Abstract
The aim of this study was to determine whether neurokinin B (NKB) or specific agonists of tachykinin NK3 receptors, [MePhe7]NKB and senktide, were able to induce airway hyperresponsiveness in guinea-pigs. The effects of these compounds were compared to those of substance P (SP), neurokinin A (NKA) and the preferential tachykinin NK1 ([Sar9, Met(02)11]SP) or NK2 ([βAla8]NKA (4-10)) receptor agonists.
In guinea-pigs pretreated with phosphoramidon (10−4 M aerosol for 10 min) and salbutamol (8.7×10−3 M for 10 min), all tachykinins administrated by aerosol (3×10−7 to 10−4 M) induced airway hyperresponsiveness 24 h later, displayed by an exaggerated response to the bronchoconstrictor effect of acetylcholine (i.v.). The rank order of potency was: [βAla8]NKA (4-10)>NKA=NKB=senktide=[MePhe7]NKB=[Sar9,Met(02)11]SP>SP.
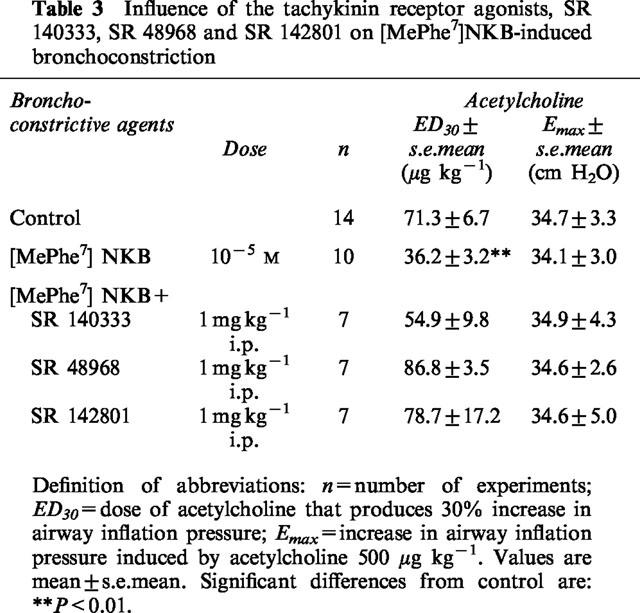
Airway hyperresponsiveness induced by [MePhe7]NKB was prevented by the tachykinin NK3 (SR 142801) and NK2 (SR 48968) receptor antagonists.
Bronchoconstriction induced by tachykinins administered by aerosol was also determined. SP, NKA, NKB and the tachykinin NK1 and NK2 receptor agonist induced bronchoconstriction. The rank order of potency was: NKA=[βAla8]NKA (4-10)>NKB=SP=[Sar9,Met(02)11]SP. Under similar conditions, and for concentrations which induce airway hyperresponsiveness, senktide and [MePhe7]NKB failed to induce bronchoconstriction.
It is concluded that tachykinin NK3-receptor stimulation can induce airway hyperresponsiveness and that this effect is not related to the ability of tachykinins to induce bronchoconstriction.
Keywords: Tachykinins, airway hyperresponsiveness, tachykinin NK3 receptors
Introduction
Tachykinins, a group of neuropeptides including substance P (SP), neurokinin A (NKA) and neurokinin B (NKB) are the transmitters of sensory neurones which, in the lung, innervate all compartments of the airway wall from the trachea down to the bronchioles (Baluk & McDonald 1998; Ellis & Undem 1994; Lundberg 1996; Lundberg & Saria 1987). The activation of C-fibre afferent nerves in airways leads to a local release of tachykinins that are responsible for several biological effects: bronchospasm, increase in microvascular permeability, vasodilatation, stimulation of glandular secretions, facilitation of cholinergic neurotransmission, recruitment and activation of inflammatory cells. Sensory nerves also mediate respiratory defence reflexes, such as coughing and sneezing (Ellis & Undem, 1994; Maggi et al., 1993; Widdicombe, 1995).
The biological actions of tachykinins are mediated via three types of receptors, denoted tachykinin NK1, NK2 and NK3 which have the highest affinity for SP, NKA and NKB, respectively. This receptor classification has been established from receptor-binding and functional studies using selective agonists or antagonists for tachykinin receptors (Regoli et al., 1994). It has now been recognized that the expression of tachykinin NK3 receptor is confined mainly to the central and peripheral nervous system, whilst tachykinins NK1 and NK2 receptors are expressed both in the nervous system and in target organs, including airways (Baluk et al., 1996; Guard & Watson, 1991; Maggi, 1993; Myers & Undem, 1993).
Several experimental reports using different animal species have suggested that tachykinins are involved in airway hyperresponsiveness, an enhanced bronchoconstrictor response to many different stimuli and a key feature of asthma. Airway hyperresponsiveness is associated with inflammation in the airways and relates closely to the severity of asthma, the frequency of symptoms, and the need for treatment (Barnes, 1989; Boushey et al., 1980; O'Byrne, 1988). Indeed, exposure to a single aerosol of SP elicited 24 h later airway hyperresponsiveness to exogenous bronchoconstrictor agents in guinea-pigs (Boichot et al., 1993). Similar data were observed in asthmatic patients (Cheung et al., 1994). NKA also enhanced methacholine response up to 4 weeks in monkeys (Tamura et al., 1989). Conversely, chronic treatment with high doses (i.p.) of capsaicin which depletes tachykinins from sensory nerves, or single pretreatments with the tachykinin NK1 [CP 96345, SR 140333], NK2 [SR 48968, MEN 10,627] or NK1+NK2 [MDL 105212; FK 224] receptor antagonists have been reported to prevent airway hyperresponsiveness in various experimental models in guinea-pigs, mice or monkeys (see reviews Advenier et al., 1997; Kraneveld et al., 1997; Spina et al., 1998).
We have recently demonstrated that the NK3 receptor antagonist, SR 142801 (Osanetant) (Emonds-Alt et al., 1995) was also able to inhibit in guinea-pig airway hyperresponsiveness induced by substance P (Daoui et al., 1997) or citric acid (Daoui et al., 1998), and to prevent in human isolated bronchi hyperresponsiveness induced by interleukin 1-β or by passive sensitization (Vincent et al., 1999). To our knowledge, no studies have been performed in order to determine if agonists for NK3 receptors may induce airway hyperresponsiveness as previously reported for substance P and NKA.
