Abstract
GABAB receptors influencing vagal pathways to the lower oesophageal sphincter and heart were investigated.
In urethane-anaesthetized ferrets, the GABAB agonist baclofen (7 μmol kg−1 i.v.) increased basal lower oesophageal sphincter (LOS) pressure. This was reversed by antagonism with CGP35348 (100 μmol kg−1 i.v.). Baclofen's effect was abolished by vagotomy, suggesting a central action, yet it was ineffective when given centrally (3–6 nmol i.c.v.).
Peripheral vagal stimulation (10 Hz, 5 s duration) caused LOS inhibition, followed by excitation, then prolonged inhibition. Bradycardia was also evoked during stimulation. Bradycardia and LOS responses were abolished after chronic supranodose vagotomy, indicating that they were due to stimulation of vagal pre-ganglionic neurones, not antidromic stimulation of afferents.
Baclofen (1–10 μmol kg−1) reduced bradycardia and enhanced LOS excitation, which was also seen in animals pretreated with atropine (400 μg kg−1 i.v.) and guanethidine (5 mg kg−1 i.v.), but not in those pretreated with L-NAME (100 mg kg−1 i.v.).
Effects of baclofen (7 μmol kg−1 i.v.) on vagal stimulation-induced LOS and cardiac responses were unchanged by the GABAB antagonists CGP35348 or CGP36742 (up to 112 μmol kg−1 i.v.), but were reversed by CGP62349 (ED50 37 nmol kg−1 i.v.) or CGP54626 (ED50 100 nmol kg−1 i.v.).
Responses of isolated LOS strips to electrical stimulation, capsaicin, NK-1, NK-2 and nicotinic receptor agonists were all unaffected by baclofen (⩽200 μ M).
We conclude that baclofen reduces vagal output at two peripheral sites: one presynaptically on pre-ganglionic neurones (CGP35348-insensitive), and another (CGP35348-sensitive) that could not be identified. This demonstrates heterogeneity of GABAB receptors through differential sensitivity to antagonists.
Keywords: Lower oesophageal sphincter, heart rate, vagus nerve, non-adrenergic non-cholinergic nerves, GABAB receptors
Introduction
γ-Amino butyric acid (GABA) is the major inhibitory transmitter in the central nervous system, for which there are three major subtypes of receptor: A, B and C. GABAB receptors are coupled via G-proteins and are present at many sites within the central and peripheral nervous systems. In particular, they are abundant pre-synaptically on vagal afferent terminals in the dorsal medulla oblongata (Bowery & Pratt, 1992), although they also mediate slow post-synaptic inhibition in the nucleus tractus solitarius (Brooks et al., 1992). GABAA and GABAC receptors, on the other hand are located mainly post-synaptically, and are coupled directly to chloride channels. GABAA receptors are found both peripherally and centrally (Ashworth Preece et al., 1997; Bowery et al., 1984), whereas GABAC receptors are found mainly in the retina (Pan & Lipton, 1995). GABAB receptor agonists have been shown to exert inhibitory effects on transmitter release in vagal nuclei (Brooks et al., 1992), and thereby to inhibit respiratory (Trippenbach, 1995), cardiovascular (Silva Carvalho et al., 1995) and gastrointestinal (Andrews et al., 1987) vagal reflexes. Peripheral effects on transmitter release from extrinsic nerves have also been shown in the airways (Belvisi et al., 1989), and from intrinsic nerves in the small intestine (Kleinrok & Kilbinger, 1983). The latter has become a useful tool in determining the activity of compounds at the GABAB receptor (Ong et al., 1994).
Our group has recently demonstrated that GABAB receptor agonists powerfully inhibit transient relaxations of the lower oesophageal sphincter (LOS) in conscious ferrets (Blackshaw et al., 1999). Transient LOS relaxations are the primary cause of gastro-oesophageal acid reflux in humans and other species including ferrets (Blackshaw et al., 1998; Mittal et al., 1995), and are therefore a pivotal target in development of new therapeutic strategies for gastro-oesophageal reflux disease. One of the barriers to further understanding of the mechanism of transient LOS relaxations is their absence in anaesthetized animals. It is believed that transient LOS relaxations are mediated via a vagal pathway and co-ordinated with other events by a central pattern generator (Mittal et al., 1995). We aimed to investigate GABAB receptors in this system by analysis of individual elements of the vagal pathway thought to be involved in transient LOS relaxations. This was undertaken by drug intervention at different points in the peripheral and central motor pathways. These points were: in the brainstem, peripherally at a presynaptic site on the vagal motor outflow, and postsynaptically in the enteric nervous system. The use of the ferret model in all of these areas has recently been established in our laboratory (Blackshaw et al., 1997; Lingenfelser et al., 1997; Smid et al., 1998a,1998b).
Methods
General surgical procedures
In vivo experiments were performed on 97 male and female ferrets (weight range 0.5–1.1 kg) anaesthetized with a single intraperitoneal dose of Urethane (1.5 g kg−1 i.p.; n=49), or sodium pentobarbitone (50 mg kg−1 i.p.; n=48). A sufficient depth of anaesthesia was checked by regular confirmation of abolition of the hindlimb pinch-withdrawal reflex. Prior to experiments ferrets were fed a standard carnivore diet with free access to water but were deprived of food for 18 h. A tracheal cannula was inserted and the right jugular vein cannulated for administration of antagonist drugs and further anaesthetic as required to maintain depth of anaesthesia. In half the experiments the vagi were kept intact, and in the other half they were dissected free of the carotid arteries, ligated and sectioned at the beginning of experiments. The left or right vagus was placed over a pair of platinum stimulating electrodes during subsequent stimulation. At other times, the nerves were kept in their original position in situ and moistened with warm isotonic NaCl. The left carotid artery was cannulated for monitoring of systemic blood pressure, which remained above 100 mmHg (mean) for the duration of experiments. In twenty experiments a 0.50 mm o.d. intracerebroventricular catheter was introduced via a small hole in the atlanto-occipital membrane and fixed with cyanoacrylate adhesive so that the tip lay at the opening of the 4th ventricle. This was used for central application of saline, baclofen and CGP35348. The position of the catheter was verified by dissection post mortem and its patency by aspiration of minute amounts of cerebrospinal fluid. Rectal temperature was maintained at 38±0.5°C with a warming pad. Animals were killed at the end of the experiment with an overdose of urethane or sodium pentobarbitone.
Manometric technique
A 5-lumen micromanometric assembly was fed down the oesophagus via the mouth and positioned during abdominal surgery so that the tip lay within the stomach. The manometric assembly consisted of a gastric pressure monitoring port, an oesophageal pressure port, and an oesophageal drainage channel to drain accumulated perfusion fluid. LOS pressure was recorded by a miniaturized sleeve sensor 2.5 cm long×1.4 mm wide. Total diameter of the assembly was 2.5–3 mm. The sleeve sensor was positioned astride the LOS by a slow pull-through from within the stomach so that the high pressure zone was monitored and adjacent side-holes recorded gastric and oesophageal body pressures. In this position, the midpoint of the sleeve lay between 17 and 22 cm from the incisors. Catheter location was confirmed post mortem. Manometric channels of the assembly were perfused with degassed distilled water at 0.1 ml min−1 per channel, which was sufficient for accurate recordings of intraluminal pressures (Blackshaw et al., 1997). For continuous drainage of manometric assembly perfusion fluid a cannula (2.5 mm OD) was inserted into the gastric corpus via the pylorus after a small laparotomy. Intraluminal and arterial pressures were displayed on an 8-channel Rikadenki RK8 chart recorder. Manometric signals were also fed into an Apple Macintosh computer fitted with a NBMIO16 A-D card (National Instruments), digitized at 0.1 s intervals and stored on hard disk. Data acquisition and subsequent off-line analysis of motility data was performed with MAD software (Royal Adelaide Hospital/C. Malbert), based on National Instruments' LabView.
