Abstract
Serotonin (5-hydroxytryptamine, 5-HT) has been shown to increase cyclic AMP production in dispersed cell aggregates from the major salivary glands of the rat. The goal of the present study was to identify the 5-HT receptor subtypes that mediate these effects in rat submandibular glands (SMG).
Among the 5-HT receptor subtypes identified in the rat, 5-HT4(a,b), 5-HT6 and 5-HT7(a,b,c) activate adenylyl cyclase (AC). We used subtype specific primers to screen rat SMG by reverse transcription-PCR. Results indicate the presence of mRNA for 5-HT4(b) and 5-HT7(a) but not for 5-HT4(a), 5-HT6 and 5-HT7(b,c).
In dispersed SMG cells, 5-carboxyamidotryptamine (5-CT), a 5-HT7 receptor agonist, stimulated cyclic AMP synthesis with higher potency (EC50=27±5 nM) but lower efficacy than 5-HT, suggesting a 5-HT7 component and an additional component in the response to 5-HT. The 5-HT7 contribution was further supported by antagonism of the 5-CT effect by metergoline, a 5-HT7 antagonist, which exhibited an affinity (Ki=50 nM) similar to that obtained at the cloned 5-HT7 receptor.
In the presence of a maximally effective concentration of 5-CT, 5-HT produced an additional increase in cyclic AMP production that was inhibited by the 5-HT4 receptor antagonist, GR113808, suggesting that the second component of cyclic AMP production is mediated by 5-HT4 receptors.
These findings indicate the presence in rat SMG of both 5-HT4(b) and 5-HT7(a) receptors positively coupled to AC.
Keywords: Adenylyl cyclase, 5-hydroxytryptamine receptors, submandibular gland
Introduction
Since the discovery of 5-HT more than 50 years ago, its actions have been well studied in many organ systems, including the gastrointestinal tract, the vasculature and the central nervous system. As currently understood, four effector systems are primarily associated with the known 5-HT receptor subtypes (Hoyer & Martin, 1996). 5-HT1A,B,D,E,F receptors are negatively-coupled to AC (via Gi); 5-HT2A,B,C are positively-coupled to phospholipase C (via Gq); 5-HT3 is a ligand-gated ion channel; and 5-HT4,6,7 are positively coupled to AC (via Gs). The effector mechanisms for 5-HT5A,B have yet to be established. Physiological effects in response to the activation of the 5-HT4,6,7 subtypes include smooth muscle relaxation (via stimulation of cyclic AMP production), smooth muscle contraction (via intracellular calcium mobilization or indirect release of acetylcholine), stimulation of chloride secretion in the rat distal colon, hormonal secretion from the adrenal cortex and modulation of cardiac function (Boess & Martin, 1994).
Our laboratory has reported that, in the isolated perfused rat SMG, 5-HT decreases acetylcholine-induced salivary flow and markedly increases saliva protein content (Turner et al., 1996). These functional effects were accompanied by an increase in cyclic AMP production in dispersed cell aggregates from the rat SMG in response to 5-HT, suggesting that 5-HT receptors positively coupled to AC are expressed in this organ. In this report, we describe results from molecular and pharmacological experiments that indicate the presence of two AC-activating 5-HT receptor subtypes in the rat SMG.
Methods
Reagents
5-HT, isobutylmethylxanthine, trichloroacetic acid, and buffer reagents were obtained from Sigma Chemical Company (St. Louis, MO, U.S.A.). [3H]-adenine (29 Ci mmol−1) was purchased from Dupont NEN (Wilmington, DE, U.S.A.). Metergoline phenylmethylester was obtained from Tocris (Ballwin, MO, U.S.A.). FSK and 5-CT were purchased from Research Biochemicals International (Natick, MA, U.S.A.). GR113808 was a generous gift provided by Glaxo Research Group (Middlesex, U.K.).
