Abstract
It has been proposed that the vagus nerve plays a role in mediating cholecystokinin-8 (CCK-8) effect on such gastric functions as motility, emptying and gastric acid secretion. To examine the contribution of the efferent pathways in realizing these effects, efferent mass activity in the ventral gastric vagal nerve in Sprague-Dawley rats was recorded.
Intravenous infusion of CCK-8 (0.1–1 nmol) suppressed the efferent activity. The effect of CCK-8 was significantly reduced in animals with total subdiaphragmatic vagotomy in comparison to those with partial vagotomy.
Intravenous infusion of CCKA receptor antagonist L-364,718 (1–100×10−6 g) blocked the response of vagal efferent activity to 0.1 nmol CCK-8, but the CCKB receptor antagonist L-365,260 (1–100×10−6 g) did not in the conditions of either partial or total vagotomy.
Intracisternal infusion of L-364,718 (1×10−6 g) blocked the response of vagal efferent activity to 0.1 nmol CCK-8 i.v.
Infusion of exogenous CCK-8 did not affect the activity of supradiaphragmatic vagal afferents.
The results suggest that the effect of systemically administered CCK-8 on vagal efferent activity is mediated by both peripherally (subdiaphragmatically) and centrally localized CCKA receptors.
Keywords: Cholecystokinin-8, CCK-8 receptors, CCKA, CCKB, vagus, visceral efferents
Introduction
Neuropeptide cholecystokinin-8 (CCK-8) which is released into the circulation during feeding (Lewis & Williams, 1990) has been shown to excite vagal gastric, hepatic as well as intestinal afferents (Cox & Randich, 1997; Kurosawa et al., 1997; Richards et al., 1996; Schwartz et al., 1994). Excitation of the vagal afferent nerve conveys satiation signals to the brain (Mayne et al., 1998), resulting in the inhibition of the food intake. At the same time the vagal afferent excitation in response to CCK-8 contributes to the reflex regulation of pancreatic secretion (Li et al., 1997), gastric motility and emptying (Raybould & Taché, 1988) as well as gastric acid secretion (Raybould & Lloyd, 1994). The vagal efferent nerve is suggested to be one of the major efferent limbs of this reflex regulation. However, there has been no study addressing the responses of the vagal efferent activity in response to CCK-8.
The effect of CCK-8 is mediated via CCKA and CCKB receptors. In general, CCKA receptors are abundant in the gastrointestinal tract while CCKB receptors are found dominantly in the brain. However, both CCKA and CCKB receptors are described on the vagal nerves (Mercer & Lawrence, 1992), the nucleus tractus solitarius (NTS) where vagal afferents terminate, and the dorsal motor nucleus of the vagal nerve (DMX) where vagal efferents emerge (Plata-Salamán et al., 1988). Although it has been shown that the gastric vagal afferent fibres are activated via the CCKA receptors in response to exogenously administered CCK-8 (Kurosawa et al., 1997; Schwartz et al., 1994), the response of the gastric vagal efferents might include the activation of both CCKA and CCKB receptors at brain stem level, including DMX and NTS (Branchereau et al., 1993).
In the present study the response of gastric vagal efferent fibres to exogenously administered CCK-8 and the mechanisms involved have been investigated. First, the activity of vagal efferents was recorded under conditions of partial or total subdiaphragmatic vagotomy to elucidate the extent of the contribution of subdiaphragmatic vagal afferents to the response. Second, the site of action of CCK-8 and its receptor mechanisms were investigated in animals with partial or total subdiaphragmatic vagotomy in order to explore mechanisms additional to those of the vagal afferent-mediated. Finally, the response of supradiaphragmatic vagal afferent activity to intravenous CCK-8 was investigated.
Methods
Animals
Male Sprague-Dawley rats (ALAB Sollentuna, Sweden) 350±70 g, were housed five per cage at 21°C, with water and food ad libitum and a 12 h light/dark cycle. Animals were deprived of food for 14–20 h before the experiment. Experiments were approved by the Karolinska Institutet local animal ethical committee.