Experimental protocols
General procedures
Initially, before assessment of responses or baseline parameters, at least 10 min were allowed for any reflexes triggered by placement of the manometric assembly to subside. An equilibration period of 5–15 min was allowed after each stimulus, or after administration of a treatment, in order that a steady basal level of sphincter pressure was reached before further tests, unless the acute effects of the treatment were under investigation. The different series of experiments described below were performed in different groups of animals unless specified otherwise.
Experiments on basal LOS pressure
In eight urethane-anaesthetized ferrets, the left vagus nerve was sectioned at the beginning of the experiment during recording of LOS pressure. This had no effect on basal LOS pressure as previously reported (Blackshaw et al., 1997). The effect of baclofen (7 μmol kg−1 i.v.) on basal LOS pressure was then observed, followed by the effect of CGP35348 (100 μmol kg−1 i.v.). In 11 additional urethane-anaesthetized animals also used for evaluation of vagal stimulation effects (described below), both vagi were sectioned at the start of experiments. Effects of baclofen and CGP35348 on basal LOS pressure were then observed. In four barbiturate-anaesthetized ferrets the vagi remained intact throughout experiments and data acquired on effects of GABAB receptor ligands on basal LOS pressure. Effects of muscimol on basal LOS pressure were observed in six experiments under barbiturate anaesthesia in which vagotomy was performed at the beginning. These were also used to evaluate effects on LOS responses to burst vagal stimulation (see below for methods).
Experiments on vagal stimulation effects: (a) Burst stimulation
In five urethane-anaesthetized ferrets, after bilateral vagotomy, the effects of increasing doses of baclofen (1–10 μmol kg−1 i.v.) on LOS responses to burst peripheral vagal stimulation (10 Hz, 0.5 ms pulses, 20 V, 5 s duration) were investigated. In separate series of experiments, after the effect of a standard dose of baclofen (7 μmol kg−1 i.v.) on the response to vagal stimulation had been established, the reversal of its effect by different GABAB receptor antagonists was studied: CGP35348 (a single dose of 100 μmol kg−1 i.v.; n=11–mentioned in Experiments on basal LOS pressure above); CGP36742 (7–112 μmol kg−1 cumulatively i.v.; n=6); CGP54626 (70 nmol−3 μmol kg−1 cumulatively i.v.; n=11) or CGP62349 (7 nmol–700 nmol kg−1 cumulatively i.v.; n=7). Urethane was used as the anaesthetic in experiments on CGP35348 and CGP36742, and barbiturate anaesthesia was used in later experiments on CGP54626 and CGP62349 according to occupational health considerations. The lack of influence of the type of anaesthetic on the effects of drugs on the response to vagal stimulation was confirmed specifically in two extra experiments in which the opposite anaesthetic/antagonist combinations to those above were used.
As far as fatigue of responses or time-dependent effects are concerned, we have previously demonstrated no change in the response to vagal stimulation over a similar time-course to these experiments (Blackshaw et al., 1997), indicating that changes in response that occurred after drug treatment were not attributable to fatigue in the response. Therefore, time controls were not performed in the present study.
In three experiments in bilaterally vagotomized animals, before treatment with baclofen, the nitric oxide synthase inhibitor L-nitro arginine methyl ester (L-NAME, 100 mg kg−1 i.v.) was administered and its effects on the response to vagal stimulation observed as reported previously (Blackshaw et al., 1997). Subsequently the effect of baclofen (7 μmol kg−1 i.v.) on the response was observed. A further six experiments in unilaterally vagotomized animals evaluated the effects of pretreatment with atropine (400 μg kg−1 i.v.) and guanethidine (5 mg kg−1 i.v.) on the response to vagal stimulation before treatment with baclofen (7 μmol kg−1 i.v.). These experiments were performed after unilateral vagotomy prior to drug treatment because the experiments were originally aimed to investigate parallel drug effects on basal LOS pressure in vagally innervated LOS. They were not repeated in bilaterally vagotomized animals for ethical reasons of animal usage.
(b) Prolonged stimulation
Instead of administering treatments between episodes of vagal stimulation, in six urethane-anaesthetized vagotomized ferrets, the effects of drug administration were measured when given after 5 min of a 15 min period of continuous low frequency vagal stimulation (0.25–0.5 Hz, 0.5 ms pulses, 20 V) in order to evaluate peripheral effects of drugs on vagal tone. The frequency of vagal stimulation was tailored for each of these studies by adjustment in a preliminary test so that it resulted in a maintained LOS pressure approximately 50% of pre-stimulation basal LOS pressure. This allowed evaluation of inhibitory or excitatory effects of drugs on vagal tone. Baclofen (7 μmol kg−1 i.v.) was given during the first period. At least 30 min after the end of the first period, another 15 min of vagal stimulation was given at the same frequency, during which CGP35348 (100 μmol kg−1 i.v.) was administered.
Experiments on central drug effects
In six animals anaesthetized with urethane and 14 animals anaesthetized with barbiturate, baclofen was administered centrally through an i.c.v. cannula in increments of 1 nmol up to a total dose of 3–6 nmol, which was sufficient to cause a significant drop in mean systemic blood pressure. This was followed by treatment with 100 nmol CGP35348 i.c.v. which restored blood pressure; its concomitant effects on basal LOS pressure were observed. Injection of appropriate volumes of saline vehicle had no effect on LOS pressure or arterial pressure in these experiments. After central drug treatments, systemic baclofen (7 μmol kg−1 i.v.) and CGP35348 (100 μmol kg−1 i.v.) were given, which had similar effects to those reported in Results under the corresponding anaesthetic; these are not illustrated.
Supranodose vagotomy studies
In two ferrets, the contribution of effects of baclofen on transmitter release from orthodromically stimulated efferent and antidromically stimulated afferent endings was determined. Vagal pre-ganglionic connections from the brainstem to the viscera were severed selectively by chronically sectioning the nerve above the nodose ganglion. This procedure is designed to leave intact only afferent fibres, whose cell bodies are in the nodose ganglion. Ferrets were anaesthetized with Halothane (2.5–4% in oxygen by inhalation). The nodose ganglion was accessed via a ventral approach after a midline cervical incision under aseptic conditions. The ganglion and vagus nerve were dissected away from the adjacent carotid artery and mobilised over a length of approximately 5 mm. The vagus was cut at least 2 mm rostral to the ganglion, after which it could be freely reflected caudally, confirming that all central connections were severed. The incision was closed with interrupted sutures and animals allowed to recover, after which unilateral Horner's syndrome was seen, indicative of the completeness of section. Experiments (without drug treatments) on LOS and cardiac responses to vagal stimulation were then performed 1 week later under barbiturate anaesthesia, using the unoperated vagus as a control. This period was observed to allow sufficient time for axons of medullary vagal neurones to degenerate (Berthoud & Powley, 1992). That vagal afferents remained viable was verified after manometric studies by electrophysiological recordings of cervical afferent fibres, which confirmed survival of single vagal afferent units with receptive fields corresponding to baroreceptors, gastric mechanoreceptors and gastric mucosal receptors, which responded with excitation of discharge to ventricular systole, gastric distension, or close intra-arterial cholecystokinin injection respectively, as described previously e.g. Blackshaw & Grundy (1990)–see also for details of electrophysiological methods.
