Abstract
Proteinase-activated receptor-2 (PAR-2) is expressed throughout the gastrointestinal tract including the pancreas, and may be involved in digestive functions. The aim of our study was to evaluate a potential role for PAR-2 in regulating salivary and pancreatic exocrine secretion in vivo.
PAR-2-activating peptides (PAR-2-APs), but not selective PAR-1-APs, administered intravenously, increased salivary secretion in the mouse or rat; this effect of the PAR-2-APs was unaffected by atropine, phentolamine, propranolol or indomethacin.
Secretion (amylase) by rat parotid gland slices in vitro was also stimulated by PAR-2-APs and trypsin, but not by activation of other PARs.
PAR-2-APs, administered to rats in vivo, caused a prompt effect on pancreatic exocrine secretion.
PAR-2 mRNA, known to be present in pancreatic tissue, was also detected in parotid tissue.
Our results indicate that in addition to a potential role in regulating cardiovascular and respiratory functions, PAR-2 may also play a general role in vivo for the direct regulation of glandular exocrine secretion.
Keywords: Proteinase (protease)-activated receptor (PAR), salivation, parotid gland, amylase, trypsin, pancreatic juice, exocrine secretion
Introduction
Proteinase-activated receptors (PARs) are unique members of the large superfamily of G-protein-coupled seven transmembrane receptors, the activation of which is achieved by an irreversible proteolytic mechanism (Dery et al., 1998). Four distinct subtypes of PARs (PARs 1 to 4) have been described (Vu et al., 1991; Nystedt et al., 1994; Ishihara, H. et al., 1997; Kahn et al., 1998; Xu et al., 1998). PAR-1, PAR-3 and PAR-4 are activated by thrombin, whereas PAR-2 is activated by trypsin or tryptase, but not by thrombin (Nystedt et al., 1994; Molino et al., 1997). Messenger RNAs for these four PARs are expressed in varying amounts in a wide variety of tissues in humans and other species (Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Kahn et al., 1998; Xu et al., 1998; Hollenberg, 1999). Remarkably, except for PAR-3, synthetic receptor-activating peptides (PAR-APs) as short as 5–6 amino acids, based on the N-terminal receptor-activating motifs of the tethered ligands, are capable of activating the receptors directly by a mechanism independent of proteolysis (e.g. the peptide, SFLLR for human PAR-1, SLIGRL for mouse and rat PAR-2 and GYPGKF for mouse PAR-4) (Vu et al., 1991; Nystedt et al., 1994; Ishihara et al., 1997; Kahn et al., 1998; Xu et al., 1998). Studies of the peptide structure-activity relationships using a variety of PAR-AP analogues have led to the development of receptor-selective agonists for PAR-1 and PAR-2 (Hollenberg et al., 1997; Kawabata et al., 1999b; Blackhart et al., 1996). As yet, receptor-selective antagonists for the PARs have not been developed.
Although a haemostatic/cardiovascular role for PARs-1, 3 and 4 can be suggested because of the activation of human and murine platelets by thrombin (Dery et al., 1998; Vu et al., 1991; Kahn et al., 1998; 1999), the physiological or pathophysiological role(s) for PAR-2 are still essentially unknown. It has been our working hypothesis that PAR-2, like PAR-1, may be involved in inflammatory or injury-response events (Cirino et al., 1996; Kawabata et al., 1998; 1999a; Vergnolle et al., 1999). PAR-2 activation is known to cause the contraction or relaxation of vascular, gastric and airway smooth muscle (Hollenberg et al., 1997; Saifeddine et al., 1996; Cocks et al., 1999). Further, activation of PAR-2 in vivo has been observed to cause hypotension in mice and other mammals (Cheun et al., 1998; Damiano et al., 1999) and to inhibit bronchoconstriction in rats (Cocks et al., 1999). Amongst other tissues, the digestive tract and pancreas have been found to express high amounts of PAR-2 mRNA (Bohm et al., 1996). In isolated tissue preparations, PAR-2-APs and trypsin have been found to modulate intestinal function (Kon et al., 1997; Corvera et al., 1997; Vergnolle et al., 1998); and PAR-2-APs have also been observed to cause amylase secretion from isolated pancreatic acinar fragments (Bohm et al., 1996). Most recently, a study of tissue in vitro has demonstrated that canine pancreatic duct cells are rich in PAR-2 and that trypsin transiently activates pancreatic duct epithelial cell ion channels by triggering PAR-2 (Nguyen et al., 1999).
Given the high levels of PAR-2 mRNA in the digestive tract (Hollenberg, 1999) and the demonstrated effects of PAR-2 activation on isolated pancreatic tissue in vitro (Bohm et al., 1996; Nguyen et al., 1999), we hypothesized that PAR-2 might play a more general role in regulating glandular exocrine function, related to the digestive process. We therefore turned our attention to the parotid gland, which plays an auxiliary digestive role. This tissue is readily accessible for studies both in vivo and in vitro. We assessed the activities of PAR-2-APs on parotid secretion in mice and rats in vivo and on rat parotid slice secretion in vitro. Further, we examined whether PAR-2 activation in vivo might trigger rat pancreatic secretion. Our data indicated that PAR-2 (but not PAR-1) may play an important role in regulating salivary and pancreatic secretion in vivo.