Experimental procedures
Rats were anaesthetized with pentobarbital sodium (60 mg kg−1, i.p.). Artificial ventilation was carried out throughout the experiments via a tracheal cannula (Harvard ventilator, model 683). Body temperature was maintained at +37.2±0.1°C (ATB-1100, Nihon Kohden, Japan). Blood pressure was continuously monitored from the femoral artery and maintained above 90 mmHg (systolic) by administration of 4% Ficoll 70. Additional pentobarbital sodium (5 mg h−1 kg−1) and muscle relaxant, gallamine triethiodide (20 mg h−1 kg−1) were injected through the right jugular vein using a syringe pump (model STC-521, Terumo, Tokyo, Japan). Experimental substances were injected through the right femoral vein (i.v.) or into the cisterna magna (i.c.m.). For the i.c.m. injections, the dura mater covering the foramen magnum was exposed. A small hole was made with a 25 gauge needle at the midline below the caudal edge of the occipital bone and 0.4–0.6 cm of polyethylene tube (PE 10, I.D. 0.28 mm, O.D. 0.61 mm; dead space 5 μl) filled with saline was inserted. To ensure the stable position of the catheter, a drop of Swebond (acrylic dental material, Svedia Dental-Industri AB, Sweden) was used. Free-leaking of clear CSF was considered as being proof of successful catheterization. The catheter was connected to an Exmire microsyringe (10 μl) using a silicone tube.
The abdomen was opened by midline section and the gastric branch of the ventral subdiaphragmatic vagal nerve was identified on the oesophagus, separated from the surrounding tissues under a binocular microscope and cut just proximal from the entrance into the stomach. The dorsal subdiaphragmatic vagus was left intact (n=8) or cut beneath the diaphragm (n=8). All branches of the ventral subdiaphragmatic vagal nerve including the hepatic, intestinal and accessory coeliac branches were cut in all animals (Figure 1). The central cut segment of the ventral gastric branch was placed on a bipolar platinum wire electrode and immersed in paraffin oil. The mass efferent activity was amplified (amplification gain 10 μV, bandpass 150–1000 Hz, time constant 0.01 s) (MEG-1200, Nihon-Kohden, Japan), counted every 2 s by a pulse counter after passing through a window discriminator (MET-1100, Nihon-Kohden, Japan) and recorded on a polygraph (WS-682G, Nihon-Kohden, Japan). The lower level of the window discriminator was always set clearly above the maximal noise peak level.
Figure 1.
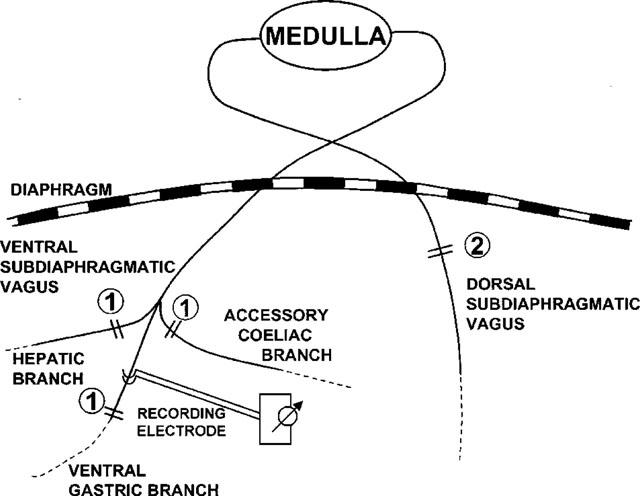
Schematic view of the two types of subdiaphragmatic vagotomies used in the present experiments. Partial subdiaphragmatic vagotomy consisted of complete transsection of hepatic, ventral gastric and accessory coeliac branches (marked 1). Total subdiaphragmatic vagotomy additionally transsected dorsal subdiaphragmatic vagus (marked 2). Electrical activity was recorded from the proximal segment of the ventral gastric branch.
The supradiaphragmatic afferent activity was recorded from the distal cut segment of a filament dissected from a bundle of the left cervical vagal nerve (n=8) in animals with total subdiaphragmatic vagotomy.
Sulphated cholecystokinin octapeptide (CCK-8) were first dissolved in saline containing 0.1% bovine serum albumine (BSA) and diluted with saline. Then it was administered intravenously as bolus injections in doses of 0.01, 0.1 or 1 nmol (ca 0.35–3.5 μg kg−1) in 0.2 ml saline given over 20 s. L-364,718, a CCKA receptor antagonist, and L-365,260, a CCKB receptor antagonist, were dissolved in 50% dimethyl sulfoxide (DMSO) at a concentration of 12.5 μmol ml−1, and then diluted with saline. Antagonists were injected intravenously as bolus injections in doses of 1, 10 and 100 μg in 0.2 ml saline 5 min before the administration of CCK-8 (0.1 nmol) (n=8). In six rats 1 μg L-364,718 in 6 μl saline was injected i.c.m. and CCK-8 (0.1 nmol) subsequently injected i.v. every 10 min over a 1 h period.
Drugs
The following drugs were used: pentobarbital sodium (Apoteksbolaget, Sweden); Ficoll 70 (Pharmacia Fine Chemicals AB, Uppsala, Sweden); gallamine triethiodide, cholecystokinin-8 (CCK 26–33), dimethyl sulfoxide (DMSO) and bovine serum albumine (BSA) from Sigma Chemical Co., St. Louis, MO, U.S.A.; paraffin oil (Kebo Lab.); L-364,718 (devazepide, or MK-329) and L-365,260 from ML Lab, London, U.K.
