Abstract
Putative receptors for CGRP and adrenomedullin have been investigated in the rat. Calcitonin Receptor-Like Receptor (CRLR), in combination with Receptor Activity Modifying Proteins (RAMPs) is hypothesized to bind either CGRP or adrenomedullin. The receptors known as RDC1 and L1 have also been shown to bind CGRP and adrenomedullin respectively.
In this study it is shown that rat CRLR cDNA specifies a CGRP receptor when co-transfected with RAMP-1 cDNA and an adrenomedullin receptor when co-transfected with either RAMP-2 or RAMP-3 cDNA in human embryonic kidney 293 cells.
CRLR, RAMP, RCD1 and L1 mRNA levels and CGRP and adrenomedullin receptor densities have been measured and correlated with each other in eight rat tissues selected for their distinctive patterns of CGRP and adrenomedullin binding.
The data are consistent with the predictions of the CRLR/RAMP model. CGRP binding correlates well with RAMP-1 mRNA levels (R=1.0, P=0.007), adrenomedullin binding shows a tendency to vary with RAMP-2 mRNA levels (R=0.85, P=0.14) and total binding is correlated with CRLR mRNA levels (R=0.94, P=0.03). The data do not support the hypothesis that RDC1 and L1 account for the majority of CGRP and adrenomedullin binding respectively.
Keywords: CGRP, adrenomedullin, CGRP receptor, adrenomedullin receptor, RAMP
Introduction
The calcitonin family of regulatory peptides consists of five known members–calcitonin (CT) itself, the calcitonin gene related peptides (α and β CGRP's), amylin and adrenomedullin. These peptides are involved in a range of potentially important biological systems including calcium homeostasis (CT, amylin), vascular tone (CGRP, adrenomedullin), food intake (amylin) and the central nervous system (CGRP, adrenomedullin). Despite the obvious pharmacological interest, progress in cloning the corresponding receptors has been slow. A CT receptor was cloned in 1991 (Lin et al., 1991) but this did not lead to the rapid identification of a family of related receptors capable of binding the other members of this family of peptides. A related receptor, the calcitonin receptor-like receptor (CRLR) was discovered (Chang et al., 1993; Fluhmann et al., 1995; Njuki et al., 1993) but these initial reports failed to identify a ligand for this receptor. Subsequently it was reported that this sequence could function as a CGRP receptor (Aiyar et al., 1996) but only when expressed in a particular cell line (Han et al., 1997). Meanwhile two other reports had indicated that an unrelated orphan receptor known as L1 had adrenomedullin receptor activity (Kapas et al., 1995) and that a related receptor known as RDC1 functioned as a CGRP receptor (Kapas & Clark, 1995) though the role of L1 as an adrenomedullin receptor has recently been questioned (Kennedy et al., 1998).
This confusing situation was clarified considerably by a report (McLatchie et al., 1998) that CRLR could function as a CGRP receptor when co-expressed with a single transmembrane domain protein known as receptor activity modifying protein (RAMP-1) and as an adrenomedullin receptor when co-expressed with either RAMP-2 or RAMP-3. Interestingly it appears that the different RAMPs influence glycosylation leading to a detectable size difference between CRLR species with CGRP and adrenomedullin receptor activity. In further work it is reported that the calcitonin receptor (CTR) can function as an amylin receptor when co-expressed with RAMP-1 or RAMP-3 (Christopoulos et al., 1999; Muff et al., 1999) and here too, receptor specificity can be correlated with receptor glycosylation (Perry et al., 1997). Thus it appears that between them, CTR and CRLR can provide receptor activities for all members of the CT family by co-expression with the appropriate RAMP protein. The position of the other putative CGRP and adrenomedullin receptors, RDC1 and L1 respectively, is unclear. Some aspects of CTR family pharmacology remain unexplained by the CRLR/CTR/RAMPs model. For instance, CGRP receptors have been subdivided into CGRP1 receptors (sensitive to the inhibitor CGRP8–37) and CGRP2 receptors (relatively insensitive to CGRP8–37) and stimulated by the agonist cys(ACM)2,7 CGRP) (Poyner, 1995). It is therefore quite possible that there are further receptors for the calcitonin family of peptides and that the RDC1 (Kapas & Clark, 1995) and L1 (Kapas et al., 1995) receptors may have a role to play.