Methods
Experimental animals
Male ddY mice weighing 20–30 g and Wistar rats weighing 200–300 g (Japan SLC. Inc.) were used with approval from the Kinki University Faculty of Pharmaceutical Sciences' Committee for the care and use of laboratory animals. Mice were employed for the detailed in vivo studies (dose-response curves; inhibitors) to economize the amount of peptides.
In vivo salivation bioassay in the mouse and rat
Rats and mice were anaesthetized with i.p. urethane (1.5 g kg−1) and the salivation bioassay was performed essentially according to the previously described method with modifications (Takeda et al., 1989). Immediately before drug challenge, saliva in the animal's oral cavity was removed using an aspirator. Then whole saliva was successively aspirated at 1 min intervals for 5 min after i.v. administration of secretagogues. The amount of saliva collected during each 1 min interval was quantitated by weight (mg).
Drug administration schedules in the in vivo salivation assay
The specific PAR-2-APs, SLIGRL-NH2 (SLp-NH2), SLIGRL (SLp-OH) and N-trans-cinnamoyl-LIGRL-ornithine-NH2 (tcLp-NH2), or their inactive control peptide LSIGRL-NH2 (LSp-NH2), the PAR-1/PAR-2 agonist SFLLR-NH2 (SFp-NH2) or its inactive control peptide FSLLR-NH2 (FSp-NH2), and the specific PAR-1-AP TFLLR-NH2 (TFp-NH2), in a dose range of 0.5–50 μmol kg−1, and carbachol at 0.08 μmol kg−1 were administered i.v. to the mouse or rat. Amastatin at 2.5 or 84 μmol kg−1 was preadministered i.v. 1 min before i.v. injection of SLp-NH2 at 0.5 μmol kg−1. Atropine at 7.2 μmol kg−1 (5 mg kg−1, phentolamine at 15.7 μmol kg−1 (5 mg kg−1), propranolol at 16.9 μmol kg−1 (5 mg kg−1) or indomethacin at 28 μmol kg−1 (10 mg kg−1) were administered i.p. 25 min before i.v. SLp-NH2 at 5 μmol kg−1. Control animals received i.v. or i.p. injection of vehicle.
Determination of composition of saliva secreted in vivo
In the mouse, the saliva secreted in response to i.v. SLp-NH2 at 5 μmol kg−1 or carbachol at 0.08 μmol kg−1 was collected and subjected to compositional analysis. Amylase activity in the collected saliva was measured spectrophotometrically using an assay kit (Amylase B-test, Wako, Japan). Sodium and potassium concentrations were determined by an automatic electrolyte analyser with ion-selective electrodes (664, Chiron, U.S.A.).
Detection of mRNA for PAR-1 and PAR-2 in rat parotid gland and pancreas by a reverse transcriptase-polymerase chain reaction
Total RNA was isolated from rat parotid gland and from rat pancreas as a positive control, using the TRIzol Reagent (Life Technologies, Inc., U.S.A.), and then mRNA was purified from the total RNA with the Oligotex-dT30 (Super) mRNA purification kit (Takara Shuzo, Japan). Essentially as described previously (Hollenberg et al., 1996), mRNA was reverse-transcribed at 42°C for 50 min, and amplified by polymerase chain reaction (RT–PCR) using the RNA LA PCR kit (AMV) ver. 1.1 (Takara Shuzo, Japan). The PCR primers for amplification of PAR-1 sequences were 5′-CCCGCTCATTTTTTCTCAGGA-3′ and 5′-GCCAATCGGTCGCGGAGAAGT-3′, leading to amplification of 394 bp fragments. The primers targeted to PAR-2 were 5′-CACCAGTAAAGGGAGAAGTCT-3′ and 5′-GGGCAGCACGTCGTGACAGGT-3′, yielding amplification of 598 bp fragments. The primers for β-actin were 5′-GTGGGGCGCCCCAGGCACCA-3′ and 5′-GTCCTTAATGTCACGCACGATTTC-3′, amplifying 537–bp fragments. Amplification was allowed to proceed for 35 cycles beginning with a 30 s denaturation period at 94°C followed by a 30 s reannealing time at 55°C and a 1 min primer extension period at 72°C. The PCR products were separated by 2% agarose gel electrophoresis and visualized by the ethidium bromide staining procedure.
In vitro assay of amylase secretion from rat parotid gland slices
Essentially according to the previously described method (Jahn et al., 1980), the rat parotid glands were removed under sodium pentobarbital (50 mg kg−1, i.p.) anaesthesia, and immediately washed with an ice-cold, gassed (95° O2 plus 5% CO2) Krebs–Henseleit buffer of the following composition (mM): NaCl, 118; KCl, 4.7; CaCl2, 2.5; MgCl2; NaHCO3, 25; KH2PO4, 1.2; glucose 10. Glands were quickly freed from adherent fat and connective tissue and cut into small pieces of about 1 mm3 in the iced buffer under a dissecting microscope. Portions of 100 mg wet weight of the slices were preincubated for 30 min in 3 ml of the same buffer that was continuously gassed with 95% O2/5% CO2 and maintained at 37°C, and a small aliquot of 30 μl was collected (background sample, BS). Then the sample solution was incubated for 10 min after addition of PAR-APs, trypsin or thrombin at various concentrations; at that time another 30 μl aliquot was collected (reaction sample, RS). Thereafter, the slices in the buffer solution were homogenized and centrifuged at 30,000 g and at 4°C for 30 min. The supernatant was used to determine the total amount of amylase present in the assay system (cells plus buffer) (total sample, TS). Amylase activity in BS, RS and TS was measured as described above, and amylase secretion (%) was calculated as follows: (RS-BS)/(TS-BS)×100.