Statistical analysis
Responses of vagal gastric efferent activity were measured over 10 s immediately before the onset of CCK-8 administration and expressed as 100% control value. This was then compared with the average count over 10 s in every 30 s (start of the injection taken as time point zero), expressed as mean±s.e.mean. Data were analysed by two-way repeated-measures ANOVA. When a significant treatment effect was found, Dunnett's test for each group and time point was performed. Comparisons of latencies of the response were made by Student's t-test, two-tailed. Probability less than 5% was considered significant.
Results
Effect of exogenous i.v. CCK-8 on vagal efferent activity in animals with partial subdiaphragmatic vagotomy
The efferent activity of the gastric vagal nerve was recorded from a branch of the central cut segment of the sectioned whole ventral subdiaphragmatic vagal nerve, i.e. the ipsilateral subdiaphragmatic vagal afferent activity was disrupted.
As shown in Figure 2, 0.1 and 1 nmol doses of CCK-8 produced a rapid, significant dose-dependent decrease in the mass activity of the gastric vagal efferent nerve (treatment × time interaction F(3, 33)=11.2, P<0.001). Administration of 0.1 nmol of CCK-8 decreased the nerve activity to 33±5% and 52±6% of the pre-administration control at 30 and 60 s, respectively. The activity returned to its basal level 2.5 min after the administration. Injection of 1.0 nmol CCK-8 further decreased the nerve activity to 28±6% and 27±6% of pre-administration control at 30 and 60 s after the administration, respectively. This decrease lasted for more than 5 min after the injection. After 1.0 nmol CCK-8 the mean latency of the response was 12±0.6 s and after 1 nmol CCK-8 10.5±0.44 s. Intravenous administration of saline or a lower dose of CCK-8 (0.01 nmol) had no effect on the mass activity of the gastric vagal efferent nerve. In other experiments, BSA or DMSO alone were also without effect on gastric vagal efferent nerve activity (data not shown).
Figure 2.
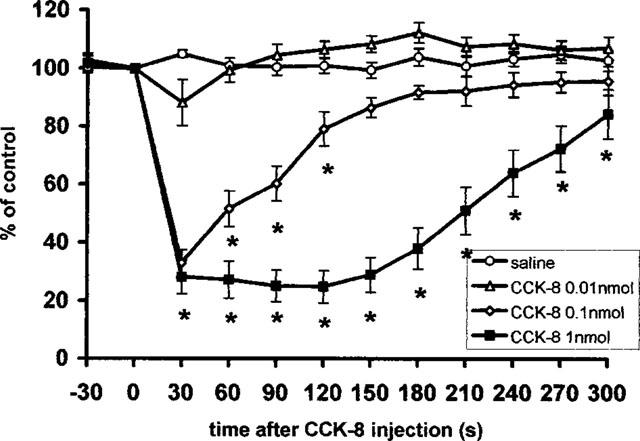
Summarized responses of mass activity in the gastric vagal efferent nerve in rats with partial vagotomy to i.v. administration of CCK-8; per cent of the pre-administration control values, mean±s.e.m., n=7–8. Time of the injection 0. *P<0.05 between CCK-8 and saline treated groups.
Effect of exogenous i.v. CCK-8 on vagal efferent activity in animals with partial subdiaphragmatic vagotomy after i.v. pretreatment with either CCKA or CCKB receptor antagonist
To study the contribution of CCKA and CCKB receptors to the response of vagal efferent activity in rats with partial subdiaphragmatic vagotomy, CCKA or CCKB receptor antagonist was administered before the injection of 0.1 nmol CCK-8. Neither the CCKA nor CCKB receptor antagonist itself affected the spontaneous efferent activity of the vagal nerve (data not shown).
Intravenous injection of L-364,718, a CCKA receptor antagonist in dose of 1 μg, 5 min prior to the injection of 0.1 nmol CCK-8 partially blocked the response of vagal efferent activity in comparison to that of CCK-8 injected after saline (Figure 3A). Larger doses of the antagonist (10 and 100 μg) completely blocked the response (treatment × time interaction F(3,33)=11.5, P<0.001).
Figure 3.