Our initial aim in these studies was to confirm that RAMPs modulated receptor activity using our own rat CRLR. Beyond this we wished to establish whether the CRLR/RAMP combination was actually responsible for the binding of CGRP and adrenomedullin in vivo. A number of complementary approaches are possible. For instance, analysis of tissue culture cells might reveal some which bind either ligand in the absence of CRLR and the appropriate RAMP. This would indicate the presence of other receptors, possibly L1 or RDC1. One could use in situ techniques to see if there was obligatory co-expression of CRLR with a member of the RAMP family coupled with binding of the predicted ligand. This could reveal other receptor systems and might also indicate the presence of other partners for CRLR and/or RAMPs in some situations although quantitation would be difficult with these techniques.
However, the approach we have chosen is to correlate transcript levels with binding activity in a series of rat tissues selected for their characteristic patterns of CGRP or adrenomedullin binding. If the CRLR/RAMP combination is responsible for most of the receptor activity in these tissues then a correlation would be expected between binding and the relevant transcripts. If either CRLR or RAMPs have other quantitatively significant functions and/or partners in these tissues or if there are other receptor systems making major contributions to ligand binding then no correlation would be expected.
Methods
Materials and animals
Adult male Wistar rats (200–220 g) were killed by decapitation and the required tissues were frozen in liquid nitrogen prior to membrane, lysate or RNA preparation. Rat adrenomedullin was obtained from Peptide Institute Inc. (Osaka, Japan) and rat [Tyr0] αCGRP was obtained from Peninsula laboratories (St Helens, Merseyside, U.K.). Rat αCGRP was custom synthesized by ASG University (Szedgel, Hungary). All peptides were checked for correct molecular weight by mass spectroscopy. Na [125I] was supplied by Amersham (Amersham Pharmacia Biotech U.K. Ltd, Bucks, U.K.). Iodogen reagent was supplied by Pierce (Rockford, Illinois, U.S.A.).
Peptide iodination
Rat adrenomedullin was iodinated by the iodogen method and purified as previously described (Owji et al., 1995). The specific activity was 10 Bq fmol−1. Rat [Tyr0] αCGRP was also iodinated by the iodogen method and purified by reverse phase h.p.l.c. (Bhogal et al., 1993). The specific activity was 35.9 Bq fmol−1 as determined by radioimmunoassay.
Cell culture and transfection
Human embryonic kidney (HEK) 293 cells were grown as previously described (Han et al., 1997) and transfected when 50% confluent. Transient transfection was carried out using Fugene-6 according to the manufacturer's instructions (Roche Diagnostics Ltd, Lewes, U.K.). The cells were grown for a further 24 h before whole cell ligand binding assays with CGRP and adrenomedullin were carried out.
Whole cell adrenomedullin and CGRP ligand binding assays
Cells were incubated for 60 min at 4°C for adrenomedullin and 22°C for CGRP in binding buffer (mM): HEPES pH 7.4 20, MgCl2 5, NaCl 100, KCl 5, EDTA 1, phosphoramidon 1 μM and 0.1% w v−1 BSA) containing 1000 Bq 125I adrenomedullin or 1000 Bq 125I [Tyr0] αCGRP. Unbound label was removed by aspiration of binding buffer and cells were washed three times with chilled binding buffer. Five hundred μl of 0.1 M NaOH was added and solubilized isotope was counted. Non-specific binding was determined in the presence of 100 nM unlabelled rat adrenomedullin or 1 μM CGRP. Specific binding was defined as total binding minus non-specific binding (Withers et al., 1996).
Membrane binding assays with 125I-Adrenomedullin and 125I-CGRP
Membranes were prepared by differential centrifugation as previously described (Bhogal et al., 1992). For adrenomedullin binding, membranes (100 μg protein) were incubated for 30 min at 4°C in 0.5 ml of binding buffer as for whole cells but containing 500 Bq (100 pM) 125I-adrenomedullin (Owji et al., 1995). For CGRP binding, membranes (200 μg protein) were incubated at 22°C for 45 min with 125I-CGRP (1000 Bq, 45 pM) in binding buffer (Bhogal et al., 1993). Bound and free labels were separated by centrifugation at 15,000×g for 2 min at 4°C. Non-specific binding was determined in the presence of 200 nM unlabelled rat peptide. Binding data were analysed by non-linear regression using the Receptor Fit programme (Lundon Software, Cleveland, Ohio, U.S.A.) to calculate the concentration of binding sites (Bmax).