The aim of this study was to determine whether NKB or the specific agonists of NK3 receptors, [MePhe7]NKB or senktide, were able to induce airway hyperresponsiveness in guinea-pigs. We also compared the potency of NKB and tachykinin NK3 agonists with those of SP, NKA and of tachykinin NK1 and NK2 receptor agonists.
Methods
Airway hyperresponsiveness to acetylcholine
Exposure to tachykinins or tachykinin receptor agonists aerosol.
Tricoloured unanaesthetized, unrestrained male or female guinea-pigs (300–400 g), were placed in Plexiglas chamber (30×25×15 cm) and exposed successively to a nebulized aqueous solution of salbutamol (8.7×10−3 M, 10 min) or phosphoramidon (10−4 M, 10 min) in order to prevent tachykinins- or tachykinin receptor agonist-induced bronchoconstriction and tachykinin metabolism; 5 min later the animals were exposed to a single aerosol of tachykinins or tachykinin receptor agonists at various concentrations (3×10−7 to 10−4 M) or vehicle solution as control group for 30 min (Figure 1). An ultrasonic nebulizer (Aerodynamic mean mass median particle diameter of 0.5 to 5 μm, NEB99, Devilbiss, Somerset, PA, U.S.A.) was used. Previous studies have shown that phosphoramidon and/or salbutamol when used alone or in combination were not able to induce airway hyperresponsiveness (Girard et al., 1996; Daoui et al., 1997, 1998).
Figure 1.
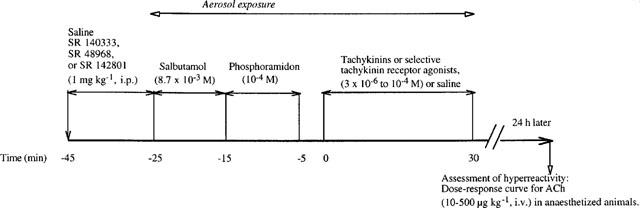
Experimental protocol of induction of airway hyperresponsiveness and measurement of airway inflation pressure in guinea-pigs.
Assessment of the in vivo bronchopulmonary reactivity.
24 h after exposure to tachykinins or tachykinin receptor agonists (Figure 1), animals were anaesthetized with urethane (1.25 g kg−1, i.p.) and placed on a heated blanket (Homeothermic blanket system, Havard Apparatus Ltd, Kent, U.K.). A jugular vein was cannulated for injection of acetylcholine. A trachea cannula was inserted and artificial ventilation was maintained by means of a constant volume ventilator (Model 7025, UGO Basile, Comerio-Varese, Italy). Airway inflation pressure was measured using a pressure transducer (P23XL, Viggo-Spectramed, Bilthoven, Netherlands) connected to the tracheal cannula via a side-arm and recorded with a recording microdynamometer (Model 7050, UGO Basile, Comerio-Varese, Italy). The tidal volume (approximately 10 ml kg−1) was adjusted to give a base-line inflation pressure of 8–10 cm H2O at the end of the inspiration. After a stabilization period of 10 min, acetylcholine was administered at increasing doses (10, 20, 50, 100, 200 and 500 μg kg−1, i.v.) 5–10 min apart. Bronchopulmonary responses were expressed as per cent response changes vs acetylcholine at 500 μg kg−1. Acetylcholine (500 μg kg−1) responses were expressed in cm H2O.
Pretreatment with drugs.
Guinea-pigs received a single dose (1 mg kg−1, i.p.) of the NK3 (SR 142801), NK2 (SR 48968) or NK1 (SR 140333) receptor antagonists, or vehicle 45 min before exposure to [MePhe7]NKB.
Bronchoconstriction
Tricoloured male or female guinea-pigs (300–400 g) were anaesthetized with urethane (1.25 g kg−1, i.p.) and placed on a heated blanket (Havard Apparatus Ltd, Kent, U.K.) which maintained body temperature at about 37°C. The left jugular vein was cannulated for injection of acetylcholine. A tracheal cannula was inserted and artificial ventilation was maintained by means of a constant volume ventilator. Animals were ventilated with room air at a rate of 60 breath per min and at a tidal volume of approximately 10 ml kg−1. Airway function was assessed by measuring changes in pleural pressure, which can be regarded as an indicator of airway resistance at least in guinea-pigs (Santing et al., 1992). Pleural pressure was determined with a catether fitted with a 16 G needle inserted into the 6th or 7th intercostal space and connected to a pressure transducer (P23XL, Viggo-Spectramed, Bilthoven, Netherlands). The pleural pressure increase was evaluated as the difference between the baseline value and the maximum response (the peak value) after tachykinin or tachykinin receptor agonist aerosol.
After a 30 min resting period, artificial ventilation was stopped, and the tracheal cannula was connected in spontaneously breathing animals directly to the nebulizer. Aerosols of tachykinin or tachykinin receptor agonist solutions (3×10−7 to 10−4 M) were administered for 2 min and about 0.3 ml of the solution was nebulized per min. After the pleural pressure was returned to baseline or stabilized, artificial ventilation was set running again. Preliminary experiments have shown that responses were reproducible at least three times at 30 min intervals. Also we have demonstrated that no significant changes were produced by 0.9% saline aerosol (0.3 ml min−1) administration. The bronchopulmonary responses were expressed as per cent changes vs acetylcholine (500 μg kg−1, i.v.) administered at the end of the experiment, in spontaneously breathing animals.
Statistical analysis of results
Data are expressed as means±s.e.mean. EC30 value is the dose which provokes an increase in airway inflation pressure of 30% of the maximal effect (Girard et al., 1996; Daoui et al., 1997, 1998). Statistical analysis of the results was assessed by a two-way analysis of variance (ANOVA) followed by a Student's t-test for paired or unpaired data. Probability values of P<0.05 were considered significant.
