Abstract
Recently, it was demonstrated that 5-HT induces relaxation of human colon circular muscle through activation of 5-HT4 receptors and 5-HT7 receptors. The aim of the current study was to develop a new in vitro bioassay of human colon that would facilitate the pharmacological analysis of 5-HT responses mediated solely by 5-HT4 receptors.
Contracting circular muscle strips with KCl (80 mM) yielded a stable contractile tension and, in contrast to muscarinic cholinoceptor agonists and histamine, a profound reduction of spontaneous contractility. This allowed the establishment of reproducible, fully-defined, agonist concentration-response curves by cumulative dosing. Under these conditions, 5-HT induced a concentration-dependent relaxation (pEC50 7.31, Hill slope 0.91).
Neither methysergide (10 μM) nor granisetron (1 μM) affected the 5-HT-induced relaxation, suggesting that 5-HT1, 5-HT2, 5-HT3, 5-ht5, 5-HT6 or 5-HT7 receptors are not involved. The lack of effect of tetrodotoxin (0.3 μM) indicated a direct effect of 5-HT on the smooth muscle.
The selective 5-HT4 receptor antagonists GR 113808, GR 125487 and RS 39604 competitively antagonized the 5-HT-induced relaxation (pKB 9.43, 10.12 and 8.53, respectively). SB 204070 (1 nM) produced a rightward shift (pA2 10.34) and depression of the 5-HT curve. These affinity estimates are similar to those previously reported for 5-HT4 receptors.
The selective 5-HT4 receptor agonists, prucalopride and R076186, induced relaxations (pEC50 7.50 and 7.57, respectively), that were blocked by GR 113808 (3 nM), yielding pA2 estimates of 9.31 and 9.21, respectively.
To summarise, in KCl (80 mM)-contracted muscle strips, 5-HT induces relaxation through activation of a homogeneous smooth muscle 5-HT4 receptor population. This new bioassay allows the focused, pharmacological characterization of human colonic 5-HT4 receptors in vitro.
Keywords: 5-HT4 receptors, relaxation, 5-hydroxytryptamine, human, colon, large intestine
Introduction
5-HT4 receptors are representatives of the seven transmembrane domain, G-protein-coupled receptor family that are positively coupled to adenylyl cyclase (Dumuis et al., 1988). They have been extensively studied in human tissues, including the brain (Reynolds et al., 1995), heart (Kaumann, 1993), urinary bladder (Candura et al., 1996), and gut (Hedge & Eglen, 1996). With respect to the upper gastrointestinal tract, 5-HT4 receptors have been found in the stomach, facilitating cholinergic neurotransmission (Schuurkes et al., 1991), and in the small intestine, involved in secretory processes (Borman & Burleigh, 1993) and relaxation (Kuemmerle et al., 1995). Recently, it was suggested that 5-HT4 receptors are involved in the initiation of the peristaltic reflex (Grider et al., 1998).
In studies of human large intestine circular muscle, Burleigh (1977) observed that 5-HT induced a methysergide- and tetrodotoxin-insensitive relaxation. This 5-HT receptor was called ‘atypical', as it could not be classified contemporarily as either the M receptor (Morphine-sensitive 5-HT receptor, corresponding to the tetrodotoxin-sensitive 5-HT3 receptor) or D receptor (Dibenzyline (phenoxybenzamine)-sensitive 5-HT receptor, corresponding to the methysergide-sensitive 5-HT2 receptor). Subsequently, the 5-HT-induced relaxation of the circular muscle was ascribed to smooth muscle 5-HT4 receptor stimulation (Tam et al., 1994; McLean et al., 1995; Meulemans et al., 1995). Additionally, it was demonstrated that the activation of 5-HT4 receptors in the human colon was associated with the stimulation of cyclic AMP formation (McLean & Coupar, 1996).
These in vitro studies have contributed to the current knowledge of the involvement of 5-HT4 receptors in the motility of the lower gastrointestinal tract. Selective 5-HT4 receptor agonists, such as prucalopride (Emmanuel et al., 1998; Briejer et al., 1998a) and SDZ HTF-919 (Appel et al., 1997a,1997b), have been demonstrated to facilitate colonic transit and increase stool frequency. In this manner, selective 5-HT4 receptor agonists might be used to treat idiopathic constipation (Briejer et al., 1999). On the other hand, it was proposed that selective 5-HT4 receptor antagonists could be applied to relieve patients suffering from irritable bowel syndrome, presumably by reducing the hypersensitive response to endogenously released 5-HT at 5-HT4 receptors in these patients (Sanger, 1996).
However, in in vitro assays of human colon, selective competitive 5-HT4 receptor antagonists did not produce simple, concentration-dependent competitive antagonism of 5-HT-induced relaxation, either in studies measuring direct relaxation (McLean & Coupar, 1995; Meulemans et al., 1995) or the inhibition of spontaneous contractility (Tam et al., 1995). It has recently been reported that this complexity is due to the concomitant stimulation of 5-HT7 receptors, for which the selective 5-HT4 receptor antagonists have negligible affinity (Prins et al., 1999b). In addition to this receptor heterogeneity, the pharmacological characterization of the 5-HT4 receptors mediating smooth muscle relaxation is further complicated by the presence of spontaneous contractility that precludes the reliable measurement of 5-HT responses under existing assay conditions. Here, we report our attempts to improve the bioassay of human colon muscle to allow selective quantitative analysis of the 5-HT4 receptor-mediated component of 5-HT-induced relaxation.