Northern blot analysis
Total RNA was prepared and analysed on formaldehyde agarose gels as previously described (Sharma et al., 1992). A 272 bp probe specific for the intracellular domain of CRLR was amplified and sub-cloned from the full-length rat CRLR cDNA. The rat L1 receptor cDNA (416 bp) probe was as previously described (Coppock et al., 1996) and the rat equivalent of the dog RDC1 sequence was a 790 bp fragment representing the first to the seventh transmembrane domains. Both L1 and RDC1 probes were gifts from Dr A.J.L. Clark, Molecular Endocrinology Laboratory, St. Bartholomew's Hospital, London. For RAMP-1 and RAMP-2, the human RAMP sequences were used to search the rat expressed sequence tag (est) database. A 475 bp fragment representing bases 17–492 of a rat RAMP-1 clone (Accession No. AI012429) and a 316 bp fragment representing bases 70–386 of a rat RAMP-2 clone (Accession No. AI012814) were amplified and cloned from a rat lung cDNA library. For RAMP-3 where there is no match in the rat 'est' database, a mouse cDNA clone (Image ID # 0761307) was obtained from the U.K. HGMP Resource Centre (Hinxton Hall, U.K.). A full-length human RAMP-3 probe was also used to confirm the hybridization pattern (McLatchie et al., 1998). In each case the insert of the clone was labelled using random primed synthesis (Feinberg & Vogelstein, 1983) and equivalent amounts were used for hybridizations.
All the Northern were quantitated on a phosphorimager using ImageQuant software (Molecular Dynamics Inc, Sunnyvale, CA, U.S.A.). The blots were then probed successively for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Gibbons et al., 1997) and β actin (Corcoran et al., 1996) mRNA and finally for the total amount of polyA+ RNA in each track using a 45mer oligo-dT probe labelled by tailing with a single dCTP residue. For each standard, hybridization in each tissue was expressed as a percentage of the total and the average figures for the three standards were used to normalize the values for the test probes.
Statistical analysis
The distributions of binding and mRNA levels in the tissues did not show a normal distribution and were therefore normalized by using natural log values to permit the use of parametric tests. The relationship of each explanatory variable (mRNA level) to each response variable (CGRP, adrenomedullin and total binding) was examined using simple linear regression. Multiple regression was then performed in a stepwise fashion to determine whether a second explanatory variable might contribute to the response variable after adjusting for the effect of the first variable. Finally each of the mRNA levels was correlated with each other using the Pearson product moment correlation to determine whether any significant unanticipated correlations could be made. Because of the large number of such correlations the Sidak adjustment was used to correct 'P values' for chance correlations.
Results
Co-transfection of CRLR and RAMP cDNAs
In earlier studies (Han et al., 1997) we have shown that transient transfection of a human embryonic kidney cell line (293 cells) with plasmids expressing rat CRLR results in these cells acquiring CGRP1 receptor activity. In transient transfections, 293 cells respond to CGRP (and to a lesser extent, adrenomedullin) with an elevation of intracellular cyclic AMP (Han et al., 1997) but receptor activity is not sufficient to permit detection of ligand binding (Figure 1). However, when 293 cells are co-transfected with RAMP-1 and CRLR cDNAs, specific CGRP binding can be seen (Figure 1a). By contrast, co-transfection of CRLR cDNA with either RAMP-2 or RAMP-3 cDNAs leads to high levels of specific adrenomedullin binding without significant levels of specific CGRP binding (Figure 1b).
Figure 1.
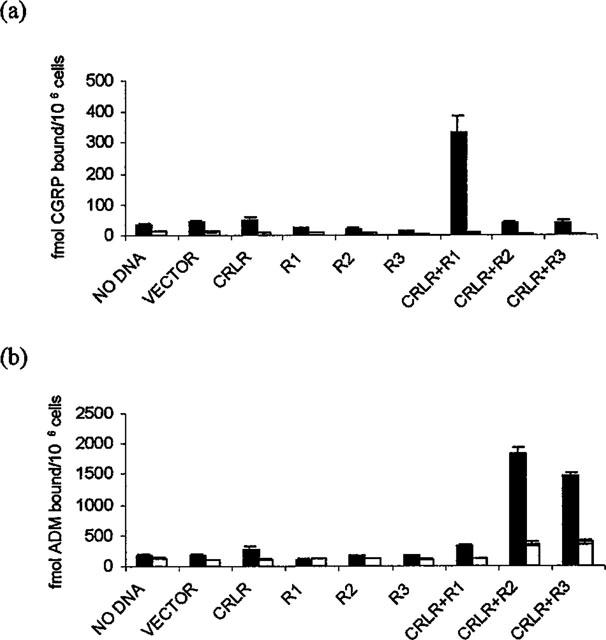
Binding of CGRP (a) or Adrenomedullin (b) to Transfected 293 Cells. Cells were grown in 24 well plates and transfected with DNA as indicated below. After 24 h, binding assays for either CGRP (a) or adrenomedullin (b) were performed in triplicate either in the absence (first column, total binding) or presence (second column, non specific binding) of unlabelled ligand and results are shown±s.e.m. Transfected DNAs are indicated on the figures with R1 indicating RAMP-1 etc.
