Abstract
A short term exposure of PC12 cells to a concentration of tert-butylhydroperoxide (tB-OOH) causing peroxynitrite-dependent DNA damage and cytotoxiticity promoted a release of arachidonic acid (AA) that was sensitive to phospholipase A2 (PLA2) inhibitors and insensitive to phospholipase C or diacylglycerol lipase inhibitors. The extent of AA release was also mitigated by nitric oxide synthase (NOS) inhibitors and peroxynitrite scavengers. Low levels (10 μM) of authentic peroxynitrite restored the release of AA mediated by tB-OOH in NOS-inhibited cells whereas concentrations of peroxynitrite of 20 μM, or higher, effectively stimulated a PLA2 inhibitor-sensitive release of AA also in the absence of additional treatments. These results are consistent with the possibility that endogenous as well as exogenous peroxynitrite promotes activation of PLA2.
Keywords: Peroxynitrite, arachidonic acid, phospholipase A2, tert-butylhydroperoxide
Introduction
Peroxynitrite, the coupling product of superoxides and nitric oxide, is a potent oxidant that induces an array of deleterious events including peroxidation of membrane lipids (Rubbo et al., 1994), depletion of glutathione (Salgo et al., 1995), DNA single strand breakage (Szabó & Ohshima, 1997), mitochondrial dysfunction (Brown, 1999) and cell death (Cookson et al., 1998; Pieper et al., 1999). All these events are thought to be directly mediated by peroxynitrite.
In the present study, we report experimental evidence consistent with the possibility that peroxynitrite may also act as a signalling molecule. In particular, endogenous as well as exogenous peroxynitrite promotes a release of arachidonic acid (AA) most likely attributable to stimulation of phospholipase A2 (PLA2).
Methods
PC12 rat pheochromocytoma cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% horse serum, 5% foetal bovine serum, penicillin (50 units ml−1) and streptomycin (50 μg ml−1), at 37°C in T-75 tissue culture flasks gassed with an atmosphere of 95% air-5% CO2. Peroxynitrite was synthesized by the reaction of nitrite with acidified H2O2 as described by Radi et al. (1991). Peroxynitrite was added as a bolous to samples and, to avoid changes in pH due to the high alkalinity of peroxynitrite stock solution, an appropriate amount of 1 N HCl was added. PC12 cells were subcultured in 6 well plates at 2×105 cells per well with [3H]-AA (0.5 μCi ml−1) and grown for 18 h. Before treatments, the cells were washed twice with saline A supplemented with 1 mg ml−1 fatty acid-free bovine serum albumin and exposed to tB-OOH or peroxynitrite, in the absence or presence of drugs, in a final volume of 1 ml of saline A. The solution was then separated and centrifuged at 12,000 r.p.m. for 1.5 min; 500 μl of the resulting supernatant were removed and radioactivity was determined in a Wallac 1409 liquid scintillation counter (Wallac, Turku, Finland). Results are shown as c.p.m.±s.e.mean values from 3–5 independent experiments.
Results and discussion
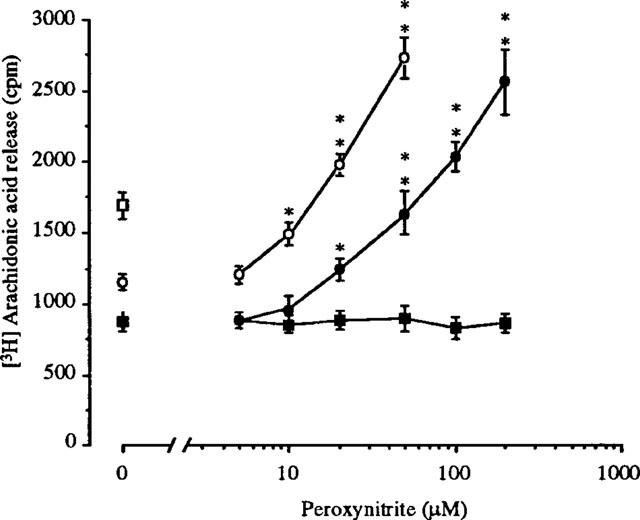
It was previously reported that tB-OOH stimulated PLA2 activity in cultured mammalian cells (Chakraborti et al., 1993; Kondakova et al., 1995). Consistently with these findings, we report (Table 1) that a 10 min exposure of PC12 cells to 200 μM tB-OOH induced a release of AA that was prevented by two general PLA2 inhibitors, mepacrine (100 μM) or 5,8,11,14-eicosatetraynoic acid (ETYA, 50 μM). Because a combined action of phospholipase C and diacylglycerol lipase has also been implicated in AA release it was of interest to determine the effect of the PLC inhibitor neomycin (1 mM) and that of 1,6-bis(cyclohexyloximonocarbonylamino)hexane (RHC-80267, 100 μM), an inhibitor of diacylglycerol lipase. The results illustrated in Table 1 indicate that none of these inhibitors affected the tB-OOH-induced