In vivo bioassay of secretion of pancreatic juice in the rat
Pancreatic exocrine secretion in the rat was assessed as described previously (Tachibana et al., 1996). Briefly, under urethane anaesthesia, the common bile-pancreatic duct of the rat was cannulated at the distal end, and then the bile duct was ligated at a distal part above the common bile-pancreatic duct and cannulated at a proximal part to the liver above the ligature. Bile was diverted via a bypass into the duodenum, and the pure pancreatic juice was collected continuously from the end of the cannula connected to the common bile-pancreatic duct using a capillary and weighed every 5 min for quantitation. After a 30 min stabilization period, the PAR-2-AP, SLp-NH2 or the control peptide LSp-NH2 at 5 μmol kg−1 in combination with amastatin at 2.5 μmol kg−1 were administered i.v. to the rat.
Peptides and other reagents
All peptides used were prepared by solid-phase synthesis either by the Peptide Synthesis Core Facility, Department of Medical Biochemistry, University of Calgary (Canada), or by ourselves. Peptide composition and purity (>98%) were ascertained by HPLC analysis, amino acid analysis and mass spectrometry. Carbachol, phentolamine hydrochloride, trypsin from porcine pancreas and human thrombin were purchased from Sigma (U.S.A.), atropine sulphate, indomethacin and tetrodotoxin were obtained from Wako (Japan), amastatin and spantide were supplied from Peptide Institute (Japan), and propranolol hydrochloride was from Tokyo Kasei (Japan).
Data analysis
Results are expressed as means±s.e.m. Statistical significance of differences between groups was analysed by Student's t-test or Tukey's multiple comparison test, and P<0.05 was accepted as significant.
Results
In vivo salivation triggered by PAR-2-APs in the mouse
SFLLR-NH2 (SFp-NH2), a thrombin/PAR-1 agonist used previously by others, that can also activate PAR-2 (Kawabata et al., 1999b), when administered i.v. at a dose of 15 μmol kg−1, evoked secretion of a small amount of saliva for 5 min in the mouse, while the inactive control peptide, FSLLR-NH2 (FSp-NH2), at the same dose had no effect. TFLLR-NH2 (TFp-NH2, 15 μmol kg−1), a highly selective PAR-1-AP that is equipotent with SFp-NH2 for activating PAR-1 (Hollenberg et al., 1997), given in the same manner, failed to trigger any salivation. In contrast, SLIGRL-NH2 (SLp-NH2), a selective PAR-2-AP, administered i.v. at 15 μmol kg−1, produced a marked salivation, although the inactive control peptide LSIGRL-NH2 (LSp-NH2) had no effect (Figure 1).
Figure 1.
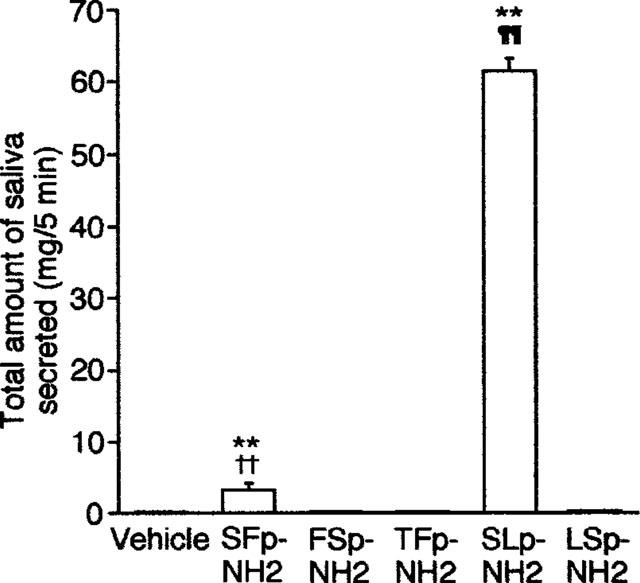
Effects of systemic (i.v.) administration of PAR-1-APs and PAR-2-APs on in vivo salivation in the mouse. The saliva secreted was collected for 5 min after i.v. administration of each peptide at 15 μmol kg−1. Data indicate the mean with s.e.mean from 4 mice. **P<0.01 vs vehicle; ††P<0.01 vs FSp-NH2; ¶¶P<0.01 vs LSp-NH2.
