Abstract
Fluoroquinolones (FQs) are associated with a low incidence of central nervous system (CNS) side effects, possibly leading to convulsions, especially when co-administered with nonsteroidal anti-inflammatory drugs (NSAIDS). Although the in vivo pro-convulsant activity of NSAIDS is essentially unknown, the convulsant potential of FQs is traditionally evaluated by in vitro γ-aminobutyric acid (GABA) binding experiments in the presence of 4-biphenyl acetic acid (BPAA), the active metabolite of fenbufen.
The aim of this study was therefore to investigate the BPAA-norfloxacin convulsant interaction in vivo.
Male Sprague-Dawley rats (n=27) were given BPAA orally, at various doses 1 h before norfloxacin infusion, which was maintained until the onset of maximal seizures, when cerebrospinal fluid (CSF) and plasma samples were collected for analysis.
An inhibitory Emax effect model with a baseline effect parameter was fitted to the norfloxacin versus BPAA concentrations in the CSF, previously shown to be part of the biophase. This model includes three parameters: the concentrations of norfloxacin in the absence of BPAA (CCSF0, Nor), and when BPAA concentration tends toward infinity (CCSFbase, Nor), and the BPAA concentration for which half of the maximal effect is observed (CCSF50, BPAA). The maximal proconvulsant effect of BPAA is given by the CCSF0, Nor / CCSFbase, Nor ratio, estimated to approximately 6 in this study.
Derived models were developed in plasma to account for the non-linear CSF diffusion of norfloxacin and protein binding of BPAA.
In conclusion this study has shown that the convulsant interaction between norfloxacin and BPAA in rats, can be adequately characterized by modelling of the CSF concentrations of the two drugs at the onset of activity, following their administration in various proportions.
Keywords: Fluoroquinolones, nonsteroïdal antiinflammatories, biphenyl acetic acid, seizures, inhibitory Emax effect model, non-linear CSF diffusion, saturable protein binding
Introduction
Fluoroquinolone (FQ) antimicrobial agents are a class of inhibitors of bacterial topoisomerases. Initially, FQs have been used in the treatment of urinary infections, but because of their activity against Gram-negative bacteria and also their potency against Gram-positive bacteria, the new FQs are used in a variety of bacterial infections such as sexually transmitted diseases, gastrointestinal or respiratory tract infections, as well as skin and bones infections (Walker & Wright, 1991; Moellering, 1996; Hopper, 1998). Because FQs can diffuse into the CNS, they have been occasionally proposed as an alternative in the treatment of CNS infections (Scheld, 1989; Hasbun & Quagliarello, 1998). They are generally well tolerated but CNS disorders including headache, confusion, hallucination, anxiety, nervousness, nightmares (Anastasio, 1988; Christ, 1990) have been reported in ∼2% of patients (Rodvold & Piscitelli, 1993). The incidence of severe CNS side effects is quite low, but seizures have been more frequently observed in patients receiving FQs in combination with NSAIDs such as fenbufen (Simpson & Brodie, 1985; Arcieri et al., 1987; Anastasio et al., 1988). It is usually admitted that the central excitatory effect of FQs results from an inhibition GABAA binding to its receptors (Tsuji et al., 1988; Akahane et al., 1989; Tsutomi et al., 1994). However in vitro GABA binding experiments have demonstrated that FQs are weak GABA antagonists (Segev et al., 1988; Akahane et al., 1989; Hori & Shimada, 1993), except in very specific situations such as in the presence of BPAA, the active metabolite of fenbufen (Halliwell et al., 1993). Therefore predicting the convulsant risk associated to FQs administration in patients, from in vitro GABA binding experiments in the presence of BPAA, as most often done and accepted by registration agencies (Akahane et al., 1989; Hori & Shimada, 1993; Tsutomi et al., 1994; Akahane et al., 1994), may not be appropriate, especially because BPAA is never associated to FQs in clinical practice. In fact we have recently demonstrated some agreement but also inconsistencies, between the convulsant risk predicted from in vitro GABA binding experiments in the presence of BPAA, and the convulsant activity actually observed in vivo in the absence of BPAA (Delon et al., 1999a). In order to elucidate these discrepancies, experiments should be conducted in vivo to compare the convulsant activities of various FQs in the absence and in the presence of BPAA. However such investigations are likely to be complicated by various phenomenon including equilibration delay (Delon et al., 1997), saturable central diffusion of the FQ (Jaehde et al., 1992) as well as non-linear protein binding of BPAA, and results are likely to vary with the observation site or/and the relative concentration of each compound. It seems therefore necessary to start with a detailed investigation of the interaction between a selected FQ such as norfloxacin and BPAA, using an experimental approach allowing distinction between the pharmacokinetic and pharmacodynamic contributions to the overall effect, after administering the two compounds at various doses, in order to describe the whole phenomenon as previously done (Levasseur et al., 1998; Delon et al., 1999b).
Methods
Animals
This work was done in accordance with the Principles of Laboratory Animal Care (NIH Publication #85-23, revised 1985), and the study protocol was approved by the local ethic committee. Male Sprague Dawley rats (n=27) from Depres Breeding Laboratories (St Doulchard, France), were housed in the Animal Breeding Facilities of the Laboratory (authorization No: 0028). Their mean body weight was equal to 254±16 g. The animals were placed in wire cages in a 12 h light-dark cycle for 5 days before the beginning of experiment to adjust to the new environment. During this period, they had free access to food (Extralabo M20, U.A.R. Laboratories, France) and water.