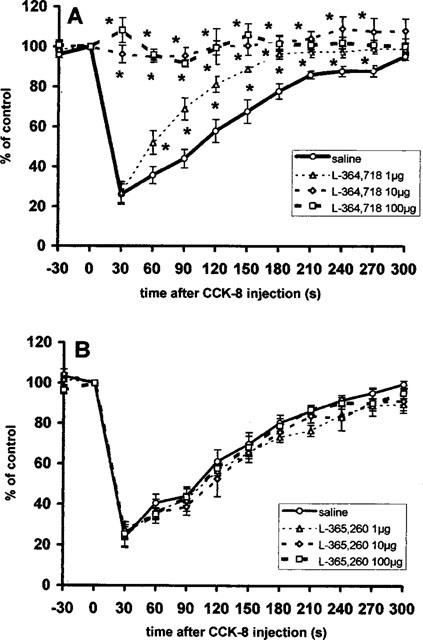
Summarized responses of the gastric vagal efferent nerve activity in rats with partial vagotomy to i.v. administration of 0.1 nmol CCK-8 5 min following i.v. injection of saline or L-364,718, a CCKA receptor antagonist (A) and saline or L-365,260, a CCKB receptor antagonist (B). Percentage of the pre-administration control values, mean±s.e.m., n=6. Time of the injection 0. *P<0.05 between saline and antagonist pre-treated groups.
Administration of L-365,260, a CCKB receptor antagonist, in the doses of 1 μg, 10 μg or 100 μg (Figure 3B) had no effect on the response of vagal efferent activity to 0.1 nmol CCK-8 (treatment × time interaction F(3,33)=0.24, P=1.0).
Effect of exogenous i.v. CCK-8 on vagal efferent activity in animals with total subdiaphragmatic vagotomy
To determine the contribution of subdiaphragmatic vagal afferent activity to the responses of the vagal efferent nerve, shown in Figure 2, the contralateral (dorsal) subdiaphragmatic vagal nerve was additionally cut. When injected 0.5 h after total vagotomy, 0.1 and 1 nmol doses of CCK-8 still produced a significant dose-dependent decrease in the gastric vagal efferent nerve activity (Figure 4) (treatment × time interaction F(3,21)=5.6, P<0.001), although the decrease in nerve activity was smaller than in the conditions where the subdiaphragmatic vagal afferent nerve was left intact. With 0.1 nmol CCK-8 the activity decreased to 82±6% of pre-administration control after 30 s and to 81±5% after 60 s, and with 1 nmol CCK-8 to 56±2% after 30 s and to 59±4% and 60 s. The activity returned to its pre-administration level 2 min after the administration of 0.1 nmol CCK-8 and 3.5 min after 1 nmol CCK-8. The mean latency of the response to CCK-8 was significantly prolonged (1 nmol CCK, 16.7±1.1 s, P<0.01) after the cutting of the dorsal subdiaphragmatic vagal nerve. Neither saline nor 0.01 nmol CCK-8 affected vagal efferent nerve activity (Figure 4).
Figure 4.
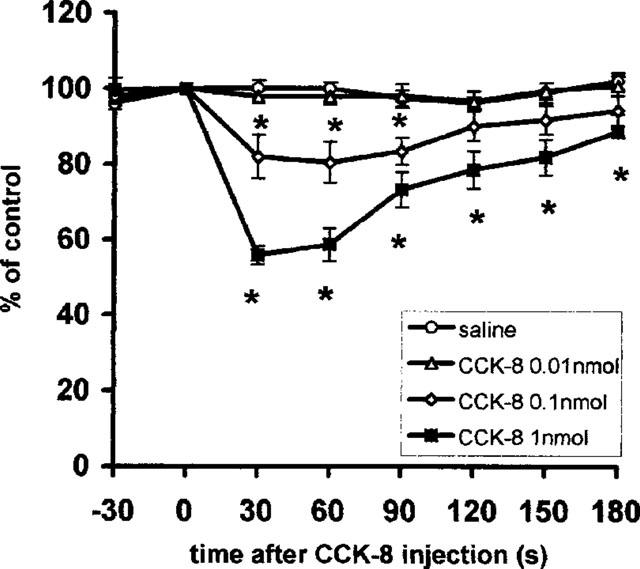
Summarized responses of mass activity in the gastric vagal efferent nerve in rats with total vagotomy to i.v. administration of CCK-8; per cent of the pre-administration control values, mean±s.e.m., n=8. Time of the injection 0. *P<0.05 between CCK-8 and saline treated groups.
Effect of exogenous i.v. CCK-8 on vagal efferent activity in animals with total subdiaphragmatic vagotomy after i.v. pretreatment with either CCKA or CCKB receptor antagonist
To study the contribution of the CCK receptors to the response of vagal efferent activity in rats with total subdiaphragmatic vagotomy, CCKA or CCKB receptor antagonist was administered prior to injection of 0.1 nmol CCK-8.
