Abstract
The role of endothelin in the initial vasoconstrictor step of hyperacute xenogeneic rejection was investigated.
Isolated rat livers were perfused in recirculation. Perfusion with human sera provided an ex vivo model of hyperacute rejection in a discordant combination.
Perfusion of 10% xenogeneic serum induced a marked (70%) and sustained reduction of the liver flow and induced the release of endothelin into the perfusion medium. In contrast, perfusion of 10% allogeneic serum or of 10% decomplemented human serum induced a weak (25%) and transient reduction of the liver flow and induced the release of minimal amounts of endothelin. The simultaneous administration of BQ 123 and BQ 788, the respective antagonists of ETA and ETB endothelin receptors, or that of bosentan, a mixed ETA/ETB antagonist, antagonized the vasoconstrictor effect of 10% xenogeneic human serum, as well as that of 10−9 M endothelin-1.
The vasoconstrictor effects of xenogeneic serum on liver circulation are, at least partly, mediated through the release of endothelin by the graft.
Keywords: Isolated perfused rat liver, endothelin, endothelin antagonists, bosentan, xenogeneic rejection, vasoconstriction
Introduction
Transplantations between discordant species are invariably followed by hyperacute rejection. Hyperacute xenogeneic rejection is initiated by the binding of natural antibodies to the graft endothelium and by complement activation. This binding leads to an immediate vasoconstriction of the graft, which is considered as the earliest step of rejection. Subsequent stages involve endothelial cell activation and cellular recruitment leading to intravascular clotting and ischemic necrosis of the graft (Auchincloss, 1988; Platt & Bach, 1991). Those phenomena may induce irreversible function damages in the graft and accelerate its rejection. A better knowledge of the mechanisms responsible for the early vasoconstrictor step could help design preventive treatments of hyperacute xenogeneic rejection, in association with inhibition of complement and antibody depletion.
The different steps of hyperacute rejection are diffi cult to study separately because of the rapid overlapping of events following graft revascularization. Isolated perfused organs are a model of choice, since the initial step of hyperacute rejection may be observed after introducing xenogeneic serum into the circuit, thus avoiding blood coagulation and cellular recruitment. Isolated kidneys (Land et al., 1971; Linn et al., 1971) and hearts (Wartenberg & Milgrom, 1978) have been used for that purpose. Isolated perfused rat liver has been widely used to study vasoactivity in the liver (Ballet et al., 1987; Gores et al., 1986; Huet et al., 1993), but rarely to study graft rejection. In the present work, isolated rat livers were perfused with xenogeneic human serum to study hyperacute xenogeneic rejection. This combination is characterized by the presence of natural anti-rat antibodies in human serum that are able to activate complement.
We hypothesized that the vasoconstriction observed at the very beginning of hyperacute rejection was mediated through the in situ production of endothelin by activated endothelial cells. Endothelins are three related vasoactive 21-amino acid polypeptides. Endothelin-1 (ET-1) is the most potent known vasoconstrictor (Yanagisawa et al., 1988). In the liver, ET-1 increases vascular resistances (Gandhi et al., 1990; Okumura et al., 1994; Tran-Thi et al., 1993) and decreases oxygen saturation of blood in sinusoids (Okumura et al., 1994). ET-1 may thus alter microcirculation and oxygen delivery to tissues and induce liver damage. It has been demonstrated that two endothelin receptors (ETA and ETB) mediate the vascular effects of endothelins (Arai et al., 1990; Sakurai et al., 1990). It is generally assumed that the vasoconstrictor action of ET-1 is mediated through ETA receptors (Robert-Levine, 1995). However, it has been recently shown that ETB receptors also exert prolonged vasoconstrictor effects in the rat liver (Zhang et al., 1995; 1997). The vasoactive effects of ET-1 may be reverted by specific antagonists of ETA (Ihara et al., 1992) or ETB receptors (Ishikawa et al., 1994; Karaki et al., 1994). More recently, bosentan has been introduced as a potent mixed, non peptide ETA/ETB receptor antagonist (Gardiner et al., 1994; Palacios et al., 1997).
To investigate whether endothelin plays a role in hyperacute xenogeneic rejection, we designed several sets of experimental studies using the isolated liver perfusion model in the discordant rat-to-human xenogeneic combination.
Methods
Animals
Male adult Sprague-Dawley rats (Janvier, Le Genest-Isle, France), weighing 250–300 g, fed on a standard pellet diet and given water ad libitum, were kept in the animal unit at least 2 days prior to the experiments. They were fasted for 12 h before the experiments, but were given free access to water (50 g l−1 glucose). All the experiments were conducted according to local institutional guidelines for the care and use of laboratory animals.
Preparation of sera
Human sera have been obtained from six healthy human donors by venous puncture after informed consent. For ex vivo experiments, human sera have been pooled. Decomplementation has been performed by heating for 40 min at 56°C. Rat blood has been obtained by arterial puncture at the iliac bifurcation. For ex vivo experiments, the sera from 20 rats have been pooled.
Drugs
ET-1 was from Sigma. BQ123, a selective ETA receptor antagonist (Ihara et al., 1992), and BQ788, a selective ETB receptor antagonist (Ishikawa et al., 1994; Karaki et al., 1994), were from Neosystem, Strasbourg, France. Bosentan, a mixed, non peptide ETA/ETB receptor antagonist (Gardiner et al., 1994; Palacios et al., 1997), was kindly provided by Produits Roche (Neuilly sur Seine, France).