Methods
Preparation
With the approval of the local ethics committee, segments of colon (ascending to sigmoid) and rectum were obtained from patients undergoing surgery for colonic cancer. The segment of colon was cut open longitudinally and luminal contents were rinsed out with Krebs-Henseleit solution (containing (mM) glucose 11.1, CaCl2 2.51, NaHCO3 25, MgSO4 1.18, KH2PO4 1.18, KCl 4.69 and NaCl 118) and the mucosa and mesentery were removed. The preparations were stored overnight at 4°C in fresh Krebs-Henseleit solution. The next day, eight circular muscle strips of approximately 2–3 cm were cut from the intertaenial area. The strips were anchored to organ bath hooks and suspended in a classical organ bath set-up for isometric measurement (20 mN tension). The 20 ml organ baths were filled with Krebs-Henseleit solution, kept at 37°C and gassed with carbogen (95% O2, 5% CO2).
Experimental protocol
The organ bath solution was replaced every 15 min for at least 60 min, until spontaneous contractility occurred. Then, treatment or vehicle were administered and left to incubate for 60 min. When included, pargyline (0.1 mM) was incubated for 30 min followed by a double replacement of the organ bath solution and a further period of 30 min. After the incubation, the strips were contracted with KCl (80 mM) and, after a stable contraction had been established (after approx. 120–180 min), agonists were added to the organ bath solution by cumulative dosing in half log unit concentration increments beginning at 1 nM. Only one curve was obtained per strip and at least one control curve to 5-HT was established per specimen of colon or rectum obtained. Approximately 16% of the total number of colon strips prepared were precluded from analysis for various reasons, such as inability to contract to KCl (inertia), decay of KCl-induced contraction and strips torn off the organ hook during contraction to KCl. Although no information was available to us concerning the state/pathology of each specimen there did not appear to be a relationship between those variables that were known to us (patient age, sex, and indication for surgery) and the failure of strips to respond.
Data analysis
The effect of compounds used to pre-contract the tissue on spontaneous contractility was expressed as the ratio of the amplitude of spontaneous contractility for a period of 5 min prior to contraction (X) over the amplitude of spontaneous contractility for 5 min prior to the addition of any relaxatory agonist (Y). In order to provide an estimate of this ratio when KCl was used as the pre-contractile agent, tracings of one muscle strip from each of six different specimens were chosen at random.
Responses to 5-HT receptor agonists were expressed as percentage of the within preparation KCl (80 mM)-induced contraction for both analysis and graphical presentation. Individual cumulative concentration-relaxation curves to agonists were fitted to the Hill equation, obtaining estimates for midpoint location pEC50, Hill slope (nH) and maximum asymptote (α). The effect of pre-treatment on these parameters was tested by one-way ANOVA (P<0.05). Antagonist affinity estimates were obtained by an iterative fitting procedure using the Schild equation, providing an estimate for pKB (according to the method described by Black et al., 1985).
If only one concentration of antagonist was tested, and the agonist curve was shifted to the right with no change in upper asymptote or slope, the antagonist affinity was expressed as a pA2 value calculated using the Schild equation. When an antagonist produced a depression of the curve, in addition to a rightward shift, the antagonist potency was expressed as an apparent pA2 value, also using the Schild equation (Arunlakshana & Schild, 1959).
Statistical analysis
To test the criteria for Schild-analysis, one-way ANOVA was performed, followed by a post-hoc Bonferroni's test for multiple comparisons. The effect of a single pretreatment was assessed by one-way ANOVA. A level of P<0.05 was considered to be significant. As some strips were precluded from subsequent analysis (see above), the number of patient tissues (denoted by n) in the control group was sometimes different from that in the treatment groups.
Compounds
The following compounds were used (with their respective suppliers given in parentheses): (1-butyl-4-piperidinyl) - methyl-8-amino-7-chloro1,4-benzodioxane-5-carboxylate HCl (SB 204070), 1-[2-[(methylsulphonyl)amino] ethyl]-4-piperidinyl-methyl 5-fluoro-2-methoxy-1H-indole-3-carboxylate (GR 125487), [1-[2-[(methylsulphonyl)amino]ethyl]-4-piperidinyl]methyl 1-methyl-1H-indole-3-carboxylate (GR 113808), granisetron HCl, 4-amino-5-chloro-2,3-dihydro-N-(1-[3-methoxypropyl]-4-piperidinyl)-7-benzofurancarboxamide HCl (prucalopride; R093877), cis-4-amino-5-chloro-N-[1- [4 - [4 - (dimethylamino)-1-piperidinyl]-4-oxo - butyl] - 3 - methoxy - 4 - piperidinyl] -2-methoxybenzamide (R076186) (Janssen Research Foundation, Belgium); carbachol, histamine HCl (Janssen Chimica, Belgium); tetrodotoxin, 5-HT creatinine sulphate, (Serva, Germany); methysergide maleate (Sandoz, Switzerland); pargyline HCl (Abbott, U.S.A.); potassium chloride (KCl; Sigma, Belgium); corticosterone, cocaine HCl (Merck, Germany); fluoxetine HCl (Tocris Cookson, U.K.); 5-methylfurmethide (James Black Foundation, U.K.) and 1-(4-amino-5-chloro-2-(3,5-dimethoxy) benzyloxyphenyl)-3-[1 -((2-methylsulphonylamino)ethyl)piperidin-4-yl]-1-propanone (RS 39604; Merck Belgolabo, Belgium).