Drugs
The substances used were: urethane (Prolabo, Paris, France); histamine dihydrochloride, phosphoramidon, salbutamol sulphate (Sigma, St Louis, MO, U.S.A.); acetylcholine hydrochloride (PCH, Paris, France); substance P, [Sar9, Met(O2)11]substance P, neurokinin A, [βAla8]neurokinin A(4-10) (Bachem, Paris, France); neurokinin B, [MePhe7]neurokinin B, senktide (Novabiochem, Paris, France); SR 48968 [(S)-N-metyl - N[(4 - acetylamino-4-phenylpiperidino-2-(3,4)dichlorophenyl) butyl]benzamide] (saredutant) used as hydrochloride, SR 140333 [(S)1-{2-[3-(3,4-dichlorophenyl)-1-(3-iso-propoxyphenylacetyl)piperidin-3-yl]etyl}-4-phenyl-1-azoniabicyclo[2.2.2]octane, chloride] (chloride of nolpitantium) and SR 142801 [(R)-(N)-(1-(3-(l-benzoyl-3-(3,4-dichlorophenyl)piperidin-3-yl) propyl)-4-phenylpiperidin-4-yl)-N-methylacetamide] (osanetant) used as hydrochloride (Sanofi Recherche, Mont-pellier, France). All drugs were dissolved in saline, except SR 48968, SR 140333 and SR 142801 which were dissolved in ethanol and then in diluted saline, and neurokinin B and [MePhe7]neurokinin B which were dissolved in acetic acid (100%) and then diluted in saline. The maximum amount of ethanol injected (20 μl per 100 g body weight) did not modify the respiratory responses to acetylcholine, the development of airway hyperresponsiveness and bronchoconstriction at the dilution used.
Results
Comparison of the ability of the tachykinins and tachykinin receptor agonists to induce hyperresponsiveness when given by aerosol
Airway hyperresponsiveness developed consistently 24 h after a single tachykinin or tachykinin receptor agonist (3×10−7 to 10−4 M) exposure in guinea-pigs pretreated with phosphoramidon, as evidenced by a significant leftward shift in dose response curves to acetylcholine-induced bronchoconstriction in comparison with matched saline controls (Figure 2). The maximum bronchoconstrictor responses to acetylcholine (Emax at 500 μg kg−1) were similar in animals exposed to tachykinins, tachykinin receptor agonists or saline (Tables 1 and 2). ED30 values for acetylcholine were significantly lower in animals pretreated with salbutamol, phosphoramidon and exposed to tachykinins or tachykinin receptor agonists than in saline exposed animals control, showing a maximal increase in sensitivity of approximately 2 fold.
Figure 2.
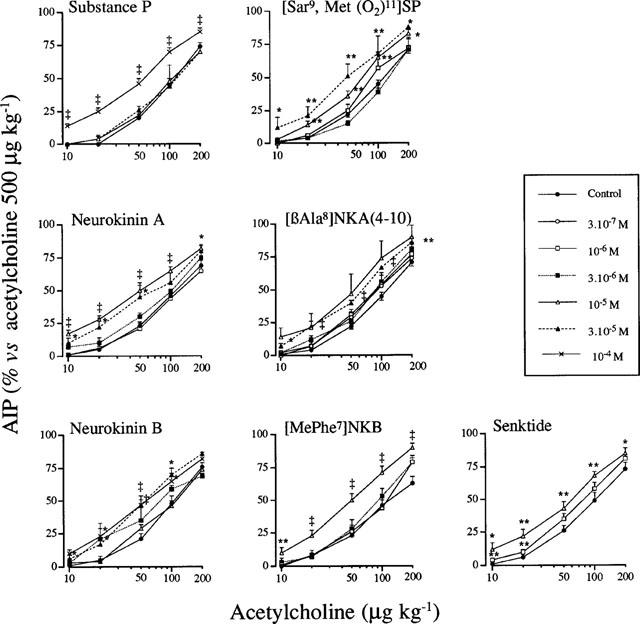
Cumulative contractile concentration-response curves for acetylcholine (10–200 μg kg−1) in anaesthetized guinea-pigs, 24 h after aerosols of salbutamol (8.7×10−3 M, 10 min), phosphoramidon (10−4 M, 10 min) and saline (NaCl 9 ‰, 30 min) or SP, NKA, NKB, [Sar9, Met(O2)11]SP, [βAla8]NKA (4-10), [MePhe7]NKB and senktide (3×10−7 to 10−4 M, 30 min). Values are means±s.e.mean, n are reported in Tables 1 and 2. AIP: airway inflation pressure. Significant difference from control shown as: *P<0.05 and **P<0.01.
Table 1.
Effects of substance P-, neurokinin A- and neurokinin B-aerosol exposure on acetylcholine-induced bronchoconstriction
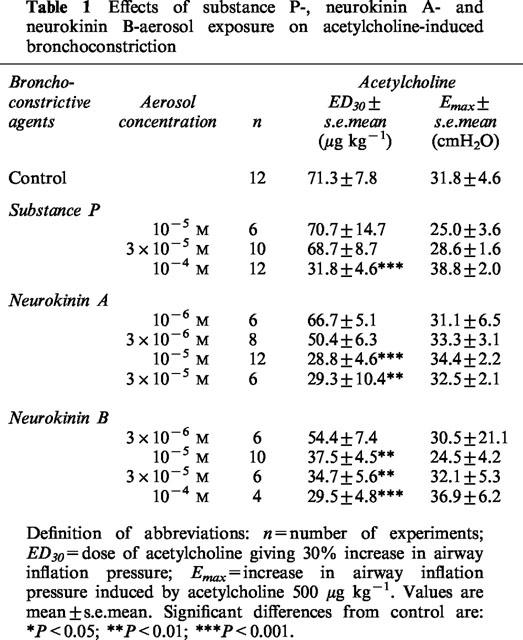
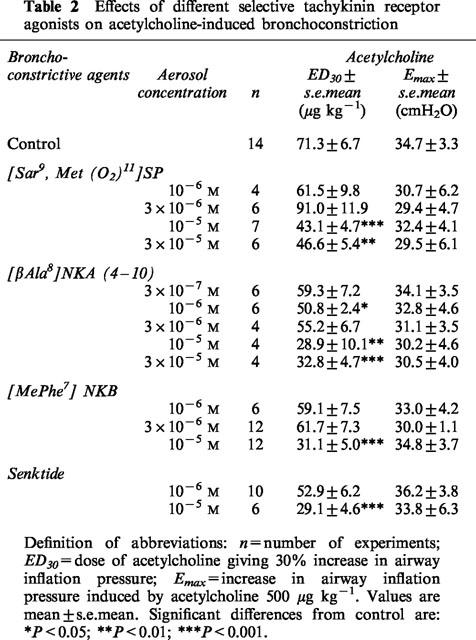
Table 2.