In vitro experiments on LOS muscle strips
Strips were obtained from seven ferrets in addition to those reported above that were killed by exsanguination under deep anaesthesia (sodium pentobarbitone, 60 mg kg−1 i.p.). The oesophagus, LOS and proximal stomach were removed and placed in ice-cold Krebs solution bubbled with carbogen (95% O2, 5% CO2). The region encompassing the gastro-oesophageal junction was located after careful dissection and a band of circular muscle dissected and divided to give two muscle strips per ferret. All muscle strips (1–2 mm wide, 5 mm long) were placed in 10 ml water-jacketed organ baths containing carbogenated Krebs solution at 37°C of the following composition (mM): NaCl 118, NaHCO3 25, KCl 4.6, MgSO4 1.2, NaH2PO4 1.3, glucose 11, CaCl2 2.5. One end of the tissue was fastened to a support while the other was attached to an isometric force transducer (FTO3, Grass, Quincy, MA, U.S.A.). A pair of platinum electrodes on either side of the tissue support was used for electrical field stimulation (EFS). Each strip was placed under an initial tension of 2 g and left to equilibrate for 60 min. In previous studies, this tension corresponded to a degree of tissue stretch of approximately 200% of the initial resting length of the muscle strip, and was within the optimal range for mechanical performance of the tissue. Only muscle strips from the gastro-esophageal junction developed a stable spontaneous tension at rest and exhibited a typical relaxation profile in response to EFS as described in our previous studies (Smid et al., 1998a,1998b).
Electrical field stimulation was delivered via rectangular wave pulses from a stimulator (S48, Grass Instruments, Quincy, MA, U.S.A.), with 3 min intervals between each stimulus. Responses of a maximum of 14 muscle strips to EFS (1–5 Hz, 50 V, 1 ms for 5 s), DMPP (nicotinic agonist; 10−5 M), capsaicin (10−6 M) and [Sar9, Met (O2)11]-substance P (NK-1 receptor-selective agonist; 10−9–10−6 M), were measured and recorded onto hard disk using Labview-based software (MAD, Charles Malbert). EFS, DMPP, capsaicin and [Sar9, Met (O2)11]-substance P-induced LOS responses were compared alone and in the presence of baclofen (3.10−5–2.10−4 M). All experiments were performed in the presence of atropine (10−6 M) and guanethidine (3.10−6 M). A non-cumulative dosing protocol showed that there was no tachyphylaxis in the responses following repeated administration of [Sar9, Met (O2)11]-substance P in the ferret LOS. The response to capsaicin was consistently reduced upon repeated administration, indicating desensitization. This reduction in response was avoided by the use of only one dose per experiment and allowing long intervals between administration (2–3 h), repeated washouts and periodic substance P administration (10−8 M every 30 min over 2–3 h). Administration of the capsaicin vehicle (see Drugs) alone in an equivalent dilution did not alter basal LOS tone. Only one dose of DMPP was used also because of desensitization that occurs with multiple doses.
Data analysis
Unless otherwise stated, sphincter pressure in vivo was assessed as the difference between recorded values of LOS pressure and gastric pressure at end-expiration. Basal LOS pressure was measured as the mean end-expiratory pressure over 1 min before and upon stabilization after treatments. Contractile responses to nerve stimulation were expressed as the peak height of contraction above the minimum sphincter pressure during the response. This was done because contraction occurred between two inhibitory phases of the response, and the peak was often below basal LOS pressure. Gastric pressure responses to nerve stimulation were not evaluated as the gastric pressure recording was used only as a reference for basal LOS pressure.
Chart speed was increased from 50 to 250 mm min−1 for observation of the effects of vagal stimulation on heart rate only in experiments on peripheral vagal stimulation in which this was specifically investigated. Analysis of heart rate responses was performed by visual inspection of chart recorder traces. Heart rate was measured over 2.5 s immediately before and for 2.5 s during the maximum effect of a 5 s burst of vagal stimulation. In order to minimize the effects of inter-animal variation, the responses are expressed as the percentage change relative to baseline.
Basal tension in LOS strips is expressed as the total tension developed after equilibration, and responses to drugs or EFS as percentage reduction of the value relative to zero. Statistical significance of differences between responses was assessed by paired Student's t-test in the case of one treatment, by repeated measures ANOVA in the case of two or more treatments, and by two-way ANOVA in the case of comparison of stimulus-response curves. Data are expressed as mean±s.e.mean, with n=number of animals.
Drugs
Drugs were prepared in isotonic saline solution. Maximum intravenous drug injection volume was 1 ml and maximum i.c.v. injection volume was 20 μl. The following drugs were used for in vivo experiments: L-NAME, guanethidine sulphate, urethane, (Sigma), CGP35348, CGP36742, CGP54626, CGP62349 (gifts from AstraZeneca R&D, Mölndal, Sweden), atropine sulphate (Astra). (±)-Baclofen was obtained from Research Biochemicals International (Natick, MA, U.S.A.). CGP62349 is a pure stereoisomer: 3-[(1R)-1-[[(2S)-2-hydroxy-3-[hydroxy[ (4 - methoxyphenyl)methyl]phosphinyl]propyl]amino]ethyl] benzoic acid. The compound henceforth referred to in this paper was in fact a diastereomeric mixture of this compound and 3-[(1S)-1-[[(2S)-2-hydroxy-3-[hydroxy[(4-methoxyphenyl)methyl]phosphinyl]propyl]amino]ethyl] benzoic acid. For muscle strip studies, [Sar9, Met (O2)11]-substance P and β-Ala8 neurokinin A [4-10] were obtained from Auspep (Melbourne, Australia). Atropine sulphate, 1,1-dimethyl-4-phenylpiperazinium iodide (DMPP), capsaicin and guanethidine sulphate were obtained from Sigma-Aldrich (Sydney, Australia). All drugs were dissolved in saline, except for capsaicin, which was dissolved in saline, ethanol and Tween 80 (8 : 1 : 1 by volume, respectively).
Ethics
All procedures in this study were performed within the guidelines for animal experimentation of the Institute for Medical and Veterinary Science, Adelaide, and approval for experiments granted by the Animal Ethics Committee of this institution.