Surgery
A polyethylene cannula (0.58 mm inside, 0.96 mm outside diameter, Plastimed Laboratories, France) was implanted in the right jugular vein of the animals the day prior to the experiment under a 60 mg kg−1 sodium pentotal (Sanofi Laboratories, France) intraperitoneal anaesthesia. Following the surgery, rats were kept under a heating lamp. After first signs of movement, the animals were placed into individual plastic cages. Food was withdrawn 12 h before the experiment, but the animals had free access to water until drug infusion.
Solutions for administration
The BPAA suspension was prepared for three different doses (10, 30, 50 mg kg−1 corresponding to 47, 141 and 236 μmol kg−1) by a mixture of BPAA (batch 125H3426, Sigma, France) in a sodium carboxymethyl cellulose (batch F17493, French Pharmaceutics Cooperation, France) 0.5% (w v−1) (Tsutomi et al., 1994). A 240 mM solution of norfloxacin hydrochloride dissolved in 5% glucose at pH 5.5, was used for intravenous administration (Delon et al., 1997).
Drugs administration
The day after surgery, the BPAA suspension was given orally by gastric tubing 1 h before the beginning of norfloxacin infusion. Three different groups were performed on the basis of the three doses of BPAA administered (n=5–8 per group). A solution of sodium carboxymethyl cellulose 0.5% devoid of BPAA was administered orally for the control group (n=8).
For norfloxacin administration, the jugular vein cannula was connected to a motor-driven syringe pump (SE400B, Vial Medical, France) containing the norfloxacin solution, at a flow rate of 960 μmol h−1. Animals were kept under a heating lamp to maintain body temperature. The infusion was stopped when the animals exhibited maximal seizures. Onset of maximal seizures was usually evidenced by tonic flexion of the forelimbs and tonic extension of the hindlimbs. Drug administration was conducted between 1400 h and 1900 h.
Samples collection
Immediately after exhibiting maximal seizures, rats were anaesthetized with an intramuscular injection of 12.5 mg of ketamin (KETALAR®, 50 mg ml−1, Parke Davis Laboratories, France) and 5 mg of xylazin hydrochloride (ROMPUN®, Bayer Laboratories, France), unless they had died following seizures. In any case CSF was collected within 3 min, as previously described (Anastasio et al., 1988; Delon et al., 1999a). Blood was subsequently withdrawn from the heart, collected in heparinized tubes (VACUTAINER®, Becton Dickinson, France) and immediately centrifuged at 3000 r.p.m. for 10 min (GR 412 model, Jouan, France). Plasma was transferred into two separate tubes. One fraction was kept frozen at −20°C until assayed. The other fraction was ultrafiltered with a Centrifree system (CF50A model, Amicon, France) for determination of unbound concentrations.
Drug analysis
Norfloxacin and BPAA concentrations were determined by h.p.l.c. The norfloxacin and BPAA assays were performed with a Kromasil C18 column (5 μm, 150×3 mm i.d.). The chromatographic system consisted of a Waters 510 model pump and a Gilson 231 autosampler connected to a Kratos 980 fluorimetric detector (excitation wavelength=280 nm, emission wavelength=445 nm) for norfloxacin and to a Waters 484 u.v. detector at the wavelength of 254 nm for the BPAA. Data were recorded and processed using a Waters 746 integrator.
For norfloxacin assay, the mobile phase consisted of 0.1 M aqueous citric acid solution containing 8% (v v−1) acetonitrile and 10 mM tetra butyl ammonium perchlorate, and the flow rate was 0.8 ml min−1. Norfloxacin was assayed in CSF and UF by direct injection after appropriate dilution (1/10 and 1/40 respectively) in a mixture of 0.1 M citrate buffer (pH=3). Plasma samples were diluted appropriately by addition of a 1.7% (v v−1) perchloric acid. The mixture was then centrifuged (3000 r.p.m., 10 min, 5°C) and 20 μl of the supernatant was injected onto the column (Delon et al., 1997).
For BPAA determination, the mobile phase consisted of a mixture of 50% (v v−1) acetonitrile, 49% (v v−1) water (Milli-Q) and 1% (v v−1) acetic acid, the flow rate was 0.8 ml min−1. BPAA was assayed in CSF and UF by direct injection after appropriate dilution (1/10) in methanol. Plasma samples were diluted appropriately by addition of a 1.7% (v v−1) perchloric acid. The mixture was then centrifuged (3000 r.p.m., 10 min, 5°C) and 20 μl of the supernatant was injected onto the column.
Theoretical analysis
Because the convulsant activity of norfloxacin is strictly related to its CSF concentration but not necessarily to its plasma concentration (Delon et al., 1997), the pharmacodynamic interaction between the two drugs was first investigated at the CSF (biophase) level, which was considered as the driving force of the system (Figure 1).
Figure 1.
