Abstract
The effects of pertussis toxin (PT) and the role of histaminergic H1, H2 and H3 receptor blockade on the actions of histamine on blood pressure, heart rate, blood gas values, and mortality were studied in anaesthetized rats.
Four days after treatment with PT, histamine dose-dependently decreased mean arterial blood pressure (MAP) and PT enhanced the histamine-induced decrease in MAP. In the PT but not in the inactivated PT (IPT) or saline treated group three out of six animals died after the highest dose of histamine (300 mg kg−1, i.v.)
In order to determine the type of histamine receptor that mediates HS, 4 days after PT the selective antagonists mepyramine (H1), cimetidine (H2) and clobenpropit (H3) were administered 20 min before the challenge with histamine. Mepyramine completely inhibited both the enhanced histamine-induced decrease in MAP and mortality brought about by PT. Cimetidine and clobenpropit had no protective effects, but rather enhanced the histamine-induced mortality elicited by PT.
The present study shows that PT caused HS in rats which is primarily mediated via H1 and secondarily via H2 and H3 receptors. These results are considered to be a first step in the elucidation of the mechanism(s) of the HS test used in the quality control of acellular pertussis vaccine.
Keywords: Histamine sensitization, pertussis toxin, histaminergic antagonists, rat, H1, H2, H3 receptors
Introduction
Current whole-cell pertussis vaccines are a compromise in which a certain level of toxicity is accepted in order to achieve a sufficient level of potency. Pertussis toxin (PT), alternatively described as leukocytosis promoting factor or histamine sensitizing factor or islet activating protein, is one of the major toxic components of Bordetella pertussis vaccines. PT has demonstrated a whole range of biological activities in various animal species: histamine sensitization (HS), leukocytosis promoting activity, release of insulin by activation of the islets of Langerhans (Parfentjev & Goodline, 1948; Pittman, 1979; Kreeftenberg et al., 1984) and autonomic and haemodynamic impairment (de Wildt et al., 1982; 1983; 1985; 1986; Van Heuven-Nolsen et al., 1989; Vleeming et al., 1993; Van Amsterdam et al., 1998a,1998b). On the other hand PT is considered to play an important role in protective immunity and therefore an ideal vaccine candidate (Sato et al., 1974).
Recently, a new generation of pertussis vaccines has been developed for vaccination against pertussis. Amongst other detoxified antigens these vaccines include pertussis toxoid, i.e. detoxified pertussis toxin (PT), in order to reduce adverse reactions of PT. In order to assure the absence of residual toxicity or reversion of pertussis toxoid to PT a safety test is required by regulatory authorities: the HS test. This test is based on the principle that mice injected with biologically active PT are sensitized to histamine (Parfentjev & Goodline, 1948). The main difficulty or limitations of laboratory methods of defining parameters for PT-induced toxicity is that the basis of toxicity is not well known. Furthermore, although simple and sensitive this test puts the animals to strong distress. Therefore, we started a study to find alternative in vitro tests based on the same principle of HS. Since mechanism(s) by which PT induces this sensitization is as yet unknown, more mechanistic studies are necessary to understand which substrates (e.g. histamine receptors and/or their signal transduction systems) and physiological systems (cardiovascular and pulmonary) are the most important targets for PT with regard to HS.
The present study was carried out in an attempt to identify the subtype of histamine receptor (H1, H2 or H3) involved in the PT-induced HS in rats. Furthermore, discrimination between cardiovascular and respiratory problems after histamine challenge in sensitized animals has been established on the basis of blood pressure and blood gas evaluations.
Methods
Animals
Male Wistar rats (250–300 g) were obtained from the SPF breeding colony of the National Institute of Public Health and Environment (RIVM). Animals were housed under constant conditions i.e. a relative humidity of 30–60% and a temperature of 22–24°C. Food and water were available ad libitum. The experiments were approved by the ethical committee of the RIVM (Bilthoven, The Netherlands).