release of AA. Thus, as it was previously observed in endothelial (Chakraborti et al., 1993) and P815 tumor (Kondakova et al., 1995) cells, AA release stimulated by tB-OOH appears to be specifically mediated by activation of PLA2 also in PC12 cells. Since the DNA-damaging (Sestili et al., 2000) and lethal (Palomba et al. unpublished) responses caused by 200 μM tB-OOH in PC12 cells are in part mediated by peroxynitrite, experiments were designed to determine if endogenous peroxynitrite was involved in the tB-OOH-induced release of AA. Cells were pre-treated with either the NOS inhibitor Nω-nitro-L-arginine methyl ester (L-NAME, 1 mM) or the peroxynitrite scavengers L-methionine (20 mM) and Trolox (1 mM) and the tB-OOH-stimulated release of AA measured. Each of these treatments significantly reduced–but did not abolish–the release of AA. Taken together, these results suggest that tB-OOH stimulates PLA2 activity in PC12 cells via peroxynitrite-dependent and -independent mechanisms. If this conclusion is correct, then addition of exogenous peroxynitrite should restore release of AA in L-NAME-supplemented cells challenged with tB-OOH. The results illustrated in Figure 1 are consistent with this possibility. Indeed, while suppression of NOS activity caused a significant reduction in the amount of AA released by tB-OOH, as low as 10 μM peroxynitrite promoted a release of AA comparable to that observed after treatment with tB-OOH in the absence of L-NAME. An even higher increase was mediated by 20 or 50 μM peroxynitrite. These responses were sensitive to inhibition by 100 μM mepacrine (not shown). It is important to note that, although 10 μM peroxynitrite did not increase the release of AA in the absence of tB-OOH, peroxynitrite concentrations of ⩾20 μM effectively stimulated this response (Figure 1). These effects were not observed using decomposed peroxynitrite. These results illustrated in Table 1 provide the additional information that (a) authentic peroxynitrite did not enhance AA release in cells pretreated with PLA2 inhibitors or peroxynitrite scavengers and that (b) the release of AA mediated by peroxynitrite is insensitive to neomycin, RHC-80267 and L-NAME. Taken together, these results strongly suggest that exogenous peroxynitrite stimulates PLA2 activity in PC12 cells. These results also emphasize the specificity of L-NAME in preventing the peroxynitrite-dependent activation of PLA2 in cells treated with tB-OOH (see above). In summary, the data presented in this study demonstrate that exogenous as well as endogenous peroxynitrite promoted release of AA in PC12 cells and strongly suggest that this response is mediated by activation of PLA2. This implies that peroxynitrite may act as a signalling messenger rather than, or in addition to, as a direct effector molecule. Whether peroxynitrite directly or indirectly activated PLA2 is a matter of future investigations. The experimental approach utilized in the present study does not provide sufficient information to speculate on the identity of the PLA2 responsive to peroxynitrite. Studies are in progress to assess the possible involvement of secretory or cellular Ca2+-dependent/independent PLA2. It will also be of importance to investigate whether the peroxynitrite-dependent release of AA is casually linked to peroxynitrite-dependent induction of DNA cleavage (Sestili et al., 2000) and toxicity (Palomba et al. unpublished) that were previously observed in PC12 cells treated with tB-OOH. As a final note, peroxynitrite was shown to progressively stimulate release of AA over a concentration-range which is compatible with the amounts of peroxynitrite produced in various pathologies; thus, the relevance of the peroxynitrite-dependent release of AA in an array of pathological conditions (including inflammation) needs to be investigated.