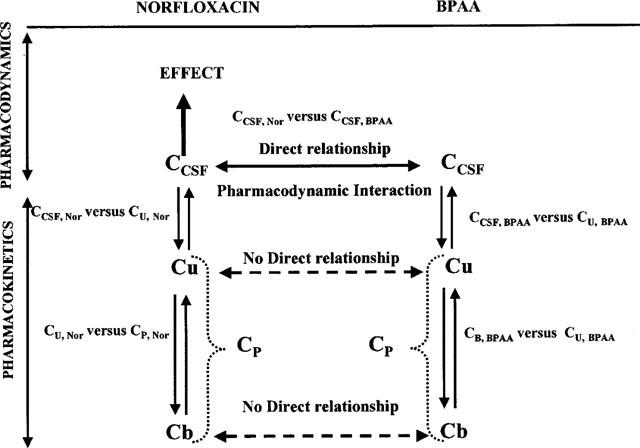
Schematic representation of the convulsant interaction between norfloxacin and BPAA with distinction between the pharmacodynamic interaction of the two compounds in CSF (biophase) and the pharmacokinetic relationships characteristic of the CSF diffusion and plasma protein binding of each compound.
CSF concentrations modelling
An inhibitory Emax effect model with a baseline effect parameter (Gabrielsson & Weiner, 1997), was fitted to the CSF norfloxacin versus BPAA concentrations (CCSF, Nor VS CCSF, BPAA). The general form of this equation is as follows:
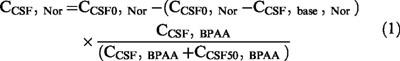 |
where CCSF0, Nor is the concentration of norfloxacin at the onset of maximal seizures in the absence of BPAA, CCSF, base, Nor is the asymptotic value of norfloxacin concentration when BPAA concentration (CBPAA) tends toward infinity, and CCSF50, BPAA is the concentration of BPAA corresponding to a norfloxacin concentration (CNor) equal to ½× (CO, Nor−Cbase, Nor).
Unbound plasma concentrations modelling
Equation 1 was also fitted to the norfloxacin versus BPAA unbound plasma concentrations (CU, Nor vs CU, BPAA). However this inhibitory Emax effect model with a baseline parameter can adequately describe both sets of data (CSF and free plasma concentrations) only if the CSF diffusion of both compounds is linear. But in these experimental conditions the CSF diffusion of norfloxacin was non-linear. The relationship between CSF and unbound plasma concentrations of norfloxacin was as follows:
where CCSFmax, Nor is the maximum concentration of norfloxacin achievable into the CSF and CU50, Nor is the unbound norfloxacin concentration that corresponds to ½ of CCSFmax, Nor.
For BPAA, the relationship between CSF and unbound plasma concentrations was linear.
where Kd is the distribution coefficient of BPAA between CSF and unbound plasma concentrations.
A new relationship (see Equation 4) could then be obtained by integrating Equations 1 and 3 into Equation 2 after reorganization.
This new equation characterizes the indirect relationship between unbound plasma concentrations of norfloxacin and BPAA, and reflects both the pharmacodynamic interaction between the two drugs, and their CSF diffusion characteristics (Figure 1).
Total plasma concentrations modelling
Equation 1 was tested again, although from the previous it was not likely to provide good data fitting. The model previously used to characterize the interaction at the unbound concentrations level (Equation 4), may describe the norfloxacin versus BPAA total plasma concentrations (CP, Nor, vs CP, BPAA), only if plasma protein binding of the two drugs is linear. However plasma protein binding of norfloxacin was linear (and actually almost negligible) but not that of BPAA.
The Langmuir equation (Behm & Wagner, 1981; Benincosa & Morris, 1993; Kochak et al., 1993) (Equation 5a), was successful in fitting BPAA bound (CB) versus free plasma (CU) concentrations:
where CBmax, BPAA is the maximal concentration of BPAA necessary to saturate 100% of binding sites, and CU50, BPAA is the unbound BPAA fraction which could saturate 50% of binding sites of BPAA.
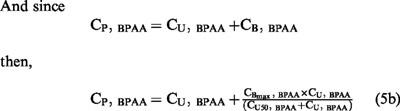 |
By re-organization of the Equation 5b, one obtains unbound concentrations (CU, BPAA) as a function of the total plasma concentration of BPAA (CP, BPAA) (see equation 5c)
where
Norfloxacin unbound versus plasma total concentrations were linearly related and fitted according to the following equation (Equation 6):
where fu is the free fraction of norfloxacin in plasma.
By integrating these plasma protein binding characteristics (Equations 5c and 6) in Equation 4, a new equation could be obtained (see equation 7), which describes the indirect relationship between total plasma concentrations of norfloxacin and BPAA, and reflects the pharmacodynamic interaction between these two compounds as well as their CSF diffusion and plasma protein binding characteristics. (See Equation 7.)
Modelling and simulations were performed with WinNonlin, version 1.0 (Scientific Consulting, Inc.). Modelling was conducted with uniform weighting. Discrimination between linear and non-linear models of CSF diffusion and protein binding was assessed from various criteria, including visual inspection, residual analysis, sum of squared residuals (SSR), correlation coefficient between observed and predicted values, and Akaïke information criteria (AIC). Data were compared by the non-parametric Kruskal-Wallis test followed by the Dunn's multiple comparison post test, with a level of significance set at P<0.05.