Intravenous injection of L-364,718, a CCKA receptor antagonist in dose of 1 μg, 5 min prior to the injection of 0.1 nmol CCK-8 partially blocked the response of vagal efferent activity in comparison to that of CCK-8 injected after saline (Figure 5A). Larger doses of the antagonist (10 and 100 μg) completely blocked the response (treatment × time interaction F(3,21)=3.9, P<0.001).
Figure 5.
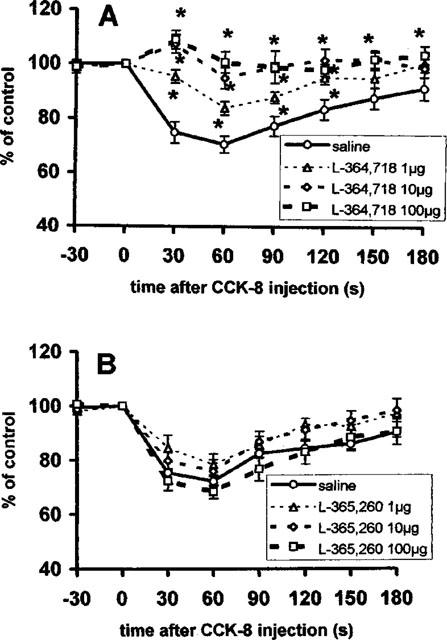
Summarized responses of the gastric vagal efferent nerve activity in rats with total vagotomy to i.v. administration of 0.1 nmol CCK-8 5 min following i.v. injection of saline or L-364,718, a CCKA receptor antagonist (A), and saline or L-365,260, a CCKB receptor antagonist (B). Percentage of the pre-administration control values, mean±s.e.m., n=6–7. Time of the injection 0. *P<0.05 between saline and antagonist pre-treated groups.
However, the effects of 0.1 nmol CCK-8 were not attenuated by the prior administration of L-365,260, a CCKB antagonist, in the doses of 1, 10 or 100 μg (Figure 5B) (treatment × time interaction F(3,21)=0.75, P=0.77).
Effect of exogenous i.v. CCK-8 on vagal efferent activity in animals with total subdiaphragmatic vagotomy after i.c.m. pretreatment with CCKA receptor antagonist
The vagal efferent activity response to 0.1 nmol CCK-8 was studied at different time intervals after the i.c.m. injection of CCKA receptor antagonist (1 μg) in animals with total subdiaphragmatic vagotomy. The reproducibility of the response of the vagal efferent activity to injections of CCK-8 at 10 min intervals was confirmed in control experiments. Ten to twenty minutes after the application of CCKA receptor antagonist the response of vagal efferent activity to CCK-8 was completely blocked (treatment × time interaction F(1,7)=3.04, P=0.007), (Figure 6). The response of i.v. CCK-8 returned after 40–50 min after i.c.m. injection of CCKA receptor antagonist (data not shown).
Figure 6.
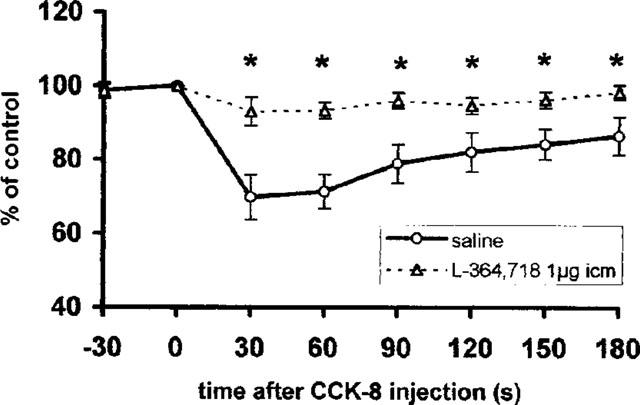
Summarized responses of the gastric vagal efferent nerve activity in rats with total vagotomy to i.v. administration of 0.1 nmol CCK-8 10–20 min following i.c.m. injection of saline or L-364,718, a CCKA receptor antagonist. Percentage of the pre-administration control values, mean±s.e.m., n=6. Time ‘0' indicates the time of injection of CCK-8. *P<0.05 between saline and L-364,718 pre-treated groups.
Effect of exogenous i.v. CCK-8 on the afferent activity of supradiaphragmatic vagal nerves
In order to elucidate the contribution of supradiaphragmatic vagal afferents in the response of vagal gastric efferent activity to CCK-8, the response of vagal afferents at the cervical level was recorded after total subdiaphragmatic vagotomy (Figure 7). No significant effect of 1.0 nmol CCK-8 was found when compared to the saline treated group.
Figure 7.