Study design
Fifty-four animals were randomly divided into nine groups of six animals each. The animals were injected intravenously (i.v.) with either 0.9 % saline (one group) or pertussis toxin (15 μg kg−1; PT; four groups) or inactivated pertussis toxin (15 μg kg−1; IPT; four groups). At 4 days after pretreatment animals were anaesthetized and systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial blood pressure (MAP), heart rate (HR) and arterial blood gas values (pH, PaO2, PaCO2) were determined. Subsequently, the effect of histamine (0–300 mg kg−1, i.v.) on the mentioned parameters was evaluated after treatment with the H1 antagonist mepyramine (3 mg kg−1, i.v.) or the H2 antagonist cimetidine (30 mg kg−1, i.v.) or the H3 antagonist clobenpropit (30 mg kg−1, i.v.) or saline.
Experimental procedures
At 4 days after pretreatment with saline, PT or IPT animals were anaesthetized with urethane (1.5 g kg−1 of body weight, intraperitoneally). SBP and DBP and HR were continuously recorded from a cannulated carotid artery via a pressure transducer connected to a HSE System (Hugo Sachs Electronic, Germany). MAP was calculated as DBP+1/3(SBP-DBP). In addition to the cannulation of the carotid artery the right femoral artery and the left jugular vein were cannulated for blood withdrawal and for administration of saline, drugs or histamine by the use of infusion pumps, respectively. Arterial blood gas values (pH, PaO2, PaCO2) were determined by a Ciba-Corning 288 Blood gas Analyser (Ciba-Corning Limited, Houten, The Netherlands). Throughout the experiments rectal temperature was monitored and the body temperature of the animals was kept between 37°C and 38°C by placing the animals on heated pads and by radiant heat.
Pertussis toxin and drugs
Pertussis toxin (RIVM standard PU 1024) was isolated and purified from Bordetella pertussis Tohama and obtained from RIVM (Bilthoven, The Netherlands). Inactivation of active pertussis toxin being the histamine-sensitizing factor (HSF) was accomplished by heating the toxine at 80°C for 30 min. Histamine was from Sigma (St. Louis, U.S.A.). Mepyramine and cimetidine were obtained from ICN Biomedicals (Zoetermeer, NL) and clobenpropit was generously provided by the Leiden-Amsterdam Centre for Drug Research (Amsterdam, The Netherlands). Urethane was obtained from Janssen (Beerse, Belgium).
Statistics
Results were tested with one-way ANOVA (95%). P<0.05 was considered to be significant. Data are presented as means±s.e.mean.
Results
Figure 1 shows DBP, SBP and HR values measured 4 days after pretreatment with saline, IPT or PT before (Figure 1a–f); t=0) as well as 10 and 20 min after administration of saline, mepyramine (3 mg kg−1, i.v), cimetidine (30 mg kg−1, i.v) or clobenpropit (30 mg kg−1, i.v) (Figure 1a–f; t=10 and t=20).
Figure 1.

Baseline (time=0) diastolic blood pressure (DBP; a and d), systolic blood pressure (SBP; b and e) and heart rate (HR; c and f) 4 days after administration of saline (a–f) or pertussis toxin (PT, 15 μg kg−1 intravenous (i.v); a–c) or inactivated pertussis toxin (IPT, 15 μg kg−1, i.v.; d–f) and the effect of saline, mepyramine (3 mg kg−1, i.v.), cimetidine (30 mg kg−1, i.v.), and clobenpropit (30 mg kg−1, i.v.) 10 and 20 min after administration in PT (a–c) or IPT (d–f) pretreated animals. Values are expressed as means±s.e.mean of six animals. The asterisks indicate P<0.05, significantly different from the compared group.
Compared with saline both PT (4 groups) and IPT (4 groups) significantly decreased DBP from 76±4 to 37±1 mmHg (mean of the four PT groups; Figure 1a, t=0) and to 42±1 mmHg (mean of the four IPT groups; Figure 1d, t=0) and significantly increased HR from 405±19 to 498±4 b.p.m. (mean of four PT groups; Figure 1c, t=0) and to 473±6 b.p.m. (mean of four IPT groups; Figure 1f, t=0). Compared with saline neither PT nor IPT changed SBP values (Figure 1b,e, t=0).