Results
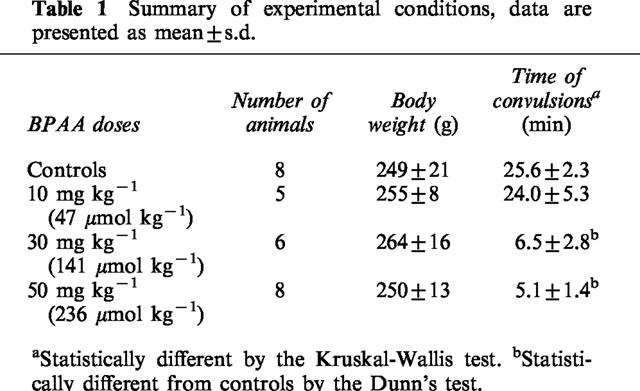
As a result of the interaction between norfloxacin and BPAA, the time of maximal seizures was significantly reduced from 25.6±2.3 min when norfloxacin was infused alone, to 5.1±1.4 min when it was co-administered with the highest oral dose of BPAA equal to 50 mg kg−1 (Table 1). Complementary experiments showed that no convulsions appeared after BPAA was administered alone even at an oral dose of 300 mg kg−1 (data not shown).
Table 1.
Summary of experimental conditions, data are presented as mean±s.d.
The inhibitory Emax effect model with a baseline effect parameter (Equation 1) was appropriate to describe the interaction between BPAA and norfloxacin in the CSF (Figure 2). The mean CSF concentration of norfloxacin at the onset of activity when given alone, was estimated by the modelling procedure (CCSF0, Nor) to 47.3±2.3 μM (CV=4.95%), which is virtually identical to the algebraic mean of individual concentrations (47.1±10.7 μM), and in agreement with results obtained from previous experiments (Delon et al., 1997; 1999a,1999b). The absence of intercept with the abscissa, characteristic of the model, is consistent with the lack of convulsant activity of BPAA administered alone, and is indicative of the existence of a maximum proconvulsant effect. The asymptotic value of norfloxacin concentration when that of BPAA tends toward infinity (CCSFbase, Nor) was estimated to 7.69±2.23 μM (CV=29%). The ratio between CCSF0, Nor and CCSFbase, Nor indicates that BPAA increases the convulsant activity of norfloxacin by approximately 6 fold at the most. Half of the maximum pro-convulsant effect was observed at a CSF concentration of BPAA (CCSF50, BPAA) equal to 0.80±0.31 μM. This last parameter however was not estimated with a great precision (CV=39%).
Figure 2.
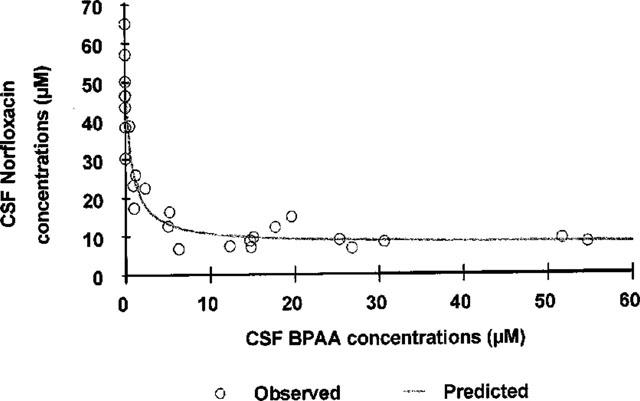
Norfloxacin versus BPAA CSF concentrations at the onset of maximal seizures. Each point represents CSF data from one rat (n=27) obtained following IV infusion of a solution of norfloxacin hydrochloride 240 mM, 1 h after oral administration of BPAA at one of the following doses: 10, 30 or 50 mg kg−1. The control group was obtained after p.o. administration of a solution of carboxymethylcellulose 0.5% devoid of BPAA. Norfloxacin versus BPAA CSF concentrations were fitted according to an Emax effect model with a baseline effect parameter (Equation 1, solid line), the three parameters estimates were: CCSF0, Nor=47.3±2.3 μM, CCSFbase, Nor=7.69± 2.23 μM, CCSF50, BPAA=0.80±0.31 μM.
This inhibitory Emax effect model did not result in a totally satisfactory fitting of the norfloxacin versus BPAA unbound plasma concentrations. The mean unbound plasma concentration of norfloxacin at the onset of activity when given alone, was correctly estimated by the modelling (CU0, Nor=1528±56, CV=3.7%), but not that of CUbase, Nor (83.3±59.1 μM, CV=71%). The ratio between these two estimates would suggest that BPAA may increase the convulsant activity of norfloxacin by up to 18 fold. Furthermore the CSF over unbound norfloxacin concentrations ratio in the absence of BPAA (CCSF0, Nor / CU0, Nor) was equal to 3.0%, when the same ratio at lower concentrations of norfloxacin (CCSFbase, Nor / CUbase, Nor) would be equal to 9.2%. This difference suggests saturable CSF diffusion of norfloxacin. In agreement with that observation, a non-linear model (Equation 2) provided better data fitting of the CSF versus unbound norfloxacin concentrations than a linear model, and lead to an estimate of a maximum achievable CSF concentration (CCSFmax, Nor) equal to 78±13 μM with a corresponding CU50, Nor value equal to 1052±358 μM (Figure 3a). In contrast the CSF diffusion of BPAA was linear (Equation 3) with a coefficient of diffusion close to unity (Kd=0.944±0.015) (Figure 3b). The indirect relationship between the unbound plasma concentrations of the two drugs was well described by Equation 4, using parameters values estimated from the three direct relationships: Equation 1 (CCSF0, Nor; CCSFbase, Nor; CCSF50, BPAA), Equation 2 (CCSFmax, Nor; CU50, Nor) and Equation 3 (Kd). In these conditions, experimental data points were almost exactly superimposed to simulated concentrations (Figure 5a).
