Abstract
Acute and chronic mechanisms of action of novel insulinotropic antidiabetic drug, BTS 67 582 (1,1-dimethyl-2-(2-morpholinophenyl)guanidine fumarate), were examined in the stable cultured BRIN-BD11 cell line.
BTS 67 582 (100–400 μM) stimulated a concentration-dependent increase (P<0.01) in insulin release at both non-stimulatory (1.1 mM) and stimulatory (8.4 mM) glucose.
Long-term exposure (3–18 h) to 100 μM BTS 67 582 in culture time-dependently decreased subsequent responsiveness to acute challenge with 200 μM BTS 67 582 or 200 μM tolbutamide at 12–18 h (P<0.001). Similarly 3–18 h culture with the sulphonylurea, tolbutamide (100 μM), also effectively suppressed subsequent insulinotropic responses to both BTS 67 582 and tolbutamide.
Culture with 100 μM BTS 67 582 or 100 μM tolbutamide did not affect basal insulin secretion, cellular insulin content, or cell viability and exerted no influence on the secretory responsiveness to 200 μM of the imidazoline, efaroxan.
While 18 h BTS 67 582 culture did not affect the insulin-releasing actions (P<0.001) of 16.7 mM glucose, 10 mM arginine, 30 mM KCl, 25 μM forskolin or 10 nM phorbol-12-myristate 13-acetate (PMA), significant inhibition (P<0.001) of the insulinotropic effects of 10 mM 2-ketoisocaproic acid (KIC) and 10 mM alanine were observed.
These data suggest that BTS 67 582 shares a common signalling pathway to sulphonylurea but not imidazoline drugs. Desensitization of drug action may provide an important approach to dissect sites of action of novel and established insulinotropic antidiabetic agents.
Keywords: BTS 67 582, tolbutamide, desensitization, clonal pancreatic beta cells, insulin release
Introduction
The guanidine-derivative, BTS 67 582 (1,1-dimethyl-2-(2-morpholinophenyl)guanidine fumarate), represents one of a new class of antidiabetic agents (Bailey et al., 1997). In addition to an ability to lower blood glucose in normal and animal models of type 2 diabetes (Kaul et al., 1995; Jones et al., 1997; Page & Bailey, 1997; Storey & Bailey, 1998), BTS 67 582 has also been demonstrated to exert similar effects in both normal and diabetic humans (Byrom et al., 1994; 1997; Skillman & Raskin, 1997). As a result of these actions, due primarily to elevations of insulin concentrations, BTS 67 582 has been proposed as a clinically useful novel oral antidiabetic drug.
Sulphonylureas remain the most important class of insulinotropic drug currently used in type 2 diabetes therapy (DeFronzo, 1998). These drugs are known to exert direct actions on the pancreatic beta cell through binding to the sulphonylurea receptor subunit (SUR1) of the two component ATP-sensitive-K+ (KATP) channel complex (Ashcroft & Gribble, 1998; Ashfield et al., 1999; Aguilar-Bryan & Bryan, 1999). This elicits membrane depolarization and elevation of cytoplasmic Ca2+ due to increased Ca2+ influx through voltage-dependent calcium channels (VDCC) (Nelson et al. 1992; Dunne et al., 1994; Ashcroft & Gribble, 1998). In addition to their ability to directly induce insulin release, sulphonylureas can also potentiate the insulinotropic actions of glucose, amino acids and 3′: 5′-cyclic monophosphate (cyclic AMP) (Ostenson et al., 1983; Sako et al., 1986). While plasma membrane SUR1 is the primary target of sulphonylurea action, additional intracellular sites are thought to exist through which these drugs may elicit insulin secretion in the absence of KATP channel function (McClenaghan & Flatt, 1999a).