In PT pretreated animals, clobenpropit caused a significant decrease in SBP (Figure 1b; t=10) and both in PT and in IPT pretreated animals clobenpropit as well as mepyramine significantly decreased HR (Figure 1c,f, t=10). Twenty minutes after drug administration all HR and SBP values were returned to baseline values thus no acute effects of histamine antagonists on blood pressure or HR existed at the start of the evaluation of the dose response of these parameters to histamine.
Figure 2 shows the effects of histamine, in the absence of histamine antagonists, 4 days after pretreatment with saline, PT or IPT on MAP, HR and blood gas values. Histamine caused in saline, PT and IPT pretreated animals a dose dependent decrease in MAP (Figure 2a). The MAP values of the IPT group were significantly lower as compared to the saline group over the whole dose-range of histamine but the slope of the curve was comparable to those of the saline group. In PT pretreated animals histamine caused a significantly more progressive decrease in MAP as compared to saline and IPT pretreated animals (Figure 2a), i.c. histamine sensitization.
Figure 2.

Effect of histamine (0–300 mg kg−1, intravenous) on mean arterial blood pressure (MAP; a) PaCO2 and PaO2 (b), heart rate (HR; c) and pH (d) 4 days after administration of saline or pertussis toxin (PT) or inactivated pertussis toxin (IPT). Values are expressed as means±s.e.mean of six animals. The asterisks indicate P<0.05, significantly different from the compared group. For doses of PT and IPT see legend of Figure 1.
After administration of 0 mg kg−1 histamine (saline) PaCO2 and PaO2 values of PT pretreated animals were significantly lower and higher, respectively, as compared to those of saline and IPT pretreated animals but after histamine there were no marked differences between the three tested groups (Figure 2b).
HR values of PT pretreated animals after 0 and 10 mg kg−1 histamine were significantly higher compared to those of saline and IPT pretreated animals. Also HR values of IPT pretreated animals after 0 and 10 mg kg−1 histamine were higher as compared to those of saline treated animals but significantly lower as compared to those of PT pretreated animals. With exception for the strong decrease in HR in the PT group after 300 mg kg−1 histamine there were no marked differences in HR values between the three tested groups (Figure 2c). Both in saline, PT and IPT pretreated animals histamine caused a dose dependent decrease in pH values without marked differences between the three tested groups (Figure 2c).
Figure 3 shows the effects of histamine, in the presence of histamine antagonists, 4 days after pretreatment with PT or IPT on MAP and HR.
Figure 3.
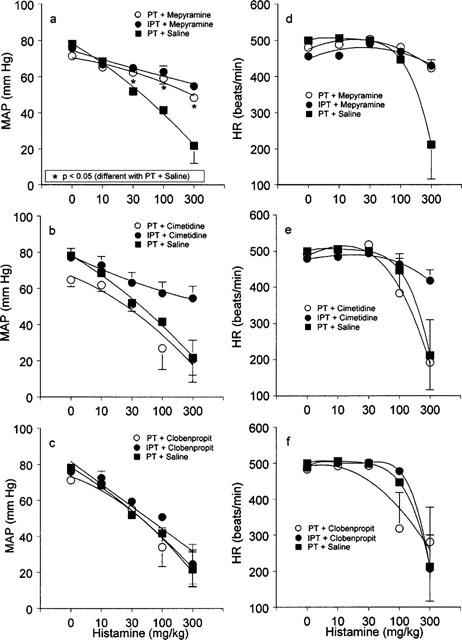
Effect of histamine (0–300 mg kg−1, intravenous; a–f) on mean arterial blood pressure (MAP; a–c) and heart rate (HR; d–f) 4 days after administration of saline or pertussis toxin (PT) or inactivated pertussis toxin (IPT) and 20–60 min after administration of saline (a–f), mepyramine (a and d), cimetidine (b and e) and clobenpropit (c and f). Values are expressed as means±s.e.mean of six animals. The asterisks indicate P<0.05, significantly different from the compared group. For doses of PT, IPT and drugs see legend of Figure 1.