Distribution of CGRP and adrenomedullin receptors in selected rat tissues
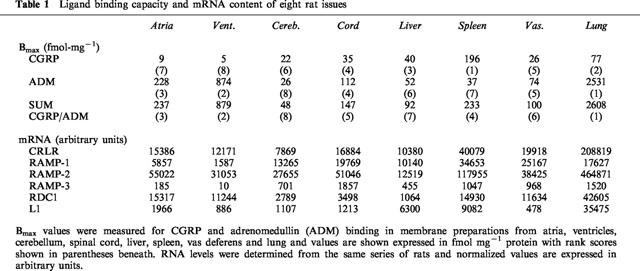
Membranes were prepared from eight rat tissues and levels of CGRP and adrenomedullin binding (Bmax values) were determined to permit correlation of receptor activity with mRNA levels in the same series of tissues (Table 1). Overall, levels of adrenomedullin binding are higher than those for CGRP with the lung and the heart having the highest concentration of specific adrenomedullin receptors and Bmax values 16–35 times higher for adrenomedullin than for CGRP. Spleen provides an example of a tissue in which CGRP receptors predominate having six times as much CGRP binding.
Table 1.
Ligand binding capacity and mRNA content of eight rat issues
Distribution of CRLR and RAMP mRNAs in selected rat tissues
In parallel with the membrane preparations, RNA was extracted from the eight tissues and analysed on four parallel Northern blots probed with CRLR, RAMP-1, RAMP-2 and RAMP-3 cDNA probes (Figure 2) and the signals were quantified using a phosphorimager. The blots were then stripped and re-probed for L1 and RDC1 and were normalized by probing successively with GAPDH, β actin and oligo dT45. The figures for the target RNAs have been normalized using the mean of these three controls (Table 1). The values are generally consistent with published data though it should be noted that we find a highly specific pattern of gene expression for RAMP-3 mRNA in the rat that was not seen in earlier work with human RNAs (McLatchie et al., 1998).
Figure 2.
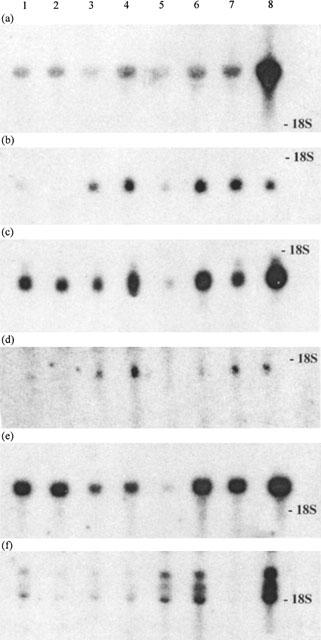
Northern blots of rat tissue RNAs hybridized with CRLR and RAMP probes. Twenty μg of total RNA from 1: atria, 2: ventricles, 3: cerebellum, 4: spinal cord, 5: liver, 6: spleen, 7: vas deferens and 8: lung was analysed by Northern blotting using probes for (a) CRLR, (b) RAMP-1, (c) RAMP-2, (d) RAMP-3, (e) RCD1 and (f) L1. After autoradiography, bands were counted in a phosphorimager.
Correlation of binding activity with mRNA levels
In Table 2, normalized values for the six mRNA levels have been correlated with the Bmax values using a series of individual regression analyses. The correlations predicted by the two models are highlighted and those which achieve significance at the P<0.05 level are marked by asterisks. In line with the predictions of the CRLR/RAMP model a significant correlation can be seen between CGRP binding and RAMP-1 mRNA and between total binding and CRLR mRNA with a tendency for adrenomedullin binding to correlate with RAMP-2 mRNA. By contrast, L1 and RDC1 mRNAs do not show a significant tendency to correlate with adrenomedullin and CGRP binding respectively and, if anything, tend to show the reverse correlations. The second stage of the analysis was to perform a multiple regression with a stepwise procedure to determine the effect on the dependent variable (binding) of the various proposed explanatory variables (mRNA levels). Using this procedure it was found that L1 mRNA correlated significantly with CGRP Bmax (R=0.36, P=0.02) after adjustment for the effect of RAMP-1. Viewed simplistically, most of the CGRP binding can be correlated with RAMP-1 mRNA levels and the residual binding not explained by RAMP-1 can be ascribed to L1 mRNA. A similar approach to the adrenomedullin and total Bmax values did not reveal any similar effects indicating that these values cannot be subdivided into fractions correlated with the presence of different mRNAs.