RT–PCR
Total RNA was isolated from rat SMG and whole rat brain to investigate 5-HT receptor subtype expression via RT–PCR. Rat brain was used as the positive control tissue for 5-HT4, 5-HT6 and 5-HT7. Total RNA was prepared from 30 mg of fresh SMG or brain tissue using an RNeasy kit (Qiagen Inc., Chatsworth, CA, U.S.A.) according to the manufacturer's protocol. Purified total RNA was treated with DNase (Boehringer Mannheim, Indianapolis, IN, U.S.A.) for 15 min at 37°C, followed by RNeasy cleanup. cDNA was synthesized from 1 μg of DNase-treated total RNA using the Advantage RT-for-PCR kit (Clontech, Palo Alto, CA, U.S.A.) according to the manufacturer's protocol. Twenty per cent of the cDNA reaction product was used for PCR as described previously (Park et al., 1997). Receptor-specific oligonucleotide primers were synthesized at the University of Missouri DNA Core Facility according to the published 5-HT4(a) and 5-HT4(b) (Gerald et al., 1995), 5-HT6 (Ruat et al., 1993a), and 5-HT7(a,b,c) (Heidmann et al., 1998; Ruat et al., 1993b) sequences (GenBank accession numbers U20906, U20907, L19656, L19654, U68489, and U68490 respectively). Primer sequences (5′-to-3′), nucleotide positions relative to the initiation codon and predicted PCR product sizes (base pairs, bp) are described in Table 1.
Table 1.
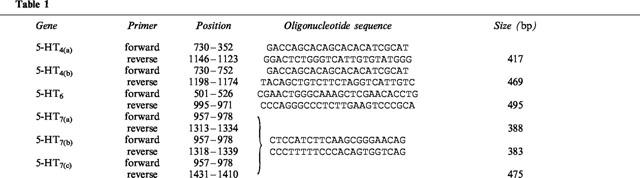
Due to the nature of the splice variations of the 5-HT7 receptor gene, the primer set shown in Table 1 would be expected to yield one or more of three different products that differ in size and carboxyterminal sequence. PCR products obtained with all primer sets were resolved by electrophoresis on 2% agarose gels, visualized with UV light, and purified using a Wizard PCR Prep DNA kit (Promega, Madison, WI, U.S.A.). The purified products were sequenced by the University of Missouri DNA Core Facility using an ABI Prism automated DNA sequencer. Oligonucleotide primers for sequencing were the same as those used in PCR. Sequence comparisons were performed using the Wisconsin Package software (Genetics Computer Group, Madison, WI, U.S.A.).
Cyclic AMP production
SMG dispersed cell aggregates were prepared from anaesthetized (sodium pentobarbitone) adult male Sprague-Dawley rats (175–225 g) as described previously (Turner & Camden, 1990). The animal use protocol for these studies has been reviewed and approved by the University of Missouri Animal Care and Use Committee. Briefly, SMG were minced and placed in 20 ml of Dulbecco's modified Eagle's medium-Ham's F12 (DMEM-F12) containing 0.2 mM CaCl2, 50 units ml−1 collagenase, 400 units ml−1 hyaluronidase and 1% (w v−1) BSA, for 40 min at 37°C. The cells were further dispersed by passage through a 10 ml transfer pipette at 20, 30 and 40 min. Dispersed cell aggregates were then washed (centrifuged at 200×g for 2 s) with buffer A [in mM: NaCl 120, KCl 4, KH2PO4 1.2, MgSO4 1.2, CaCl2 1, glucose 10, and HEPES 15 (pH 7.4)] containing 1% (w v−1) BSA, then resuspended in 20 ml of buffer A containing 0.1% BSA (Buffer B). Cyclic AMP production in the cell aggregates was assessed by the [3H]-adenine pre-loading procedure, as described previously (Turner et al., 1996). Dispersed cell aggregates were incubated with 5 μCi ml−1 [3H]-adenine for 30 min at 37°C, washed, pelleted and resuspended in 3 ml of ice cold buffer B. The 5 min stimulation step was initiated by the addition of 50 μl of the cell aggregate suspension to test tubes containing agonist (± antagonist) and 1 μM FSK in a final volume of 500 μl of buffer B. FSK was included throughout because the magnitude of the cyclic AMP responses to 5-HT receptor agonists is synergized by inclusion of 1 μM FSK without affecting the potency of the agonists (Turner et al., 1996). The stimulation was terminated by addition of 25 μl of 100% trichloroacetic acid. The tubes were centrifuged (5 min at 1800×g) and the supernatants were passed over Dowex and alumina columns, as described previously (Turner & Camden, 1990). The collected [3H]-ATP and [3H]-cyclic AMP fractions were quantitated on a Beckman LS5000TD liquid scintillation counter. Data are expressed as per cent [3H]-ATP to [3H]-cyclic AMP conversion:
Data analysis
Data from the cyclic AMP experiments are reported as the mean±s.e. mean. Agonist potencies (expressed as EC50 values) were calculated using a nonlinear curve fitting procedure (GraphPad Prism, San Diego, CA, U.S.A.) based on the sigmoidal concentration-response relationship with an unconstrained slope. Antagonist affinity (Ki) was determined via Schild analysis.