Isolated perfused liver experiments
Isolated perfused rat livers
Rats were anaesthetized with ether, and the liver was prepared according to a standard technique (Marteau et al., 1989). Briefly, the bile duct was cannulated with polyethylene tubing (ID 0.30 mm) (Biotrol, Paris, France). One ml of saline (9 g l−1) containing 1000 IU of heparin (Laboratoire Léo, Neuilly, France) was injected into the penis vein. The portal vein was then cannulated with a large polyethylene catheter (ID 2 mm, length 2 cm) (Biotrol, Asnières, France) and the hepatic artery ligated. The liver was immediately perfused with approximately 20 ml of the perfusion solution, excised and transferred to the perfusion chamber. The entire procedure took less than 10 min. The liver was perfused through portal vein in a recirculating system slightly modified from Miller (Marteau et al., 1989; Miller, 1973), with 100 ml of Krebs-Ringer bicarbonate phosphate buffer with 1% bovine serum albumin (Fraction V, Calbiochem, Los Angeles, U.S.A.). The perfusion solution was supplemented with 1.5 g l−1 of glucose and 2.5 mM calcium. The perfusate was oxygenated with a mixture of 95% O2 and 5% CO2. The pH value of perfusate was maintained at 7.40±0.05 by adjusting CO2 flow. The perfusion was performed at 37°C in a thermostatically controlled cabinet. Portal pressure was equal to the height of the column of medium perfusing the liver and was maintained constant at the physiological value of 11.5±0.15 cm H2O (8.5 mmHg) by overflow of the perfusate into the reservoir. Portal venous flow was determined after diversion of the outflow by measuring the volume of perfusion medium collected over 1 min. All values were expressed as ml min−1 g liver−1. After an equilibration period of 15 min, the outflow from hepatic vein was measured every 5 min. Only experiments where the initial portal flow was higher than 3 ml min−1 g−1 of liver tissue were selected.
Standard criteria were used to assess the viability of the perfused liver (7,8): normal gross appearance; stable pH; bile flow>1 μl min−1 g−1 liver; ALT activity in the venous outflow stable and less than 10 IU l−1 in the controls; rate of increase in potassium in the venous outflow less than 0.3 μM per hour, and oxygen consumption>2 μmol min−1 g−1. The haemodynamic stability of the perfused liver in the basal state was monitored by measuring portal blood flow for 65 min after the equilibration period. This corresponded to the longest experimental period of time, during which the portal blood flow did not vary significantly. The bile flow also remained stable during the control experiments.
During each experiment, liver biopsies were performed before adding serum and then at 5 and 25 min after serum (allo- or xenogeneic) had been introduced into the perfusion medium. At the end of the experiment, the liver was perfused for 10 min with Trypan blue (200 μmol l−1), in order to assess cell viability (Vaubourdolle et al., 1993).
Experimental protocol
In the first set of experiments (five experiments in each group), the effects of different concentrations of ET-1 (Sigma) (10−10 to 10−8 M) on portal circulation were determined. In the control group, the same volume of saline was added at 20 min.
In the second set of experiments (five experiments in each group), the respective roles of ETA and ETB endothelin receptors on the vasoconstrictor effects of ET-1 were assessed. BQ 123 and BQ 788 were introduced 10 min prior to ET-1. Group 1 received 10−9 M ET-1; group 2, 10−9 M ET-1 combined with 10−6 M BQ123 and 10−6 M BQ 788; group 3 (control group) received the same volume of saline.
In the third set of experiments (five experiments in each group), eight experimental groups were designed. After the perfusion flow had been allowed to stabilize for 20 min, the experimental groups were perfused as follows: (1) buffer solution alone; (2) rat allogeneic serum at the final concentration of 10%; (3) human xenogeneic serum at various final concentrations (2.5, 5, 10 and 20%); since the effect of 20% xenogeneic serum was not significantly different from that obtained with 10%, this latter concentration was used in further experiments; (4) decomplemented human xenogeneic serum at the final concentration of 10%; (5) 10% human xenogeneic serum + 10−6 M BQ123 and 10−6 M BQ788; in this group, BQ123 and BQ788 were added into the perfusate 10 min prior to human serum.
In the fourth set of experiments (five experiments in each group), the respective roles of ETA and ETB endothelin receptors on the vasoconstrictor effects of human serum were assessed. After the perfusion flow had been allowed to stabilize for 20 min, the experimental groups were perfused as follows: (1) buffer solution alone; (2) 10% human xenogeneic serum; (3) 10% human xenogeneic serum +10−6 M BQ123; (4) 10% human xenogeneic serum +10−6 M BQ788; BQ123 and BQ788 were added into the perfusate 10 min prior to human serum.
In the fifth set of experiments (five experiments in each group), five experimental groups were designed. After the perfusion flow had been allowed to stabilize for 20 min, the experimental groups were perfused as follows: (1) buffer solution alone; (2) 10% human xenogeneic serum; (3) 10% human xenogeneic serum + 10−5 M bosentan (added to the perfusate 10 min prior to the human serum).
Histological study and immunohistochemistry
A portion of each liver biopsy and of the liver obtained at the end of the experiment was immediately fixed in Bouin's solution then embedded in paraffin. Sections were stained with haematoxylin-eosin-safran for histological examination. The remaining portion of each biopsy was snap-frozen in liquid nitrogen and stored at −80°C for immunohistochemical analysis. Optically, a mild and non specific pattern of injury was constantly observed in the groups perfused with xenogeneic serum as well as in those perfused with allogeneic or decomplemented xenogeneic sera. Periportal hepatocytes were slightly clarified, contrasting with an increased eosinophilic staining of peripheral cells. Only a few endothelial cells (<5%) of sinusoids and portal and central veins were stained after perfusion with Trypan blue (Figure 1).
Figure 1.