In the time-course experiments, i.v. administration of SLp-NH2 at 5 μmol kg−1 rapidly evoked salivation, the effect peaking after 1 min and disappearing after 3 min, although vehicle (saline) or LSp-NH2 at the same dose, given i.v., did not induce any saliva secretion responses throughout 5 min (Figure 2A). The effect of SLp-NH2 was dose-dependent in a range of 0.5–5 μmol kg−1. SLp-NH2 at the largest dose tested, 50 μmol kg−1, was less effective than that at 5 μmol kg−1. The dose-response curve for the effect of this compound thus exhibited a bell shape. The control peptide LSp-NH2 remained ineffective throughout the same dose range. SLIGRL (SLp-OH), administered i.v., also evoked salivation in a dose-dependent manner, but its effective dose range was high (5–50 μmol kg−1), relative to the potency of SLp-NH2, in agreement with the previous studies demonstrating a higher potency of SLp-NH2 relative to SLp-OH for activating PAR-2 in vitro and in vivo (Kawabata et al., 1998; Saifeddine et al., 1996) (Figure 2B). Another PAR-2-AP derivative, N-trans-cinnamoyl-LIGRL-ornithine-NH2 (tcLp-NH2) at 0.5–1.5 μmol kg−1 caused salivation equivalent to that caused by the same dose of SLp-NH2. In this assay, tcLp-NH2 appeared to be a ‘partial agonist', since tcLp-NH2 at doses higher than 5 μmol kg−1 was unable to give the maximum effect (Figure 2B). Amastatin, an inhibitor of aminopeptidase that is a major enzyme responsible for degradation of PAR-related peptides (Saifeddine et al., 1996; Hollenberg et al., 1993), when preadministered i.v. at 2.5 or 84 μmol kg−1, dramatically enhanced the salivation response to a dose of SLp-NH2 (0.5 μmol kg−1) that was ineffective by itself, although amastatin at those doses alone was incapable of inducing salivation. Amastatin at the two distinct doses was almost equipotent in terms of facilitation of the effect of SLp-NH2, indicating that the effect of amastatin at 2.5 μmol kg−1 was already maximal (Figure 2C).
Figure 2.
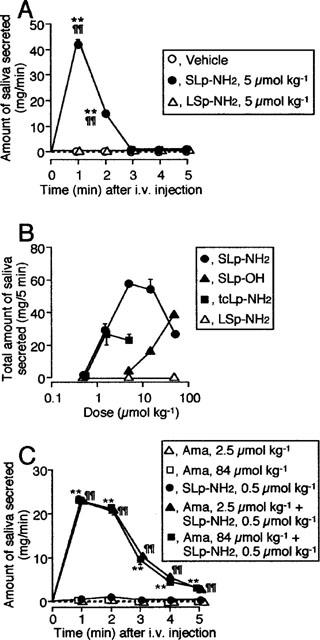
Salivation evoked by PAR-2-APs, administered i.v., in the mouse. (A) Time course to i.v. SLp-NH2 or LSp-NH2 at 5 μmol kg−1. The amount of saliva secreted was quantitated every minute for 5 min. (B) Dose-related effects of i.v. SLp-NH2, SLp-OH, tcLp-NH2 or LSp-NH2 on saliva secretion for 5 min. (C) Potentiation by amastatin of SLp-NH2-induced salivation. Amastatin (Ama) at 2.5 or 84 μmol kg−1 was administered i.v. 1 min before i.v. SLp-NH2 at 0.5 μmol kg−1. Data indicate the mean with s.e.mean from four mice. **P<0.01 vs vehicle, and ¶¶P<0.01 vs LSp-NH2 in (A); **P<0.01 vs Ama at 84 μmol kg−1, and ¶¶P<0.01 vs Ama at 2.5 μmol kg−1 in (C).
Atropine (7.2 μmol kg−1), phentolamine (15.7 μmol kg−1), propranolol (16.9 μmol kg−1) and indomethacin (28 μmol kg−1), preadministered i.p., failed to attenuate or enhance salivation evoked by i.v. SLp-NH2 at 5 μmol kg; the amount of saliva secreted (mg per 5 min) in the mice pretreated with vehicle, atropine, phentolamine, propranolol and indomethacin was 48.8±4.3, 57.6±6.8, 54.4±6.2, 66.5±10.0 and 50.5±8.9 (n=4), respectively.
Comparison of composition of the saliva secreted in response to the PAR-2-AP, SLp-NH2, and by carbachol in the mouse
To characterize salivation induced by activation of PAR-2 in the mouse, the composition of the saliva (total amount: 37.4±1.4 mg per 5 min, n=4) secreted in response to i.v. SLp-NH2 at 5 μmol kg−1 was compared to saliva secreted in response to i.v. carbachol at a dose (0.08 μmol kg−1) that induced an equivalent amount of saliva (total amount: 41.4±4.3 mg per 5 min, n=4). The concentrations of Na+ and K+ ions (95.0±15.1 and 72.5±4 , mEq L−1), and the activity of amylase (981.8±35.5×103 IU L−1) in the saliva secreted due to SLp-NH2 were comparable to those due to carbachol (96.3±3.1 and 92.5±5.2 mEq L−1; 723.8± 122.6×103 IU L−1), respectively, although a minor significant (P<0.05) difference was seen in the K+ ion levels, being consistent with the previous hypothesis that PAR-2, like the muscarinic receptor, is likely coupled to Gq thereby stimulating phospholipase C in some tissues or cells (Dery et al., 1998).