Results
Molecular identification of 5-HT receptor subtype-specific mRNA
Among the identified 5-HT receptor subtypes, 5-HT4, 5-HT6 and 5-HT7 have been shown to be positively coupled to AC (Hoyer & Martin, 1996; Lucas & Hen, 1995). We thus made the initial assumption that 5-HT-induced cyclic AMP formation in the rat SMG is through one or more of these known receptor subtypes, with a novel 5-HT receptor being an alternative explanation. Our first approach therefore was to use RT–PCR with subtype specific oligonucleotide primers to screen rat SMG mRNA for expression of 5-HT4, 5-HT6 and 5-HT7 receptor messages. The results shown in Figure 1 indicate that rat SMG expresses mRNA for the 5-HT4(b) and 5-HT7(a) receptors. No cDNA product was obtained when primers for the short form of 50HT4, 5-HT4(a) and 5-HT6 were used, although these latter two primer sets yielded products from the positive control tissues. No product of the appropriate size for the 5-HT7(c) receptor mRNA was obtained and sequencing of the 5-HT7 PCR product gave no indication of the presence of the 5-HT7(b) splice variant. Nucleotide sequence comparisons between the RT–PCR products generated from SMG mRNA and published nucleotide cDNA sequences demonstrated 100% identity with both the 5-HT4(b) and 5-HT7(a) receptors, suggesting that these two 5-HT receptor subtypes are expressed in rat SMG.
Figure 1.
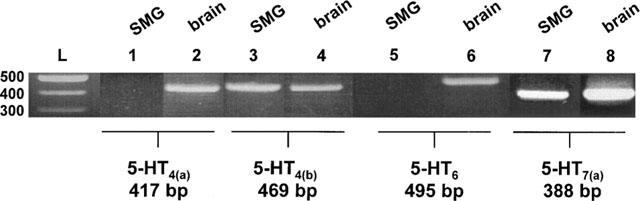
RT–PCR analysis of 5-HT receptor subtype mRNA expression in the rat SMG. 5-HT receptor subtype-specific oligonucleotide primers were designed and used in the RT–PCR as described in Methods. Migration of 500, 400 and 300 base pair (bp) ladder bands (L) in 2% agarose gel electrophoresis are shown on the left and labels above each lane indicate the cDNA source. Primers used and size of product obtained are indicated below each band. Absence of genomic DNA contamination in the cDNA samples was verified by PCR without prior incubation with reverse transcriptase.
Pharmacological characterization of 5-HT induced cyclic AMP production
Functional studies in SMG dispersed cell aggregates were performed to confirm the molecular data. As shown in Figure 2, cyclic AMP production increased in dispersed cell aggregates upon treatment with both 5-HT, as reported previously (Turner et al., 1996), and 5-CT, a 5-HT7 receptor-selective (with respect to 5-HT4) full agonist with nM affinity (Markstein et al., 1999; Tsou et al., 1994; Plassat et al., 1993; Ruat et al., 1993b). 5-CT (EC50=27±5 nM) was more potent than 5-HT (EC50=700±100 nM) but was less efficacious, producing a maximal response that was only about 50% of that elicited by 5-HT. This pharmacological profile, indicative of co-expression of the 5-HT4 and 5-HT7 receptors, has been observed recently in studies with rat brain (Markstein et al., 1999). These data suggest two components of the cyclic AMP response to 5-HT in rat SMG: one component that is mediated by 5-HT7(a) receptors and mimicked with 5-CT and a second, non-5-HT7(a) component that is not sensitive to 5-CT and which, based on the RT–PCR results, would be predicted to be mediated by 5-HT4(b) receptors.