Figure 3.
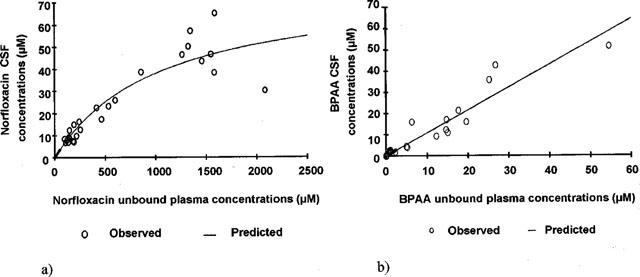
Norfloxacin (a) and BPAA (b) CSF versus unbound plasma concentrations. Each point represents data from one rat (n=27). (a) Pairs of data were fitted according to a non-linear model (Equation 2, solid line), the two parameters CCSFmax, Nor and CU50, Nor were respectively estimated to 78±13 μM and 1052±358 μM. (b) Pairs of data were fitted according to a linear model (Equation 3, solid line), with a coefficient of diffusion of BPAA (Kd) equal to 0.944±0.015.
Figure 5.
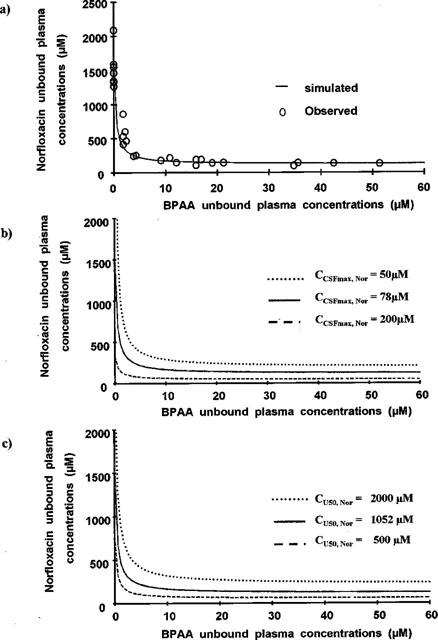
Norfloxacin versus BPAA concentrations in UF. (a) represents the superimposition of the simulated curve from Equation 4 and experimental data points. Norfloxacin versus BPAA unbound plasma concentrations were simulated for various values of the parameters characteristic of the non-linear CSF diffusion of norfloxacin: CCSFmax, Nor from 50 μM (top, right) to 200 μM (bottom, left) (b) and CU50, Nor from 500 μM (bottom, left) to 2000 μM (top, right) (c).
The initial Emax effect model (Equation 1) did not provide adequate fitting of the total plasma concentrations sets of data at all. In particular a very poor estimate of CPbase, Nor was obtained (50±76 μM, CV=154%), with a ratio between CP0, Nor and CPbase, Nor suggesting a pro-convulsant effect by up to 30 fold. BPAA plasma protein binding was extensive (>96%) and non-linear, with parameters estimated from the Langmuir equation (equation 5a) equal to 932±62 μM (CBmax, BPAA) and 12.7±2.1 μM (CU50, BPAA) (Figure 4a). Plasma protein binding of norfloxacin was linear (equation 6) and virtually negligible with an unbound fraction fu=0.99±0.03 estimated from the slope of the regression (Figure 4b). The indirect relationship between the total plasma concentrations of the two drugs was relatively well described by Equation 7, with parameters values estimated from three corresponding direct relationships (Equations 4, 5a, 6), as illustrated on Figure 6a.
Figure 4.
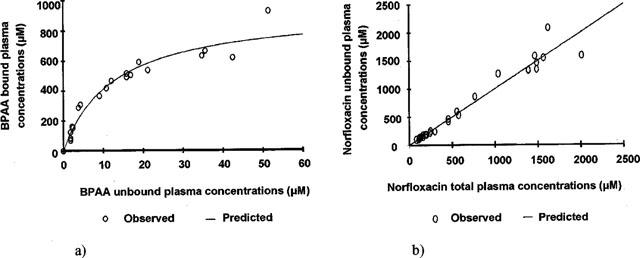
BPAA (a) and norfloxacin (b) plasma protein binding. Each point represents data from one rat (n=27). (a) Plasma protein binding of BPAA followed a non linear model and pairs of data were fitted according to a Langmuir equation (Equation 5a, solid line) with parameters estimates equal to 932±62 μM (CBmax, BPAA) and 12.7±2.1 μM (CU50, BPAA). (b) Pairs of data were fitted according to a linear model (Equation 6, solid line) with the free fraction of norfloxacin (fu) estimated to 0.99±0.03.
Figure 6.