A number of other potentially important oral antidiabetic agents also act through targeting the KATP channel including: imidazolines (e.g. efaroxan, phentolamine); the phenylalanine-dervivative, A-4166; and the benzoic acid-derivative, repaglinide (Chan et al., 1991; Akiyoski et al., 1995; Rustenbeck et al., 1995; Zaitsev et al., 1996; Proks & Ashcroft, 1997; Fuhlendorff et al., 1998). Closure of the KATP channel by these structurally diverse drugs is believed to result primarily from direct interaction with the channel pore (Kir6.2) and/or SUR1 subunits (Chan et al., 1991; Akiyoski et al., 1995; Rustenbeck et al., 1995; Zaitsev et al., 1996; Proks & Ashcroft, 1997; Fuhlendorff et al., 1998). Characterization of the principal mechanisms underlying the insulin-releasing actions of BTS 67 582 also identified the KATP channel as the primary site of action (Dickinson et al., 1997; Louchami et al., 1998; McClenaghan et al., 1998c). However, while previous studies indicate common signalling pathways utilized by BTS 67 582 and sulphonylureas (McClenaghan et al., 1998c), relatively little is known to date about the precise sites of action of BTS 67 582 in the pancreatic beta cell. Recent observations indicate that unlike sulphonylureas, which stimulate a characteristic monophasic insulinotropic response, BTS 67 582 may exert a biphasic insulin-releasing effect (Storey & Bailey, 1998).
Chronic hyperglycaemia in type 2 diabetes is associated with glucose desensitization and glucose toxicity (Ward et al., 1984; Portha et al., 1994; Ammon, 1997; Yki-Jarvinen, 1998; Zawalich et al., 1998). While glucose desensitization has obvious implications in type 2 diabetes, this phenomenon may also extend to other physiological and pharmacological secretagogues, including the sulphonylureas (Grunberger, 1993; Morgan et al., 1994; Chapman et al., 1999). Indeed, the decline in sulphonylurea activity during long term application has been suggested to be directly attributable to a densensitization of the pancreatic beta cell to the action of these drugs (Dunbar & Foa, 1974; Filiponni et al., 1983; Karam et al., 1986; Grunberger, 1993). Due to the widespread use of these drugs, it is perhaps surprising that little attention has been devoted to the mechanisms underlying sulphonylurea densensitization (Gullo et al., 1991; Rabuazzo et al., 1992).
The present study exploits the stable cultured pancreatic BRIN-BD11 cell line (McClenaghan et al., 1996a) to study the acute and chronic mechanisms of action of BTS 67 582 on the pancreatic beta cell. These results demonstrate for the first time that prolonged exposure to BTS 67 582 can induce desensitization to the acute actions of both BTS 67 582 and tolbutamide. Distinct and common modes of action between BTS 67 582, tolbutamide and efaroxan are revealed together with consequences of BTS 67 582 desensitization on the regulation of insulin secretion by other agents. In addition, this study highlights how induction of drug desensitization may be a useful model system to discriminate between the modes of action of a range of therapeutically relevant insulinotropic agents.
Methods
Clonal pancreatic BRIN-BD11 cells (passage 28–35) were used in this study. Characteristics of this glucose-responsive cell line, derived from electrofusion of RINm5F cells with rat pancreatic beta cells, have been detailed elsewhere (McClenaghan et al., 1996a,1996b,1996c, 1998b; McClenaghan & Flatt, 1999a). BRIN-BD11 cells typically retain their functional features in extended culture and thus offer an attractive alternative to cultured pancreatic beta cells, which exhibit an inherently short functional lifespan.
BRIN-BD11 cells were grown in RPMI-1640 tissue culture medium containing 11.1 mM glucose, 0.3 g l−11 L-glutamine, and supplemented with 10% (v v−1) foetal calf serum, 100 IU ml−1 penicillin and 0.1 g l−1 streptomycin (Gibco, Paisley, Strathclyde, U.K.). Cells were washed with Hank's balanced saline solution prior to detachment from tissue culture flasks with the aid of 0.025% trypsin containing 1 mM EDTA. Cells were then seeded at 1.5×105 cells per well into 24-multiwell plates (Nunc, Rolkilde, Denmark). Monolayers of BRIN-BD11 cells were cultured (3–18 h) in the absence (standard culture conditions) or presence of either 100 μM BTS 67 582 (Knoll Pharmaceuticals Research and Development, Nottingham, U.K.) or tolbutamide (Sigma Chemical Company, Poole, U.K.) at 37°C. After the appropriate period, culture medium was replaced with 1 ml of a Krebs Ringer Bicarbonate (KRB) buffer supplemented with 0.1% bovine serum albumin and 1.1 mM glucose (McClenaghan et al., 1996c). Following a 40 min preincubation step at 37°C, to allow the cells to equilibrate with the KRB buffer, the preincubation buffer was replaced with 1 ml of KRB test buffer containing 1.1 or 8.4 mM glucose plus test agents as detailed in the legends to the Figures. After 20 min incubation at 37°C, aliquots of test buffer were removed and stored at −20°C for insulin radioimmunoassay (Flatt & Bailey, 1981). Insulin release (ng per 106 cells 20 min−1) is expressed as mean±standard error of the mean (s.e.mean) of six independent observations. Groups were compared by Student's unpaired t-test and differences considered significant if P<0.05.