Figure 2.
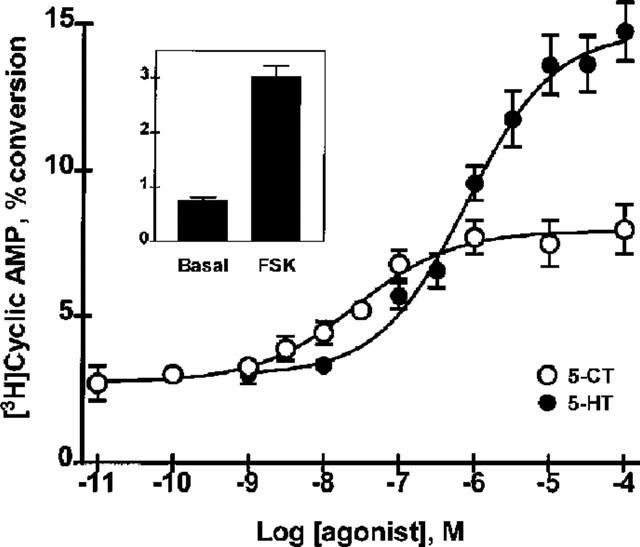
Concentration-dependent effects of 5-CT and 5-HT on cyclic AMP production in rat SMG dispersed cell aggregates. Production of cyclic AMP in response to 5-HT receptor agonists (5 min incubation) was measured by the [3H]-adenine loading procedure as outlined in Methods. EC50 values for the 5-CT and 5-HT concentration-response curves were 27±5 and 700±100 nM, respectively. Per cent conversion values of basal and 1 μM FSK-treated alone (inset) were 0.8±0.1 and 3.1±0.2 respectively (n=22). Each concentration-response curve is the mean±s.e. mean of at least four experiments in the presence of 1 μM FSK.
To support the contention that 5-HT7(a) receptors are responsible for the cyclic AMP response to 5-CT, we performed a Schild analysis on 5-CT-stimulated cyclic AMP production using the 5-HT7 antagonist, metergoline phenylmethylester (Plassat et al., 1993; Ruat et al., 1993b). As shown in Figure 3A, metergoline, present at fixed concentrations of 50, 100, 250 and 500 nM, shifted the 5-CT concentration-response curve (EC50=20±4 nM) dextrally to yield EC50 values of 44±17 nM, 99±21 nM, 1.1±0.2 μM and 5±2 μM, respectively. Metergoline had no effect on FSK-stimulated cyclic AMP production (Figure 3B). The calculated Ki value for metergoline was 50 nM (Figure 3C), in agreement with published values for this antagonist at the cloned and expressed 5-HT7 receptor (Plassat et al., 1993; Ruat et al., 1993b). Methiothepin, another 5-HT7-selective (relative to 5-HT4) antagonist, inhibited the 5-HT receptor-stimulated response with a potency (data not shown) in agreement with earlier reports (Tsou et al., 1994). We also observed that the 5-HT4-selective antagonist, GR113808, when used at concentrations up to 10 μM, did not inhibit the 5-CT effect (data not shown), whereas GR113808 was found to be effective against the 5-CT-independent component of the 5-HT effect (see below).
Figure 3.