Table 2.
Correlation of binding data with mRNA levels
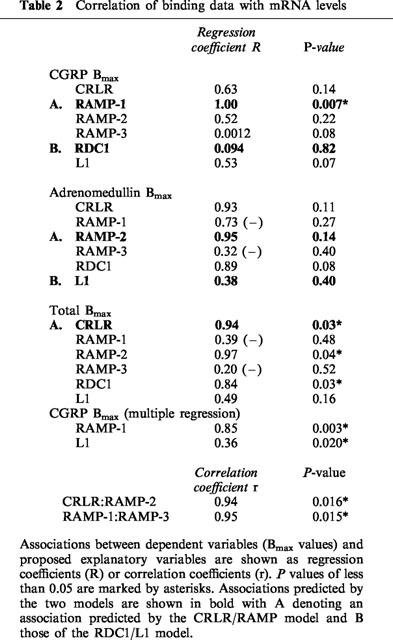
Finally the data was analysed for evidence of unexpected correlations between the variables. As this part of the analysis is not guided by the predictions of a model the ‘P values' have to be adjusted to take account of the large number of individual correlations being made. Despite this adjustment, two of the correlations are highly significant. CRLR expression is strongly correlated with RAMP-2 (r=0.94, P=0.016) and RAMP-1 is strongly correlated with RAMP-3 (r=0.95, P=0.015).
Discussion and conclusions
Transfection of CRLR in combination with RAMP-1 leads to the expression of CGRP receptor activity in HEK 293 cells and co-transfection of CRLR with RAMP-2 or RAMP-3 similarly leads to expression of adrenomedullin receptor activity (Figure 1). During preparation of this manuscript, similar results were reported by Buhlmann et al. (1999) using both rat UMR 106 osteoblasts and COS7 cells and by Kamitani et al. (1999) using 293 EBNA and Hela EBNA cells, although the latter group finds less specificity with the CRLR/RAMP-1 and CRLR/RAMP-3 combinations than do the others. The general conclusion that CRLR and RAMPs can combine to produce a functional receptor seems now to be well established. What is less clear is whether this combination operates in vivo to provide a significant fraction of the binding seen in whole tissues.
Accordingly, eight tissues were chosen to reflect the range of adrenomedullin/CGRP receptor activities seen in the body. The adrenomedullin binding data is largely comparable with our previous study (Owji et al., 1995) but there are some discrepancies with the literature in terms of CGRP binding. Levels of binding in the cerebellum are often quoted as the highest of any rat tissue (Wimalawansa, 1996) whereas we find relatively low levels in this tissue. Interestingly Stangl and co-workers (Stangl et al., 1993) also report lower levels of CGRP binding in the rat cerebellum (61 fmol mg−1 protein) compared to liver and spleen, with spleen showing the highest binding (221 fmol mg−1 protein) as in this study. Our binding in the liver is low compared to that reported by Chantry et al., 1991 (580 fmol mg−1 protein), (Chantry et al., 1991). However, these workers used liver plasma membranes and our results compare reasonably with Stangl and co-workers (103 fmol mg−1 protein) who used a similar crude membrane preparation (Stangl et al., 1993). Levels of binding detected in the atria (Bmax 9 fmol mg−1 protein) are very low and agree with published results for guinea-pig atria (6 fmol mg−1 protein, (Dennis et al., 1990).
The analysis of mRNA levels (Figure 2) largely confirms the distribution seen previously (Kapas et al., 1995; Kapas & Clark, 1995; McLatchie et al., 1998; Njuki et al., 1993) except that in the rat, we find that RAMP-3 is only expressed at low levels. This contrasts with the apparently high and relatively uniform expression level reported in human tissues (McLatchie et al., 1998). This is not a function of the probe used as we obtained exactly the same distribution when using a human probe on the same series of rat RNA blots and the result is not significantly influenced by the choice of internal control used to normalize the blots.
It is important to consider the biological significance of any correlations seen between the parameters measured. It would not be correct to conclude that just because the level of an mRNA was significantly correlated (statistically) with a ligand binding activity that this implies that the encoded protein is required for the binding. The mRNA might be expressed in the same cells as the receptor and/or its expression might be co-regulated with that of the actual receptor. Conversely a lack of correlation does not mean that the mRNA does not encode the receptor or a component thereof. If there are multiple receptors for a ligand then a simple correlation with binding would only be expected for components of the dominant receptor system. Finally it should also be appreciated that the level of an mRNA will not be proportional to the activity of the encoded receptor in all tissues and in all situations. One would expect a rough correlation but this might not be strong enough to achieve significance in our experiments.