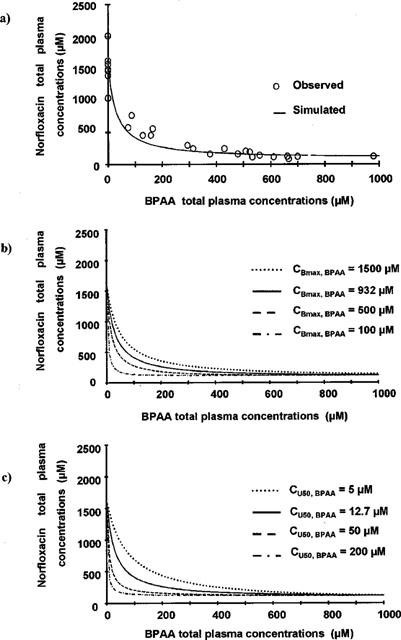
Norfloxacin versus BPAA concentrations in total plasma. (a) was obtained by superimposition of simulated curve obtained from Equation 7 and experimental data points. Norfloxacin versus BPAA total plasma concentrations were simulated for various values of the parameters characteristic of the non-linear protein binding of BPAA: CBmax, BPAA from 100 μM (bottom, left) to 1500 μM (top, right) (b) and CU50, BPAA from 5 μM (top, right) to 200 μM (bottom, left) (c).
Discussion
Drug interactions may have pharmacokinetic or/and pharmacodynamic origins, and for that reason pharmacodynamic interactions alone are difficult to distinguish in vivo. However an interesting situation occurs when drug concentrations can be measured within the biophase at the onset of activity, because it makes possible the differentiation between the pharmacokinetic and pharmacodynamic contributions to the observed effect. The cyclosporine-theophylline convulsant interaction has been investigated with such an approach (Hoffman et al., 1994). However cyclosporine was administered at a fixed dose, precluding full characterization of the interaction, as could be done with more complete experimental protocols (Zhi & Levy, 1990; Levasseur et al., 1998; Delon et al., 1999b).
The convulsant interaction between BPAA and norfloxacin was therefore investigated with an experimental approach similar to that previously used for the characterization of the pefloxacin-theophylline (Levasseur et al., 1998) and pefloxacin-norfloxacin (Delon et al., 1999b) convulsant interactions. The basic concept consists in measuring the concentrations of the two drugs in CSF, which was previously shown to be part of the biophase (Delon et al., 1997) at the onset of activity. However in these previous studies, the two compounds had a convulsant effect on their own, whereas in the present situation only norfloxacin may induce convulsions, BPAA having only a pro-convulsant effect. Therefore the isobolographic approach previously used, was not appropriate to investigate the BPAA-norfloxacin interaction and a new model had therefore to be developed.
The pro-convulsant effect of BPAA was well characterized in the biophase (CSF) by an inhibitory Emax effect model with a baseline effect parameter. At first glance this model (Equation 1) could also apply to free plasma concentrations, except for the CUbase, Nor which could not be estimated accurately. Furthermore, this modelling would suggest that the pro-convulsant effect of BPAA increases the convulsant activity of norfloxacin by up to 18 times (ratio between CU0, Nor and CUbase, Nor) when CSF data indicated an increase by 6 fold at the most (ratio between CCSF0, Nor and CCSFbase, Nor). This is because the same structural model (Equation 1) can apply to both CSF and free plasma concentration data sets, only if the CSF diffusion of the two compounds are linear, which was not the case (Figure 3). Therefore a new relationship (Equation 4) had to be found between unbound plasma concentrations, taking into account the linear CSF diffusion of BPAA and the non-linear CSF diffusion of norfloxacin. The originality of this approach summarized on Figure 1, was to consider that although CSF concentrations are determined by plasma concentrations (Delon et al., 1997), the effect (convulsions) appears when BPAA and norfloxacin concentrations reach certain values in CSF (biophase) independently of plasma levels. Therefore there is a direct relationship between the CSF concentrations of the two drugs at the onset of activity, which in fact constitutes the driving force of the system. Plasma concentrations are only indirectly related.
The apparent non-linear CSF diffusion of norfloxacin (Equation 2 and Figure 3a), may have several origins, including the existence of some active transport systems (Jaehde et al., 1992), a pharmacokinetic (CSF diffusion) interaction with BPAA (Ichikawa et al., 1992), and a relatively slow distribution of norfloxacin into CSF (Delon et al., 1997) together with a decrease in infusion time when BPAA doses increased (Table 1). This effect of the non-linear CSF diffusion of norfloxacin on the relationship between the unbound plasma concentrations of norfloxacin and BPAA was further investigated using computer simulations with various values of CCSFmax, Nor and CU50, Nor (Figure 5b,c). Simulations showed that changes in CCSFmax, Nor or CU50, Nor do not really affect the general shape of the curve, which is only shifted to the top (or right side) when CCSFmax, Nor decreases (Figure 5b) or when CU50, Nor increases (Figure 5c). This observation is consistent with the fact that a reasonably good data fitting of unbound plasma concentrations could be obtained with Equation 1. However as can also be observed and deduced from Equation 4, the ratio CU0, Nor/CUbase, Nor does not change with CU50, Nor, but varies with CCSFmax, Nor. This explains the inconsistency between the estimates of the CCSF0, Nor/CCSFbase, Nor and CU0, Nor/CUbase ratios, obtained when Equation 1 was applied to both sets of data.