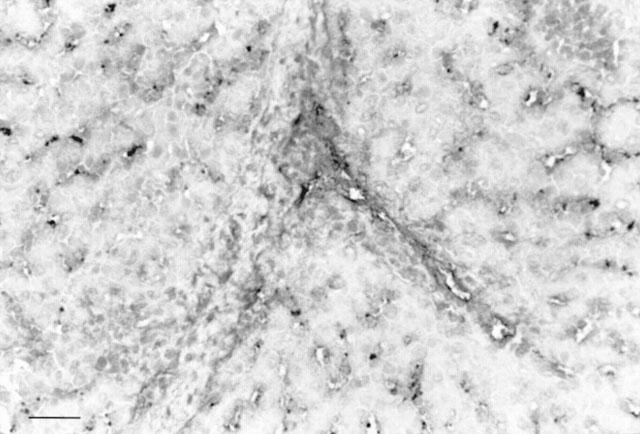
Histological study. Minimal injury was constantly observed in the group perfused with xenogeneic serum. Only a few endothelial cells (<5%) of sinusoids and portal and central veins were stained after perfusion with trypan blue. Haematoxylin-eosin-safran staining. Bar=50 μm.
Cryostat sections from each biopsy, 5 μm thick, were mounted on polylysine-coated slides (CML-CBE). Immunoglobulin and complement expression was evaluated by an indirect immunofluorescence method. After air drying, cryostat sections were successively refixed in acetone for 5 min, incubated with phosphate buffered saline (BioMérieux), pH 7.6, for 10 min, washed, then incubated with mouse monoclonal primary antibody for 1 h in a moist chamber at 37°C. The primary antibodies were anti-human IgG (1:50), anti-human IgM (1:20), or anti-human C3c, C3bi, C4d or factor Bb (1:100)(Byk). The slides were then incubated with 1:100 fluorescein isothiocyanate-labeled goat anti-mouse immunoglobulin (Tebu) as second antibody for 30 min, at room temperature. The method to detect human immunoglobulins and complement was then the same as above. In negative controls, the primary antibody was replaced by a non-relevant antibody at the same dilution. Stained sections were examined using an epifluorescent microscope (Leitz).
Endothelin production
Endothelin production by the perfused liver was estimated by the endothelin levels in the perfusion medium, determined by an ELISA from Cayman Chemical (Ann Arbor, MI, U.S.A.), according to the manufacturer's instructions. The intra- and interassay coefficients of variation are less than 10%. The specificity of the assay for ET-1, ET-2 and ET-3 is 100%, whereas it is <0.01% for big endothelin. The threshold of detection of endothelin in culture media is 5 pg ml−1.
Statistical analysis
Data are expressed as means±s.e.mean. Statistical analysis was done using Statview™ IV software (Abacus Concepts, Berkeley, CA, U.S.A.) on a Macintosh computer. Analysis of variance (ANOVA) and Scheffe's test was used.
Results
Haemodynamic studies
In the control group, the liver flow was 3.12±0.19 ml min−1 g−1 of liver tissue during the first 5 min and reached a maximal level of 3.23±0.22 ml min−1 g−1 after 15 min. The liver flow remained stable at 3.04±0.30 ml min−1 g−1 at the end of the 65 min period of observation (Figure 2). Variations in the liver flow were not significant during the experiment.
Figure 2.
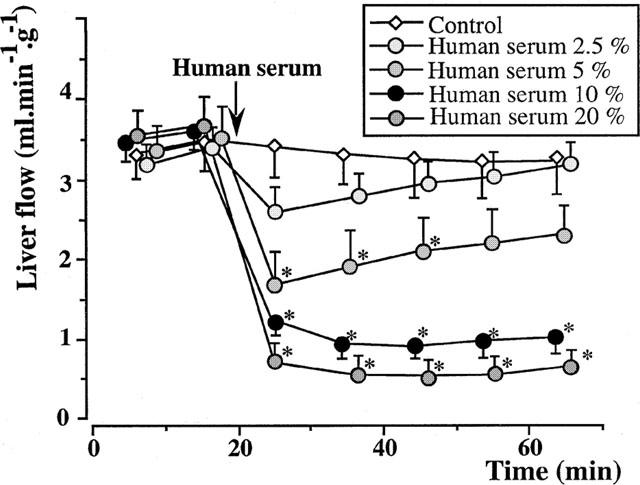
Isolated rat livers perfused with human serum (five experiments in each group). In the control group (perfusion with buffer solution alone), the liver flow remained stable and higher than 3 ml min−1 g−1 during the 65 min period of observation. After perfusion with 2.5, 5, 10 and 20% fresh human serum, the liver flow dropped by 23, 53, 66 and 80%, respectively. *:P<0.05 vs controls.
In the group perfused with 2.5% fresh human serum, the liver flow decreased from 3.35±0.25 ml min−1 g−1 at 15 min, to 2.59±0.29 ml min−1 g−1, 5 min after injection of serum into the medium (decrease by 23% of the initial value), whereas the perfusion with 5% fresh human serum decreased the liver flow by 53%, from 3.50±0.36 to 1.65±0.34 ml min−1 g−1, that with 10% fresh human serum by 66%, from 3.55±0.21 to 1.24±0.14 ml min−1 g−1, and that with 20% fresh human serum by 80%, from 3.69±0.35 to 0.72±0.22 ml min−1 g−1 (Figure 2). In the two latter cases, no tendency to recovery was observed. The concentration of 10% was used in the following experiments.
Perfusion of 10% fresh rat serum led to a transient liver vasoconstriction, with a flow dropping from 3.72±0.22 to 2.45±0.35 ml min−1 g−1 (P<0.05 vs control group). The liver flow returned to 3.56±0.39 ml min−1 g−1, 10 min later, and remained stable until the end of the 65 min period of observation (Figure 3).
Figure 3.