With these points in mind we will now discuss our findings and their bearing on the putative L1/RDC1 and CRLR/RAMP adrenomedullin and CGRP receptors. L1 expression is not strongly or significantly correlated with adrenomedullin binding activity in the tissues we have studied (R=0.38, P=0.40) and similarly, RDC1 does not correlate with CGRP binding (R=0.09, P=0.82). In fact there is a perverse tendency for L1 to correlate with CGRP binding (R=0.53, P=0.07) and for RDC1 to correlate with adrenomedullin binding (R=0.89, P=0.08) and the correlation of RDC1 with total binding achieves significance (R=0.75, P=0.03). Thus our data does not support the original ligand assignments made for these two receptors but is not inconsistent with the possibility that RDC1 might play a role in the binding of adrenomedullin and/or that L1 might play a role in the 'non RAMP-1' binding of CGRP (R=0.36, P=0.02). We note that in rat aortic vascular smooth muscle cells which bind adrenomedullin, RDC1 is expressed but not CRLR or L1 (Autelitano & Tang, 1999). We have recently tested the L1 sequence (kindly provided by Dr S. Kapas, St Bartholomew's and the Royal London School of Medicine and Dentistry). We have been unable to detect binding of either adrenomedullin or CGRP in 293 cells transfected either by L1 alone or in combination with RAMPs. It is possible that the activity of L1 is dependent on cell specific accessory factors as has proved to be the case with CRLR.
The naive predictions of the CRLR/RAMP model are that CRLR should correlate with total (i.e. adrenomedullin+CGRP) binding, RAMP-1 with CGRP binding and RAMP-2+RAMP-3 with adrenomedullin binding. Since RAMP-3 levels are low, RAMP-3 is unlikely to affect binding significantly in any tissue. When the Bmax values are compared with the levels of each mRNA it is apparent that CRLR expression is significantly correlated with the combined Bmax for CGRP and adrenomedullin (R=0.94, P=0.03) and that RAMP-1 expression is significantly correlated with CGRP Bmax (R=1.00, P=0.007) as predicted by the model. The model predicts a similar correlation between adrenomedullin binding and RAMP-2 and/or RAMP-3 mRNA levels but this correlation, though strong (R=0.95), did not achieve significance in our study (P=0.14). Thus our data is consistent with the CRLR/RAMP model for CGRP and adrenomedullin receptors but one should always bear in mind the limitations inherent in this type of experiment. Furthermore, despite these correlations, mRNA levels are not strictly proportional to binding activity in all cases. We note in particular that in the ventricles, the level of CRLR/RAMP-2 seems insufficient to account for the Bmax for adrenomedullin suggesting the possibility of other factors contributing to binding.
Two unexpected correlations in our data which are both strong and significant are those between CRLR and RAMP-2 mRNA levels (r=0.94, P=0.016) and between RAMP-1 and RAMP-3 mRNA levls (r=0.95, P=0.015). In fact, although not an a priori prediction of the model, some association between CRLR and RAMP-2 would be expected given the other data from Table 1. Since adrenomedullin binding represents 71% of the observed binding and RAMP-3 hybridization is quantitatively insignificant it follows that RAMP-2 should be correlated with total binding and hence with CRLR mRNA levels. The surprising feature is that the correlation with total binding is higher than that with adrenomedullin binding implying RAMP-2 co-expression with CRLR in situations where CGRP binding is seen.
The association of RAMP-1 and RAMP-3 mRNA levels could not have been similarly predicted. Both have recently been shown to combine with the calcitonin receptor to produce high affinity amylin binding (Christopoulos et al., 1999; Muff et al., 1999). However, calcitonin receptor mRNA levels are too low to detect by Northern blotting in the tissues we have studied (Njuki et al., 1993) so distortion of our data by RAMPs co-expressed with the caltitonin receptor seems unlikely. We are unable to explain this strong and totally unexpected correlation and suggest that there are significant features of this system which remain to be elucidated.
Acknowledgments
We thank the British Heart Foundation (Project Grant PG 97091) and the Institut de Recherche Jouveinal/Parke-Davis for support of these studies and Dr S. Foord, Glaxo Wellcome, Stevenage and co-workers for gifts of human RAMP clones. We thank Mr Paul Bassett (ICSM-Hammersmith, Statistics Department) for the statistical analysis of our data.