Effects of different selective tachykinin receptor agonists on acetylcholine-induced bronchoconstriction
Relative potency of agonists
The relative potencies of the tachykinins and their selective analogues were compared using data derived from ED30 values (Tables 1 and 2). Significant increases in airway responsiveness were observed from 10−6 M for [βAla8]NKA (4-10), from 10−5 M for NKA, NKB, [MePhe7]NKB, senktide and [Sar9, Met(O2)11]SP and for 10−4 for SP. The rank order of potency was: [βAla8]NKA (4-10)≈thinsp;4 NKA = NKB = [MePhe7]NKB = senktide = [Sar9, Met(O2)11]SP>SP (Tables 1 and 2).
Effects of SR 140333 (NK1 antagonist), SR 48968 (NK2 antagonist) or SR 142801 (NK3 antagonist) on [MePhe7]NKB- induced hyperreactivity
Airway hyperresponsiveness to acetylcholine following exposure to [MePhe7]NKB was abolished by a single dose of the tachykinin NK2 or NK3 receptor antagonists SR 48968 and SR 142801 (1 mg kg−1, i.p.), administered 45 min before [MePhe7]NKB exposure (Table 3). In contrast after the administration of the selective NK1 antagonist SR 140333 (1 mg kg−1, i.p.), the ED30 for acetylcholine did not appear significantly different between control animals and animals pretreated with [MePhe7]NKB. Only a partial inhibition was observed.
Table 3.
Influence of the tachykinin receptor agonists, SR 140333, SR 48968 and SR 142801 on [MePheM7]NKB-induced bronchoconstriction
Comparison of the bronchoconstrictor response to tachykinin and selective tachykinin receptor agonists in guinea-pigs in vivo
In control animals that received successive administrations of a 0.9 % NaCl aerolized solution no significant changes in the baseline was observed. The effects of various tachykinins and selective receptor agonists were then examined. Without prior treatment with phosphoramidon (10−4 M, aerosol for 10 min), the guinea-pigs did not respond to tachykinin or tachykinin receptor agonist aerosolized solution at concentrations up to 3×10−5 M. After pretreatment with phosphoramidon, the guinea-pigs responded to SP, NKA, NKB, [Sar9, Met(O2)11]SP and [βAla8]NKA (4-10) with an immediate bronchoconstriction. The rank order of potency to induce bronchoconstriction was: [βAla8]NKA (4-10)=NKA>NKB=SP>[Sar9, Met(O2)11]SP. Under similar conditions [MePhe7]NKB and senktide had no effect (Figure 3).
Figure 3.
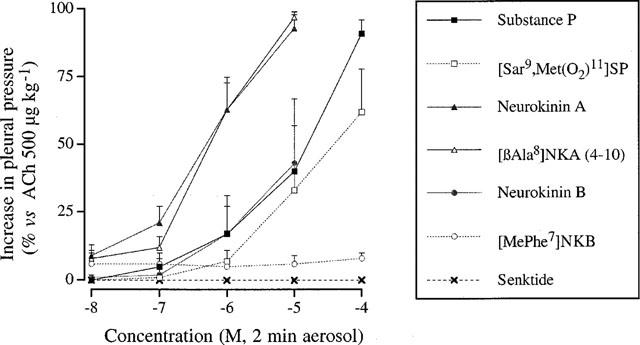
Bronchoconstriction induced by various tachykinins (SP, NKA, NKB) and specific tachykinin receptor agonists [Sar9, Met(O2)11]SP, [βAla8]NKA (4-10), [MePhe7]NKB or senktide (3×10−7 to 10−4 M, aerosol) in anaesthetized guinea-pigs. Results are expressed as per cent increase of the bronchoconstriction induced by acetylcholine 500 μg kg−1 i.v.
Discussion
Tachykinin NK3 receptor stimulation induces airway hyperresponsiveness
Tachykinins induce various effects in the bronchopulmonary system and they are recognised to be involved in the pathogenesis of airway hyperresponsiveness in animals models (see introduction and reviews of Advenier et al., 1997; Kraneveld et al., 1997; Spina et al., 1998). Indeed, it has been previously reported that aerosol exposure of SP induces the development of airway hyperresponsiveness in guinea-pigs (Boichot et al., 1993; Daoui et al., 1997). Similar results were obtained in asthmatic patients (Cheung et al., 1994). Moreover, NKA also elicits airway hyperresponsiveness in monkeys (Tamura et al., 1989) but no studies have been conducted to date that evaluate the capacity for NK3 receptor agonists to induce the development of airway hyperresponsiveness.
In the present study, we found that NKB and the tachykinin NK3 receptor agonists, [MePhe7]NKB (4-10) and senktide are also able to induce airway hyperresponsiveness in guinea-pigs pretreated with phosphoramidon. However, in terms of potency, the selective agonist for tachykinin NK2 receptor [βAla8]NKA (4-10) appears the most effective, since it significantly elicits a significant leftward shift of the dose-reponse curve to ACh, from the concentration of 10−6 M. We also observed that [MePhe7]NKB and senktide showed a similar effect as NKA, whereas NKB and [Sar9, Met (O2)11]SP were more effective than SP. The activity of NKB could be mediated through the effect on NK1 and/or NK2 receptors regarding the nonselective activity of this compound on NK3 receptors (Regoli et al., 1994). Nevertheless, the involvement of NK3 receptors in the effects of [MePhe7]NKB and senktide is strongly suggested by the high selectivity of these agonists on NK3 receptors in several radioligand binding and functional assays (Regoli et al., 1994).
The development of bronchial hyperresponsiveness induced by tachykinins and selective agonists for tachykinin receptors is not related to their bronchoconstrictor effects. Indeed, in this study the guinea-pigs are pretreated by the potent bronchodilator drug, salbutamol, in order to avoid all spasmogenic activities during the aerosol administration of tachykinin and tachykinin selective agonist. Moreover, [MePhe7]NKB and senktide do not elicit a bronchoconstrictor response by themselves. The dissociation between the bronchoconstrictor activity and the induction of bronchial hyperresponsiveness is also demonstrated by the use of [βAla8]NKA (4-10) and NKA. Both compounds elicit similar bronchoconstrictor effects (Chan et al., 1994; Yuan et al., 1994 and this study), but [βAla8]NKA (4-10) induced a more marked leftward shift of the dose-response curve to ACh than NKA. Similar observations and conclusions may be proposed in view of the respective efficacy of [Sar9, Met(O2)11]SP and SP in the induction of bronchoconstriction and airway hyperresponsiveness.