All compounds were dissolved in 0.9% NaCl solution, except for GR 113808 and R076186, which were dissolved in 0.9% NaCl acidified in the stock solution with tartaric acid, and pargyline, which was dissolved in distilled water with 10% cyclodextrin in the stock solution. The solvents had no effect on the baseline tension or the curves to 5-HT, and the total volume of compound solution added to the organ bath never exceeded 1 ml, to avoid significant dilution of the KCl concentration. The solutions were prepared freshly on the day of the experiment and all dilutions were prepared using 0.9% NaCl solution.
Results
In order to study relaxations to low concentrations of agonists and to construct reproducible concentration-relaxation curves, a stable basal contractile state of the muscle strips is required. The spontaneous contractility, that progressively developed while replacing the organ bath solution every 15 min, was subject to variability both in amplitude and frequency. This variation was observed within one strip, between the strips of one specimen, and also between the strips of different specimens. Accordingly, the spontaneous contractility was not used as a contractile state from which to measure relaxatory responses to 5-HT and further preliminary experimentation was performed in order to obtain reproducible and stable precontractions. The non-selective muscarinic cholinoceptor agonists, carbachol (1 μM, spontaneous contractility ratio 0.8±0.1; n=6 specimens) and 5-methylfurmethide (1 μM, ratio 0.4±0.2; n=2 specimens), produced stable increases in contractile tension but they also enhanced spontaneous contractions decreasing the assay signal-to-noise ratio. Histamine (10 μM, ratio 0.9±0.3; n=2 specimens) had a similar effect and was thus also considered unsuitable for these studies. In contrast, KCl (80 mM) induced a contraction that reached an initial peak within about 30 min and then faded to a variable extent over 120–180 min to attain a plateau. This was maintained for at least a further 120 min, with reduced spontaneous contractility (Figure 1; ratio 16±7). As a consequence, KCl was chosen to contract the strips in all further experiments.
Figure 1.
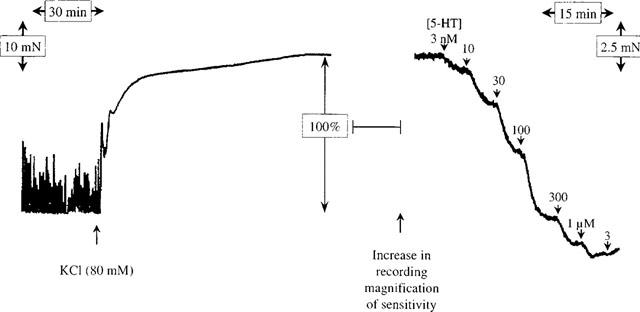
Representative chart-recorder tracing of an experiment with human isolated colonic circular muscle. Shown are the KCl-induced contraction (left), which, after a stabilization time and an increase in the recording magnification of sensitivity, is followed by adding 5-HT in half log unit ascending cumulative concentrations (right).
5-HT induced a concentration-dependent relaxation of KCl contracted muscle strips, yielding a monophasic sigmoidal concentration-response curve (Figure 1 and Table 1), consistent with a single-site interaction. The 23 specimens used in the experiments were distributed down the large intestine as follows; ascending colon (four specimens), transverse colon (one), descending colon (one), sigmoid colon (16) and rectum (one). The midpoint location, slope and upper asymptotes of the 5-HT curves obtained in the two regions for which there sufficient samples to make a formal comparison, the ascending colon, (pEC50=7.34±0.10, nH=0.92±0.16, α=44±7%) and the sigmoid colon (pEC50=7.35±0.05, nH=1.06±0.11, α=31±3%), were indistinguishable. Similarly, there were no other obvious differences in the 5-HT curves obtained on tissues from the other regions. Accordingly, the data from all regions were pooled for subsequent analysis.
Table 1.
Effects of tetrodotoxin, receptor antagonists and inhibitiors of re-uptake and breakdown, on mean curve parameters of 5-HT in human colon circular muscle assay
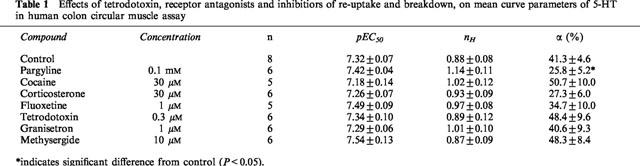
Inhibitors of uptake-1 (cocaine, 30 μM), uptake-2 (corticosterone, 30 μM) or selective serotonin re-uptake (fluoxetine, 1 μM) had no significant effect on the 5-HT curve parameters. Irreversible and non-selective inhibition of monoamine oxidase by pargyline (0.1 mM) significantly reduced the maximum response to 5-HT without increasing the potency. Hence, inhibitors of breakdown or re-uptake of 5-HT were not used. Tetrodotoxin (0.3 μM) did not affect the curve to 5-HT, neither did it change the precontraction induced by KCl. The data relating to the effects of the above-mentioned compounds on the 5-HT-induced relaxation are presented in Table 1.
Agonists
5-HT and the selective 5-HT4 receptor agonists, prucalopride and R076186, produced concentration-dependent relaxations (Figures 1 and 2, Table 2). The midpoint location, slope and upper asymptote estimates for the 5-HT, prucalopride and R076186 curves were not significantly different.