Results
Basal LOS pressure
Under urethane anaesthesia, vagally intact animals showed levels of basal LOS pressure similar to those we have reported previously (Blackshaw et al., 1997). This was unaffected upon section of the left cervical vagus, but was significantly increased following complete bilateral vagal section (Figure 1). In intact barbiturate-anaesthetized ferrets, levels of basal LOS pressure were significantly higher than with urethane (Figure 1), and showed little or no change following bilateral vagotomy (data not illustrated). Baclofen (7 μmol kg−1 i.v.) increased basal LOS pressure only in urethane-anaesthetized animals with an intact vagus. This effect was reversible with the GABAB receptor antagonist CGP35348 (100 μmol kg−1 i.v.; Figure 1). The GABAA receptor agonist muscimol at a dose of 0.15 μmol kg−1 had no maintained effect on basal LOS pressure in six ferrets; there was an initial reduction of 50±8% which returned to baseline in 1.1±0.2 min. Higher doses of muscimol (up to 1.5 μmol kg−1 i.v.) induced profound hypotension, but little change was seen in the effect on LOS pressure (data not shown).
Figure 1.
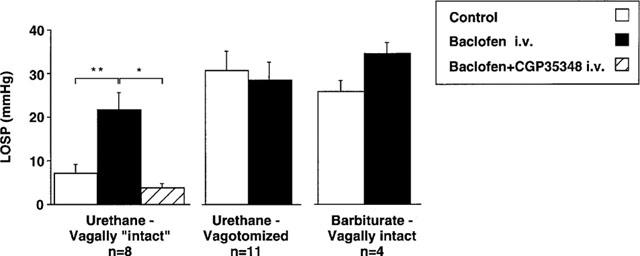
Effects of GABAB receptor ligands on basal LOS pressure (LOSP). This was evaluated in separate series of experiments in urethane-anaesthetized ferrets with one intact vagus nerve (left graph, n=8), in urethane-anaesthetized ferrets following bilateral cervical vagotomy (centre graph, n=11), and in vagally intact barbiturate anaesthetized ferrets (right graph, n=4). Baclofen (7 μmol kg−1 i.v.) significantly increased basal LOS pressure (**P<0.01) only in urethane-anaesthetized ‘intact' ferrets. This effect was reversible with the selective GABAB receptor antagonist CGP35348 (100 μmol kg−1 i.v. *P<0.05).
In order to determine if baclofen's action on basal LOS pressure was central or peripheral, the drug was administered (3–6 nmol) into the fourth ventricle in a separate series of experiments. It had no effect on LOS pressure in either urethane- or barbiturate-anaesthetized ferrets (Figure 2), and yet significantly reduced arterial pressure under both anaesthetics (125±8 mmHg before, 87±9 mmHg after baclofen, P<0.01–urethane data; 139±4 mmHg before, 113±7 mmHg after baclofen, P<0.01–barbiturate data). These effects were reversible with CGP35348 (100 nmol kg−1 i.c.v.) (120±4 mmHg, P<0.05–urethane data; 149±4 mmHg, P<0.001–barbiturate data).
Figure 2.
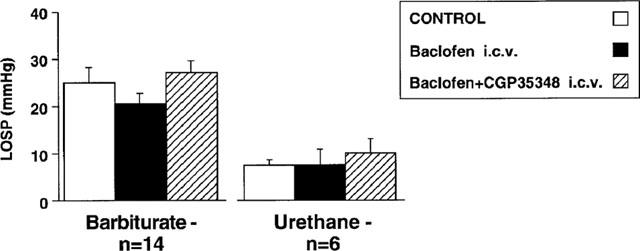
Effects of intracerebroventricular (i.c.v.) administration of GABAB receptor ligands on basal LOS pressure (LOSP) in ferrets anaesthetized with barbiturate or urethane. Neither baclofen (3–6 nmol i.c.v.) nor subsequent CGP35348 (100 nmol i.c.v.) had any effect on basal LOS pressure.
LOS and cardiac responses to peripheral vagal stimulation
Having established from observations described above that an intact vagal pathway was necessary for the effect of systemically administered baclofen on basal LOS pressure, we further investigated the effect of baclofen on peripheral vagal output after vagotomy by restoring it artificially through electrical vagal stimulation. This was done either in bursts (2–10 Hz, 20 V, 0.5 ms pulses, 5 s duration), or as a prolonged, low frequency background (0.25–0.5 Hz, 20 V, 0.5 ms pulses, 15 min duration), the latter being designed to mimic the spontaneous discharge in vagal efferent fibres, and the former to mimic bursts of vagal discharge that may induce profound relaxation during, for example, swallowing. Baclofen had reproducible effects on LOS responses to both stimulation paradigms as described below, whereas muscimol (0.15 μmol kg−1 i.v.) was without any effect on the response to 5 or 10 Hz burst vagal stimulation (27.3±4.0 mmHg contraction before, 26.9±1.7 mmHg contraction after; n=6; data for 10 Hz, 5 s duration).
Burst vagal stimulation-effects on LOS
Figure 3A shows a typical response of the LOS to burst vagal stimulation, the details of which have been reported previously (Blackshaw et al., 1997). The response is triphasic, with an initial drop in LOS pressure during stimulation, followed by a frequency-dependent (Figure 3B, P=0.001 2-way ANOVA) increase still during stimulation, followed by a prolonged reduction usually to zero (gastric pressure) which returned to basal levels over several minutes. Although baclofen often affected the later recovery phase (as seen in Figure 3), this was not consistent. It did, on the other hand, have a highly consistent and significant effect on the contractile phase of the response. This enhancement of LOS contraction was seen at all frequencies of stimulation and was dose-dependent from 1–10 μmol kg−1 (Figure 3B, P<0.01, 2-way ANOVA). Under conditions of adrenergic and cholinergic blockade achieved by treatment with atropine (400 μg kg−1 i.v.) and guanethidine (5 mg kg−1 i.v.), baclofen (7 μmol kg−1 i.v.) still enhanced slightly but significantly the LOS contractile response (Figure 3C, P<0.05). Thus an effect on inhibitory NANC pathways to the LOS was likely, and this was confirmed with the nitric oxide synthase inhibitor L-NAME. L-NAME potently increased the contractile phase of the response as described previously (Blackshaw et al., 1997), after which baclofen had no effect (Figure 3D). Although data are shown only for 10 Hz stimulation, this lack of effect of baclofen was seen also at 5 Hz, after which smaller contractions occurred.
Figure 3.