Results
Insulinotropic responses at non-stimulatory and stimulatory glucose concentrations
Acute static incubation with 100–400 μM BTS 67 582 (EC50 150) evoked a 1.2–2.6 fold concentration-dependent increase (P<0.01 to P<0.001) in insulin release at non-stimulatory (1.1 mM) and stimulatory (8.4 mM) glucose (Figure 1). Characteristic of the BRIN-BD11 cells, raising the glucose concentration from 1.1 to 8.4 mM stimulated a 1.6 fold (P<0.001) insulin-secretory response, and resulted in an additive 1.1–1.5 fold (P<0.05 to P<0.001) potentiation of BTS 67 582-induced insulin release (Figure 1). BTS 67 582 exhibited a similar secretory profile to tolbutamide (maximal response 400 μM; EC50 160; unpublished observations) consistent with an almost equipotent effect in rat islets (Louchami et al., 1998).
Figure 1.
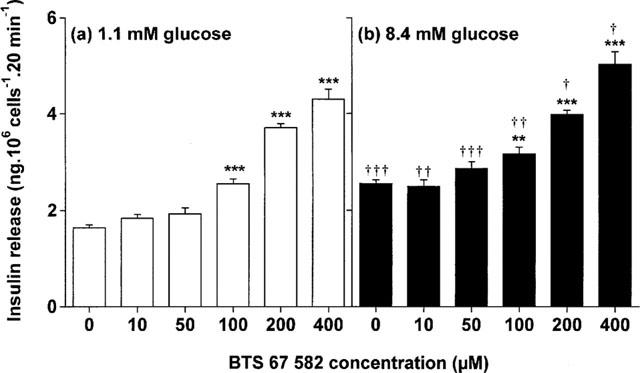
Effects of 0–400 μM BTS 67 582 at (a) non-stimulatory (1.1 mM) or (b) stimulatory (8.4 mM) glucose. Following 40 min of preincubation with a buffer containing 1.1 mM glucose, effects of BTS 67 582 were tested during a 20 min incubation period. Values are mean±s.e.mean for six separate observations. **P<0.01, ***P<0.001 compared with respective effects in the absence of BTS 67 582. †P<0.05, ††P<0.01, †††P<0.001 compared with respective effects at 1.1 mM glucose.
Responsiveness to insulinotropic drugs following exposure to BTS 67 582 and tolbutamide in culture
As shown in Figure 2a, BTS 67 582, efaroxan and tolbutamide (each at 200 μM) elicited respective 2.1-, 2.5- and 1.9 fold (P<0.001) insulin secretory responses from the BRIN-BD11 cells after culturing for 18 h in standard RPMI-1640 media. However, 18 h culture with 100 μM BTS 67 582 abolished subsequent secretory responses to both BTS 67 582 and tolbutamide (Figure 2a). Notably the response to efaroxan was unaffected (Figure 2a), indicating different sites of action of BTS 67 582 and imidazoline drugs. A similar pattern emerged following 18 h culture with 100 μM tolbutamide, with an abolition of both BTS 67 582 and tolbutamide-induced insulin release, whilst the insulinotropic actions of efaroxan remained intact (Figure 2b).
Figure 2.