Figure 2.
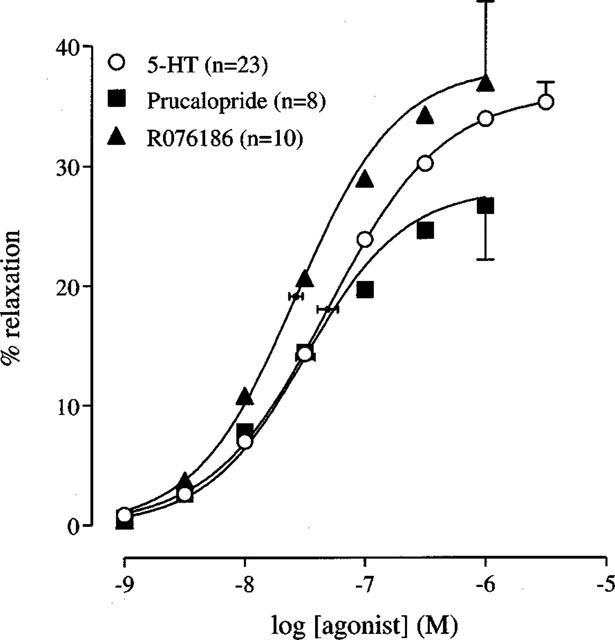
Concentration-response curves to 5-HT, prucalopride and R076186 in the human isolated colonic circular muscle. The curves shown superimposed on the mean experimental data points represent simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope, that were obtained from the iterative fitting procedure.
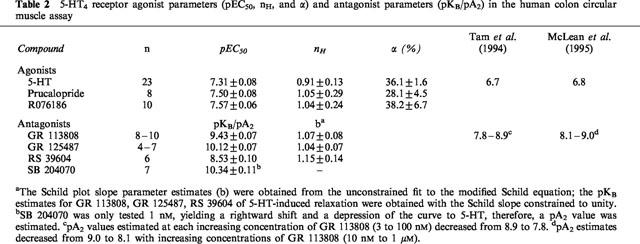
Table 2.
5-HT4 receptor agonist parameters (pEC50, nH, and α) and antagonist parameters (pKB/pA2) in the human colon circular muscle assay
Antagonists
The selective 5-HT3 receptor antagonist granisetron (1 μM; Sanger & Nelson, 1989) did not alter the curve to 5-HT, indicating that 5-HT3 receptors were not involved (Table 1). Similarly, the 5-HT1, 5-HT2, 5-ht5, 5-HT6, 5-HT7 receptor antagonist methysergide (10 μM; Gommeren et al., 1998) did not affect the curve to 5-HT. None of the selective 5-HT4 receptor antagonists GR 113808, GR 125487 and RS 39604 significantly affected the maximal response or the Hill slope of the 5-HT curve and thus they all behaved as simple competitive antagonists (pKB 9.43±0.07; 10.12±0.07 and 8.53±0.10, respectively; Figure 3; Table 2). Another selective 5-HT4 receptor antagonist, SB 204070, was only tested at 1 nM. This concentration shifted the 5-HT curve to the right (apparent pA2=10.34±0.11) but also reduced the maximal response to 5-HT (Figure 4). Additionally, GR 113808 (3 nM) produced a parallel rightward shift of prucalopride and R076186 curves associated with pA2 values of 9.31±0.14 and 9.21±0.12, respectively (Figure 5).
Figure 3.
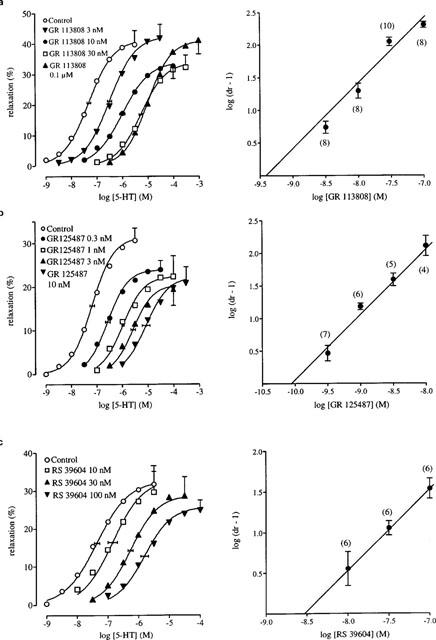
Left panel: The effect of (a) GR 113808 (b) GR 125487 and (c) RS 39604 on 5-HT-induced relaxation in the human isolated colonic circular muscle. The curves shown superimposed on the mean experimental data points represent simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope, that were obtained from the iterative fitting procedure. Right panel: Schild plots of GR 113808 (a); GR 125487 (b) and RS 39604 (c) with the Schild slope constrained to unity. The number depicted in parentheses above each average log (dr-1) point represents the number of curves constructed with that concentration of antagonist.
Figure 4.