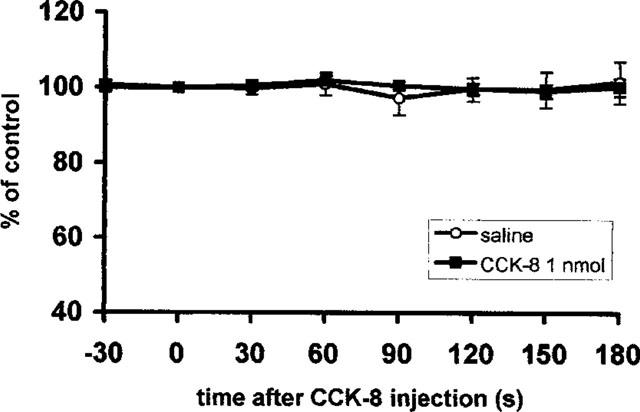
Summarized responses of mass activity in the cervical vagal afferent nerve in rats with total subdiaphragmatic vagotomy to i.v. administration of CCK-8; per cent of the pre-administration control values, mean±s.e.m., n=8. Time of the injection 0.
Discussion
The results show that administration of exogenous 0.1–1.0 nmol CCK-8 (ca 0.35–3.5 μg kg−1) significantly decreased the gastric vagal efferent nerve activity. This effect of CCK-8 on gastric vagal efferent activity was mediated by CCKA receptors, localized both peripherally and centrally.
In the present study exogenous CCK-8 induced a decrease of gastric vagal efferent activity and this effect of CCK-8 was significantly diminished after total subdiaphragmatic vagotomy, suggesting that the main site of action of CCK-8 was the subdiaphragmatic vagal afferents. These results are supported by Schwartz et al. (1994) and Kurosawa et al. (1997) who have demonstrated that intravenous injection of CCK-8 increases the vagal gastric afferent activity dose-dependently. The present vago-vagal neural mechanism underlies the reflex responses to CCK-8 of the gastric motility and emptying (Raybould & Taché, 1988; Grundy et al., 1995; Rogers et al., 1995), although the results can not exclude the possibility that the intact contralateral subdiaphragmatic vagal efferent nerve augments the response of the recorded efferent nerve activity to CCK-8 via some unknown mechanisms.
The finding that CCK-8 still had an effect on efferent activity after total subdiaphragmatic vagotomy suggests that CCK-8 also might stimulate supradiaphragmatic vagal afferents, and/or act directly on central vagal sites. We had excluded the involvement of supradiaphragmatic vagal afferents in the response of gastric vagal efferents to exogenous CCK-8, since intravenous injection of 1 nmol of CCK-8, a dose significantly affecting both subdiaphragmatic vagal afferent and efferent activity, had no effect on the activity of supradiaphragmatic vagal afferents. Although it is generally accepted that CCK does not readily cross the blood-brain barrier in the time course of the current experiments (Oldendorf, 1981), recent studies suggest that CCK and related peptides may have access to specific brain sites where the blood-brain barrier is incomplete (Harro et al., 1993; Hagino & Moroji, 1994). In addition, larger doses of CCK-8 (4–16 μg kg−1) may activate non-vagal sympathetic afferents as reduction in food intake has been observed in rats with subdiaphragmatic vagal deafferentation (Moran et al., 1997).
CCK-8 exerts its physiological effects via either CCKA or CCKB receptors. Studies by Schwartz et al. (1994) and Kurosawa et al. (1997) demonstrated that the gastric vagal afferents are activated via CCKA receptors, indicating that the profound decrease in the gastric vagal efferent activity in animals with partial subdiaphragmatic vagotomy is mainly produced via stimulation of the CCKA receptors located on the vagal afferent terminals (Zarbin et al., 1981).
In order to further determine the type of CCK receptors involved in the inhibition of gastric vagal efferent nerve activity and the localization of the receptors being activated in rats with total subdiaphragmatic vagotomy, CCKA (i.v. or i.c.m.) or CCKB (i.v.) receptor antagonist were injected prior to the administration of CCK-8. Systemically administered L-364,718 and L-365,260 have been shown to be able to cross the blood-brain barrier and to reduce the central responses of peripherally administered CCK-8 and CCK-8-related peptides (Hagino & Moroji, 1994; Ladurelle et al., 1997; Lin & Lin, 1990). However, this occurs slowly and is unlikely to account for the effects observed in current experiments. Intravenous injection of a CCKA receptor antagonist blocked the response of vagal efferent activity while that of a CCKB receptor antagonist had no effect on the response, indicating that the effect of CCK-8 on vagal efferent activity after total vagotomy was mediated through CCKA, but not CCKB receptors. Moreover, i.c.m. administration of 1 μg of the CCKA receptor antagonist, a dose which showed a partial block when administered intravenously, completely blocked the response of the vagal efferent activity to peripherally administered CCK-8. These results suggest that the response of the vagal efferent activity to CCK-8 in rats with total subdiaphragmatic vagotomy are mainly mediated via the centrally located CCKA receptors (Branchereau et al., 1993), possibly those in area postrema etc. where the blood-brain barrier is lacking (Gross et al., 1990; Mönnikes et al., 1997; Moran et al., 1986). In accordance with our findings, it has been shown (Mönnikes et al., 1997) that the activation of some of the nuclei in the brain such as the paraventricular nucleus, NTS and especially the area postrema by systemic administration of CCK-8 was not totally dependent on the vagal afferent excitation, suggesting the contribution of central CCK receptors. The central CCKA receptor would further augment the responses of the vagal efferent nerve activity in animals with intact vagal afferents (Barber et al., 1995).