Tachykinin NK2 and NK3 receptor antagonists prevent NK3-induced airway hyperresponsiveness
The present results showed that airway hyperresponsiveness induced by the selective NK3 receptor agonist [MePhe7]NKB was abolished by the tachykinin NK3 receptor antagonist, SR 142801 (osanetant), but also by the tachykinin NK2 receptor antagonist, SR 48968 (saredutant). Similar observations were previously reported on airway hyperresponsiveness induced by SP or citric acid (Daoui et al., 1997, 1998). This later compound has been demonstrated to release endogenous tachykinins from capsaicin sensitive nerve endings (Belmonte et al., 1990; Fox et al., 1995; Geppetti et al., 1991; Steen et al., 1992). In contrast to the inhibitory activity of SR 142801, the effect of SR 48968 and other NK2 receptor antagonists on the development of bronchial hyperresponsiveness have been clearly demonstrated in sensitized and challenged guinea-pigs (Boichot et al., 1995; Kudlacz et al., 1996) or after exposure to toluene diisocyanate (Marek et al., 1996); cold air (Yoshihara et al., 1996); ozone (Masson et al., 1996) or PAF (Perretti & Manzini, 1993) in guinea-pigs.
The tachykinin NK1 receptor antagonist, SR 140333, only elicit a partial inhibition of [MePhe7]NKB. In previous reports, we observed that SR 140333 did not prevent in guinea-pigs airway hyperresponsiveness induced by SP (Boichot et al. 1996) or by an allergen challenge in sensitized animals (Boichot et al., 1995). The effects of SR 140333 is however debated since Schuiling et al. (1999) reported a preventive effect of this drug in the latter model. These discrepancies could be explained by the different protocols and spasmogenic agents used in both cases.
Hypothesis on the mechanism of action of tachykinin NK3 agonists
To date, it is difficult to provide the exact mechanism and the site of action of NK3 agonists in the development of airway hyperresponsiveness. Indeed, airway hyperresponsiveness is a complex process which involves multiple cell interactions and several mediators. Firstly, it seems that the action of NK3 agonists is mainly due to the NK3 receptor activation pathway. Secondly, this activity may involve neuronal receptors rather than post-junctional effector sites. Thirdly, a cascade of physiopathological process associated with the successive stimulation of tachykinin receptors may also be proposed.
The activity of NKB, [MePhe7]NKB and senktide involve the NK3 receptor pathway as suggested by the high selectivity of these agonists on this receptor subtype, but also by the inhibitory activity of the selective NK3 antagonist, SR 142801. The compound selectivity has been demonstrated by radioligand binding and well characterized in vitro functional assays for tachykinin receptors (Beaujouan et al., 1997; Daoui et al., 1997; Emonds-Alt et al., 1995; Nguyen-Le et al., 1996; Oury-Donat et al., 1995; Patacchini et al., 1995). In vivo, the selectivity of SR 142801 is clearly suggested by two assays: in contrast to SR 48968, SR 142801 (1 mg kg−1) did not inhibit bronchoconstriction induced by [Nle10]NKA (4-10) in anaesthetized guinea-pigs (Daoui et al., 1997); and unlike SR 140333, SR 142801 (1 mg kg−1) failed to inhibit the hypotension induced by [Sar9, Met(O2)11]SP in guinea-pigs and dogs (Emonds-Alt et al., 1993, 1995; Roccon et al., 1996).
A direct priming effect of NK3 receptor stimulation on target cells is unlikely, since a low number of NK3 tachykinin receptor has been identified in lung (Baluk et al., 1996). In agreement with previous studies (Ellis et al., 1993; Killingsworth & Shore, 1995; Maggi et al., 1991), our results demonstrating that [MePhe7]NKB and senktide do not present bronchoconstrictor activity in the guinea-pig suggest that NK3 receptor stimulation does not lead to the contraction of airway smooth muscle. A direct participation of NK3 receptors in the impairment of vessels and endothelial cells, leading to microvascular leakage and airway obstruction is also excluded since SR 142801 failed to inhibit tachykinin and capsaicin-induced plasma extravasation which is mainly mediated by NK1 receptors and suppressed by SR 140333 (Inoue et al., 1996). Finally, no effect of tachykinin NK3 receptor stimulation has been demonstrated in mucus or in inflammatory cells (Ellis & Undem, 1994; Maggi et al., 1993; Rogers, 1995).
Tachykinin NK3 receptor may increase neuronal activity and responsiveness of target cells. Several electrophysiological studies have reported that tachykinins elicit an important activity on the control of various neuronal and ganglionic potentials at the periphery and, among tachykinins, neurokinin B and stimulation of tachykinin NK3 receptors seems to play a predominant role. This has been suggested by studies showing that substance P and neurokinin B (but not neurokinin A) induced depolarization of guinea-pig bronchial parasympathetic ganglion neurones, and that neurokinin B was 60-fold more potent and five time more efficient than substance P (Myers & Undem, 1993). Neurokinin B and [Asp5,6, metylPhe8]SP(5-11) (a selective agonist for NK3 receptors) induced a decrease in membrane resistance (Myers & Undem, 1993). Interestingly, the capsaicin-evoked slow excitatory postsynaptic potential of guinea-pig bronchial and tracheal parasympathetic ganglion neurons (Myers et al., 1996) and the relaxation of the guinea-pig trachea elicited by antidromic stimulation of capsaicin-sensitive vagal afferent nerves were reduced by SR 142801 (Canning et al., 1998). A similar control of neuronal transmission and reflexes by NK3 receptor has also been evidenced in gastrointestinal tract (Johnson et al., 1996, 1998; Mawe, 1995; Zhao et al., 1995). On the basis of these data, however it is not clear whether peripheral neuronal electric activities mediated by tachykinin NK3 receptors may lead to the modulation of airway hyperresponsiveness to ACh. Hence, the importance of the role of tachykinins in nodose ganglia has been recently strengthened in the airway hyperresponsiveness induced in the guinea-pig (Fischer et al., 1996; Weinreich et al., 1997), but until now no evidence for a role of NK3 at this site of action has been demonstrated.
Finally, the mechanism of the prevention of airway hyperresponsiveness induced either by tachykinin NK3 agonists (the present study), SP (Daoui et al., 1997) or citric acid (Daoui et al., 1998) by SR 48968 and SR 142801 is unclear and suggest the involvement of various physiopathological serial process. For example, a costimulation of NK3+NK2 receptor or NK3+NK1 receptor has been reported for intestinal motility (Croci et al., 1995). An interrelationship between different tachykinins has been also suggested. Indeed, Schmid et al. (1998) showed that NK3 receptors mediate enhancement of substance P release from rat capsaicin-sensitive spinal cord afferent terminals.