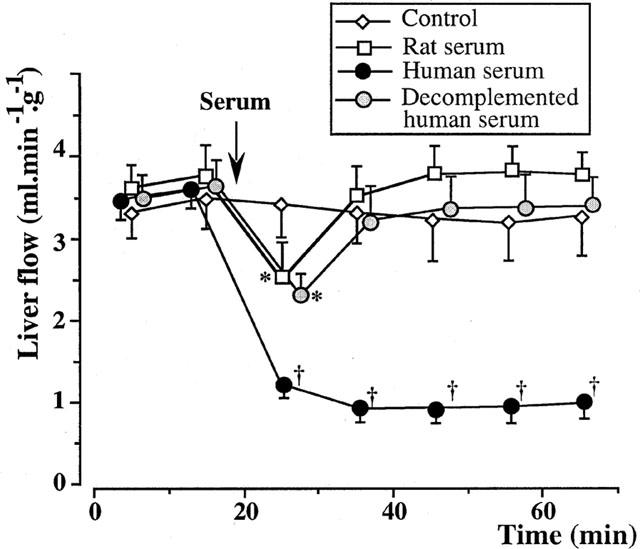
Isolated rat livers perfused with rat or decomplemented human serum (five experiments in each group). In the group perfused with 10% fresh rat serum, a transient liver vasoconstriction was induced. The liver flow recovered to basal value within 10 min, and remained stable until the end of the 65 min period of observation. In the group perfused with 10% decomplemented human serum, a transient liver vasoconstriction was also observed. *:P<0.05 vs controls; †:P<0.01 vs controls.
Decomplemented human serum at 10% also induced a transient liver vasoconstriction, with a flow dropping from 3.62±0.22 to 2.22±0.15 ml min−1 g−1 (P<0.05 vs control group). The liver flow returned to its initial level 10 min later, then remained stable until the end of the experimentation (Figure 3).
The administration of 10−6 M BQ 123 and 10−6 M BQ 788, 10 min before the perfusion of 10% xenogeneic serum, partially reversed the vasoconstrictor effects of the serum (Figure 4). The liver flow decreased from 3.61±0.17 ml min−1 g−1 at 15 min to 2.15±0.26 ml min−1 g−1 at 25 min and 1.71±0.13 ml min−1 g−1 at 35 min. A level of 2.46±0.21 ml min−1 g−1 had been recovered at the end of perfusion. All the values in this group were higher than the corresponding data in the group perfused with fresh human serum alone (P<0.05).
Figure 4.
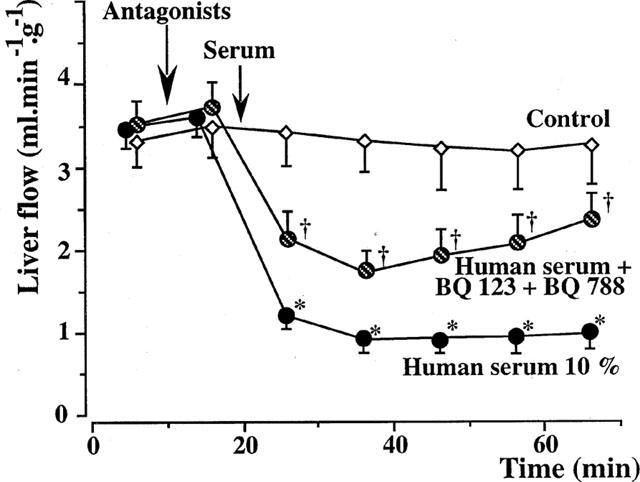
Isolated rat livers perfused with human serum. Effect of combined endothelin antagonists (five experiments in each group). The administration of 10−6 M BQ 123 and 10−6 M BQ 788, 10 min before the serum perfusion, partially reversed the effects of xenogeneic human serum. *:P<0.01 vs controls; †:P<0.05 vs livers perfused with fresh human serum alone.
In order to analyse the contribution of each subtype of endothelin receptor in vasoconstriction during xenogeneic rejection, BQ 123 and BQ 788 (10−6 M) have also been used separately. BQ 123 partially reversed the vasoconstrictor effects of the serum, and BQ 788 had only marginal effects on this vasoconstriction (Figure 5).
Figure 5.
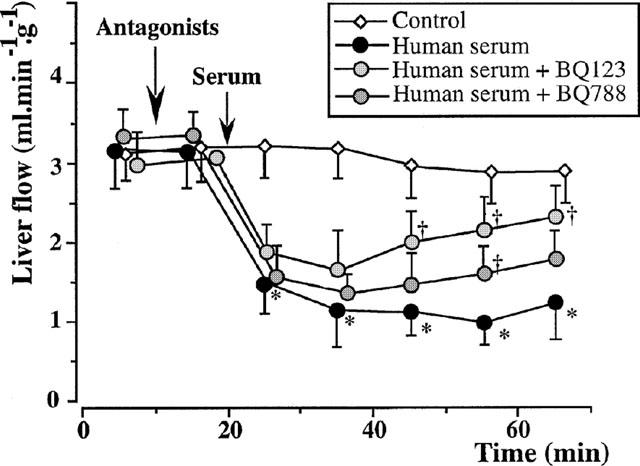
Isolated rat livers perfused with human serum. Individual effects of endothelin antagonists (five experiments in each group). The administration of 10−6 M BQ 123 and 10 min before the serum perfusion, partially reversed the effects of xenogeneic human serum, whereas that of 10−6 M BQ 788 had only marginal effect. *:P<0.01 vs controls; †:P<0.05 vs livers perfused with fresh human serum alone.
The administration of 10−5 M bosentan, 10 min before the perfusion of 10% xenogeneic serum, completely reversed the vasoconstrictor effects of the serum (Figure 6). The liver flow decreased from 3.11±0.57 ml min−1 g−1 at 15 min to 2.55±0.56 ml min−1 g−1 at 25 min and 2.78±0.63 ml min−1 g−1 at 35 min. A level of 2.86±0.61 ml min−1 g−1 had been recovered at the end of perfusion. None of the values in this group were different from those of the control group. Bosentan alone had no significant effect on the liver flow (data not shown).