Mepyramine did not affect the histamine-induced decrease in MAP in IPT pretreated animals (for comparison to IPT treatment without mepyramine see Figure 2a). However, mepyramine significantly reduced the histamine-induced decrease in MAP in PT pretreated animals, i.e. histamine sensitization, as compared to saline treatment of PT pretreated animals (Figure 3a). After treatment with mepyramine the histamine-induced decrease in MAP in PT pretreated animals was comparable to those observed in the IPT pretreated group (Figure 3a).
Cimetidine did not reduce the histamine-induced decrease in MAP neither in PT nor in IPT pretreated animals (Figure 3b). Clobenpropit enhanced the histamine-induced decrease in MAP in IPT pretreated animals to values observed in PT pretreated animals with or without clobenpropit (Figure 3c). Neither histamine nor the used drugs did clearly affect HR values except for the highest doses of histamine in some groups (Figure 3d–f).
Figure 4 shows the effects of histamine, after treatment with histamine antagonists, 4 days after pretreatment of PT or IPT on blood gas values and pH. Neither histamine nor the used drugs did clearly affect PaCO2 and PaO2 values except for the PaO2 values after clobenpropit in PT pretreated animals (Figure 4c). This decrease in PaO2 values across the full dose range of histamine can be attributed to the clobenpropit-induced decrease in initial values (PaO2 values decreased from 89.5±3.3–77.7±5.2 mmHg measured before and 20 min after administration of clobenpropit, respectively). Histamine caused a decrease in pH values which was reduced by mepyramine and enhanced by cimetidine as compared to saline in PT pretreated animals (Figure 4d–f).
Figure 4.

Effect of histamine (0–300 mg kg−1, intravenous; a–f) on PaCO2 and PaO2 (a–c), and pH (d–f) 4 days after administration of saline or pertussis toxin (PT) or inactivated pertussis toxin (IPT) and 20–60 min after administration of saline (a–f), mepyramine (a and d), cimetidine (b and e) and clobenpropit (c and f). Values are expressed as means±s.e.mean of six animals. The asterisks indicate P<0.05, significantly different from the compared group. For doses of PT, IPT and drugs see legend of Figure 1.
Table 1 shows the histamine-induced mortality and the effects of the three tested histamine antagonists on this parameter in saline, PT or IPT pretreated animals. Histamine evoked no mortality in the saline group. In PT pretreated animals, after treatment with saline, cimetidine or clobenpropit, the highest dose of histamine (300 mg kg−1, i.v.) evoked the death of 3–4 out of six animals, however, mepyramine completely inhibited the histamine-induced mortality. Cimetidine and clobenpropit enhanced the histamine-induced mortality as compared to saline since 2–3 out of six PT-pretreated animals treated with cimetidine or clobenpropit died after histamine 100 mg kg−1, i.v. while all animals treated with saline survived this dose of histamine. Histamine evoked no mortality in IPT pretreated animals treated with saline, mepyramine or cimetidine but in the group treated with clobenpropit three out of six animals died after the highest dose of histamine.
Table 1.
Histamine-induced mortality

Discussion
The demonstration of the absence of biological active PT in acellular vaccines is of utmost importance (Cameron, 1987). Various assay methods now available: the mouse-weight-gain test, histamine sensitization (HS) test, the leukocytosis promoting assay and the Chinese hamster ovary (CHO) test.
Sensitization for histamine-induced mortality in mice (Parfentjev & Goodline, 1984, Morse & Morse, 1976) has been accepted by regulatory authorities in the quality control of acellular pertussis vaccine. However, the mechanism(s) by which PT induces histamine sensitization is still unclear. In order to get more insight in the underlying mechanism(s) we investigated in rats the effects of PT and the role of H1, H2 and H3 receptor blockade on the actions of histamine using the following parameters: blood pressure, heart rate, blood gas values, and mortality.