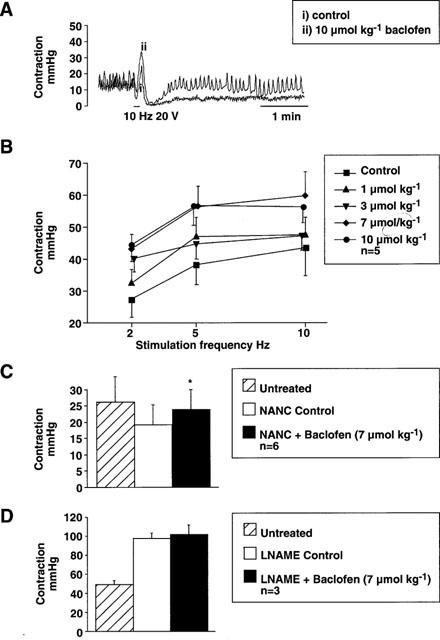
Original recordings showing LOS response to a burst of vagal stimulation (0.5 ms pulses, 20 V, 10 Hz, 5 s duration) before (i) and after (ii) treatment with baclofen (10 μmol kg−1 i.v.). Basal LOS pressure is unaffected, but the excitatory phase of the response is enhanced. In this example, baclofen also reveals a more rapid recovery of LOS pressure after the later, inhibitory phase of the response. Respiratory movements are superimposed on the LOS trace in both recordings. (B) Dose- and frequency-dependence of the effect of baclofen on the second (excitatory) phase of the response (2–10 Hz, 0–10 μmol kg−1 i.v.) in bilaterally vagotomized animals. Both stimulation frequency and drug dosage effects were significant (P<0.001 and P<0.01 respectively). (C) Effect of atropine (400 μg kg−1 i.v.) and guanethidine (5 mg kg−1 i.v.)–NANC control–on the LOS contractile response to vagal stimulation (0.5 ms pulses, 20 V, 10 Hz, 5 s duration) in unilaterally vagotomized animals. Baclofen (7 μmol kg−1 i.v.) administered subsequently significantly enhanced the response (*P<0.05) as seen in untreated animals (B). (D) Effect of L-NAME (100 mg kg−1) on the LOS contractile response to vagal stimulation (0.5 ms pulses, 20 V, 10 Hz, 5 s duration) in bilaterally vagotomized animals. Baclofen (7 μmol kg−1 i.v.) administered subsequently had no further effect in three experiments.
From the effects of baclofen on basal LOS pressure described earlier, we expected that the vagal stimulation-induced responses would be reversible by CGP35348. This antagonist (100 μmol kg−1 i.v.) had no effect on the enhancement of LOS contraction by baclofen (Figure 4). Another GABAB receptor antagonist CGP36742 was therefore tested in a separate series of experiments, but this was also ineffective in reversing baclofen's effect at a range of doses up to 112 μmol kg−1 at two stimulation frequencies (5 and 10 Hz). On the other hand, the selective GABAB antagonists CGP62349 and CGP54626 reversed the effects of baclofen, with mean ED50's of 37 and 100 nmol kg−1 respectively.
Figure 4.
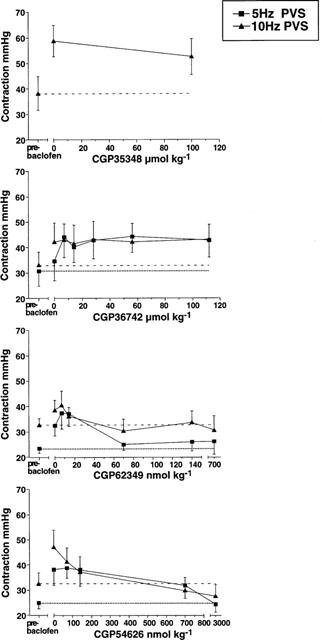
Effects of four different GABAB receptor antagonists (CGP35348 100 μmol kg−1, CGP36742 7–112 μmol kg−1, CGP62349 7–700 nmol kg−1 and CGP54626 70 nmol–3 μmol kg−1) on the baclofen (7 μmol kg−1 i.v.)-induced change in LOS contraction after vagal stimulation (PVS, 0.5 ms pulses, 20 V, 5 and 10 Hz, 5 s duration). The increase in response after baclofen was unchanged by CGP35348 or CGP36742, but was potently antagonized by CGP62349 or CGP54626 (mean ED50s 37 and 100 nmol kg−1 respectively).
A week after supranodose vagotomy in two ferrets, stimulation of the operated cervical vagus (up to 50 V, 50 Hz) had no effect on LOS pressure, heart rate or blood pressure, whereas stimulation of the intact vagus had similar effects to those reported in unoperated animals. We confirmed that afferent neurones were viable in these preparations by electrophysiological recordings of single vagal afferent fibres which revealed baroreceptors, gastric mechanoreceptors and mucosal receptors which responded with excitation of discharge to ventricular systole, gastric distension, or close intra-arterial cholecystokinin respectively, as described previously e.g. (Blackshaw & Grundy, 1990).
Burst vagal stimulation-effects on heart rate
Along with the effects of vagal stimulation on LOS pressure described above, there was a concomitant bradycardia which was measured in experiments conducted specifically for this purpose using rapid chart recorder paper speeds. This occurred only during the period of stimulation, and was frequency-dependent (Figure 5). Bradycardia was significantly reduced by baclofen. Baclofen had no effects on resting heart rate (data not illustrated). Baclofen's effect on the bradycardia response to vagal stimulation was unaffected by CGP36742, but was reversed by CGP62349 or CGP54626 (Figure 5) in a similar way to the effects on LOS responses described above. Data were not recorded for effects of CGP35348.
Figure 5.
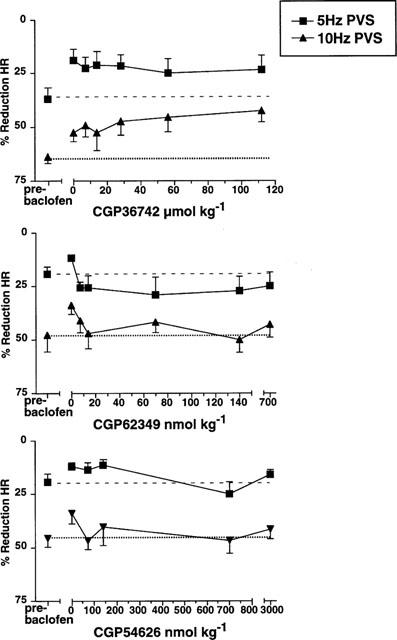
Effects of three different GABAB receptor antagonists (CGP36742 7–112 μmol kg−1, CGP62349 7–700 nmol kg−1 and CGP54626 70 nmol–3 μmol kg−1 i.v.) on the baclofen (7 μmol kg−1 i.v.) induced change in bradycardia after vagal stimulation (PVS, 0.5 ms pulses, 20 V, 5 and 10 Hz, 5 s duration). A similar pharmacological profile was seen to effects on LOS responses illustrated in Figure 4.
Prolonged vagal stimulation-effects on LOS
Figure 6 shows the mean LOS and blood pressure responses to prolonged vagal stimulation. LOS pressure showed a rapid reduction upon commencement of the period of stimulation which was maintained for the 5 min before administration of baclofen. Baclofen (7 μmol kg−1 i.v.) caused a significant (P=0.01) increase in LOS pressure during stimulation, but had only a brief effect on blood pressure in the first minute post-baclofen. At the end of the period of vagal stimulation, LOS pressure returned to similar levels to those observed before stimulation. In a subsequent period of vagal stimulation, CGP35348 (100 μmol kg−1 i.v.) had no effect on LOS pressure, but enhanced blood pressure significantly (P=0.01). This effect on blood pressure was maintained beyond the period of stimulation, and was seen also in other experiments in which the drug was given without vagal stimulation (data not illustrated).
Figure 6.