Figure 6.
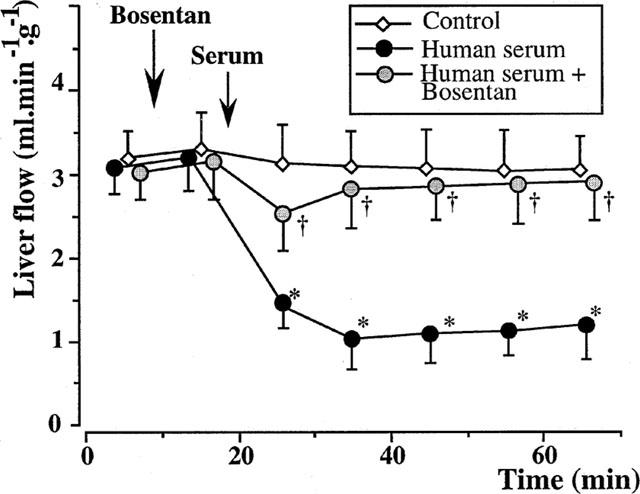
Isolated rat livers perfused with human serum. Effect of bosentan (five experiments in each group). The administration of 10−5 M bosentan, 10 min before the serum perfusion, completely reversed the effects of xenogeneic human serum. *:P<0.01 vs controls; †:P<0.05 vs livers perfused with fresh human serum alone.
The administration of 10−6 M BQ 123 and 10−6 M BQ 788 inhibited the transient liver vasoconstriction induced by the perfusion of 10% rat serum (Figure 7).
Figure 7.
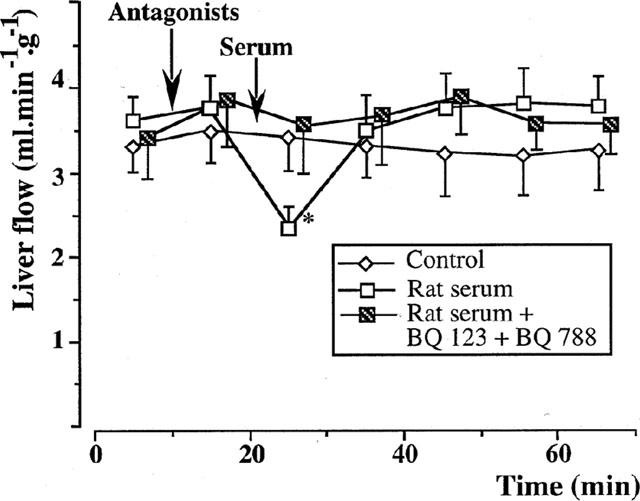
Isolated rat livers perfused with rat serum. Effect of endothelin antagonists (five experiments in each group). The administration of 10−6 M BQ 123 and 10−6 M BQ 788, 10 min before the serum perfusion, inhibited the vasoconstrictive effect of rat serum. *:P<0.05 vs controls.
Endothelin production
Endothelin was undetectable in normal rat and human sera. Adding fresh human xenogeneic sera into the perfusate produced a marked ET-like immunoreactivity production after 5 min, which was significantly higher than during perfusion of decomplemented human sera or allogeneic sera (Figure 8).
Figure 8.
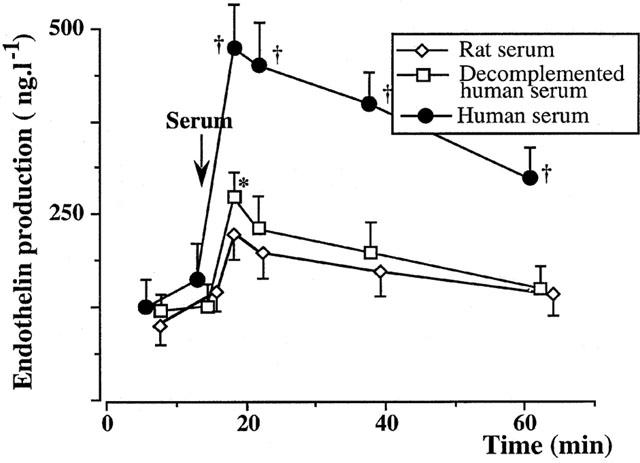
Endothelin production in the isolated perfused rat liver experiments (five experiments in each group). Adding fresh human xenogeneic serum into the perfusate produced a marked endothelin production after 5 min, which was significantly higher than during perfusion of decomplemented human serum or allogeneic serum. *:P<0.05 vs controls perfused with buffer solution.
Immunohistochemistry
On liver biopsies taken 5 and 25 min after adding xenogeneic serum, immunofluorescence studies showed the deposition of human IgM (Figure 9a), C3bi (data not shown), and factor Bb (Figure 9b) along the sinusoidal endothelial cells. In contrast, such deposits were not observed with allogeneic serum or in control experiments (data not shown). After adding decomplemented human serum, only IgM deposits were observed (Figure 9c).
Figure 9.
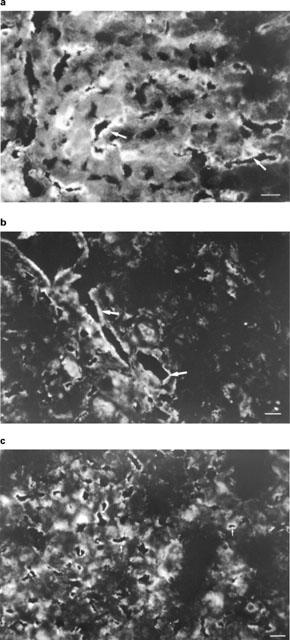
Immunohistochemistry in the isolated perfused rat liver experiments. On liver biopsies taken 5 and 25 min after adding xenogeneic serum, immunofluorescence studies showed the deposition of human IgM (a) and factor Bb (b) along sinusoidal endothelial cells (patterns obtained from one representative experiment out of six). After adding decomplemented human serum, only IgM deposits were observed (c). Bar=10 μm. Deposits are indicated by arrows.