As for unbound concentrations, an indirect relationship was derived (Equation 7) between total plasma concentrations of the two drugs (Figure 6a), taking into account the various direct relationships identified and illustrated on Figure 1, in particular the nonlinear plasma protein binding of BPAA (equation 5a). Parameters characteristic of this non-linear protein binding (CBmax, BPAA and Cu50, BPAA) were then let to vary in order to estimate their effect on the indirect relationship between the total plasma concentrations of the two drugs. As illustrated on Figure 6b,c, CP0, Nor and CPbase, Nor are not affected by changes in CBmax, BPAA or Cu50, BPAA However the general shape of the CP, Nor versus CP, BPAA curves is modified. The curvature becomes less pronounced as CB max, BPAA increases or Cu50, BPAA decreases. Therefore the saturable protein binding of the pro-convulsant compound is the main reason why the initial inhibitory model (Equation 1) was not appropriate to fit the total plasma concentrations data sets.
In conclusion the pro-convulsant effect of BPAA on norfloxacin as a representative FQ, has been investigated for the first time in vivo, with an approach distinguishing between the pharmacokinetics and the pharmacodynamics of this interaction. It was shown that the pharmacodynamic interaction could be well and best characterized from the CSF (biophase) concentration measurements at the onset of activity, providing that the two drugs had been administered in a sufficiently large range of doses in order to estimate adequately the three parameters characteristic of the interaction model. Plasma concentrations alone can hardly provide such information, because of pharmacokinetic complexities such as the non-linear plasma protein binding of BPAA, or the apparent non-linear CSF diffusion of the FQ. Interestingly unbound plasma concentrations, which are frequently considered as the ‘active form' of the drugs, do not properly reflect the interaction in this particular situation as well. Ignoring these pharmacokinetic complexities would lead to false conclusions when plasma concentrations are considered alone, and to major inconsistencies when they are compared to the analysis of CSF concentrations. The integrated pharmacokinetic-pharmacodynamic modelling procedure, successfully developed to reconcile these apparently conflicting data, is now being used in our laboratory to compare the pro-convulsant effect of BPAA on various FQs.
Acknowledgments
Anne Boulanger and Isabelle Martineau are gratefully acknowledged for their excellent assistance in perfoming the experiments.
Abbreviations
- BPAA
4-biphenyl acetic acid
- CNS
central nervous system
- CSF
cerebrospinal fluid
- FQs
fluoroquinolones
- GABA
γ-aminobutyric acid
- h.p.l.c.
high performance liquid chromatography, NSAIDs, nonsteroidal anti-inflammatory drugs
References
- AKAHANE K., KIMURA Y., TSUTOMI Y., HAYAKAWA I. Possible intermolecular interaction between quinolones and biphenylacetic γ-aminobutyric acid receptor sites. Antimicrob. Agents Chemother. 1994;38:2323–2329. doi: 10.1128/aac.38.10.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AKAHANE K., SEKIGUCHI M., UNE T., OSADA Y. Structure-epileptogenicity relationship of quinolones with special reference to their interaction with γ-aminobutyric acid receptor sites. Antimicrob. Agents Chemother. 1989;33:1704–1708. doi: 10.1128/aac.33.10.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANASTASIO G.D., MENSCER D., LITTLE J.M. Norfloxacin and seizures. Ann. Intern. Med. 1988;109:169–170. doi: 10.7326/0003-4819-109-2-169. [DOI] [PubMed] [Google Scholar]
- ARCIERI G., GRIFFITH E., GRUENWALDT G., HEYD A., O'BRIEN B., BACKER N., AUGUST R. Ciprofloxacin: an update on clinical experience. Am. J. Med. 1987;82 Suppl. 4A:381–386. [PubMed] [Google Scholar]
- BEHM H.L., WAGNER J.G. Parabolic equation relating free and total drug concentrations in cases of nonlinear plasma protein binding. J. Pharmacol. Sci. 1981;70:802–803. doi: 10.1002/jps.2600700725. [DOI] [PubMed] [Google Scholar]
- BENINCOSA L.J., MORRIS M.E. Nonlinear pharmacokinetic and protein binding of tiaprofenic acid in female Lewis rats. J. Pharmacol. Sci. 1993;82:429–430. doi: 10.1002/jps.2600820418. [DOI] [PubMed] [Google Scholar]
- CHRIST W. Central nervous system toxicity of quinolones: human and animal findings. J. Antimicrob. Chemother. 1990;26:219–225. doi: 10.1093/jac/26.suppl_b.219. [DOI] [PubMed] [Google Scholar]
- DELON A., BOUQUET S., HUGUET F., BRUNET V., COURTOIS P., COUET W. Pharmacokinetic-pharmacodynamic contributions to the convulsant activity of fluoroquinolones in rats. Antimicrob. Agents Chemother. 1999a;43:1511–1515. doi: 10.1128/aac.43.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELON A., HUGUET F., COURTOIS P., VIERFOND J.-M., BOUQUET S., COUET W. Pharmacokinetic-pharmacodynamic contributions to the convulsant activity of pefloxacin and norfloxacin in rats. J. Pharmacol. Exp. Ther. 1997;280:983–987. [PubMed] [Google Scholar]