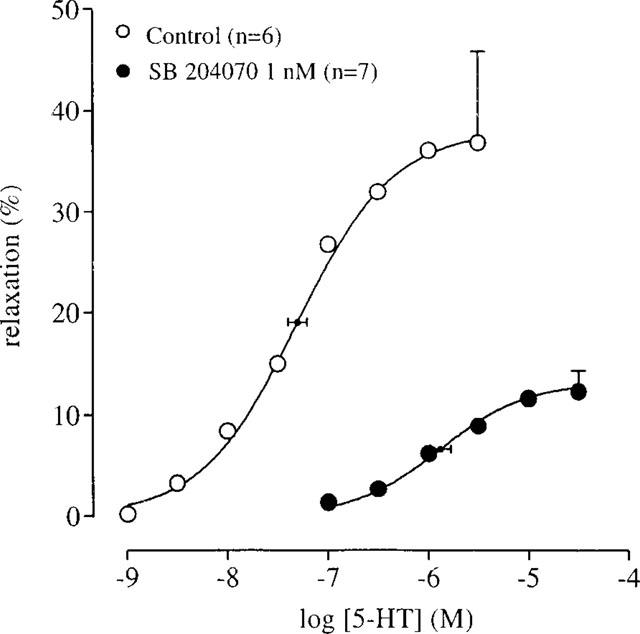
The effect of SB 204070 on 5-HT-induced relaxation of human isolated colonic circular muscle. The curves shown superimposed on the mean experimental data points represent simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope, that were obtained from the iterative fitting procedure.
Figure 5.
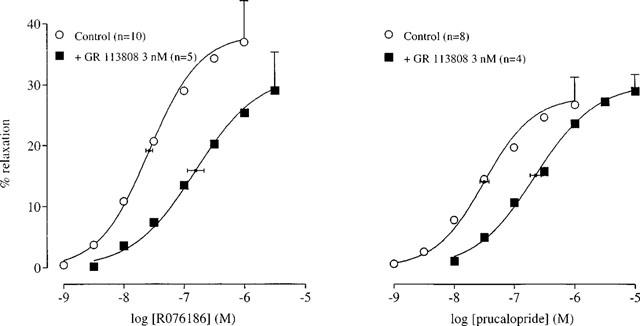
The effect of the selective 5-HT4 receptor antagonist GR 113808 on the relaxation curve to prucalopride and R076186 in the human isolated colonic circular muscle. The curves shown superimposed on the mean experimental data points represent simulations using the Hill-equation and the parameters for midpoint location (with horizontal standard error bars), upper asymptote location (with vertical standard error bars) and the Hill slope, that were obtained from the iterative fitting procedure.
Discussion
The present data indicate that when KCl (80 mM) is used to pre-contract human colonic circular smooth muscle, the relaxation induced by 5-HT is mediated solely by a homogeneous population of 5-HT4 receptors. Prior to this study, relaxations to 5-HT were assessed in bioassays measuring inhibition of spontaneous contractility (Tam et al., 1994), relaxation of tone either in the absence or presence of carbachol (McLean et al., 1995) or relaxation of histamine-induced contractions (Meulemans et al., 1995). However, in our hands, it was this spontaneous contractility, and, moreover, the lack of a stable tone that complicated the establishment of reproducible cumulative concentration-response curves. The use of KCl minimized the spontaneous contractility and induced a slowly equilibrating but stable tone so that reproducible, fully-defined, agonist concentration-response curves could be obtained by cumulative dosing. It has been demonstrated that KCl-induced contractions are suitable for the observation of relaxations to various agonists in tissues, including the bladder (Hedge et al., 1997), stomach (Molderings et al., 1998) and colon (MacDonald et al., 1996).
Tetrodotoxin did not alter the relaxation to 5-HT, indicating that the relaxant 5-HT4 receptor is located on the smooth muscle. Furthermore, fluoxetine, cocaine, corticosterone and pargyline did not alter the potency of 5-HT (although pargyline reduced the maximal response to 5-HT), demonstrating that the exogenously administered 5-HT was not subject to significant pre-junctional (re-)uptake or metabolism. If pargyline was producing an effect on 5-HT disposition, then a leftward shift of the midpoint location of 5-HT curve might be expected rather than the depression of the upper asymptote which was obtained. Therefore, this effect of pargyline was probably not due to an effect on inhibition of monoamine oxidase or 5-HT uptake.
The selective 5-HT4 receptor agonists, prucalopride (Briejer et al., 1998b) and R076186 (Briejer et al., 1993) induced relaxation of the muscle strips at 5-HT4 receptor-selective concentrations. Previously, we showed that prucalopride and R076186 also induce 5-HT4 receptor-mediated relaxation of canine rectal circular smooth muscle (Prins et al., 1999a). The small standard errors associated with the pEC50 estimates of all of the agonists tested, either in the absence or presence of antagonists, illustrates the improved reproducibility and increased signal-to-noise ratio in the new assays compared to those used previously (Tam et al., 1994; McLean et al., 1995).
The affinity estimates obtained for the selective 5-HT4 receptor antagonists GR 113808 (Gale et al., 1994b; Grossman et al., 1993; Kaumann, 1993), GR 125487 (Gale et al., 1994a), RS 39604 (Hedge et al., 1995) and SB 204070 (Wardle et al., 1994) were similar to previously reported values obtained in human, rat and/or guinea-pig tissues (see corresponding references). The antagonist potency for GR 113808 was independent of the agonist used since the pA2 values estimated from the interaction with prucalopride and R076186 were indistinguishable from the pKB value estimated using 5-HT as agonist. The insurmountable antagonism expressed by SB 204070 is consistent with previous reports of its behaviour as a slowly dissociating antagonist in assays of guinea-pig, rat and canine tissues (Wardle et al., 1994; Zeitung et al., 1998; Leung et al., 1995; Prins et al., 1999a). In these studies, similar apparent pA2 values were reported, varying between 10 and 11.