Abbreviations
- ADM
adrenomedullin
- CGRP1 and CGRP2
CGRP receptor subtypes
- CRLR
calcitonin receptor-like receptor
- CT
calcitonin
- CTR
calcitonin receptor
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HEK
human embryonic kidney
- R
regression coefficient
- r
correlation coefficient
- RAMP
receptor activity modifying protein
References
- AIYAR N., RAND K., ELSHOURBAGY N.A., ZENG Z., ADAMOU J.E., BERGSMAN D.J., LI Y. A cDNA encoding the Calcitonin Gene-related Peptide Type 1 Receptor. J. Biol. Chem. 1996;271:11325–11329. doi: 10.1074/jbc.271.19.11325. [DOI] [PubMed] [Google Scholar]
- AUTELITANO D.J., TANG F. Co-expression of prepro-adrenomedullin with a putative adrenomedullin receptor gene in vascular smooth muscle. Clin. Sci. Colch. 1999;96:493–498. [PubMed] [Google Scholar]
- BHOGAL R., SMITH D.M., BLOOM S.R. Investigation and characterization of binding sites for islet amyloid polypeptide in rat membranes. Endocrinology. 1992;130:906–913. doi: 10.1210/endo.130.2.1310282. [DOI] [PubMed] [Google Scholar]
- BHOGAL R., SMITH D.M., PURKISS P., BLOOM S.R. Molecular identification of binding sites for calcitonin gene-related peptide (CGRP) and islet amyloid polypeptide (IAPP) in mammalian lung: species variation and binding of truncated CGRP and IAPP. Endocrinology. 1993;133:2351–2361. doi: 10.1210/endo.133.5.8404688. [DOI] [PubMed] [Google Scholar]
- BUHLMANN N., LEUTHAUSER K., MUFF R., FISCHER J.A., BORN W. A receptor activity modifying protein (RAMP)2-dependent adrenomedullin receptor is a calcitonin gene-related peptide receptor when coexpressed with human RAMP1. Endocrinology. 1999;140:2883–2890. doi: 10.1210/endo.140.6.6783. [DOI] [PubMed] [Google Scholar]
- CHANG C.P., PEARSE R.V. , II, O'CONNELL S., ROSENFELD M.G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993;11:1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- CHANTRY A., LEIGHTON B., DAY A.J. Cross-reactivity of amylin with calcitonin-gene-related peptide binding sites in rat liver and skeletal muscle membranes. Biochem. J. 1991;277:139–143. doi: 10.1042/bj2770139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPOULOS G., PERRY K.J., MORFIS M., TILAKARATNE N., GAO Y., FRASER N.J., MAIN M.J., FOORD S.M., SEXTON P.M. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol. Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- COPPOCK H.A., OWJI A.A., BLOOM S.R., SMITH D.M. A rat skeletal muscle cell line (L6) expresses specific adrenomedullin binding sites but activates adenylyl cyclase via calcitonin gene related peptide receptors. Biochem. J. 1996;318:241–245. doi: 10.1042/bj3180241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORCORAN C.M., FRASER P., MARTINI G., LUZZATTO L., MASON P.J. High level, position independent expression of the human G6PD gene in transgenic mice. Gene. 1996;173:241–246. doi: 10.1016/0378-1119(96)00094-7. [DOI] [PubMed] [Google Scholar]
- DENNIS T., FOURNIER A., CADIEUX A., POMERLEAU F., JOLICOEUR F.B., STPIERRE S., QUIRION R. hCGRP8-37, a calcitonin gene-related peptide antagonist revealing calcitonin gene-related peptide receptor heterogeneity in brain and periphery. J. Pharmacol. Exp. Ther. 1990;254:123–128. [PubMed] [Google Scholar]
- FEINBERG A.P., VOGELSTEIN B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal. Biochem. 1983;132:6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- FLUHMANN B., MUFF R., HUNZIKER W., FISCHER J.A., BORN W. A human orphan calcitonin receptor-like structure. Biochem. Biophys. Res. Commun. 1995;206:341–347. doi: 10.1006/bbrc.1995.1047. [DOI] [PubMed] [Google Scholar]
- GIBBONS A.H., LEGON S., WALKER M.M., GHATEI M., CALAM J. The effect of gastrin-releasing peptide on gastrin and somatostatin messenger RNAs in humans infected with Helicobacter pylori. Gastroenterology. 1997;112:1940–1947. doi: 10.1053/gast.1997.v112.pm9178686. [DOI] [PubMed] [Google Scholar]