PAR-2-APs-induced parotid gland secretion in vivo and in vitro in the rat, and RT–PCR detection of PAR-2 mRNA
In the rat, as observed in the mouse, the specific PAR-2-AP, SLp-NH2, but not its inactive control LSp-NH2 or the specific PAR-1-AP, TFp-NH2, when administered in vivo (5 μmol kg−1), triggered significant salivation, with an effect peaking at 1 min and lasting up to 4 min (Figure 3A).
Figure 3.
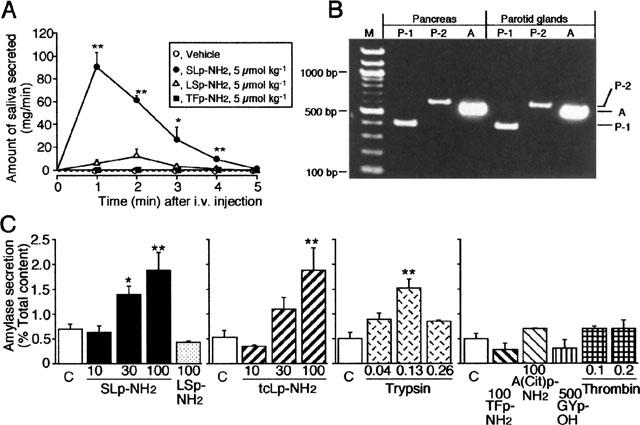
PAR-2-mediated in vivo and in vitro salivation in the rat, and RT–PCR detection of PAR-2 mRNA in the rat salivary gland. (A) In vivo salivation induced by PAR-2 activation in the rat. SLp-NH2, LSp-NH2 and TFp-NH2 were administered i.v. to the rat. The amount of saliva secreted was quantitated every minute for 5 min. Data indicate the mean with s.e.mean from four rats. *P<0.05, **P<0.01 vs vehicle. (B) RT–PCR analysis of mRNA harvested from the rat parotid gland and from the rat pancreas for detection of PAR-2. Primers were designed to PAR-1 (lane P-1), PAR-2 (lane P-2) and actin (lane A). The positions of the predicted PCR products are shown on the right. M, marker. (C) In vitro secretion of amylase from the rat parotid slices. The slices were incubated at 37°C for 10 min, with peptides or enzymes at various concentrations (μM). Data indicate the mean with s.e.mean from nine control (C) and 4–6 test experiments. *P<0.05, **P<0.01 vs the control.
The selective PAR-2-APs, SLp-NH2 and tcLp-NH2 at 10–100 μM evoked secretion of amylase from rat parotid slices in vitro, in a concentration-dependent manner, while LSp-NH2 at 100 μM had no effect. The PAR-2-activating enzyme trypsin at 0.13 μM, also induced significant secretion of amylase from the slices, although a larger concentration, 0.26 μM, of trypsin produced a smaller effect. The selective PAR-1-APs, TFp-NH2 or A(parafluoro-)FR-(cyclohexyl-)A-citrulline-Y-NH2 [A(Cit) p-NH2] at 100 μM, the PAR-4-AP, GYPGKF (GYp-OH) at 500 μM and thrombin at 0.1–0.2 μM did not trigger amylase secretion from the parotid slices (Figure 3C).
In the rat pancreas, used as the positive control, mRNAs for PAR-1 and for PAR-2 were readily detected by RT-PCR (Figure 3B, left, lanes P-1 and P-2). The RT–PCR analysis of mRNA harvested from the rat parotid gland also yielded PCR products of the predicted size, 394 bp for PAR-1 and 598 bp for PAR-2, respectively (Figure 3B, right, lanes P-1 and P-2), indicating expression of both PAR-1 and PAR-2 in the rat parotid gland.
In vivo pancreatic exocrine secretion responses to PAR-2 activation by SLp-NH2 in the rat
Finally, as a comparative experiment, we examined if the administration of PAR-2-APs in vivo would, as for the parotid gland, also trigger pancreatic exocrine secretion. The PAR-2-AP, SLp-NH2 at 5 μmol kg−1, when co-administered i.v. with amastatin at 2.5 μmol kg−1, produced a prompt increase in pancreatic juice secretion for 0–5 min after the administration, followed by a pronounced and transient suppression of pancreatic secretion for 5–10 min, with a subsequent long-lasting increase in secretion that lasted for 15–60 min (Figure 4).
Figure 4.
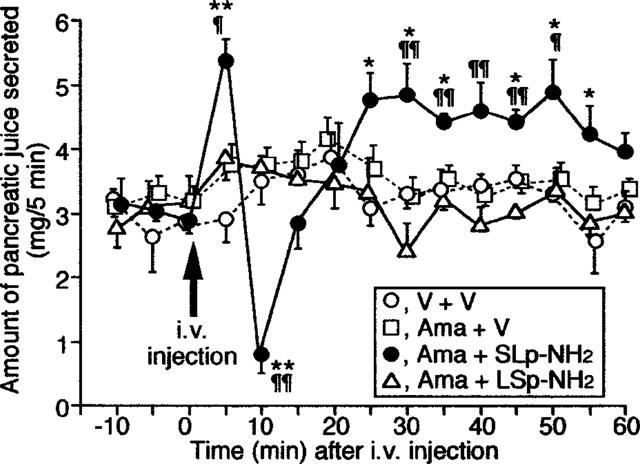
Effect of the PAR-2-AP, SLp-NH2, administered i.v., on in vivo pancreatic juice secretion in the rat. SLp-NH2 or LSp-NH2 at 5 μmol kg−1, in combination with amastatin at 2.5 μmol kg−1, were administered i.v. to the rat. The amount of the juice was quantitated every 5 min. V: Vehicle. Data indicate the mean with s.e.mean from four rats. *P<0.05, **P<0.01 vs V+V; ¶P<0.05, ¶¶P<0.01 vs Ama+LSp-NH2.