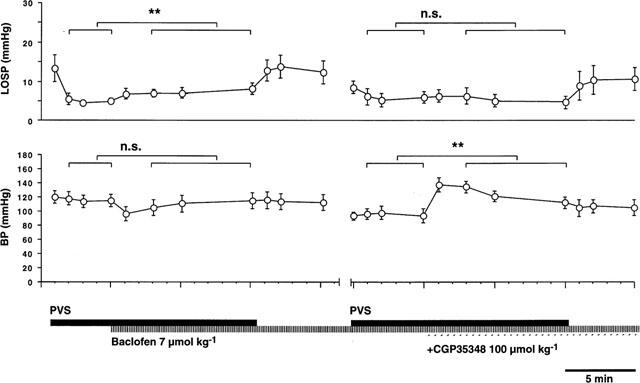
Effects of GABAB receptor ligands on mean LOS pressure (LOSP) and arterial blood pressure (BP) during low frequency peripheral vagal stimulation (PVS, 0.5 ms pulses, 20 V, 15 min duration) at frequencies (0.25–0.5 Hz) sufficient to achieve approximately 50% inhibition of basal LOS pressure before drug administration. Baclofen (7 μmol kg−1 i.v.), given after 5 min of PVS, attenuated the reduction of basal LOS pressure by PVS (**P<0.01). At the end of the stimulation period, LOS pressure returned to prestimulation levels. Subsequently, CGP35348 (100 μmol kg−1 i.v.) administered during a second 15 min period of vagal stimulation at the same frequency had no effect on LOS pressure. Again, LOS pressure returned to prestimulation levels on cessation of stimulation. Changes in LOS responses were not paralleled by changes in BP. Baclofen did not significantly affect BP during stimulation, whereas CGP35348 significantly enhanced BP (**P<0.01).
Isolated LOS strip responses to NK-1 and nicotinic receptor agonists, field stimulation and capsaicin
Isolated LOS muscle strips responded to electrical field stimulation with pure inhibition (Figure 7A). The NK-1 receptor agonist [Sar9, Met (O2)11]-substance P (selective NK-1 agonist) induced relaxation which was concentration-dependent up to 10−7 M (Figure 7B). The nicotinic receptor agonist DMPP (10−5 M) also caused relaxation (Figure 7C).
Figure 7.
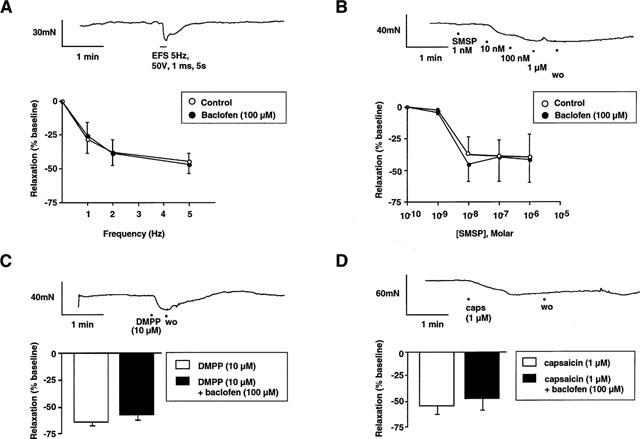
Representative trace of the LOS muscle strip response to electrical field stimulation (EFS; 5 Hz, 50 V, 1 ms duration for 5 s). The response consists of a rapid relaxation during the stimulus, with baseline tone restored within 1 min post-stimulus. Stimulus-response curves show LOS muscle strip responses to EFS (1–5 Hz, 50 V, 1 ms duration for 5 s) in control and baclofen-pretreated tissue. Relaxation is frequency-dependent up to 5 Hz. Baclofen (10−4 M) did not alter EFS-induced relaxation. (B) Representative trace of the LOS muscle strip response to cumulative concentrations of [Sar9, Met (O2)11]-substance P (10−9–10−6 M). The response consisted of a rapid dose-dependent relaxation, with baseline restored 5–6 min after washout. Concentration-response curves show LOS muscle strip responses to [Sar9, Met (O2)11]-substance P form control and baclofen-pretreated tissues. Baclofen (10−4 M) did not alter the dose-response profile of the NK-1 receptor agonist in LOS muscle strips. (C) Representative trace of the LOS muscle strip response to the nicotinic receptor agonist DMPP (10−5 M). The response consisted of rapid relaxation, with baseline tone restored within 1 min post-stimulus. Histogram shows group data for LOS muscle strip responses to DMPP (10−5 M) in control and baclofen-treated tissues. Baclofen (10−4 M) did not affect LOS relaxation to DMPP. (D) Representative trace of the LOS muscle strip response to capsaicin (10−6 M). The response consisted of a slow-onset relaxation, with baseline restored approximately 10 min after washout. Concentration-response histogram shows LOS strip responses to capsaicin (10−6 M), alone and in the presence of baclofen (10−4 M). Relaxation was unaltered following pretreatment with baclofen.
Transmitter release from collaterals of mainly spinal afferents was induced by bath application of capsaicin (Smid et al., 1998a), which induced a rapid and prolonged relaxation of LOS strips (Figure 7D). The relaxation response to 10−6 M capsaicin was reproducible in amplitude in repeated trials when performed in accordance with the protocol described in detail in Methods.
Responses to electrical field stimulation, [Sar9, Met (O2)11]-substance P, DMPP and capsaicin were all unaffected by baclofen (10−4 M) (Figure 7). Also, development of LOS basal tone and contractile responses to the NK-2 receptor agonist β-Ala8 neurokinin A [4-10] were similarly unaffected by baclofen (data not illustrated).
Discussion and Conclusions
Vagal output to the viscera may be maintained tonically active by central excitatory drive to pre-ganglionic motorneurones. This tonic output may be directed towards either excitatory or inhibitory post-ganglionic pathways or both, depending on the effector and the circumstances. Changes in the final output through these pathways may be made at six stages: effects on afferent activation peripherally, release of transmitters from the central endings of afferents which provide input to reflexes, within the central nervous system, pre-synaptically on peripheral transmitter release from vagal pre-ganglionic efferents, within the post-ganglionic plexuses, and pre-junctionally on transmitter release from intrinsic neurones. Additionally, vagal reflexes may be mediated at the level of axon collaterals of primary afferent neurones. Thus a peripheral stimulus may influence the effector directly without central involvement. This study shows that systemically applied baclofen at least acts at one stage in the pathway–presynaptically on transmitter release from vagal efferent neurones onto post-ganglionic plexuses. This accounts for baclofen's actions on responses of the LOS and heart to vagal stimulation, but the influence of baclofen on basal LOS pressure is more complex; it originates peripherally because systemic, not central baclofen lowered basal LOS pressure. We can also deduce that baclofen's action was not via elements of the enteric nervous system due to its lack of effect in vagotomized animals, or in isolated preparations of the LOS.