In the present study histamine dose-dependently decreased MAP. PT but not saline enhanced this histamine-induced decrease in MAP. This indicates an involvement of a PT related histamine sensitizing factor (HSF) in the enhanced histamine-induced decrease in MAP after PT. This HSF seems to be a heat-labile component of PT since, like saline, IPT (PT heated during 30 min at 80°C) did not enhance the histamine-induced decrease in MAP. In previous studies we demonstrated that a heat-labile component of PT was also the predominant cause of an impairment of cholinergic and adrenergic responsiveness in rats (De Wildt et al., 1982; Vleeming et al., 1993; Van Amsterdam et al., 1998a,1998b).
In order to determine the subtype of histamine receptor that mediates the enhanced histamine-induced decrease in MAP after PT the selective antagonists mepyramine (H1), cimetidine (H2) and clobenpropit (H3) (see Hill, 1990; Hill et al., 1997) were used in our experimental system. After PT, mepyramine inhibited the enhanced histamine-induced decrease in MAP but cimetidine and clobenpropit were not effective in this respect. This observation suggests that after PT H1 but not H2 or H3 receptors are predominantly involved in PT-induced HS.
Furthermore, after PT but not after saline or IPT the highest dose of histamine (300 mg kg−1, i.v.) evoked the death of 3–4 out of six animals. Mepyramine completely inhibited this PT related histamine-induced mortality and again cimetidine and clobenpropit were ineffective. This confirms that H1 but not H2 or H3 receptors are directly involved in the PT-induced histamine sensitization in rats. However, an indirect role of H3 receptors in PT-induced HS cannot be excluded since PT also blocks H3 receptor signalling (via inactivation of G proteins) and thereby the H3 receptor mediated feedback on histamine release.
Histaminergic H1 receptors are coupled via PT insensitive G-proteins to the phospholipase C (PLC) system and to the nitric oxide (NO) synthase system (Hill, 1990; Hill et al., 1997). The latter system leads via a Ca2+/calmodulin-dependent pathway and subsequent activation of guanylate cyclase to vasodilatation (Leurs et al., 1991; Van de Voorde et al., 1998). In rats it has been shown that H1-agonists including histamine cause a decrease in blood pressure most likely by the release of nitric oxide (NO) from endothelial cells since this effect could be inhibited or abolished by the NO synthase inhibitor L-NAME (Nilsson et al., 1997; Malinowska et al., 1999; Magos et al., 1999). Translated to our experiments this suggests that the NO synthase system might be involved in PT-induced HS.
Histaminergic H2-receptors are coupled via PT insensitive G-proteins to the adenylate cyclase system and activation of this system results in cyclic AMP accumulation and vasodilatation (Powell & Shamel, 1979; Johnson 1992; Hill, 1990; Coruzzi et al., 1996; Hill et al., 1997; Van de Voorde et al., 1998; Kishi et al., 1998). There is also some evidence that H2-receptors are coupled to the PLC system (Hill et al., 1997). In the present study the H2 receptor antagonist cimetidine had no protective effect on PT induced HS. In contrast, the list of animals that died after histamine (Table 1) shows that cimetidine enhanced the histamine-induced mortality as compared to saline. It is known that cimetidine blocks the histamine-induced rise in intracellular cyclic AMP levels and as consequence it also blocks cyclic AMP-induced inhibition of H1-mediated agonistic activity (Hill 1990, Beukers et al., 1997). This latter indirect activity of cimetidine may result in an increase H1-mediated agonistic activity. This observation can be an explanation for the enhanced histamine-induced mortality after cimetidine as compared to saline.