Discussion
Although discovered in 1994 (Nystedt et al.) by reduced stringency cloning from a murine cDNA library, the physiological and/or pathophysiological role that PAR-2 may play in vivo has yet to be defined. The main finding of our study was that the activation of PAR-2 in vivo, with the use of receptor-selective activating peptides, led to both salivary and pancreatic secretion. Data obtained with the parotid slice preparation in vitro paralleled exactly the results obtained in vivo. Our work thus adds the regulation of exocrine glandular secretion to the list of possible roles that the novel PAR-2 system might subserve in vivo. Our data obtained in intact animals are in complete accord with results obtained with isolated pancreatic acini and cultured pancreatic duct epithelial cells in vitro (Bohm et al., 1996; Nguyen et al., 1999); but our data obtained in vivo suggest a more complex regulation of pancreatic secretion by PAR-2 (multi-phasic response) than was suggested by the studies done in vitro (Bohm et al., 1996; Nguyen et al., 1999). Comparable studies done in vivo with PAR-2-activating reagents have also pointed to a role for PAR-2 in the regulation of the cardiovascular (Cheun et al., 1998; Damiano et al., 1999) and respiratory (Cocks et al., 1999) functions.
It is of note that the highly selective PAR-1-AP, TFp-NH2, did not cause salivary secretion in vivo, and that neither thrombin nor the selective PAR-1-APs [TFp-NH2 and A(Cit)p-NH2] and PAR-4-AP (GYp-NH2) caused amylase release from rat parotid slices in vitro (Figure 3C). In contrast, the PAR-2-selective activating peptides, SLp-NH2 and tcLp-NH2, were equipotent in this regard, whereas the partial-reverse-sequence PAR-2-derived peptide, LSp-NH2, known not to activate PAR-2, was inactive. It is recognized that SLp-NH2 is unable to activate PAR-1, PAR-3 or PAR-4, since this peptide cannot, as do either thrombin or the PAR-1- and PAR-4-APs, cause aggregation of human (PAR-1 plus PAR-4) or murine (PAR-3 plus PAR-4) platelets (Vu et al., 1991; Ishihara et al., 1997; Kahn et al., 1998; 1999). Further, our salivary secretion data, showing that SLp-NH2 and tcLp-NH2 were equipotent in vivo as well as in vitro, support the conclusion that the effects were due to PAR-2 itself, and not to other pharmacologically distinct receptors that can also be activated by SLp-NH2 (Vergnolle et al., 1998; Roy et al., 1998; Saifeddine et al., 1998). The low, but detectable activity in the salivation assay of one of the originally-described thrombin-receptor (PAR-1) activating peptides [SFp-NH2 (formerly designated TRAP); Figure 1] can be understood in terms of its ability to activate PAR-2 (Kawabata et al., 1999b). The ball-shaped dose-response curve for the salivation effect of SLp-NH2 in vivo (Figure 2B) might suggest the presence of unknown negative feedback systems or the non-specific actions of this peptide at large doses, although the detailed mechanisms remain to be investigated. The pharmacological profile for the salivary secretion assay, in vivo as well as in vitro [trypsin >> SLp-NH2=tcLp-NH2; LSp-NH2, A(Cit)p-NH2 and TFp-NH2 inactive] parallels exactly the structure-activity profile for the agonists in stimulating calcium signalling in rat PAR-2-expressing KNRK cells (Vergnolle et al., 1998). Further, the range of concentration over which trypsin caused amylase secretion from parotid slices in vitro was comparable to the concentrations of trypsin that cause PAR-2-mediated intestinal prostaglandin secretion (Kong et al., 1997), canine pancreatic duct epithelial cell ion channel activation (Nguyen et al., 1999) and the suppression of colonic motility (Corvera et al., 1997). Our data thus strongly support a role for the trypsin/tryptase-activated PAR-2 in regulating salivary secretion, rather than the thrombin-activated receptors, PARs-1,3 or 4.