Our early data appeared to indicate a single mechanism for baclofen's action, both on basal LOS pressure in intact animals and on vagal stimulation-evoked LOS responses, which was by acting presynaptically to reduce vagal output onto inhibitory post-ganglionic pathways that in turn release nitric oxide onto smooth muscle. However, an important discrepancy was revealed on comparison of the action of the GABAB receptor antagonist CGP35348 in these two situations. Whereas the effect of systemic baclofen on basal LOS pressure in urethane-anaesthetized animals was completely reversed by CGP35348, this antagonist did not change baclofen's effect on LOS responses to vagal stimulation, although it was reversed by other antagonists. This is best explained by there being two sites of peripheral GABAB receptors: one on vagal pre-ganglionic endings that is insensitive to CGP35348, and another elsewhere that is sensitive to CGP35348. Other instances of specific sites of GABAB receptors that are CGP35348-sensitive and -insensitive are evident from studies of two areas of the central nervous system: the cortex and the medulla oblongata. Baclofen inhibits the release of a number of transmitters from synaptosomes of human cortical neurones (Bonanno et al., 1996; 1997). Its effects on release of somatostatin and glutamate are reversible with CGP35348, whereas its effect on release of GABA (via autoreceptors) is not. Trippenbach & Lake (1994) showed that in the medullary control of respiration in rats, baclofen inhibited several aspects of the respiratory pattern, but not all of these were reversible by CGP35348. Clues to the location of the CGP35348-sensitive site in our studies may arise from our results using two different anaesthetics. Marked effects of baclofen on basal LOS pressure were seen in animals anaesthetized with urethane but not in those anaesthetized with barbiturate (in which LOS pressure was higher initially). It is established that these two anaesthetic agents differ substantially in at least two respects: firstly their effects on gastric motility (Grundy, 1990), and secondly their ability to release somatostatin from the gastrointestinal mucosa (Yang et al., 1990). Regarding the latter, high circulating levels of somatostatin are reached under urethane anaesthesia in rats, but not under barbiturate anaesthesia.
As just described, baclofen inhibits somatostatin release which is reversible with CGP35348 (Bonanno et al., 1996). If somatostatin were involved in the action of baclofen in our experiments, this could be mediated either peripherally on pathways independent of vagal motor influences, or within the central nervous system, having crossed the blood-brain-barrier. We found that the somatostatin analogue octreotide, injected i.c.v. caused profound LOS relaxation in vagally-intact animals (Blackshaw, unpublished observation), which at least demonstrates there are receptors for somatostatin on central vagal motor pathways. Regarding the first option of effects of anaesthetics via gastric motility, considerably higher levels of gastric motility are seen under urethane than under barbiturate in ferrets (Grundy, 1990). These higher levels of motility lead to increased activation of gastric vagal mechanoreceptors (Blackshaw & Grundy, 1990). Activation of vagal afferents in turn may cause reflex inhibition of basal LOS pressure (Kawahara et al., 1994). It has recently been shown that baclofen has a powerful peripheral inhibitory action on the mechanical responsiveness of gastric vagal mechanoreceptors (Page & Blackshaw, 1999), which is reversible with antagonists including CGP35348 in vivo (Blackshaw & Partosoedarso, 1999).
We are therefore left with one demonstrable mechanism of action of baclofen in the system under study on vagal output onto inhibitory enteric pathways, as demonstrated by changes in vagal stimulation-induced responses. The other mechanism of action involved in effects on basal LOS pressure remains elusive and is the subject of further study.
Because the vagus was electrically stimulated, it may be that LOS responses resulted from antidromic activation of gastro-oesophageal afferent fibres in addition to orthodromic activation of efferents. Transmitter release from afferent axon collaterals has been previously implicated in control of gastric motility (Delbro et al., 1982) and LOS relaxation (Blackshaw & Dent, 1997; Blackshaw et al., 1997; Smid et al., 1998a,1998b), although the latter four studies from our laboratory indicated that this phenomenon was evident following activation of spinal and not vagal afferent collaterals. The contribution of antidromically activated afferents to LOS responses observed in the present study was investigated by unilateral supranodose vagotomy in two animals. This procedure allows afferent and efferent pathways in one vagus to be kept intact, and the efferent pathways to be selectively removed in the other vagus. Supranodose vagotomy separates the somata of vagal pre-ganglionic neurones from their peripheral axons, which degenerate rapidly (Berthoud & Powley, 1992), whereas the somata of vagal afferents remain in continuity with their axons. Following selective antidromic afferent stimulation even at extreme levels, no effect was seen on LOS or cardiovascular parameters, showing not only that actions of GABAB receptors were restricted to vagal pre-ganglionic neurones, but also that vagal afferent collaterals have no functional connection with motor pathways to the LOS or heart.
Baclofen has a potential therapeutic effect in reducing transient LOS relaxations and gastro-oesophageal reflux (Blackshaw et al., 1999; Lehmann et al., 1999; Lidums et al., 2000). It was noted that the same order of antagonist potency against the effects of baclofen seen in this study was also observed in our parallel study on transient LOS relaxations in conscious ferrets (Blackshaw et al., 1999). This study therefore demonstrates not only a site of action of GABAB receptors along vagal reflex pathways, but also a robust model in which possible subtypes of GABAB receptor may be researched. This is significant from the point of view of drug development for gastro-oesophageal reflux disease.
Acknowledgments
AstraZeneca R&D Mölndal are gratefully acknowledged for their financial support of this project, and their collaboration with the supply of compounds (Dr A. Lehmann and Dr S. von Unge).
Abbreviations
- GABA
γ-amino butyric acid
- LOS
lower oesophageal sphincter
References
- ANDREWS P.L., BINGHAM S., WOOD K.L. Modulation of the vagal drive to the intramural cholinergic and non-cholinergic neurones in the ferret stomach by baclofen. J Physiol (Lond.) 1987;388:25–39. doi: 10.1113/jphysiol.1987.sp016599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHWORTH PREECE M., KRSTEW E., JARROTT B., LAWRENCE A.J. Functional GABAA receptors on rat vagal afferent neurones. Br. J. Pharmacol. 1997;120:469–475. doi: 10.1038/sj.bjp.0700909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELVISI M.G., ICHINOSE M., BARNES P.J. Modulation of non-adrenergic, non-cholinergic neural bronchoconstriction in guinea-pig airways via GABAB-receptors. Br. J. Pharmacol. 1989;97:1225–1231. doi: 10.1111/j.1476-5381.1989.tb12582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHOUD H.R., POWLEY T.L. Vagal afferent innervation of the rat fundic stomach: Morphological characterization of the gastric tension receptor. J. Comp. Neurol. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- BLACKSHAW L.A., DENT J. Lower oesophageal sphincter responses to noxious oesophageal chemical stimuli in the ferret: involvement of tachykinin receptors. J. Auton. Nerv. Syst. 1997;66:189–200. doi: 10.1016/s0165-1838(97)00083-0. [DOI] [PubMed] [Google Scholar]