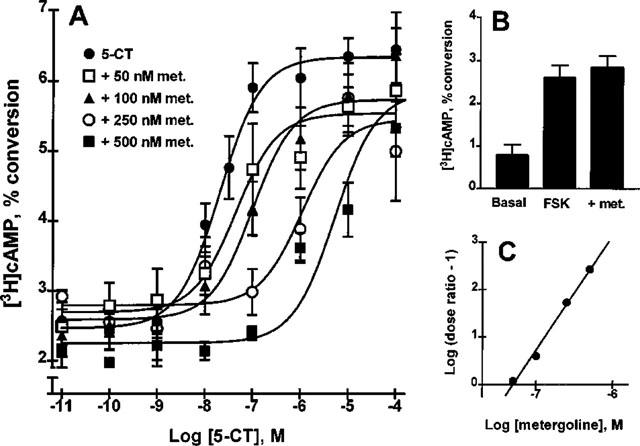
Effect of 5-HT7 antagonist, metergoline, on 5-CT-stimulated cyclic AMP production. (A) 5-CT concentration-responses displayed a rightward shift in EC50 when metergoline, a 5-HT antagonist selective for 5-HT7 vs 5-HT4 receptors, was added to SMG dispersed cell aggregates at concentrations of 50, 100, 250 or 500 nM. (B) Treatment of the dispersed cell aggregates with 500 nM metergoline alone had no effect on the 1 μM FSK-stimulated response. (C) A Schild plot of the data in Figure 3A yielded a Ki value for metergoline of 50 nM. Data are the mean±s.e. mean of at least four replicate experiments.
As demonstrated in Figure 2, the 5-CT concentration-dependent increase in cyclic AMP production exhibited a maximal plateau at a concentration of 1 μM. An additional increase in cyclic AMP production was observed in response to 5-HT (EC50=730±90 nM) in the presence of 1 μM 5-CT, (Figure 4A), further supporting the presence of two components of cyclic AMP production. This second, 5-HT-induced component of cyclic AMP production was inhibited by GR113808 (Figure 4B), a 5-HT4-selective antagonist (Saxena, 1995; Grossman et al., 1993). Taken together, these data indicate that the cyclic AMP response to 5-HT in rat SMG is due to activation of both 5-HT4(b) and 5-HT7(a) receptors.
Figure 4.
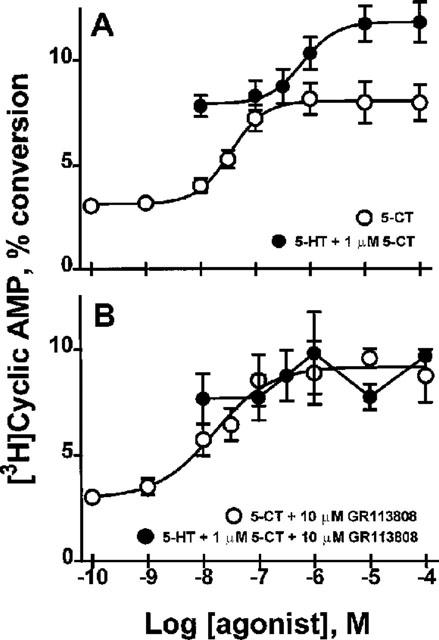
Two components of 5-HT-stimulated cyclic AMP production. 5-HT-stimulated cyclic AMP production was characterized as either 5-CT-dependent or 5-CT-independent. (A) The high potency component of this two-component response was induced by 5-CT (0.1 nM–100 μM, EC50=35±3 nM) and reached a maximum at 1 μM. With 1 μM 5-CT present, the low potency component was elicited with 5-HT (0.01–100 μM, EC50=730±90 nM. (B) This two component concentration-response study was repeated in the presence of a 5-HT4-selective antagonist, GR113808 (10 μM), in which the 5-CT-dependent component (EC50=16±5 nM) was insensitive to GR113808, while the 5-CT-independent response was attenuated by GR113808. (A) and (B) are the mean±s.e.mean of at least four replicate experiments each.
Discussion
The key finding of this study is that the rat SMG expresses two 5-HT receptor subtypes, 5-HT4(b) and 5-HT7(a), both of which are coupled to increased cyclic AMP production. This finding extends our previous observations (Turner et al., 1996) of 5-HT-induced elevations of cyclic AMP levels in the submandibular, sublingual and parotid salivary glands of the rat and of modulation by 5-HT of SMG saliva flow and protein content.