Conclusion
The effect of exogenous CCK-8 on vagal efferent activity was realized mainly through peripheral CCKA receptors located on the subdiaphragmatic vagal afferents, but not through CCKB. The response of the efferent activity can be partly mediated through the central CCKA receptors. Our present results are in line with earlier studies showing that CCKA receptors play a major role in regulating the afferent and efferent gastric vagal activity in response to CCK-8. These neural mechanisms underlie the involvement of CCK-8 in the reflex regulation of gastric motility, emptying, and acid secretion.
Acknowledgments
The authors acknowledge the technical help and advice of Mr Lennart Löfqvist and editorial expertise of Mrs Audrey Singh, and ML Lab (London, U.K.) for providing L-364,718 and L-365,360. This work was supported by the Karolinska Institutet Foundation, Wenner-Gren Center foundation, the Foundation for Acupuncture and Alternative Treatment Methods and a SRF Grant for Biomedical Research.
Abbreviations
- CCKA
type A CCK receptor
- CCKB
type B CCK receptor
- CCK-8
cholecystokinin-8 (26–33)
- DMX
dorsal motor nucleus of the vagus nerve
- NTS
nucleus tractus solitarius
- L-364,718
type A CCK antagonist
- L-365,260
type B CCK antagonist
References
- BARBER W.D., YUAN C.-S., BURKS T.F., FELDMAN J.L., GREER J.J. In vitro brainstem- gastric preparation with intact vagi for study of primary visceral afferent input to dorsal vagal complex in caudal medulla. J. Autonom. Nerv. Syst. 1995;51:181–189. doi: 10.1016/0165-1838(94)00129-8. [DOI] [PubMed] [Google Scholar]
- BRANCHEREAU P., CHAMPAGNAT J., DENAVIT-SAUBIÉ M. Cholecystokinin-gated currents in neurons of the rat solitary complex in vitro. J. Neurophysiol. 1993;70:2584–2595. doi: 10.1152/jn.1993.70.6.2584. [DOI] [PubMed] [Google Scholar]
- COX J.E., RANDICH A. CCK-8 activates hepatic vagal C-fibre afferents. Brain Res. 1997;776:189–194. doi: 10.1016/s0006-8993(97)01036-6. [DOI] [PubMed] [Google Scholar]
- GROSS P.M., WALL K.M., PANG J.J., SHAVER S.W., WAINMAN D.S. Microvascular specializations promoting rapid interstitial solute dispersion in nucleus tractus solitarius. Am. J. Physiol. 1990;259:R1131–R1138. doi: 10.1152/ajpregu.1990.259.6.R1131. [DOI] [PubMed] [Google Scholar]
- GRUNDY D., BAGAEV V., HILLSLEY K. Inhibition of gastric mechanoreceptor discharge by cholecystokinin in the rat. Am. J. Physiol. 1995;268:G355–G360. doi: 10.1152/ajpgi.1995.268.2.G355. [DOI] [PubMed] [Google Scholar]
- HAGINO Y., MOROJI T. Effects of systemically administered ceruletide on the in vivo release and metabolism of dopamine in the medial prefrontal cortex of awake, freely moving rats: an in vivo microdialysis study. Brain Res. 1994;644:40–46. doi: 10.1016/0006-8993(94)90344-1. [DOI] [PubMed] [Google Scholar]
- HARRO J., VASAR E., BRADWEJN J. CCK in animal and human research of anxiety. Trends Pharmacol. Sci. 1993;14:244–257. doi: 10.1016/0165-6147(93)90020-k. [DOI] [PubMed] [Google Scholar]
- KUROSAWA M., UVNÄS-MOBERG K., MIYASAKA K., LUNDEBERG T. Interleukin-1 increases activity of the gastric vagal afferent nerve partly via stimulation of type A CCK receptor in anesthetized rat. J. Autonom. Nerv. Syst. 1997;62:72–78. doi: 10.1016/s0165-1838(96)00111-7. [DOI] [PubMed] [Google Scholar]
- LADURELLE N., KELLER G., BLOMMAERT A., ROQUESM B.P., DAUGÉ V. The CCK-B agonist, BC264, increases dopamine in the nucleus accumbens and facilitates motivation and attention after intraperitoneal injection in rats. Eur. J. Neurosci. 1997;9:1804–1814. doi: 10.1111/j.1460-9568.1997.tb00747.x. [DOI] [PubMed] [Google Scholar]