Given the unusual proteinase-mediated mechanism for the activation of PAR-2 and an absence of a conventional circulating ‘hormonal' ligand, an interesting question to pose is: under what circumstances might this receptor become activated, so as to subserve a physiological/pathophysiological role? Our working hypothesis has been that the PARs in general play roles in the setting of an inflammatory response, injury or during organ development (Hollenberg et al., 1999). In this context, it can be pointed out that exposure of rats to an intestinal antigenic challenge can result in the release of mast cell proteinases into the circulation (Scudamore et al., 1995) and that elevated levels of α-tryptase can be detected in the blood of individuals with systemic mastocytosis (Schwartz et al., 1995). Our data showing that PAR-2 activation stimulates pancreatic exocrine secretion would imply that the sensitivity of pancreatic PAR-2 to circulating α-tryptase might account for the increased incidence of peptic ulcer disease in individuals with systemic mastocytosis. PAR-2 activation might also account for other symptomatology in such individuals (diarrhoea, hypotension and urticaria), an area that merits further study. Whether circulating mast cell tryptases (human) or chymases (rat) may account for a stimulation of exocrine glandular secretion remains an interesting topic for further exploration. That our data imply a general effect of PAR-2 activation on exocrine glandular secretion suggests that potential of generalized systemic effect that could be brought about by the release (due to inflammation or other causes) into the circulation of PAR-2-targeted proteinases. Taken together with its other roles in gastrointestinal tract (Hollenberg et al., 1997; Saifeddine et al., 1996; Kong et al., 1997; Corvera et al., 1997), PAR-2 appears to play a key role in the digestive systems, and may provide a novel scope for drug development.
Acknowledgments
The work described here was supported in part by research grant No. 11672283 from the Japanese Ministry of Education, Science and Culture.
Abbreviations
- A(Cit)p-NH2
A(parafluoro-)FR-(cyclohexyl-)A-citrulline-Y-NH2
- FSp-NH2
FSLLR-NH2
- GYp-OH
GYPGKF
- LSp-NH2
LSIGRL-NH2: PAR, proteinase-activated receptor
- PAR-AP
PAR-activating peptide
- RT–PCR
reverse transcriptase-polymerase chain reaction
- SFp-NH2
SFLLR-NH2
- SLp-NH2
SLIGRL-NH2
- SLp-OH
SLIGRL
- tcLp-NH2
N-trans-cinnamoyl-LIGRL-ornithine-NH2
- TFp-NH2
TFLLR-NH2
References
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. doi: 10.1074/jbc.271.28.16466. [DOI] [PubMed] [Google Scholar]
- BOHM S.K., KONG W., BROMME D., SMEEKENS S.P., ANDERSON D.C., CONNOLLY A., KAHN M., NELKEN M.A., COUGHLIN S.R., PAYAN D.G., BUNNETT N.W. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEUN W.-M., ANDRADE-GORDON P., DERIAN C.K., DAMIANO B.P. Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can. J. Physiol. Pharmacol. 1998;76:16–25. doi: 10.1139/cjpp-76-1-16. [DOI] [PubMed] [Google Scholar]
- CIRINO G., CICALA C., BUCCI M.R., SORRENTINO L., MARAGANORE J.M., STONE S.R. Thrombin functions as an inflammatory mediator through activation of its receptor. J. Exp. Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKS T.M., FONG B., CHOW J.W., ANDERSON G.P., FARAUMAN A.G., GOLDIE R.G., HENRY P.J., CARR M.J., HAMILTON J.R., MOFFATT J.D. A protective role for protease-activated receptors in the airways. Nature (London) 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- CORVERA C.U., DERY O., MCCONALOGURE K., BOHM S.K., KHITIN L.M., CAUGHEY G.H., PAYA D.G., BUNNETT N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Invest. 1997;100:1383–1393. doi: 10.1172/JCI119658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMIANO B.P., CHEUNG W.-M., SANTULLI R.J., FUNG-LEUNG W.-P., NGO K., YE R.D., DARROW A.L., DERIAN C.K., GARAVILLA L.D., ANDRADE-GORDON P. Cardiovascular responses mediated by protease-activated receptor-2 (PAR-2) and thrombin receptor (PAR-1) are distinguished in mice deficient in PAR-2 or PAR-1. J. Pharmacol. Exp. Ther. 1999;288:671–678. [PubMed] [Google Scholar]
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. doi: 10.1152/ajpcell.1998.274.6.C1429. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D. Protease-activated receptors: PAR4 and counting: how long is the course. Trends Pharmacol. Sci. 1999;20:271–273. doi: 10.1016/s0165-6147(99)01333-4. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D., LANIYONU A.A., SAIFEDDINE M., MOORE G.J. Role of the amino- and carboxyl-terminal domains of thrombin receptor-derived polypeptides in biological activity in vascular endothelium and gastric smooth muscle: evidence for receptor subtypes. Mol. Pharmacol. 1993;43:921–930. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B. Proteinase-activated receptor-2 in rat aorta: Structural requirements for agonist activity of receptor-activating peptides. Mol. Pharmacol. 1996;49:229–233. [PubMed] [Google Scholar]
- HOLLENBERG M.D., SAIFEDDINE M., AL-ANI B., KAWABATA A. Proteinase-activated receptors: structural requirements for activity, receptor cross-reactivity, and receptor selectivity of receptor-activating peptides. Can. J. Physiol. Pharmacol. 1997;75:832–841. [PubMed] [Google Scholar]