Both of the 5-HT receptor subtypes identified in rat SMG have been characterized (Eglen et al., 1997; Hegde & Eglen, 1996). The 5-HT7 receptor cDNA was cloned from rat brain (Ruat et al., 1993b) as well as the rat proximal convoluted tubule (Shen et al., 1993). In addition, splice variants of the rat and human receptors have been identified (Heidmann et al., 1997; 1998; Eglen et al., 1997; Stam et al., 1997; Jasper et al., 1997). The pharmacological profile of this receptor subtype includes the observation by several groups that 5-CT is a full agonist with high potency and selectivity with respect to 5-HT4 (Plassat et al., 1993; Ruat et al., 1993b). In addition, 5-HT antagonists (e.g., metergoline and methiothepin) with selectivity for 5-HT7 receptors vs 5-HT4 receptors, have been used to study the tissue distribution of the 5-HT7 subtype (Eglen et al., 1997). The observation that 5-CT stimulates cyclic AMP production with high potency (Figure 2) and that this stimulation is blocked by metergoline (Figure 3) complements the RT–PCR data (Figure 1) to indicate that functional 5-HT7(a) receptors are expressed in the rat SMG.
The 5-HT4 receptor, initially characterized pharmacologically (Dumuis et al., 1988), was subsequently cloned, expressed and further characterized (Gerald et al., 1995). Two 5-HT4 receptor splice variants, 5-HT4(a) and 5-HT4(b) for short and long forms, respectively, were found (Gerald et al., 1995). Pharmacological characteristics of the 5-HT4 receptor include a relatively low potency of 5-HT compared with other subtypes (Clarke et al., 1989) and high selectivity of the antagonist, GR113808 (Hegde & Eglen, 1996). The 5-CT-independent component of 5-HT-stimulated cyclic AMP production in rat SMG exhibits both of these characteristics and, combined with the RT–PCR results (Figure 1), strongly suggests that the 5-CT-independent component of cyclic AMP production is mediated by 5-HT4(b) receptors. Together, these results indicate that the activity of these two receptor subtypes can account for the entire increase in cyclic AMP production in response to 5-HT.
As reviewed previously (Eglen et al., 1997; Hegde & Eglen, 1996; Boess & Martin, 1994), the 5-HT7 receptor is expressed in various regions of the brain (Heidmann et al., 1998). In the guinea-pig, 5-HT7 receptors are coupled to ileal relaxation (Carter et al., 1995). In addition, evidence suggests that this subtype may be involved in the regulation of vascular tone (Terron, 1997), γ-aminobutyric acid-mediated neurotransmission (Kawahara et al., 1994) and tachycardia (Villalon et al., 1997). 5-HT4 receptors are abundantly expressed in the central nervous system and in the periphery (Hegde & Eglen, 1996). In smooth muscle, 5-HT4 receptor activation has been shown to either induce relaxation or constriction in the rat oesophagus or guinea-pig ileum, respectively. In addition, 5-HT4 receptors stimulate chloride secretion in the rat colon, enhance hormone release from frog adrenocortical cells and produce tachycardia in a porcine model (Boess & Martin, 1994).
Definitive in vivo studies demonstrating a physiological role for 5-HT on mammalian salivary gland function have not been performed, although previous reports have suggested roles for 5-HT in the regulation of the salivary glands of the blowfly (Calliphora erythrocephala, Berridge & Heslop, 1981) and the rat (Turner et al., 1996; Chernick et al., 1989). There is abundant evidence that cyclic AMP-mobilizing agonists have key roles in modulating saliva volume and content, particularly protein content. Norepinephrine, through the activation of β-adrenergic receptors, has the predominant role in modulating salivary secretion through regulation of cyclic AMP levels (Turner & Camden, 1990). However, agonists at other AC-coupled receptors, including vasoactive intestinal peptide (VIP, Turner & Camden, 1990) and prostanoids (Martinez et al., 1987), are also important in the regulation of salivary gland function. It is worth noting that, while less effective than β-adrenergic receptor agonists in stimulating cyclic AMP production in the salivary glands (Turner et al., 1996), 5-HT (Figure 2) is as effective as VIP (Turner & Camden, 1990) and the prostanoids (Martinez et al., 1987), supporting the idea that 5-HT may also be important in salivary gland regulation. A key extension of the current studies is to define the precise, and perhaps distinct, roles that 5-HT4(b) and 5-HT7(a) receptors play in salivary gland physiology. Ongoing studies are focused on defining the distribution of the 5-HT4(b) and 5-HT7(a) receptor subtypes among the various cell types within the gland, identifying the endogenous sources of the 5-HT involved in the activation of these receptors, and assessing the effects of subtype-specific antagonists on salivary function in vivo. The list of agents shown to be involved in the regulation of salivary gland function has increased steadily in recent years. The identification of two AC-coupled 5-HT receptor subtypes in rat SMG suggests that this list may eventually include 5-HT.