Table 1.
tB-OOH and peroxynitrite promote a release of AA sensitive to PLA2 inhibitors
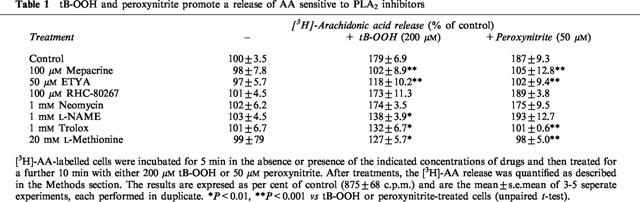
Figure 1.
Release of AA mediated by increasing concentrations of peroxynitrite in the absence or presence of L-NAME/tB-OOH. [3H]-AA-labelled cells were exposed for 5 min to 1 mM L-NAME and then treated for a further 10 min with 200 μM tBOOH in the absence or presence of increasing concentrations of peroxynitrite (open circles). The open square indicates the [3H]-AA release measured in cells treated with tB-OOH alone. Also shown are the results obtained in cells exposed for 10 min to authentic (closed circles) or decomposed (closed squares) peroxynitrite in the absence of additional treatments. After treatments, the [3H]-AA release was quantified as described in the Methods section. Each point is the mean±s.e.mean of 3–5 separate experiments, each performed in duplicate. *P<0.05, **P<0.01 vs untreated or tB-OOH/L-NAME-treated cells (ANOVA followed by Dunnet's test).
Acknowledgments
The financial support of Telethon-Italy (Grant no. 1110) is gratefully acknowledged.
Abbreviations
- AA
arachidonic acid
- ETYA
5,8,11,14-eicosatetraynoic acid
- L-NAME
Nω-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- PLA2
phospholipase A2
- RHC-80267
1,6-bis(cyclohexyloximonocarbonylamino)hexane
- tB-OOH
tert-butylhydroperoxide
References
- BROWN G.C. Nitric oxide and mitochondrial respiration. Biochim. Biophys. Acta. 1999;1411:351–369. doi: 10.1016/s0005-2728(99)00025-0. [DOI] [PubMed] [Google Scholar]
- CHAKRABORTI S., MICHAEL J.R., GURTNER G.H, , GHOSH S.S., DUTTA G., MERKER A. Role of a membrane-associated serine esterase in the oxidant activation of phospholipase A2 by t-butyl hydroperoxide. Biochem. J. 1993;292:585–589. doi: 10.1042/bj2920585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKSON M.R., INCE P.G., SHAW P.J. Peroxynitrite and hydrogen peroxide induced cell death in the NSC34 neuroblastoma x spinal cord cell line: role of poly (ADP-ribose) polymerase. J. Neurochem. 1998;70:501–508. doi: 10.1046/j.1471-4159.1998.70020501.x. [DOI] [PubMed] [Google Scholar]
- KONDAKOVA I.V., PEIRETTI F., NALBONE G., LAFONT H. Phospholipase A stimulation in tumor cells by subtoxic concentration of tert-butyl hydroperoxide. Biochim. Biophys. Acta. 1995;1258:297–302. doi: 10.1016/0005-2760(95)00133-w. [DOI] [PubMed] [Google Scholar]
- PIEPER A.A., VERMA A., ZHANG J., SNYDER S.H. Poly(ADP-ribose)polymerase, nitric oxide and cell death. Trends Pharmacol. Sci. 1999;20:171–181. doi: 10.1016/s0165-6147(99)01292-4. [DOI] [PubMed] [Google Scholar]
- RADI R., BECKAM J.S., BUSH K.M., FREEMAN B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- RUBBO H., RADI R., TRUJILLO M., KALYANARAMAN B., BARNES S., KIRK M., FREEMAN B.A. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J. Biol. Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- SALGO M.G., BERMUDEZ E., SQUADRITO G.L., PRYOR W.A. Peroxynitrite causes DNA damage and oxidation of thiols in rat thymocytes. Arch. Biochem. Biophys. 1995;322:500–505. doi: 10.1006/abbi.1995.1493. [DOI] [PubMed] [Google Scholar]
- SESTILI P., CLEMENTI E., GUIDARELLI A., SCIORATI C., CANTONI O. Endogenous and exogenous nitric oxide enhance the DNA stran scission induced by tert-butylhydroperoxide in PC12 cells via peroxynitrite-dependent and independent mechanisms, respectively. Eur. J. Neurosci. 2000;12:145–154. doi: 10.1046/j.1460-9568.2000.00891.x. [DOI] [PubMed] [Google Scholar]
- SZABÓ C., OHSHIMA H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]