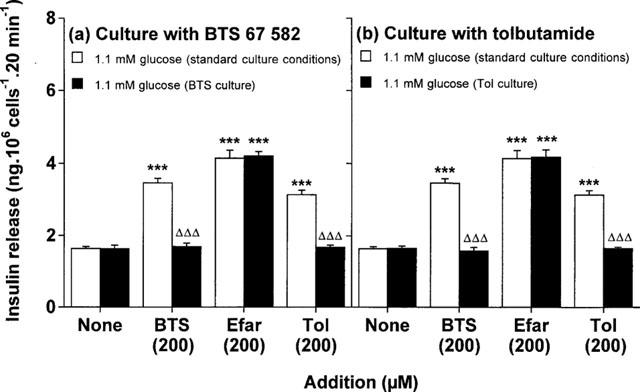
Effects of culture with (a) BTS 67 582 or (b) tolbutamide on BTS 67 582-, efaroxan- and tolbutamide-induced insulin secretion. After 18 h culture in the absence (standard culture conditions) or presence of either 100 μM BTS 67 582 (BTS culture) or 100 μM tolbutamide (Tol culture), cells were preincubated for 40 min before 20 min acute incubation with a buffer containing 1.1 mM glucose in the absence or presence of 200 μM BTS 67 582 (BTS), 200 μM efaroxan (Efar) or 200 μM tolbutamide (Tol). Values are mean±s.e.mean for six separate observations. ***P<0.001 compared with respective effects in the absence of addition. ΔΔΔP<0.001 compared with respective effects after standard culture conditions.
Time-dependency of downregulation of insulinotropic actions of BTS 67 582 and tolbutamide
Subsequent responsiveness to BTS 67 582 or tolbutamide was examined following 3, 6, 12 and 18 h to examine the possible time-dependency of downregulation. As shown in Figure 3a, there was a progressive decline in the insulin secretory effects of BTS 67 582 following 3–18 h culture with 100 μM BTS 67 582, reaching significance at 12 h (61% decrease, P<0.001) and with a maximum suppression (84% decrease, P<0.001) by 18 h. Subsequent responsiveness to tolbutamide after 3–18 h exposure to BTS 67 582 (Figure 3b) followed a similar pattern, also reaching significance at 12 h (73% decrease, P<0.001) and with a maximal reduction (95% decrease, P<0.001) by 18 h. As shown in Figure 3c, 12–18 h culture with 100 μM tolbutamide was also effective at suppressing (by 89–96%, P<0.001) subsequent responsiveness to BTS 67 582. These conditions similarly suppressed (by 44–58%, P<0.01 to P<0.001) the subsequent insulinotropic action of tolbutamide (data not shown). Culture for 3–18 h with BTS 67 582 or tolbutamide did not affect basal insulin secretion (at 1.1 mM glucose), cellular insulin content (64–71 ng 106 cells−1), or cell viability (assessed using trypan blue). Prolonged exposure to efaroxan (6–18 h) did not alter subsequent secretory responsiveness to either BTS 67 582 or tolbutamide (unpublished observations).
Figure 3.
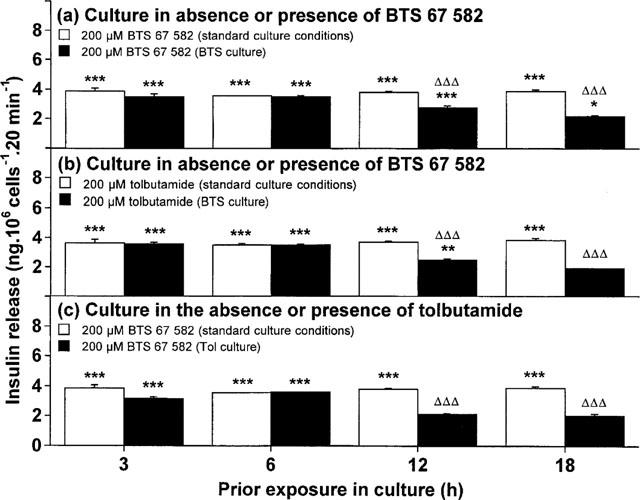
Time-dependent effects of culture with (a,b) BTS 67 582 or (c) tolbutamide on subsequent insulin-secretory responsiveness to (a,c) BTS 67 582 and (b) tolbutamide. After 3, 6, 12 or 18 h culture in the absence (standard culture conditions) or presence of either 100 μM BTS 67 582 (BTS culture) or 100 μM tolbutamide (Tol culture), cells were preincubated for 40 min before 20 min acute incubation with a buffer containing 1.1 mM glucose in the absence or presence of either 200 μM BTS 67 582 (BTS) or 200 μM tolbutamide (Tol). Values are mean±s.e.mean for six separate observations. Insulin-secretory responses to control (1.1 mM glucose) remained constant from 3–18 h regardless of culture condition employed (in ng per 106 cells 20 min−1: 1.90±0.11 for standard culture conditions; 1.97±0.12 for BTS culture; 1.88±0.09 for Tol culture). *P<0.05, **P<0.01, ***P<0.001 compared with respective effects of control (1.1 mM glucose alone). ΔΔΔP<0.001 compared with respective effects after standard culture conditions.