- BLACKSHAW L.A., GRUNDY D. Effects of cholecystokinin (CCK-8) on two classes of gastroduodenal vagal afferent fibre. J. Auton. Nerv. Syst. 1990;31:191–202. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- BLACKSHAW L.A., PARTOSOEDARSO E.R.Baclofen inhibits mechanical sensitivity of gastrointestinal vagal afferents in vivo Gastroenterology 1999116A959(Abstract) [Google Scholar]
- BLACKSHAW L.A., HAUPT J.A., OMARI T., DENT J. Vagal and sympathetic influences on the ferret lower oesophageal sphincter. J. Auton. Nerv. Syst. 1997;66:179–188. doi: 10.1016/s0165-1838(97)00082-9. [DOI] [PubMed] [Google Scholar]
- BLACKSHAW L.A., STAUNTON E., DENT J., HOLLOWAY R.H., MALBERT C.H. Mechanisms of gastro-oesophageal reflux in the ferret. Neurogastroenterol. Motil. 1998;10:49–56. doi: 10.1046/j.1365-2982.1998.00085.x. [DOI] [PubMed] [Google Scholar]
- BLACKSHAW L.A., STAUNTON E., LEHMANN A., DENT J. Inhibition of transient LES relaxations and reflux in ferrets by GABAb receptor agonists. Am. J. Physiol. 1999;277:G867–G874. doi: 10.1152/ajpgi.1999.277.4.G867. [DOI] [PubMed] [Google Scholar]
- BONANNO G., FASSIO A., SCHMID G., SEVERI P., SALA R., RAITERI M. Pharmacologically distinct GABAB receptors that mediate inhibition of GABA and glutamate release in human neocortex. Br. J. Pharmacol. 1997;120:60–64. doi: 10.1038/sj.bjp.0700852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONANNO G., GEMIGNANI A., SCHMID G., SEVERI P., CAVAZZANI P., RAITERI M. Human brain somatostatin release from isolated cortical nerve endings and its modulation through GABAB receptors. Br. J. Pharmacol. 1996;118:1441–1446. doi: 10.1111/j.1476-5381.1996.tb15558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWERY N.G., PRATT G.D. GABAB receptors as targets for drug action. Arzneimittelforschung. 1992;42:215–223. [PubMed] [Google Scholar]
- BOWERY N.G., PRICE G.W., HUDSON A.L., HILL D.R., WILKIN G.P., TURNBULL M.J. GABA receptor multiplicity. Visualization of different receptor types in the mammalian CNS. Neuropharmacology. 1984;23:219–231. doi: 10.1016/0028-3908(84)90063-7. [DOI] [PubMed] [Google Scholar]
- BROOKS P.A., GLAUM S.R., MILLER R.J., SPYER K.M. The actions of baclofen on neurones and synaptic transmission in the nucleus tractus solitarii of the rat in vitro. J Physiol (Lond.) 1992;457:115–129. doi: 10.1113/jphysiol.1992.sp019367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELBRO D., FANDRIKS L., LISANDER B., ANDERSSON S.A. Gastric atropine-sensitive excitation by peripheral vagal stimulation after hexamethonium. Antidromic activation of afferents. Acta Physiol. Scand. 1982;114:433–440. doi: 10.1111/j.1748-1716.1982.tb07006.x. [DOI] [PubMed] [Google Scholar]
- GRUNDY D. The effect of surgical anaesthesia on antral motility in the ferret. Exp Physiol. 1990;75:701–708. doi: 10.1113/expphysiol.1990.sp003448. [DOI] [PubMed] [Google Scholar]
- KAWAHARA H., BLACKSHAW L.A., NISYRIOS V., DENT J. Transmitter mechanisms in vagal afferent-induced reduction of lower esophageal sphincter (LOS) pressure in the rat. J. Auton. Nerv. Syst. 1994;49:69–80. doi: 10.1016/0165-1838(94)90022-1. [DOI] [PubMed] [Google Scholar]
- KLEINROK A., KILBINGER H. gamma-Aminobutyric acid and cholinergic transmission in the guinea-pig ileum. Naunyn. Schmiedebergs. Arch. Pharmacol. 1983;322:216–220. doi: 10.1007/BF00500768. [DOI] [PubMed] [Google Scholar]
- LEHMANN A., ANTONSSON M., BREMNER-DANIELSEN M., FLÄRDH M., HANSSON-BRANDEN L., KÄRRBERG L. Activation of the GABAb receptor inhibits transient lower esophageal sphincter relaxations in the dog. Gastroenterology. 1999;117:1147–1154. doi: 10.1016/s0016-5085(99)70400-2. [DOI] [PubMed] [Google Scholar]
- LIDUMS I., LEHMANN A., CHECKLIN H., DENT J., HOLLOWAY R.H. Control of transient lower esophageal sphincter relaxations and reflux by the GABAB agonist baclofen in normal subjects. Gastroenterology. 2000;118:7–13. doi: 10.1016/s0016-5085(00)70408-2. [DOI] [PubMed] [Google Scholar]
- LINGENFELSER T., BLACKSHAW L.A., SUN W., DENT J. Pyloric motor response to central and peripheral nitric oxide in the ferret. Neurogastroenterol. & Motil. 1997;9:167–175. doi: 10.1046/j.1365-2982.1997.d01-39.x. [DOI] [PubMed] [Google Scholar]
- MITTAL R.K., HOLLOWAY R.H., PENAGINI R., BLACKSHAW L.A., DENT J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- ONG J., KERR D.I., LACEY G., CURTIS D.R., HUGHES R., PRAGER R.H. Differing actions of nitropropane analogs of GABA and baclofen in central and peripheral preparations. Eur. J. Pharmacol. 1994;264:49–54. doi: 10.1016/0014-2999(94)90634-3. [DOI] [PubMed] [Google Scholar]
- PAGE A.J., BLACKSHAW L.A. GABAB receptors inhibit mechanosensitivity of primary afferent endings. J. Neurosci. 1999;19:8597–8602. doi: 10.1523/JNEUROSCI.19-19-08597.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Z.H., LIPTON S.A. Multiple GABA receptor subtypes mediate inhibition of calcium influx at rat retinal bipolar cell terminals. J. Neurosci. 1995;15:2668–2679. doi: 10.1523/JNEUROSCI.15-04-02668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVA CARVALHO L., DAWID MILNER M.S., SPYER K.M. The pattern of excitatory inputs to the nucleus tractus solitarii evoked on stimulation in the hypothalamic defence area in the cat. J Physiol (Lond.) 1995;487:727–737. doi: 10.1113/jphysiol.1995.sp020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMID S., LYNN P.A., TEMPLEMAN R., BLACKSHAW L.A. Activation of non-adrenergic non-cholinergic inhibitory pathways by endogenous and exogenous tachykinins in the ferret lower oesophageal sphincter. Neurogastroenterol. & Motil. 1998a;10:149–156. doi: 10.1046/j.1365-2982.1998.00092.x. [DOI] [PubMed] [Google Scholar]
- SMID S., PAGE A.J., O'DONNELL T.A., LANGMAN J., ROWLAND R., BLACKSHAW L.A. Oesophagitis-induced changes in capsaicin-sensitive tachykininergic pathways in the ferret lower oesophageal sphincter. Neurogastroenterol. & Motil. 1998b;10:403–411. doi: 10.1046/j.1365-2982.1998.00118.x. [DOI] [PubMed] [Google Scholar]
- TRIPPENBACH T. Baclofen-induced block of the Hering-Breuer expiratory-promoting reflex in rats. Can. J. Physiol. Pharmacol. 1995;73:706–713. doi: 10.1139/y95-091. [DOI] [PubMed] [Google Scholar]
- TRIPPENBACH T., LAKE N. Excitatory cardiovascular and respiratory effects of baclofen in intact rats. Can. J. Physiol. Pharmacol. 1994;72:1200–1207. doi: 10.1139/y94-170. [DOI] [PubMed] [Google Scholar]
- YANG H., WONG H., WU V., WALSH J.H., TACHE Y. Somatostatin monoclonal antibody immunoneutralization increases gastrin and gastric acid secretion in urethane-anesthetized rats. Gastroenterology. 1990;99:659–665. doi: 10.1016/0016-5085(90)90952-w. [DOI] [PubMed] [Google Scholar]