The striking observation of this study was that the 5-HT-induced relaxation appeared to be entirely mediated by 5-HT4 receptor stimulation, as was demonstrated by the concentration-dependent rightward shift of the curve to 5-HT by GR 113808, GR 125487 and RS 39604 (Figure 3) over dose ratios greater than 100. The results with GR 113808 contrast with previous reports in the human colon, measuring inhibition of spontaneous contractility, in which the profile of GR 113808-induced antagonism was not consistent with competition at a single class of receptor. Thus, GR 113808 shifted the curve to 5-HT in parallel to the right at 3 nM (pA2 of 8.9), however, at 10–100 nM GR 113808 produced little to no further rightward shift (Tam et al., 1995; Table 2). Similarly, McLean et al. (1995), measuring direct relaxation, demonstrated that GR 113808 (10 nM) produced a parallel rightward displacement of the curve to 5-HT (pA2 9.0), but that this displacement did not linearly increase with the concentration of GR 113808 (100 nM; pA2 8.7 and 1 μM; pA2 8.1). In line with these observations, McLean & Coupar (1995) demonstrated that the selective 5-HT4 receptor antagonist SB 207710 shifted the curve to 5-HT rightward, but the Schild slope (0.5) was significantly less than unity.
These reported deviations from expected competitivity of the selective 5-HT4 receptor antagonists, pointed to the possibility of simultaneous stimulation of another 5-HT receptor for which they have lower affinity. Interestingly, in the combined presence of methysergide and ondansetron (both at 10 μM; a treatment that will not produce significant block of 5-HT4 receptors), the Schild slope of SB 207710 changed to a value not significantly different from unity, consistent with simple competitive antagonism (McLean & Coupar, 1995). The pKB value obtained (pKB 10.1) was consistent with those observed at 5-HT4 receptors on guinea-pig colon (apparent pA2 10.0; Brown et al., 1993) and human atrium (pKB 10.0; Kaumann et al., 1994). An explanation of this phenomenon was found very recently, in a study in our laboratory, demonstrating that in addition to 5-HT4 receptors, smooth muscle 5-HT7 receptors also mediate relaxation of human colon (Prins et al., 1999b). Therefore, it is most likely that the potent 5-HT7 receptor antagonist methysergide (Carter et al., 1995; Gommeren et al., 1998) was inhibiting the effect of 5-HT7 receptors in the study by McLean & Coupar (1995).
The bioassay developed here encounters none of these problems, as the necessity to include antagonists to observe 5-HT4 receptor-mediated effects alone is absent, as shown by the parallel rightward shift by GR 125487, GR 113808 and RS 39604 of the curve to 5-HT. Presumably, the 5-HT7 receptor-mediated component is sensitive to the nature and the level of pre-contraction. Why the 5-HT7 receptor is not operational when the tissues are contracted with KCl, remains to be determined. The advantage of this new bioassay is that it facilitates the clear-cut, quantitative pharmacological analysis of 5-HT4 receptors in the human colon. Furthermore, the minimization of spontaneous contractility allows the observation of agonism at low agonist concentrations and may increase the probability of observing responses to low-efficacy agonists.
In conclusion, it has been demonstrated that in KCl-precontracted human colonic circular muscle strips, the 5-HT-induced relaxation is due to smooth muscle 5-HT4 receptor stimulation. The 5-HT-induced relaxation is exclusively mediated by 5-HT4 receptors, with no involvement of 5-HT7 receptors, that presumably complicated previous characterizations of colonic 5-HT4 receptors. Thus, this improved bioassay allows the quantitative, pharmacological characterization of human colonic 5-HT4 receptors.