Similarly, clobenpropit enhanced the histamine-induced mortality and in IPT treated animals it had an unfavourable PT-like effect on the histamine-induced decrease in MAP. It can be speculated that this PT-like HS effect of clobenpropit in IPT occurs since both PT and clobenpropit blocks H3-mediated agonistic activity, however, by a different mechanism of action. Clobenpropit prevents H3-mediated signal development as receptor antagonist while PT prevents H3-mediated signal transmission by inactivation of the G protein coupled to the H3 receptor (Clark & Hill, 1996). It is well known that H3 receptors can function as inhibitory auto-receptors (see Hill, 1990; Hill et al., 1997). Blocking of these pre-synaptically located auto-receptors by clobenpropit results in an enhanced synthesis and release of endogenous histamine (Mochizuki et al., 1991; Ito et al., 1997; Timm et al., 1998; Kanamaru et al., 1998). This might be an explanation for the enhanced histamine-induced decrease in MAP and increase in mortality after clobenpropit as compared to saline.
In addition to the identification of the subtype of histamine receptor involved in the PT-induced HS in rats we were also interested whether the cardiovascular or the pulmonary system is mainly inflicted in the HS due to PT. Although we found a prominent HS within the cardiovascular system, PT-induced HS did not markedly affect the respiratory system in rats. It should be noted that respiratory functions after histamine challenge in sensitized rats has only been established on the basis of blood gas evaluations. In order to investigate a contribution of HS within the respiratory system further research should also be focused on lung mechanics especially in a sensitive model like the guinea-pig.
In conclusion, PT caused HS in rats. Mepyramine inhibited while cimetidine and clobenpropit enhanced this effect. These findings indicate that primarily H1 and secondarily H2 and H3 receptors are involved PT-induced HS in rats. This results are considered to be a first step in the elucidation of the mechanism(s) of HS test in mice as developed by Parfentjev & Goodline and used in the quality control of acellular pertussis vaccine.
Acknowledgments
This study was financially supported by the Institutional Centre for Alternatives to Animal Testing (ICAAT). Jan-Dirk te Biesebeek kindly helped us with the data analysis.
Abbreviations
- DBP
diastolic blood pressure
- H
histamine
- HR
heart rate
- HS
histamine sensitization
- HSF
histamine-sensitizing factor
- I.V.
intravenously
- IPT
inactivated pertussis toxin
- MAP
mean arterial blood pressure
- PLC
phospholipase C
- PT
pertussis toxin
- SBP
systolic blood pressure
References
- BEUKERS M.W., KLAASSEN C.H., DE GRIP W.J., VERZIJL D., TIMMERMAN H., LEURS R. Heterologous expression of rat epitope-tagged histamine H2 receptors in insect Sf9 cells. Br. J. Pharmacol. 1997;122:867–874. doi: 10.1038/sj.bjp.0701466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMERON J. Safer acellular and whole-cell pertussis vaccines [letter] Lancet. 1987;1:1427–1428. doi: 10.1016/s0140-6736(87)90611-8. [DOI] [PubMed] [Google Scholar]
- CLARK E.A., HILL S.J. Sensitivity of histamine H3 receptor agonist-stimulated [35S]GTP gamma[S] binding to pertussis toxin. Eur. J. Pharmacol. 1996;296:223–225. doi: 10.1016/0014-2999(95)00800-4. [DOI] [PubMed] [Google Scholar]