- ISHIHARA H., CONNOLLY A.J., ZENG D., KAHN M.L., ZHENG Y.W., TIMMONS C., TRAM T., COUGHLIN S.R. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- JAHN R., UNGER C., SOLING H.-D. Specific protein phosphorylation during stimulation of amylase secretion by β-agonists or dibutyryl adenosine 3′,5′-monophosphate in the rat parotid gland. Eur. J. Biochem. 1980;112:345–352. doi: 10.1111/j.1432-1033.1980.tb07211.x. [DOI] [PubMed] [Google Scholar]
- KAHN M.L., NAKANISHI-MATSUI M., SHAPIRO M.J., ISHIHARA H., COUGHLIN S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAHN M.L., ZHENG Y.-W., HUANG W., BIGORNIA V., ZENG D., MOFF S., FARESE R.V., Jr, TAM C., COUGHLIN S.R. A dual thrombin receptor system for platelet activation. Nature (London) 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., MINAMI T., KATAOKA K., TANEDA M. Increased vascular permeability by a specific agonist of protease-activated receptor-2 in rat hindpaw. Br. J. Pharmacol. 1998;125:419–422. doi: 10.1038/sj.bjp.0702063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., KURODA R., NISHIKAWA H., ASAI T., KATAOKA K., TANEDA M. Enhancement of vascular permeability by specific activation of protease-activated receptor-1 in rat hindpaw: a protective role of endogenous and exogenous nitric oxide. Br. J. Pharmacol. 1999a;126:1856–1862. doi: 10.1038/sj.bjp.0702513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWABATA A., SAIFEDDINE M., AL-ANI B., LEBLOND L., HOLLENBERG M.D. Evaluation of proteinase-activated receptor-1 (PAR1) agonists and antagonists using a cultured cell receptor desensitization assay: activation of PAR2 by PAR1 targeted ligands. J. Pharmacol. Exp. Ther. 1999b;228:358–370. [PubMed] [Google Scholar]
- KONG W., MCCONALOGUE K., KHITIN L.M., HOLLENBERG M.D., PAYAN D.G., BOHM S.K., BUNNETT N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8884–8889. doi: 10.1073/pnas.94.16.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLINO M., BARNATHAN E.S., NUMEROF R., CLARK J., DREYER M., CUMASHI A., HOXIE J.A., SCHECHTER N., WOOLKALIS M., BRASS L.F. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J. Biol. Chem. 1997;272:4043–4049. doi: 10.1074/jbc.272.7.4043. [DOI] [PubMed] [Google Scholar]
- NGUYEN T.D., MOODY M.W., STEINHOFF M., OKOLO C., KOH D.-S., BUNNET N.W. Trypsin activates pancreatic duct epithelial cell ion channels. J. Clin. Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYSTEDT S., EMILSSON K., WAHLESTEDT C., SUNDELIN J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:9208–9212. doi: 10.1073/pnas.91.20.9208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY S.S., SAIFEDDINE M., LOUTZENHISER R., TRIGGLE C.R., HOLLENBERG M.D. Dual endothelium-dependent vascular activities of proteinase-activated receptor-2-activating peptides: evidence for receptor heterogeneity. Br. J. Pharmacol. 1998;123:1434–1440. doi: 10.1038/sj.bjp.0701726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., AL-ANI B., CHENG C.-H., WANG L., HOLLENBERG M.D. Rat proteinase-activated receptor-2 (PAR-2): cDNA sequence and activity of receptor-derived peptides in gastric and vascular tissue. Br. J. Pharmacol. 1996;118:521–530. doi: 10.1111/j.1476-5381.1996.tb15433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAIFEDDINE M., ROY S.S., AL-ANI B., TRIGGLE C.R., HOLLENBERG M.D. Endothelium-dependent contractile actions of proteinase-activated receptor-2-activating peptides in human umbilical vein: release of a contracting factor via a novel receptor. Br. J. Pharmacol. 1998;125:1445–1454. doi: 10.1038/sj.bjp.0702213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ L.B., SAKAI K., BRADFORD T.R., REN S., ZWEIMAN B.A., COROBEC A.S., METCALFE D.D. The α form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J. Clin. Invest. 1995;96:2702–2710. doi: 10.1172/JCI118337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCUDAMORE C.L., THORNTON E.M., MCMILLAN L., NEWLANDS G.F.J., MILLER H.R.P. Release of the mucosal mast cell granule chymase, rat mast cell protease-II, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J. Exp. Med. 1995;182:1871–1881. doi: 10.1084/jem.182.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACHIBANA I., KANAGAWA K., YAMAMOTO Y., OTSUKI M. Pharmacological profile of a new serine derivative cholecystokinin receptor antagonist TP-680 on pancreatic, biliary and gastric function. J. Pharmacol. Exp. Ther. 1996;279:1404–1412. [PubMed] [Google Scholar]
- TAKEDA Y., KRAUSE J.E. Neuropeptide K potently stimulates salivary gland secretion and potentiates substance P-induced salivation. Proc. Natl. Acad. Sci. U.S.A. 1989;86:392–396. doi: 10.1073/pnas.86.1.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br. J. Pharmacol. 1999;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., MACNAUGHTON W.K., AL-ANI B., SAIFEDDINE M., WALLACE J.L., HOLLENBERG M.D. Proteinase-activated receptor 2 (PAR2)-activating peptides: identification of a receptor distinct from PAR2 that regulates intestinal transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7766–7771. doi: 10.1073/pnas.95.13.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VU T.-K.H., HUNG D.T., WHEATON V.I., COUGHLIN S.R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanisms of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- XU W.-F., ANDERSEN H., WHITMORE T.E., PRESNELL S.R., YEE D.P., CHING A., GILBERT T., DAVIE E.W., GOSTER D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. U.S.A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]