In conclusion, the present study demonstrates the potent ability of neurokinin B and tachykinin receptor agonists [MePhe7]NKB and senktide to induce airway hyperresponsiveness and the inhibitory effect of the selective NK3 receptor antagonists (SR 142801) on [MePhe7]NKB-induced hyperresponsiveness in guinea-pigs. For concentrations that induced airway hyperresponsiveness, senktide and [MePhe7]NKB failed to induce bronchoconstriction. Our data suggest that NK3 receptor stimulation can induce airway hyperresponsiveness and this effect is not related to the ability of tachykinins to induce bronchoconstriction.
Abbreviations
- NKA
neurokinin A
- NKB
neurokinin B
- SP
substance P
References
- ADVENIER C., LAGENTE V., BOICHOT E. The role of tachykinin receptor antagonists in the prevention of bronchial hyperresponsiveness, airway inflammation and cough. Eur. Respir. J. 1997;10:1892–1906. doi: 10.1183/09031936.97.10081892. [DOI] [PubMed] [Google Scholar]
- BALUK P., MCDONALD D.M.Proinflammatory peptides in sensory nerves of the airways Proinflammatory and antiinflammatory peptides 1989112New York: Marcel Dekker; 45–87.In: Said SS (ed) [Google Scholar]
- BALUK P., NIGEL W., MCDONALD B., MCDONALD D.M. Localization of tachykinin NK1, NK2, and NK3 receptors in airways by immunohistochemistry. Am. J. Respir. Crit. Care. Med. 1996;153:A161. [Google Scholar]
- BARNES P.J. New concepts in the pathogenesis of bronchial hyperreponsiveness and asthma. J. Allergy Clin. Immunol. 1998;83:1013–1026. doi: 10.1016/0091-6749(89)90441-7. [DOI] [PubMed] [Google Scholar]
- BEAUJOUAN J.C., SAFFFROY M., TORRENS Y., GLOWINSKI J. Potency and selectivity of the tachykinin NK3 receptor antagonist SR 142801. Eur. J. Pharmacol. 1997;319:307–316. doi: 10.1016/s0014-2999(96)00848-5. [DOI] [PubMed] [Google Scholar]
- BELMONTE C., GALLAR J., POZO M.A., REBELLO I. Excitation by irritant chemical substances of sensory afferent units in the cats cornea. J. Physiol. 1990;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOICHOT E., BIYAH K., GERMAIN N., EMONDS-ALT X., LAGENTE V., ADVENIER C. Involvement of tachykinin NK1 and NK2 receptor in substance P-induced microvascular leakage hypersensitivity and airway hyperresponsiveness. Eur. Respir J. 1996;9:1445–1450. doi: 10.1183/09031936.96.09071445. [DOI] [PubMed] [Google Scholar]
- BOICHOT E., GERMAIN N., LAGENTE V., ADVENIER C. Prevention by the tachykinin NK2 receptor antagonist, SR48968, of antigen-induced airway hyperresponsiveness in sensitized guinea-pigs. Br. J. Pharmacol. 1995;114:259–261. doi: 10.1111/j.1476-5381.1995.tb13220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOICHOT E., LAGENTE V., PAUBERT-BRAQUET M., FROSSARD N. Inhaled substance P induces activation of alveolar macrophages and increases airway responses in the guinea-pig. Neuropeptides. 1993;25:307–313. doi: 10.1016/0143-4179(93)90048-f. [DOI] [PubMed] [Google Scholar]
- BOUSHEY H.A., HOLTZMAN M.J., SHELLER J.R., NADEL J.A. Bronchial hyperreactivity. Am. Rev. Respir Dis. 1980;121:389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- CANNING B.J., FISCHER A., UNDEM J. Pharmacological analysis of the tachykinin receptors that mediate activation of nonadrenergic, noncholinergic relaxant nerves that innervate guinea-pig trachealis. J. Pharmacol. Exp. Ther. 1998;284:370–377. [PubMed] [Google Scholar]
- CHAN C.C., TOUSIGNANT C., HO E., BRIDEAU C., SAVOIE C., RODGER I.W. Evaluation of bronchoconstriction by neurokinins and its inhibition by selective nonpeptide antagonists in conscious guinea-pigs, using a double-chamber plethysmograph technique. Can. J. Physiol. Pharmacol. 1994;72:11–18. doi: 10.1139/y94-003. [DOI] [PubMed] [Google Scholar]
- CHEUNG D., VAN DER VEEN H., HARTIGH J.D., DIJKMAN J.H., STERK P.J. Effects of inhaled substance P on airway responsiveness to methacholine in asthmatic subjects in vivo. J. Appl. Physiol. 1994;77:1325–1332. doi: 10.1152/jappl.1994.77.3.1325. [DOI] [PubMed] [Google Scholar]
- CROCI T., LANDI M., EMONDS-ALT X., LE FUR G., MANARA L. Neuronal NK3-receptors in guinea-pig ileum and taenia caeci: in vitro characterization by their first non-peptide antagonist, SR 142801. Life Sci. 1995;57:361–366. doi: 10.1016/0024-3205(95)02211-z. [DOI] [PubMed] [Google Scholar]
- DAOUI S., COGNON C., NALINE E., EMONDS-ALT X., ADVENIER C. Involvement of tachykinin NK3 receptors in citric acid-induced cough and bronchial responses in guinea-pigs. Am. J. Respir. Crit. Care. Med. 1998;158:42–48. doi: 10.1164/ajrccm.158.1.9705052. [DOI] [PubMed] [Google Scholar]
- DAOUI S., CUI Y.Y., LAGENTE V., EMONDS-ALT X., ADVENIER C. A tachykinin NK3 receptor antagonist, SR 142801 (Osanetant), prevents substance P-induced bronchial hyperractivity in guinea-pigs. Pulm. Pharmacol. Ther. 1997;10:261–270. doi: 10.1006/pupt.1998.0104. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J. Pharmacology of nonadrenergic, noncholinergic nerves in airway smooth muscle. Pulm. Pharmacol. 1994;7:205–223. doi: 10.1006/pulp.1994.1024. [DOI] [PubMed] [Google Scholar]
- ELLIS J.L., UNDEM B.J., KAYS J.S., GHANEKAR S.V., BARTHLOW H.G., BUCKNER C.K. Pharmacological examination of receptors mediating contractile responses to tachykinins in airways isolated from human, guinea-pig and hamster. J. Pharmacol. Exp. Ther. 1993;267:95–101. [PubMed] [Google Scholar]