- CORUZZI G., GAMBARELLI E., BERTACCINI G., TIMMERMAN H. Cardiovascular effects of the novel histamine H2 receptor agonist amthamine: interaction with the adrenergic system. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:417–422. doi: 10.1007/BF00261438. [DOI] [PubMed] [Google Scholar]
- DE WILDT D.J., DE JONG Y., NIJKAMP F.P., KREEFTENBERG J.G. Pharmacological evaluation of purified component and whole-cell pertussis vaccine in the cardiovascular system of rats. J. Pharmacol. Exp. Ther. 1985;232:541–544. [PubMed] [Google Scholar]
- DE WILDT D.J., DE JONG Y., NIJKAMP F.P., KREEFTENBERG J.G. Vascular beta-adrenoceptor blocking activity of endotoxin and pertussis toxin from Bordetella pertussis in rats. Eur. J. Pharmacol. 1986;127:205–210. doi: 10.1016/0014-2999(86)90365-1. [DOI] [PubMed] [Google Scholar]
- DE WILDT D.J., KREEFTENBERG H.G., NIJKAMP F.P. Impaired autonomic responsiveness of the cardiovascular system of the rat induced by a heat-labile component of Bordetella pertussis vaccine. Infect. Immun. 1983;41:476–481. doi: 10.1128/iai.41.2.476-481.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WILDT D.J., KREEFTENBERG J.G., NIJKAMP F.P. Severe impairment of cholinergic and adrenergic responsiveness in Bordetella pertussis vaccinated rats. Eur. J. Pharmacol. 1982;86:315–316. doi: 10.1016/0014-2999(82)90337-5. [DOI] [PubMed] [Google Scholar]
- HILL S.J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol. Rev. 1990;42:45–83. [PubMed] [Google Scholar]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- ITO C., ONODERA K., YAMATODANI A., YANAI K., SAKURAI E., SATO M., WATANABE T. The effect of haloperidol on the histaminergic neuron system in the rat brain. Tohoku. J. Exp. Med. 1997;183:285–292. doi: 10.1620/tjem.183.285. [DOI] [PubMed] [Google Scholar]
- KANAMARU M., IWASE M., HOMMA I. Autoregulation of histamine release in medulla oblongata via H3-receptors in rabbits. Neurosci. Res. 1998;31:53–60. doi: 10.1016/s0168-0102(98)00023-6. [DOI] [PubMed] [Google Scholar]
- JOHNSON C.L.Histamine receptors and cyclic nucleotides The Histamine Receptor 1992Wiley-Liss: New York; 129–143.Schwartz, J.C. & Hass, H. (eds.) [Google Scholar]
- KISHI F., NAKAYA Y., ITO S. Histamine H2-receptor-mediated nitric oxide release from porcine endothelial cells. J. Cardiovasc. Pharmacol. 1998;32:177–182. doi: 10.1097/00005344-199808000-00002. [DOI] [PubMed] [Google Scholar]
- KREEFTENBERG J.G., VAN STRAATEN VAN DE KAPPELLE I., DE WILDT D.J., TERLIGEN J.B., PETERS W.J., WALVOORT H.C. A biphasic serum glucose response in mice to inoculation with pertussis vaccine. J. Biol. Stand. 1984;12:151–157. doi: 10.1016/s0092-1157(84)80048-7. [DOI] [PubMed] [Google Scholar]
- LEURS R., BROZIUS M.M., JANSEN W., BAST A., TIMMERMAN H. Histamine H1-receptor-mediated cyclic GMP production in guinea-pig lung tissue is an L-arginine-dependent process. Biochem. Pharmacol. 1991;42:271–277. doi: 10.1016/0006-2952(91)90713-f. [DOI] [PubMed] [Google Scholar]
- MAGOS G.A., VIDRIO H., REYNOLDS W.F., ENRIQUEZ R.G. Pharmacology of Casimiroa edulis IV. Hypotensive effects of compounds isolated from methanolic extracts in rats and guinea pigs. J. Ethnopharmacol. 1999;64:35–44. doi: 10.1016/s0378-8741(98)00101-9. [DOI] [PubMed] [Google Scholar]
- MALINOWSKA B., PISZCZ J., SCHLICKER E., KRAMER K., ELZ S., SCHUNACK W. Histaprodifen, methylhistaprodifen, and dimethylhistaprodifen are potent H-1-receptor agonists in the pithed and in the anaesthetized rat. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:11–16. doi: 10.1007/pl00005316. [DOI] [PubMed] [Google Scholar]