Abbreviations
- D receptor
dibenzyline (phenoxybenzamine)-sensitive 5-HT receptor
- M receptor
morphine-sensitive 5-HT receptor
References
- APPEL S., KUMLE A., HUBERT M., DUVAUCHELLE T. First pharmacokinetic-pharmacodynamic study in humans with a selective 5-hydroxytryptamine4 receptor agonist. J. Clin. Pharmacol. 1997a;37:229–237. doi: 10.1002/j.1552-4604.1997.tb04785.x. [DOI] [PubMed] [Google Scholar]
- APPEL S., KULME A., MEIER R. Clinical pharmacodynamics of SDZ HTF 919, a new 5-HT4 receptor agonist, in a model of slow colonic transit. Clin. Pharmacol. Ther. 1997b;62:546–555. doi: 10.1016/S0009-9236(97)90050-3. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–54. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK J.W., LEFF P., SHANKLEY N.P. Further analysis of anomalous pKB values for histamine H2-receptor antagonists on the mouse isolated stomach assay. Br. J. Pharmacol. 1985;86:581–587. doi: 10.1111/j.1476-5381.1985.tb08934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Evidence for the involvement of a 5-HT4 receptor in the secretory response of human small intestine to 5-HT. Br. J. Pharmacol. 1993;110:927–928. doi: 10.1111/j.1476-5381.1993.tb13901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRIEJER M.R., AKKERMANS L.M.A., MEULEMANS A.L., LEFEBVRE R.A., SCHUURKES J.A.J. Cisapride and a structural analogue, R 76,186, are 5-hydroxytryptamine4 (5-HT4) receptor agonists on the guinea-pig colon ascendens. Naunyn-Schmiedebergs' Arch. Pharmacol. 1993;347:464–470. doi: 10.1007/BF00166736. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., GHOOS E., EELEN J., SCHUURKES J.A.J. Serotonin 5-HT4 receptors mediate the R093877-induced changes in contractile patterns in the canine colon. Gastroenterology. 1998a;112:A705. [Google Scholar]
- BRIEJER M.R., MEULEMANS A.L., BOSMANS J.-P., VAN DAELE P., SCHUURKES J.A.J. In vitro pharmacology of the novel enterokinetic R093877. Gastroenterology. 1998b;112:A704. [Google Scholar]
- BRIEJER M.R., SCHUURKES J.A.J., SARNA S.K. Idiopathic constipation: two few stools and too little knowledge. Trends Pharmacol. Sci. 1999;20:1–3. doi: 10.1016/s0165-6147(98)01278-4. [DOI] [PubMed] [Google Scholar]
- BROWN A.M., YOUNG T.J., PATCH T.L., CHEUNG C.W., KAUMANN A.J., GASTER L., KING F.D. [125I]-SB207710, a potent, selective radioligand for 5-HT4 receptors. Br. J. Pharmacol. 1993;110:10P. [Google Scholar]
- BURLEIGH D.E. Evidence for more than one type of 5-hydroxytryptamine receptor in the human colon. J. Pharm. Pharmacol. 1997;29:538–541. doi: 10.1111/j.2042-7158.1977.tb11391.x. [DOI] [PubMed] [Google Scholar]
- CANDURA S.M., MESSORI E., FRANCESCHETTI G.P., D'AGOSTINO G., VICINI D., TAGLIANI M., TONINI M. Neural 5-HT4 receptors in the human isolated detrusor muscle: effects of indole, benzimidazolone and substituted benzamide agonists and antagonists. Br. J. Pharmacol. 1996;118:1965–1970. doi: 10.1111/j.1476-5381.1996.tb15631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTER D., CHAMPNEY M., HWANG B., EGLEN R.M. Characterization of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur. J. Pharmacol. 1995;280:243–250. doi: 10.1016/0014-2999(95)00195-q. [DOI] [PubMed] [Google Scholar]
- DUMUIS A., BOUHELAL R., SEBBEN M., CORY R., BOCKAERT J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol. Pharmacol. 1988;34:880–887. [PubMed] [Google Scholar]
- EMMANUEL A.V., KAMM M.A., ROY A.J., ANTONELLI K. Effect of a novel prokinetic drug, R093877, on gastrointestinal transit in healthy volunteers. Gut. 1998;42:511–516. doi: 10.1136/gut.42.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., DARTON J., BUNCE K.T., WHITEHEAD J.W.F., KNIGHT J., PARKHOUSE T.J., OXFORD A.W., HUMPHREY P.P.A. GR125487: A selective and high affinity 5-HT4 receptor antagonist. Br. J. Pharmacol. 1994a;113 Suppl.:120P. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE J.D., GROSSMAN C.J., WHITEHEAD J.W.F., OXFORD A.W., BUNCE K.T., HUMPHREY P.P. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br. J. Pharmacol. 1994b;111:332–338. doi: 10.1111/j.1476-5381.1994.tb14064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOMMEREN W., RENDERS J., VAN GOMPEL P., LESAGE A., LEYSEN J., JURZAK M. Extensive pharmacological study of the G protein-coupled fraction of human 5-HT receptors using agonist radioligand binding. Naunyn-Schmiedebergs' Arch. Pharmacol. 1998;358:P8.42. [Google Scholar]
- GRIDER J.R., FOXX-ORENSTEIN A.E., JIN J.G. 5-Hydroxytryptamine4 receptor agonists initiate the peristaltic reflex in human, rat, and guinea-pig intestine. Gastroenterology. 1998;115:370–380. doi: 10.1016/s0016-5085(98)70203-3. [DOI] [PubMed] [Google Scholar]