- LEWIS L.D., WILLIAMS J.A. Regulation of cholecystokinin secretion by food, hormones, and neural pathways in the rat. Am. J. Physiol. 1990;258:G512–G518. doi: 10.1152/ajpgi.1990.258.4.G512. [DOI] [PubMed] [Google Scholar]
- LI Y., HAO Y., OWYANG C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am. J. Physiol. 1997;273:G679–G685. doi: 10.1152/ajpgi.1997.273.3.G679. [DOI] [PubMed] [Google Scholar]
- LIN T.-H., LIN J.H. Effects of protein binding and experimental disease states on brain uptake of benzodiazepines in rats. J. Pharmacol. Exp. Therap. 1990;253:45–50. [PubMed] [Google Scholar]
- MAYNE R.G., ARMSTRONG W.E., CROWLEY W.R., BEALER S.L. Cytoarchitectonic analysis of Fos-immunoreactivity in brainstem neurons following visceral stimuli in conscious rats. J. Neuroendocrinol. 1998;10:839–847. doi: 10.1046/j.1365-2826.1998.00271.x. [DOI] [PubMed] [Google Scholar]
- MERCER J.G., LAWRENCE C.B. Selectivity of cholecystokinin (CCK) receptor antagonists, MK-329 and L-365,260, for axonally-transported CCK binding sites on the rat vagus nerve. Neurosci. Lett. 1992;137:229–231. doi: 10.1016/0304-3940(92)90410-9. [DOI] [PubMed] [Google Scholar]
- MÖNNIKES H., LAUER G., ARNOLD R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res. 1997;770:277–288. doi: 10.1016/s0006-8993(97)00865-2. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., BALDESSARINI A.R., SALORIO C.F., LOWERY T., SCHWARTZ G.J. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- MORAN T.H., ROBINSON P.H., GOLDRICH M.S., MCHUGH P.R. Two brain cholecystokinin receptors: implications for behavioural actions. Brain Res. 1986;362:175–179. doi: 10.1016/0006-8993(86)91413-7. [DOI] [PubMed] [Google Scholar]
- OLDENDORF W.F. Blood-brain barrier permeability to peptides: pitfalls in measurement. Peptides. 1981;2:109–111. doi: 10.1016/0196-9781(81)90020-6. [DOI] [PubMed] [Google Scholar]
- PLATA-SALAMÁN C.R., FUKUDA A., OOMURA Y., MINAMI T. Effects of sulphated cholecystokinin octapeptide (CCK-8) on the dorsal motor nucleus of the vagus. Brain Res. Bull. 1988;21:839–842. doi: 10.1016/0361-9230(88)90054-8. [DOI] [PubMed] [Google Scholar]
- RAYBOULD H.E., LLOYD K.C.K. Integration of postprandial function in the proximal gastrointestinal tract. Ann. N.Y. Acad. Sci. 1994;713:143–156. doi: 10.1111/j.1749-6632.1994.tb44061.x. [DOI] [PubMed] [Google Scholar]
- RAYBOULD H.E., TACHÉ Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am. J. Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- RICHARDS W., HILLSLEY K., EASTWOOD C., GRUNDY D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J. Physiol. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS R.C., MCTIGUE D.M., HERMANN G.E. Vagovagal reflex control of digestion: afferent modulation by neural and ‘endoneurocrine' factors. Am. J. Physiol. 1995;268:G1–G10. doi: 10.1152/ajpgi.1995.268.1.G1. [DOI] [PubMed] [Google Scholar]
- SCWARTZ G.J., MCHUGH P.R., MORAN T.H. Pharmacological dissociation of responses to CCK and gastric loads in rat mechanosensitive vagal afferents. Am. J. Physiol. 1994;267:R303–R308. doi: 10.1152/ajpregu.1994.267.1.R303. [DOI] [PubMed] [Google Scholar]
- ZARBIN M.A., WAMSLEY J.K., INNIS R.B., KUHAR M.J. Cholecystokinin receptors: presence and axonal flow in the rat vagus nerve. Life Sci. 1981;29:697–705. doi: 10.1016/0024-3205(81)90022-9. [DOI] [PubMed] [Google Scholar]