- MOCHIZUKI T., YAMATODANI A., OKAKURA K., TAKEMURA M., INAGAKI N., WADA H. In vivo release of neuronal histamine in the hypothalamus of rats measured by microdialysis. Naunyn Schmiedebergs Arch. Pharmacol. 1991;343:190–195. doi: 10.1007/BF00168609. [DOI] [PubMed] [Google Scholar]
- MORSE S.I., MORSE J.H. Isolation and properties of the leukocytosis- and lymphocytosis-promoting factor of Bordetella pertussis. J. Exp. Med. 1976;143:1483–1502. doi: 10.1084/jem.143.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON B.O., KOCKUM I., ROSENGREN E. Effects of aminoguanidine and L-NAME on histamine-induced blood pressure drop in the rat. Acta Physiol. Scand. 1997;161:339–344. doi: 10.1046/j.1365-201X.1997.00240.x. [DOI] [PubMed] [Google Scholar]
- PARFENTJEV L.A., GOODLINE M.A. Histamine shock in mice sensitized with Hemophilus pertussis vaccin. J. Pharmacol. Exp. Ther. 1948;42:411–413. [PubMed] [Google Scholar]
- PITTMAN M. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough. A hypothesis. Rev. Infect. Dis. 1979;1:401–412. doi: 10.1093/clinids/1.3.401. [DOI] [PubMed] [Google Scholar]
- POWELL J.R., SHAMEL L.B. Characterization of vascular histamine receptors in the rat. Br. J. Pharmacol. 1979;66:517–520. doi: 10.1111/j.1476-5381.1979.tb13688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO Y., ARAI H., SUZUKI K. Leukocytosis-promoting factor of Bordetella pertussis. 3. Its identity with protective antigen. Infect. Immun. 1974;9:801–810. doi: 10.1128/iai.9.5.801-810.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TIMM J., MARR I., WERTHWEIN S., ELZ S., SCHUNACK W., SCHLICKER E. H2 receptor-mediated facilitation and H3 receptor-mediated inhibition of noradrenaline release in the guinea-pig brain. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357:232–239. doi: 10.1007/pl00005162. [DOI] [PubMed] [Google Scholar]
- VAN AMSTERDAM J.G., TE BIESEBEEK J.D., VAN DE KUIL T., VAN DER LAAN J.W., DE WILDT D.J., VLEEMING W. Repeated administration of whole-cell and acellular pertussis vaccines affects haemodynamics and autonomic responsiveness. Vaccine. 1998a;16:1668–1674. doi: 10.1016/s0264-410x(98)00058-9. [DOI] [PubMed] [Google Scholar]
- VAN AMSTERDAM J.G., TE BIESEBEEK J.D., VAN DE KUIL T., VAN DER LAAN J.W., WEMER J., DE WILDT D.J., VLEEMING W. The effect of pertussis toxin and whole-cell pertussis vaccine on haemodynamics and autonomic responsiveness in the rat depends on route of administration and age. Vaccine. 1998b;16:666–671. doi: 10.1016/s0264-410x(97)00257-0. [DOI] [PubMed] [Google Scholar]
- VAN DE VOORDE J., DELAEY C., DEPYPERE H., VANHEEL B. Mechanisms involved in the vasorelaxing influence of histamine on isolated human subcutaneous resistance arteries. Eur. J. Pharmacol. 1998;349:61–66. doi: 10.1016/s0014-2999(98)00179-4. [DOI] [PubMed] [Google Scholar]
- VAN HEUVEN NOLSEN D., SCHOUTE E., VERDOUW P.D., DE WILDT D.J., NIJKAMP F.P. In vivo beta 2-adrenoceptor hyporesponsiveness in the cardiovascular system of the pig after Bordetella pertussis. Eur. J. Pharmacol. 1989;162:135–141. doi: 10.1016/0014-2999(89)90613-4. [DOI] [PubMed] [Google Scholar]
- VLEEMING W., WEMER J., RIEZEBOS J., VAN AMSTERDAM J.G., DE WILDT D.J., PORSIUS A.J. Modulation by pertussis toxin of salbutamol- and arecoline-induced effects in the isolated heart and aorta of the rat. Eur. J. Pharmacol. 1993;250:415–422. doi: 10.1016/0014-2999(93)90028-g. [DOI] [PubMed] [Google Scholar]