Discussion
This report shows for the first time the role played by endothelin in hyperacute rejection of discordant xenografts. Vasoconstriction of the graft is considered as the earliest step that initiates hyperacute rejection of discordant xenografts (Auchincloss, 1988; Platt & Bach, 1991). Our results support the concept that this vasoconstriction is mediated through the in situ production of endothelin by activated endothelial cells. This finding could help prevent that phenomenon which burdens the functional future of the graft and accelerates its rejection.
Ex vivo perfusion of isolated liver is a model of choice to investigate the vasoactive effects of xenogeneic rejection. In contrast to in vivo models, where all components of xenogeneic rejection act simultaneously, ex vivo experiments allow to discriminate the various factors involved in the rejection process. We used a constant pressure system, because it provides a stable basal vascular resistance and allows direct and sensitive measurement of flow rates (Ballet et al., 1987; Huet et al., 1993; Marteau et al., 1989) for at least 65 min. Perfusion pressure was set at a value within the physiological range of portal pressure values (Vorobioff et al., 1984; Ossenberg et al., 1974). Since the resistance of the perfusion system is a constant factor, we assume that variations in the liver flow reflect variations in the intrahepatic resistance.
The experimental rat-to-human combination was used to investigate the vasoactive effects of hyperacute xenogeneic rejection, because many reagents are readily available to detect human antibodies and complement components in the liver graft. This is a discordant combination, since natural human anti-rat antibodies that can activate complement and lyse rat endothelial cells are present in human sera (data not shown). In the present study, natural human anti-rat antibodies and C3bi and Bb deposits were detected along sinusoidal endothelial cells from rat livers perfused with fresh human serum.
Perfusion of rat livers with fresh human serum induced a dramatic reduction in liver flow, when compared to perfusion with allogeneic serum or to controls. In contrast, we found no significant increase in the release of liver enzymes (alanine aminotransferase, aspartate aminotransferase, lacticodehydrogenase and creatine kinase) after perfusion of human serum, compared to allogeneic or control groups. Moreover, the isoenzyme creatin kinase-BB, a marker of liver sinusoidal damage (Vaubourdolle et al., 1993), remained constantly undetectable in the perfusion medium. Those results suggest that endothelial cells were activated rather than lysed, and that there was no significant parenchymal damage. Mild liver lesions observed under light microscopy were in agreement with the absence of significant increase in liver enzyme production along the experiments.
In contrast, decomplemented human serum and allogeneic sera had minimal effect on liver flow. Those data suggest that complement activation is necessary to efficiently induce the release of vasoconstrictor mediators by stimulated endothelial cells. Both direct and alternative pathway have been activated in this model; no further attempt has been made to determine the respective roles of these two pathways in the release of vasoactive substances.
Several vasoconstrictor mediators could be involved in the phenomenon observed: serotonin, neuropeptide Y, angiotensine II, platelet activating factor, endothelin as well as an inhibition of synthesis of vasodilators such as NO or vasodilatory prostaglandins. In preliminary reports, we and others have shown the early production of endothelin in a discordant xenogeneic combination (Massault et al., 1994; Terajima et al., 1996). In the liver, endothelin seems to be one of the most powerful vasoconstrictor substances. Indeed, we have shown in a previous work that the liver flow decreased by 50% after a perfusion of 10−9 M ET-1 and was interrupted following the perfusion of 10−8 M ET-1 (Zhang et al., 1997).
Our present data strongly suggest that endothelin is an important mediator of the vasoconstriction induced during the initial step of hyperacute xenogeneic rejection: (a) ET-like immunoreactivity was released a few minutes after introducing fresh xenogeneic serum; in contrast, ET-like immunoreactivity release was minimal after introducing allogeneic or decomplemented xenogeneic serum, which have weak vasoconstrictor activities; (b) the vasoconstriction induced by xenogeneic serum was inhibited by previous injection of ETA and ETB receptor antagonists into the perfusate. The inhibition obtained by the combination of ETA and ETB receptor antagonists BQ123 and BQ 788 was only partial, as was the inhibition of the vasoconstriction induced by 10−9 M ET-1 under the conditions used in the present work. In contrast, reversal was complete when bosentan, a mixed ETA/ETB receptor antagonist, was used at 10−5 M. Although we cannot rule out that other vasoconstrictor substances are simultaneously released during the activation of the endothelial cells, this reversal strongly suggests that ET-1 is one of the main substances mediating vasoconstriction during xenogeneic rejection.
It has been previously shown that, in rat liver, ETA and ETB receptors coexist in equal proportions (Journeaux et al., 1994). ETA receptors are usually considered as the mediators of vasoconstriction, whereas ETB receptors mediate vasodilatation in most tissues. However, recent data (Zhang et al., 1995; 1997) suggest that ET-1 modulates hepatic microcirculation via both ETA and ETB receptors. Vascular smooth muscle cells contain both ETA and ETB receptors. ET-1 may cause contraction of presinusoidal portions of the portal vein, artery, and pericentral hepatic veins (Gondo et al., 1993). Moreover, the presence of endothelin receptors has also been demonstrated on stellate cells and sinusoidal endothelial cells (Mallat et al., 1995; Rockey, 1995), and increased production of ET-1 leading to reduced liver blood flow has been shown in the liver after liver allograft rejection (Watschinger et al., 1991).