- GROSSMAN C.J., KILPATRICK G.J., BUNCE K.T. Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br. J. Pharmacol. 1993;109:618–624. doi: 10.1111/j.1476-5381.1993.tb13617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- HEGDE S.S., BONHAUS D.W., JOHNSON L.G., LEUNG E., CLARK R.D., EGLEN R.M. RS 39604: a potent, selective and orally active 5-HT4 receptor antagonist. Br. J. Pharmacol. 1995;115:1087–1095. doi: 10.1111/j.1476-5381.1995.tb15922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDGE S.S., CHOPPIN A., BONHAUS D., BRIAUD S., LOEB M., MOY T.M., LOURY D., EGLEN R.M. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br. J. Pharmacol. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. Blockade of human atrial 5-HT4 receptors by GR 113808. Br. J. Pharmacol. 1993;110:1172–1174. doi: 10.1111/j.1476-5381.1993.tb13937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., GASTER L.M., KING F.D., BROWN A.M. Blockade of human atrial 5-HT4 receptors by SB 207710, a selective and high affinity 5-HT4 receptor antagonist. Naunyn-Schmiedebergs' Arch. Pharmacol. 1994;349:546–548. doi: 10.1007/BF00169146. [DOI] [PubMed] [Google Scholar]
- KUEMMERLE J.F., MURTHY K.S., GRIDER J.R., MARTIN D.C., MAKHLOUF G.M. Coexpression of 5-HT2A and 5-HT4 receptors coupled to distinct signaling pathways in human intestinal muscle cells. Gastroenterology. 1995;109:1791–1800. doi: 10.1016/0016-5085(95)90745-9. [DOI] [PubMed] [Google Scholar]
- LEUNG E., BLISSARD D., JETT M.F., EGLEN R.M. Investigation of the 5-hydroxytryptamine receptor mediating the ‘transient' short-circuit current response in guinea-pig ileal mucosa. Naunyn-Schmiedebergs' Arch. Pharmacol. 1995;351:596–602. doi: 10.1007/BF00170158. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., MCLAUGHLIN D.P., FULTON J., MACDONALD E., SCOTT P.J. Effects of catecholamines on isolated human colonic smooth muscle. J. Auton. Pharmacol. 1996;16:213–220. doi: 10.1111/j.1474-8673.1996.tb00425.x. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. 5-HT4 receptor antagonist affinities of SB207710, SB205008, and SB203186 in the human colon, rat oesophagus, and guinea-pig ileum peristaltic reflex. Naunyn-Schmiedebergs' Arch. Pharmacol. 1995;352:132–140. doi: 10.1007/BF00176766. [DOI] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M. Further investigation into the signal transduction mechanism of the 5-HT4-like receptor in the circular smooth muscle of human colon. Br. J. Pharmacol. 1996;118:1058–1064. doi: 10.1111/j.1476-5381.1996.tb15506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEAN P.G., COUPAR I.M., MOLENAAR P. A comparative study of functional 5-HT4 receptors in human colon, rat oesophagus and rat ileum. Br. J. Pharmacol. 1995;115:47–56. doi: 10.1111/j.1476-5381.1995.tb16318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEULEMANS A.L., GHOOS E., CHEYNS P., SCHUURKES J.A.J. 5-HT-induced relaxations of human sigmoid colon are mediated via 5-HT4 receptors. Pflüger's Arch. Eur. J. Physiol. 1995;429:R9. [Google Scholar]
- MOLDERINGS G.J., DONECKER K., BURIAN M., SIMON W.A., SCHRODER D.W., GOTHERT M. Characterization of I2 imidazoline and sigma binding sites in the rat and human stomach. J. Pharmacol. Exp. Ther. 1998;285:170–177. [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., VAN BERGEN P.J.E., AKKERMANS L.M.A., SCHUURKES J.A.J. Evidence for 5-HT7 receptors mediating relaxation of human colon circular smooth muscle. Br. J. Pharmacol. 1999b;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., VAN HASELEN J.F.W.R., LEFEBVRE R.A., BRIEJER M.R., AKKERMANS L.M.A., SCHUURKES J.A.J. Pharmacological characterisation of 5-HT4 receptors mediating relaxation of canine isolated rectum circular smooth muscle. Br. J. Pharmacol. 1999a;127:1431–1437. doi: 10.1038/sj.bjp.0702665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS G.P., MASON S.L., MELDRUM A., DE KECZER S., PARNES H., EGLEN R.M., WONG E.H. 5-Hydroxytryptamine (5-HT)4 receptors in post mortem human brain tissue: distribution, pharmacology and effects of neurodegenerative diseases. Br. J. Pharmacol. 1995;114:993–998. doi: 10.1111/j.1476-5381.1995.tb13303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER G.J. 5-Hydroxytryptamine and functional bowel disorders. Neurogastroenterol. Mot. 1996;8:319–331. doi: 10.1111/j.1365-2982.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- SANGER G.J., NELSON D.R. Selective and functional 5-hydroxytryptamine3 receptor antagonism by BRL 43694 (granisetron) Eur. J. Pharmacol. 1989;159:113–124. doi: 10.1016/0014-2999(89)90695-x. [DOI] [PubMed] [Google Scholar]
- SCHUURKES J.A.J., MEULEMANS A.L., OBERTOP H., AKKERMANS L.M.A. 5-HT4 receptors on the human stomach. J. Gastrointest. Mot. 1991;3:199. [Google Scholar]
- TAM F.S., BUNCE K.T., HILLIER K., GROSSMAN C. Characterization of the 5-hydroxytryptamine receptor type involved in inhibition of spontaneous activity on human isolated colonic circular muscle. Br. J. Pharmacol. 1994;113:143–150. doi: 10.1111/j.1476-5381.1994.tb16186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAM F.S., HILLIER K., BUNCE K.T., GROSSMAN C. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br. J. Pharmacol. 1995;115:172–176. doi: 10.1111/j.1476-5381.1995.tb16335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLE K.A., ELLIS E.S., BAXTER G.S., KENNETT G.A., GASTER L.M., SANGER G.J. The effects of SB 204070, a highly potent and selective 5-HT4 receptor antagonist, on guinea-pig distal colon. Br. J. Pharmacol. 1994;112:789–794. doi: 10.1111/j.1476-5381.1994.tb13148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEITUNG K.D., PERKINS L.A., HSU S., BONHAUS D., EGLEN R.M., WONG A.G., LEUNG E. Comparison of GR 113808 and SB 204070 at 5-HT4 receptors in vitro. FASEB. 1994;8:A92–533. [Google Scholar]