- EMONDS-ALT X., BICHON D., DUCOUX J.P., HEAULME M., MILOUX B., PONCELET M., PROIETTO V., VAN BROECK D., VILAIN P., NELIAT G., SOUBRIÉ P., LE FUR G., BRELIÈRE J.C. SR 142801, the first potent non-peptide antagonist of the tachykinin NK3 receptor. Life Sci. 1995;56:L27–L32. doi: 10.1016/0024-3205(94)00413-m. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH JD., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.F., PROIETTO V., BROECK D.V., SOUBRIÉ P., LE FUR G., BRELIÈRE J.C. In vitro and in vivo biological activities of SR 140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- FISCHER A., MCGREGOR G.P., SARIA A., PHILIPPIN B., KUMMER W. Induction of tachykinin gene and peptide expression in guinea-pig nodose primary afferent neurons by allergic airway inflammation. J. Clin. Invest. 1996;98:2284–2291. doi: 10.1172/JCI119039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX A.J., URBAN L., BARNES P.J., DRAY A. Effects of capsazepine against capsaicin-and proton-evoked excitation of single airway c-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–752. doi: 10.1016/0306-4522(95)00115-y. [DOI] [PubMed] [Google Scholar]
- GEPPETTI P., DELBIANCO E., PATACCHINI R., SANTICIOLI P., MAGGI C.A., TRAMONTANA M. Low pH-induced release of CGRP from capsaicin-sensitive sensory nerves. Mechanism of action and biological response. Neuroscience. 1991;41:295–301. doi: 10.1016/0306-4522(91)90218-d. [DOI] [PubMed] [Google Scholar]
- GIRARD V., YAVO J.C., NALINE, EMONDS-ALT X., ADVENIER C. The tachykinin NK2 receptor antagonist SR 48968 inhibits citric acid-induced airway hyperresponsiveness in guinea-pigs. Am. J. Respir. Crit. Care. Med. 1996;153:1496–1502. doi: 10.1164/ajrccm.153.5.8630592. [DOI] [PubMed] [Google Scholar]
- GUARD S., WATSON S.P. Tachykinin receptor types: classification and membrane signalling mechanisms. Neurochem. Int. 1991;18:149–165. doi: 10.1016/0197-0186(91)90180-l. [DOI] [PubMed] [Google Scholar]
- INOUE H., NAGATA N., KOSHIHARA Y. Involvement of tachykinin receptors in oedema formation and plasma extravasation induced by substance P, neurokinin A, and neurokinin B in mouse ear. Inflamm. Res. 1996;45:316–323. doi: 10.1007/BF02252943. [DOI] [PubMed] [Google Scholar]
- JOHNSON P.J., BORNSTEIN JC., BURCHER E. Roles of neuronal NK1 and NK3 receptors in synaptic transmission during motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 1998;124:1375–1384. doi: 10.1038/sj.bjp.0701967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON P.J., BORNSTEIN J.C., YUAN S.Y., FURNESS J.B. Analysis of contributions of acetylcholine and tachykinins to neuro-neuronal transmission in motility reflexes in the guinea-pig ileum. Br. J. Pharmacol. 1996;118:973–983. doi: 10.1111/j.1476-5381.1996.tb15495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLINGSWORTH C.R., SHORE S.A. Tachykinin receptors mediating contraction of guinea-pig lung strips. Regulatory Peptides. 1995;57:149–161. doi: 10.1016/0167-0115(95)00032-7. [DOI] [PubMed] [Google Scholar]
- KRANEVELD A.D., FOLKERTS G., VAN OOSTERHOUT A.J.M., NIJKAMP F.P. Airway hyperresponsiveness: first eosinophils and then neuropeptides. Int. J. Immunopharmac. 1997;19:517–527. doi: 10.1016/s0192-0561(97)00085-4. [DOI] [PubMed] [Google Scholar]
- KUDLACZ E.M., SHATZER S.A., KNIPPENBERG R.W., LOGAN D.E., POIROT M., VAN GIERSBERGEN P.L.M., BURKHOLDER T.P. In vitro and in vivo characterization of MDL 105,212A, a nonpeptide NK1/NK2 tachykinin receptor antagonist. J. Pharmacol. Exp. Ther. 1996;277:840–851. [PubMed] [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: Integrative aspects on amines, neuropeptides adenosine triphosphate, amino-acids and nitricoxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- LUNDBERG J.M., SARIA A. Polypeptide-containing neurons in airway smooth muscle. Ann. Rev. Physiol. 1987;49:557–572. doi: 10.1146/annurev.ph.49.030187.003013. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinin receptors and airway pathophysiology. Eur. Respir J. 1993;6:735–742. [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., QUARTARA L. Tachykinin receptors in guinea-pig isolated bronchi. Eur. J. Pharmacol. 1991;197:167–174. doi: 10.1016/0014-2999(91)90517-t. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., ROVERO P., GIACHETTI A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- MAREK W., POTTHAST J.J.W., MARCZYNSKI B., BAUR X. Role of substance P and neurokinin A in toluene diisocyanate-induced increased airway responsiveness in rabbits. Lung. 1996;174:83–97. doi: 10.1007/BF00177703. [DOI] [PubMed] [Google Scholar]
- MASSON P., FLUCKIGER J.P., RODGER I.W. Ozone induced airways hyperresponsiveness in conscious unrestrained guinea-pigs: effects of PDE IV inhibitors and neurokinin antagonists. Am. J. Respir. Crit. Care. Med. 1996;153:A627. [Google Scholar]
- MAWE G.M. Tachykinins as mediators of slow EPSPs in guinea-pig gall-bladder ganglia: involvement of neurokinin-3 receptors. J. Physiol. 1995;485:513–524. doi: 10.1113/jphysiol.1995.sp020747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS A., UNDEM B. Electrophysiological effects of tachykinins and capsaicin on guinea-pig bronchial parasympathetic ganglion neurones. J. Physiol. 1993;470:665–679. doi: 10.1113/jphysiol.1993.sp019881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYERS A., UNDEM B., KUMMER W. Anatomical and electrophysiological comparison of the sensory innervation of bronchial and tracheal parasympathetic ganglion neurons. J. Autonom. Nerv. System. 1996;61:162–168. doi: 10.1016/s0165-1838(96)00081-1. [DOI] [PubMed] [Google Scholar]