Whilst both ETA and ETB receptors mediate vasoconstriction to exogenously applied ET-1 in this model of isolated perfused rat liver, it is unclear which subtype is involved in the response to xenogeneic serum, where endothelin is presumed to be released endogenously from endothelial cells. Indeed, exogenously applied endothelin probably targets ETB receptors present on sinusoidal endothelial cells before ETA constrictor receptors. In contrast, endogenously generated endothelin is likely to meet first the ETA constrictor receptors, to which it binds immediately and dissociates slowly, leaving less interaction with ETB subtype. Our results support this hypothesis, since ETB receptor antagonist BQ788 had marginal effects on the vasoconstrictor effects of human serum, whilst ETA receptor antagonist BQ123 significantly reversed this vasoconstriction.
A weak and transient vasoconstriction was also observed in the groups perfused with rat serum or with decomplemented human serum. This phenomenon has been noticed for a long time (Brauer et al., 1953). It could be triggered by endothelium-activating substances, either present in the peripheral blood, or released during the clotting process required to obtain the serum. Those endothelium-activating substances, which seem to be cleared by the liver with an apparent half-life of 10–15 min, remain to be defined. Reversal of the vasoconstriction induced by rat serum by endothelin antagonists suggests that endothelin may be involved. However, the short duration of this vasoconstriction does not allow to conclude, since spontaneous reversal, related to hepatic clearance of other vasoactive mediators, could be involved as well.
In conclusion, intrahepatic release of endothelin occurs very early during hyperacute xenogeneic rejection, following the engagement of the complement cascade and endothelial cell activation. Our data suggest that endothelin is an important mediator of the vasoconstrictor step of hyperacute xenogeneic rejection of the liver. The use of the endothelin antagonists, such as bosentan, in association with inhibitors of complement activation could have synergistic beneficial effects in the prevention of xenogeneic rejection.
Acknowledgments
This study was supported by a grant from INSERM (CRI 94-03) and from the Ministère de la Recherche (ACC-SV). We thank Ms Marie Mianowski for reviewing the manuscript.
Abbreviations
- ET
endothelin
References
- ARAI H., HORI S., ARAMORI I., OHKUBO H., NAKANISHI S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- AUCHINCLOSS H. Xenografting: a review. Transplantation. 1988;46:1–20. doi: 10.1097/00007890-198807000-00001. [DOI] [PubMed] [Google Scholar]
- BALLET F., CHRÉTIEN Y., REY C., POUPON R. Norepinephrine: a potential modulator of the hepatic transport of taurocholate. A study in the isolated perfused rat liver. J. Pharmacol. Exp. Ther. 1987;240:303–307. [PubMed] [Google Scholar]
- BRAUER R.W., LEONG G.F., PESSOTTI R.L. Vasomotor activity in the isolated perfused rat liver. Am. J. Physiol. 1953;174:304–312. doi: 10.1152/ajplegacy.1953.174.2.304. [DOI] [PubMed] [Google Scholar]
- GANDHI C.R., STEPHENSON K., OLSON M.S. Endothelin, a potent peptide agonist in the liver. J. Biol. Chem. 1990;265:17432–17435. [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Effects of bosentan (Ro 47-0203), an ETA-, ETB-receptor antagonist, on regional haemodynamic responses to endothelins in conscious rats. Br. J. Pharmacol. 1994;112:823–830. doi: 10.1111/j.1476-5381.1994.tb13153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDO K., UENO T., SAKAMOTO M., SAKISAKA S., SATA M., TANIKAWA K. The endothelin-1 binding site in rat liver tissue: light- and electron-microscopic autoradiographic studies. Gastroenterology. 1993;104:1745–1749. doi: 10.1016/0016-5085(93)90654-u. [DOI] [PubMed] [Google Scholar]
- GORES G.J., KOST L.J., LARUSSO N.F. The isolated perfused rat liver. Conceptual and practical considerations. Hepatology. 1986;6:511–517. doi: 10.1002/hep.1840060331. [DOI] [PubMed] [Google Scholar]
- HUET P.M., KASSISSIA J., SEMRET J.G.Hemodynamics of isolated perfused liver Perfused liver 1993Paris: Editions INSERM and John Libbey & Co; 39–50.ed Ballet F. & Thurman R.G. [Google Scholar]
- IHARA M., NOGUCHI K., SAEKI T., FUKURODA T., TSUCHIDA S., KIMURA S., FUKAMI T., ISHIKAWA K., NISHIKIBE N., YANO M. Biological profile of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50:247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA K., IHARA M., NOGUCHI K., MASE T., MINO N., SAEKI T., FUKURODA T., FUKAMI T., OZAKI T., NAGASE T., NISHIKIBE M., YANO M. Biochemical and pharmacological profile of a potent and selective endothelin B- receptor antagonist, BQ-788. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4892–4896. doi: 10.1073/pnas.91.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOUNEAUX C., MALLAT A., SERRADEIL-LE GAL C., GOLDSMITH P., HANOUNE J., LOTERSZTAJN S. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J. Biol. Chem. 1994;269:1845–1851. [PubMed] [Google Scholar]