Acknowledgments
This work was supported by National Institute for Dental and Craniofacial Research grant DE07389.
Abbreviations
- 5-CT
5-carboxyamidotryptamine
- FSK
forskolin
- SMG
submandibular gland
References
- BERRIDGE M.J., HESLOP J.P. Separate 5-hydroxytryptamine receptors on the salivary gland of the blowfly are linked to the generation of either cyclic adenosine 3′,5′-monophosphate or calcium signals. Br. J. Pharmacol. 1981;73:729–738. doi: 10.1111/j.1476-5381.1981.tb16809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- CARTER D., CHAMPNEY M., HWANG B., EGLEN R.M. Characterization of a postjunctional 5-HT receptor mediating relaxation of guinea-pig isolated ileum. Eur. J. Pharmacol. 1995;280:243–250. doi: 10.1016/0014-2999(95)00195-q. [DOI] [PubMed] [Google Scholar]
- CHERNICK W., BOBYOCK E., BRADFORD P. 5-Hydroxytryptamine modulation of rat parotid salivary gland secretion. J. Dent. Res. 1989;68:59–63. doi: 10.1177/00220345890680010901. [DOI] [PubMed] [Google Scholar]
- CLARKE D.E., CRAIG D.A., FOZARD J.R. The 5-HT4 receptor: naughty, but nice. Trends Pharmacol. Sci. 1989;10:385–386. doi: 10.1016/0165-6147(89)90177-6. [DOI] [PubMed] [Google Scholar]
- DUMUIS A., BOUHELAL R., SEBBEN M., CORY R., BOCKAERT J. A nonclassical 5-hydroxytryptamine receptor positively coupled with adenylate cyclase in the central nervous system. Mol. Pharmacol. 1988;34:880–887. [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. Trends Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- GERALD C., ADHAM N., KAO H.T., OLSEN M.A., LAZ T.M., SCHECHTER L.E., BARD J.A., VAYSSE P.J., HARTIG P.R., BRANCHEK T.A., WEINSHANK R.L. The 5-HT4 receptor: molecular cloning and pharmacological characterization of two splice variants. EMBO J. 1995;14:2806–2815. doi: 10.1002/j.1460-2075.1995.tb07280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSMAN C.J., KILPATRICK G.J., BUNCE K.T. Development of a radioligand binding assay for 5-HT4 receptors in guinea-pig and rat brain. Br. J. Pharmacol. 1993;109:618–624. doi: 10.1111/j.1476-5381.1993.tb13617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGDE S.S., EGLEN R.M. Peripheral 5-HT4 receptors. FASEB J. 1996;10:1398–1407. doi: 10.1096/fasebj.10.12.8903510. [DOI] [PubMed] [Google Scholar]
- HEIDMANN D.E., METCALF M.A., KOHEN R., HAMBLIN M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- HEIDMANN D.E., SZOT P., KOHEN R., HAMBLIN M.W. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology. 1998;37:1621–1632. doi: 10.1016/s0028-3908(98)00070-7. [DOI] [PubMed] [Google Scholar]
- HOYER D., MARTIN G.R. Classification and nomenclature of 5-HT receptors: a comment on current issues. Behav. Brain. Res. 1996;73:263–268. doi: 10.1016/0166-4328(96)00109-x. [DOI] [PubMed] [Google Scholar]
- JASPER J.R., KOSAKA A., TO Z.P., CHANG D.J., EGLEN R.M. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7b) Br. J. Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAHARA F., SAITO H., KATSUKI H. Inhibition by 5-HT7 receptor stimulation of GABAA receptor-activated current in cultured rat suprachiasmatic neurones. J. Physiol. (Lond) 1994;478:67–73. doi: 10.1113/jphysiol.1994.sp020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCAS J.J., HEN R. New players in the 5-HT receptor field: genes and knockouts. Trends Pharmacol. Sci. 1995;16:246–252. doi: 10.1016/s0165-6147(00)89034-3. [DOI] [PubMed] [Google Scholar]
