and cardiovascular disease. N. Engl. J. Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- SUDHIR K., KO E., ZELLNER C., WONG H.E., HUTCHISON S.J. Physiological concentrations of estradiol attenuate endothelin-1- induced coronary vasoconstriction in vivo. Circulation. 1997;96:3626–3632. doi: 10.1161/01.cir.96.10.3626. [DOI] [PubMed] [Google Scholar]
- TEOH H., LEUNG S.W.S., MAN R.Y.K. Short-term exposure to physiological levels of 17β-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc. Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- TEOH H., MAN R.Y.K. Progesterone modulates estradiol actions: Acute effects at physiological concentrations. Eur. J. Pharmacol. 1999;378:57–62. doi: 10.1016/s0014-2999(99)00438-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.K., ADAMS M.R., HERRINGTON D.M., CLARKSON T.B. Short-term administration of estrogen and vascular responses of atherosclerotic coronary arteries. J. Am. Coll. Cardiol. 1992;20:452–457. doi: 10.1016/0735-1097(92)90116-5. [DOI] [PubMed] [Google Scholar]