- DELON A., LEVASSEUR M.L., GIRAUDON M., BOUQUET S., COUET W. Antagonistic interaction between the convulsant activities of pefloxacin and its main metabolite norfloxacin in rats. Pharm. Res. 1999b;16:1895–1898. doi: 10.1023/a:1011967813140. [DOI] [PubMed] [Google Scholar]
- GABRIELSON J., WEINER D. Pharmacokinetic and pharmacodynamic data analysis concepts and applications 1997Swedish Pharmaceutical Press: Stockholm, Sweden; 182–194.2nd edn [Google Scholar]
- HALLIWELL R.F., DAVEY P.G., LAMBERT J.J. Antagonism of GABAA receptors by 4-quinolones. J. Antimicrob. Chemother. 1993;31:457–462. doi: 10.1093/jac/31.4.457. [DOI] [PubMed] [Google Scholar]
- HASBUN R., QUAGLIARELLO V.J.Use of the quinolones in the treatment of bacterial meningitis The Quinolones 1998New York, N.Y.: Academic Press; 287–301.2nd ednCh 11 [Google Scholar]
- HOFFMAN A., PINTO E., AFARGAN M., SCHATTNER A. Cyclosporine enhances theophylline neurotoxicity in rats. J Pharm. Sci. 1994;83:559–561. doi: 10.1002/jps.2600830423. [DOI] [PubMed] [Google Scholar]
- HOOPER D.C. Clinical applications of quinolones. Biochim. Biophys. Acta. 1998;1400:45–61. doi: 10.1016/s0167-4781(98)00127-4. [DOI] [PubMed] [Google Scholar]
- HORI S., SHIMADA J.Effects of quinolones on the central nervous system Quinolones Antimicrobial Agents 1993Washington D.C.: American Society for Microbiology; 513–518.2nd edn. edHooper, D.C. & Wolfon, J.SCh. 27, pp [Google Scholar]
- ICHIKAWA N., NAORA K., HAYASHIBARA M., KATAGIRI Y., IWAMOTO K. Effect of fenbufen on the entry of the new quinolones, norfloxacin and ofloxacin, into the central nervous system in rats. J. Pharm. Pharmacol. 1992;44:915–920. doi: 10.1111/j.2042-7158.1992.tb03236.x. [DOI] [PubMed] [Google Scholar]
- JAEHDE U., LANGEMEIJER M.W.E., DE BOER A.G., BREIMER D.D. Cerebrospinal fluid transport and disposition of the quinolones ciprofloxacin and pefloxacin in rats. J. Pharmacol. Exp. Ther. 1992;263:1140–1146. [PubMed] [Google Scholar]
- KOCHAK G.M., SUDHAKAR P., ROBERT I., HONC F., KACHMAN D., PERRINO P., EGGER H. Prinomide tromethamide pharmacokinetics: Mutually dependent saturable and competitive protein binding between primonide and its own metabolite. Pharm. Res. 1993;10:49–55. doi: 10.1023/a:1018916811904. [DOI] [PubMed] [Google Scholar]
- LEVASSEUR L.M., DELON A., GRECO W.R., FAURY P., BOUQUET S., COUET W. Development of a new quantitative approach for the isobolographic assessment of the convulsant interaction between pefloxacin and theophylline in rats. Pharm. Res. 1998;15:1069–1076. doi: 10.1023/a:1011938429379. [DOI] [PubMed] [Google Scholar]
- MOELLERING R.C. The place of quinolones in every clinical practice. Chemotherapy. 1996;42:54–61. doi: 10.1159/000239492. [DOI] [PubMed] [Google Scholar]
- RODVOLD K.A., PISCITELLI S.C. New oral macrolide and fluoroquinolone antibiotics: an overview of pharmacokinetics, interactions, and safety. Clin. Infect. Dis. 1993;17:S192–S199. doi: 10.1093/clinids/17.supplement_1.s192. [DOI] [PubMed] [Google Scholar]
- SCHELD W.M. Quinolone therapy for infections of the central nervous system. Rev. Infect. Dis. 1989;11:S1194–S1202. doi: 10.1093/clinids/11.supplement_5.s1194. [DOI] [PubMed] [Google Scholar]
- SEGEV S., REHAVI M., RUBINSTEIN E. Quinolones, theophylline and diclofenac interactions with the γ-aminobutyric acid receptor. Antimicrob. Agents Chemother. 1988;32:1624–1626. doi: 10.1128/aac.32.11.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON K.J., BRODIE M.J. Convulsions related to enoxacin. Lancet. 1985;ii:161. doi: 10.1016/s0140-6736(85)90270-3. [DOI] [PubMed] [Google Scholar]
- TSUJI A., SATO H., KUME Y., TAMAI I., OKEZAKI E. Inhibitory effects of quinolone antibacterial agents on γ-aminobutyric acid binding to receptor sites in rat brain membranes. Antimicrob. Agents Chemother. 1988;32:190–194. doi: 10.1128/aac.32.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUTOMI Y., MATSUBAYASHI K., AKAHANE K. Quantification of GABAA receptor inhibition required for quinolone-induced convulsions in mice. J. Antimicrob. Chemother. 1994;34:737–746. doi: 10.1093/jac/34.5.737. [DOI] [PubMed] [Google Scholar]
- WALKER R.C., WRIGHT A.J. The fluoroquinolones. Mayo Clin. Proc. 1991;66:1249–1259. doi: 10.1016/s0025-6196(12)62477-x. [DOI] [PubMed] [Google Scholar]
- ZHI J., LEVY G. Isobolographic assessment of the convulsant interaction between theophylline and caffeine or pentylenetetrazol in rats. J. Pharm. Sci. 1990;79:678–681. doi: 10.1002/jps.2600790805. [DOI] [PubMed] [Google Scholar]