- MARKSTEIN R., MATSUMOTO M., KOHLER C., TOGASHI H., YOSHIOKA M., HOYER D. Pharmacological characterisation of 5-HT receptors positively coupled to adenylyl cyclase in the rat hippocampus. Naunyn Schmiedebergs' Arch. Pharmacol. 1999;359:454–459. doi: 10.1007/pl00005375. [DOI] [PubMed] [Google Scholar]
- MARTINEZ J.R., CASSITY N., BARKER S. Differential effects of prostaglandins and isoproterenol on cAMP content and Na, K pump activity in rat submandibular acini. Experientia. 1987;43:1013–1015. doi: 10.1007/BF01952223. [DOI] [PubMed] [Google Scholar]
- PARK M.K., GARRAD R.C., WEISMAN G.A., TURNER J.T. Changes in P2Y1 nucleotide receptor activity during the development of rat salivary glands. Am. J. Physiol. 1997;272:C1388–C1393. doi: 10.1152/ajpcell.1997.272.4.C1388. [DOI] [PubMed] [Google Scholar]
- PLASSAT J.L., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., ARRANGE J.M., TARDIVEL-LACOMBE J., DIAZ J., LEURS R., SCHWARTZ J.C. A novel rat serotonin (5-HT6) receptor: molecular cloning, localization and stimulation of cAMP accumulation. Biochem. Biophys. Res. Commun. 1993a;193:268–276. doi: 10.1006/bbrc.1993.1619. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.M., SCHWARTZ J.C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAXENA P.R. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol. Ther. 1995;66:339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J.J., METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- STAM N.J., ROESINK C., DIJCKS F., GARRITSEN A., VAN HERPEN A., OLIJVE W. Human serotonin 5-HT7 receptor: cloning and pharmacological characterisation of two receptor variants. FEBS Lett. 1997;413:489–494. doi: 10.1016/s0014-5793(97)00964-2. [DOI] [PubMed] [Google Scholar]
- TERRON J.A. Role of 5-HT7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br. J. Pharmacol. 1997;121:563–571. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU A.P., KOSAKA A., BACH C., ZUPPAN P., YEE C., TOM L., ALVAREZ R., RAMSEY S., BONHAUS D.W., STEFANICH E., JAKEMAN L.B., EGLEN R.M., CHANG H.W. Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylyl cyclase. J. Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- TURNER J.T., CAMDEN J.M. The influence of vasoactive intestinal peptide receptors in dispersed acini from rat submandibular gland on cyclic AMP production and mucin release. Arch. Oral. Biol. 1990;35:103–108. doi: 10.1016/0003-9969(90)90170-f. [DOI] [PubMed] [Google Scholar]
- TURNER J.T., SULLIVAN D.M., ROVIRA I., CAMDEN J.M. A regulatory role in mammalian salivary glands for 5-hydroxytryptamine receptors coupled to increased cyclic AMP production. J. Dent. Res. 1996;75:935–941. doi: 10.1177/00220345960750031101. [DOI] [PubMed] [Google Scholar]
- VILLALON C.M., HEILIGERS J.P., CENTURION D., DE VRIES P., SAXENA P.R. Characterization of putative 5-HT7 receptors mediating tachycardia in the cat. Br. J. Pharmacol. 1997;121:1187–1195. doi: 10.1038/sj.bjp.0701260. [DOI] [PMC free article] [PubMed] [Google Scholar]