- NGUYEN-LE X.K., NGUYEN Q.T., GOBEIL F., PHENG L.H., EMONDS-ALT X., BRELIÈRE J.C., REGOLI D. Pharmacological characterization of SR 142801: A new non-peptide antagonist of the neurokinin NK3 receptor. Pharmacology. 1996;52:283–291. doi: 10.1159/000139393. [DOI] [PubMed] [Google Scholar]
- O'BYRNE P.M. Allergen-induced airway hyperresponsiveness. J. Allergy Clin. Immunol. 1988;81:119–127. doi: 10.1016/0091-6749(88)90230-8. [DOI] [PubMed] [Google Scholar]
- OURY-DONAT F., CARAYON P., THURNEYSSEN O., PAILHON V., EMONDS-ALT X., SOUBRIÉ P., LE FUR G. Functional characterization of the nonpeptide neurokinin-3 (NK3) receptor antagonist, SR 142801 on the human NK3 receptor expressed in chinese hamster ovary cells. J. Pharmacol. Exp. Ther. 1995;274:148–164. [PubMed] [Google Scholar]
- PATACCHINI R., BARTHO L., HOLZER P., MAGGI C.A. Activity of SR 142801 at peripheral tachykinin receptors. Eur. J. Pharmacol. 1995;278:17–25. doi: 10.1016/0014-2999(95)00090-8. [DOI] [PubMed] [Google Scholar]
- PERRETTI F., MANZINI S. Activation of capsaicin-sensitive sensory fibers modulates PAF-induced bronchial hyperresponsiveness in anesthetized guinea-pigs. Am. Rev. Respir. Dis. 1993;148:927–931. doi: 10.1164/ajrccm/148.4_Pt_1.927. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BOUDON A., FAUCHÈRE J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–594. [PubMed] [Google Scholar]
- ROCCON A., MARCHIONNI D., NISATO D. Study of SR 142801, a new potent non-peptide NK3 receptor antagonist on cardiovascular responses in conscious guinea-pig. Br. J. Pharmacol. 1996;118:1095–1102. doi: 10.1111/j.1476-5381.1996.tb15511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROGERS D.F. Neurokinin receptors subserving airways secretion. Can. J. Physiol. Pharmacol. 1995;73:932–939. doi: 10.1139/y95-129. [DOI] [PubMed] [Google Scholar]
- SANTING R.E., MEURS H., VAN DER MARK T.W., REMIE R., OOSTEROM W.C., BROUWER F., ZAAGSMA J. A novel method to assess airway function parameters in chronically instrumented, unrestrained guinea-pigs. Pulm. Pharmacol. 1992;5:265–272. doi: 10.1016/0952-0600(92)90069-s. [DOI] [PubMed] [Google Scholar]
- SCHMID G., CARITA F., BONANNO G., RAITERI M. NK3 receptor mediate enhancement of substance P release from capsaicin-sensitive spinal cord afferent terminals. Br. J. Pharmacol. 1998;125:621–626. doi: 10.1038/sj.bjp.0702093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUILING M., ZUIDHOF A.B., ZAAGSMA J., MEURS H. Involvement of tachykinin NK1 receptor in the development of allergen-induced airway hyperreactivity and airway inflammation in conscious, unrestrained guinea-pigs. Am. J. Respir. Crit. Care. Med. 1999;159:423–430. doi: 10.1164/ajrccm.159.2.9804125. [DOI] [PubMed] [Google Scholar]
- SPINA D., PAGE C.P., MORLEY J.Sensory neuropeptides and bronchial hyperresponsiveness Proinflammatory and antiinflammatory peptides 1998New York: Marcel Dekker; 89–145.In: Said SS edVol 112 [Google Scholar]
- STEEN K.H., REEH P.W., ANTON F., HANDWERKER H.O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin in vitro. J. Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA G., SAKAI K., TANIGUCHI Y. Neurokinin A-induced bronchial hyperresponsiveness to metacholine in japanese monkeys. Tohoku. J. Exp. Med. 1989;159:69–73. doi: 10.1620/tjem.159.69. [DOI] [PubMed] [Google Scholar]
- VINCENT F., NALINE E., MOLIMARD M., DAOUI S., VILAIN P., EMONDS-ALT X., ADVENIER C. Hyperresponsiveness to the tachykinin NK1 receptor agonist [Sar9, Met(O2)11]SP after IL-1β pretreatment or passive sensitization of human isolated bronchi: effect of tachykinin NK2 and NK3 receptor antagonists. Am. J. Respir. Crit. Care. Med. 1999;159:A281. [Google Scholar]
- WEINREICH D., KIMBERLY A., MOORE A., TAYLOR G.E. Allergic inflammation in isolated vagal sensory ganglia unmasks silent NK2 tachykinin receptors. J. Neurosci. 1997;17:7683–7693. doi: 10.1523/JNEUROSCI.17-20-07683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDICOMBE J.G. Neurophysiology of the cough reflex. Eur. Respir. J. 1995;8:1193–1202. doi: 10.1183/09031936.95.08071193. [DOI] [PubMed] [Google Scholar]
- YOSHIHARA S., GEPPETTI P., LINDEN A., HARA M., CHAN B., NADEL J.A. Tachykinins mediate the potentiation of antigen-induced bronchoconstriction by cold air in guinea-pigs. J. Allergy Clin. Immunol. 1996;97:756–760. doi: 10.1016/s0091-6749(96)80152-7. [DOI] [PubMed] [Google Scholar]
- YUAN L., BURCHER E., NAIL B.S. Use of selective agonists and antagonists to characterize tachykinin receptors mediating airway responsiveness in anesthetized guinea-pigs. Pulm. pharmacol. 1994;7:169–178. doi: 10.1006/pulp.1994.1020. [DOI] [PubMed] [Google Scholar]
- ZHAO F., SAITO K., YOSHIOKA K., GUO J.Z., MURAKOSHI T., KONISHI S., OTSUKA M. Subtypes of tachykinin receptors on tonic and phasic neurones in coeliac ganglion of the guinea-pig. Br. J. Pharmacol. 1995;115:25–30. doi: 10.1111/j.1476-5381.1995.tb16315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