- HAN Z.Q., COPPOCK H.A., SMITH D.M., VAN NOORDEN S., MAKGOBA M.W., NICHOLL C.G., LEGON S. The interaction of CGRP and adrenomedullin with a receptor expressed in the rat pulmonary vascular endothelium. J. Mol. Endocrinol. 1997;18:267–272. doi: 10.1677/jme.0.0180267. [DOI] [PubMed] [Google Scholar]
- KAMITANI S., ASAKAWA M., SHIMEKAKE Y., KUWASAKO K., NAKAHARA K., SAKATA T. The RAMP2/CRLR complex is a functional adrenomedullin receptor in human endothelial and vascular smooth muscle cells. FEBS Lett. 1999;448:111–114. doi: 10.1016/s0014-5793(99)00358-0. [DOI] [PubMed] [Google Scholar]
- KAPAS S., CATT K.J., CLARK A.J.L. Cloning and expression of cDNA encoding a rat adrenomedullin receptor. J. Biol. Chem. 1995;270:25344–25347. doi: 10.1074/jbc.270.43.25344. [DOI] [PubMed] [Google Scholar]
- KAPAS S., CLARK A.J.L. Identification of an orphan receptor gene as a type 1 calcitonin gene-related peptide receptor. Biochem. Biophys. Res. Commun. 1995;217:832–838. doi: 10.1006/bbrc.1995.2847. [DOI] [PubMed] [Google Scholar]
- KENNEDY S.P., SUN D., OLEYNEK J.J., HOTH C.F., KONG J., HILL R.J. Expression of the rat adrenomedullin receptor or a putative human adrenomedullin receptor does not correlate with adrenomedullin binding or functional response. Biochem. Biophys. Res. Commun. 1998;244:832–837. doi: 10.1006/bbrc.1998.8349. [DOI] [PubMed] [Google Scholar]
- LIN H.Y., HARRIS T.L., FLANNERY M.S., ARUFFO A., KAJI E.H., GORN A., KOLAKOWSKI L.F., Jr, LODISH H.F., GOLDRING S.R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991;254:1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MUFF R., BUHLMANN N., FISCHER J.A., BORN W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- NJUKI F., NICHOLL C.G., HOWARD A., MAK J.C., BARNES P.J., GIRGIS S.I., LEGON S. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin. Sci. Colch. 1993;85:385–388. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- OWJI A.A., SMITH D.M., COPPOCK H.A., MORGAN D.G., BHOGAL R., GHATEI M.A., BLOOM S.R. An abundant and specific binding site for the novel vasodilator adrenomedullin in the rat. Endocrinology. 1995;136:2127–2134. doi: 10.1210/endo.136.5.7720662. [DOI] [PubMed] [Google Scholar]
- PERRY K.J., QUIZA M., MYERS D.E., MORFIS M., CHRISTOPOULOS G., SEXTON P.M. Characterization of amylin and calcitonin receptor binding in the mouse alpha-thyroid-stimulating hormone thyrotroph cell line. Endocrinology. 1997;138:3486–3496. doi: 10.1210/endo.138.8.5312. [DOI] [PubMed] [Google Scholar]
- POYNER D.R. Pharmacology of receptors for calcitonin gene-related peptide and amylin. Trends Pharmacol. Sci. 1995;16:424–428. doi: 10.1016/s0165-6147(00)89093-8. [DOI] [PubMed] [Google Scholar]
- SHARMA S.K., AUSTIN C., HOWARD A., LO G., NICHOLL C.G., LEGON S. Characterization of rat gastric inhibitory peptide cDNA. J. Mol. Endocrinol. 1992;9:265–272. doi: 10.1677/jme.0.0090265. [DOI] [PubMed] [Google Scholar]
- STANGL D., MUFF R., SCHMOLCK C., FISCHER J.A. Photoaffinity labeling of rat calcitonin gene-related peptide receptors and adenylate cyclase activation: identification of receptor subtypes. Endocrinology. 1993;132:744–750. doi: 10.1210/endo.132.2.8381072. [DOI] [PubMed] [Google Scholar]
- WIMALAWANSA S. Calcitonin gene related peptide and its receptors: molecular genetics, physiology, pathophysiology and therapeutic potentials. Endocrine Reviews. 1996;17:533–585. doi: 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- WITHERS D.J., COPPOCK H.A., SEUFFERLEIN T., SMITH D.M., BLOOM S.R., ROZENGURT E. Adrenomedullin stimulates DNA synthesis and cell proliferation via elevation of cAMP in Swiss 3T3 cells. FEBS Lett. 1996;378:83–87. doi: 10.1016/0014-5793(95)01427-6. [DOI] [PubMed] [Google Scholar]