- KARAKI H., SUDJARWO S., HORI M. Novel antagonist of endothelin ETB1 and EBT2 receptors, BQ 788: effects on blood vessel and small intestine. Biochem. Biophys. Res. Commun. 1994;205:168–173. doi: 10.1006/bbrc.1994.2645. [DOI] [PubMed] [Google Scholar]
- LAND W., SCHILLING A., ALDENHOFF J., LAMERZ R., PIELSTICKER K., MENDLER N., BRENDEL W. In vitro studies on the mechanism of hyperacute xenograft rejection. Transplant. Proc. 1971;3:888–890. [PubMed] [Google Scholar]
- LINN B.S., JENSEN J.A., PARDO V., DAVIES D., FRANKLIN L. Relationship between structural and functional changes in rejecting renal xenograft. Transplant. Proc. 1971;3:527–530. [PubMed] [Google Scholar]
- MALLAT A., FOUASSIER L., PRÉAUX A., SERRADEIL-LE GAL C., RAUFASTE D., ROSENBAUM J., DHUMEAUX D., JOUNEAUX C., MAVIER P., LOTERSZTAJN S. Growth inhibitory properties of endothelin-1 in human hepatic myofibroblastic Ito cells. J. Clin. Invest. 1995;96:42–49. doi: 10.1172/JCI118052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTEAU P., BALLET F., CHAZOUILLÈRES O., CHRÉTIEN Y., REY C., PETIT D., POUPON R. Effect of vasodilators on hepatic microcirculation in cirrhosis: a study in the isolated perfused rat liver. Hepatology. 1989;9:820–823. doi: 10.1002/hep.1840090605. [DOI] [PubMed] [Google Scholar]
- MASSAULT P.P., CALMUS Y., CARAYON A., CHERRUAU B., LEGENDRE C., WEILL B., HOUSSIN D. Early endothelin production during hyperacute xenogeneic rejection of the liver. Transplant. Proc. 1994;26:1078. [PubMed] [Google Scholar]
- MILLER L.L.Technique of isolated rat liver perfusion Isolated liver perfusion and its applications 1973New York: Raven Press; 11–52.ed. Bartosek, I., Guatain, A. & Miller, L.L. pp [Google Scholar]
- OKUMURA S., TAKEI Y., KAWANO S., NAGANO K., MASUDA E., GOTO M., TSUJI S., MICHIDA T., CHEN S.S., KASHIWAGI T. Vasoactive effect of endothelin-1 on rat liver in vivo. Hepatology. 1994;19:155–161. doi: 10.1016/0270-9139(94)90067-1. [DOI] [PubMed] [Google Scholar]
- OSSENBERG F.W., DENIS P., BENHAMOU J.P. Hepatic blood flow in the rat: effect of portocaval shunt. J. Appl. Physiol. 1974;37:806–808. doi: 10.1152/jappl.1974.37.6.806. [DOI] [PubMed] [Google Scholar]
- PALACIOS B., LIM S.L., PANG C.C. Subtypes of endothelin receptors that mediate venous effects of endothelin-1 in anaesthetized rats. Br. J. Pharmacol. 1997;122:993–998. doi: 10.1038/sj.bjp.0701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLATT J.L., BACH F.H. The barrier to xenotransplantation. Transplantation. 1991;52:937–947. doi: 10.1097/00007890-199112000-00001. [DOI] [PubMed] [Google Scholar]
- ROBERT-LEVINE E. Endothelins. N. Engl. J. Med. 1995;333:356–363. doi: 10.1056/NEJM199508103330607. [DOI] [PubMed] [Google Scholar]
- ROCKEY D.C. Characterization of endothelin receptors mediating rat hepatic stellate cell contraction. Biochem. Biophys. Res. Comm. 1995;207:725–731. doi: 10.1006/bbrc.1995.1247. [DOI] [PubMed] [Google Scholar]
- SAKURAI T., YANAGISAWA M., TAKUWA Y., MIYAZAKI S., GOTO K., MASAKI T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- TERAJIMA H., YAGI T., SHIRAKATA Y., SHINOHARA H., SATOH S., ARIMA Y., MASHIMA S., HIROSE T., GOMI T., IKAI I., MORIMOTO T., INAMOTO T., YAMAOKA Y. Assessment of hyaluronate clearance and endothelin production during extracorporeal xenogeneic pig liver perfusion. Transplant. Proc. 1996;28:633–634. [PubMed] [Google Scholar]
- TRAN-THI T., KAWADA N., DECKER K. Regulation of endothelin-1 action on the perfused rat liver. FEBS Lett. 1993;318:353–357. doi: 10.1016/0014-5793(93)80544-5. [DOI] [PubMed] [Google Scholar]
- VAUBOURDOLLE M., CHAZOUILLERES O., POUPON R., BALLET F., BRAUNWALD J., LEGENDRE C., BAUDIN B., KIRN A., GIBOUDEAU J. Creatin Kinase-BB: A marker of liver sinusoidal damage in ischemia-reperfusion. Hepatology. 1993;17:423–428. [PubMed] [Google Scholar]
- VOROBIOFF J., BREDFELDT J.E., GROSZMANN R.J. Increased blood flow through the portal system in cirrhotic rats. Gastroenterology. 1984;87:1120–1126. [PubMed] [Google Scholar]
- WARTENBERG J., MILGROM F. ‘Rejection' of heart perfused in vitro by allotransplantation serum. Transplantation. 1978;26:340–345. doi: 10.1097/00007890-197811000-00014. [DOI] [PubMed] [Google Scholar]
- WATSCHINGER B., VYCHYTIL A., SCHULLER M., HARTTER E., TRAINDL O. The pathophysiologic role of endothelin in acute vascular rejection after renal transplantation. Transplantation. 1991;52:743–746. doi: 10.1097/00007890-199110000-00035. [DOI] [PubMed] [Google Scholar]
- YANAGISAWA M., KURIHARA H., KIMURA S., TOMOBE Y., KOBAYASHI M., MITSUI Y., YAZAKI Y., GOTO K., MASAKI T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- ZHANG B., CALMUS Y., WEN L., SOGNI P., LOTERSZTAJN S., HOUSSIN D., WEILL B. Endothelin-1 induces liver vasoconstriction through both ETA and ETB receptors. J. Hepatol. 1997;26:1104–1110. doi: 10.1016/s0168-8278(97)80119-5. [DOI] [PubMed] [Google Scholar]
- ZHANG J.X., BAUER M., CLEMENS M.G. Vessel- and target cell-specific actions of endothelin-1 and endothelin-3 in rat liver. Am. J. Physiol. 1995;269:G269–G277. doi: 10.1152/ajpgi.1995.269.2.G269. [DOI] [PubMed] [Google Scholar]