Reversibility of downregulation of insulinotropic actions of BTS 67 582
Possible recovery of the insulinotropic capacities of BTS 67 582 and tolbutamide after drug exposure were assessed after recovery periods of 6 and 12 h. Returning BRIN-BD11 cells to standard culture conditions following 18 h culture with 100 μM BTS 67 582 resulted in a 418% increase (P<0.001; 56% of maximal response) insulin-secretory responsiveness to BTS 67 582 at 6 h, with a complete recovery (712% increase, P<0.001) at 12 h (Figure 4a). Likewise, a significant 326% increase (P<0.001; 43% of maximal response) in secretory responsiveness to tolbutamide was observed at 6 h, with full responsiveness (667% increase, P<0.001) being achieved 12 h post-BTS 67 582 culture (Figure 4b). After tolbutamide culture, secretory responsiveness to BTS 67 582 returned at 6 h (544% increase, P<0.001; 55% of maximal response) with a complete restoration (908% increase, P<0.001) at 12 h (Figure 4c). Secretory responsiveness to tolbutamide similarly returned at 6 h (P<0.001) and was fully restored at 12 h (unpublished observations).
Figure 4.
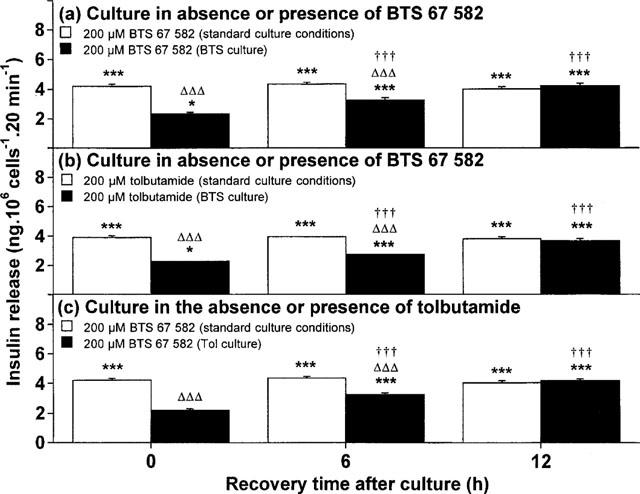
Time-dependent recovery of insulin-secretory responsiveness to (a,c) BTS 67 582 or (b) tolbutamide after 18 h culture with (a,b) 100 μM BTS 67 582 or (c) tolbutamide. After 18 h culture in the absence (standard culture conditions) or presence of either 100 μM BTS 67 582 (BTS culture) or 100 μM tolbutamide (Tol culture), cells were cultured in the absence of BTS 67 582 or tolbutamide for 0, 12 or 18 h before preincubation (40 min) and subsequent 20 min incubation with a buffer containing 1.1 mM glucose in the absence or presence of 200 μM BTS 67 582 (BTS) or 200 μM tolbutamide (Tol). Values are mean±s.e.mean for six separate observations. Insulin-secretory responses to control (1.1 mM glucose) remained constant from 3–18 h regardless of culture condition employed (in ng per 106 cells 20 min−1: 1.92±0.08 for standard culture conditions; 2.00±0.08 for BTS culture; 1.96±0.11 for Tol culture). *P<0.05, ***P<0.001 compared with respective effects of control (1.1 mM glucose). ΔΔΔP<0.001 compared with respective effects after standard culture conditions. †††P<0.001 compared with respective effects at 0 h.
Downregulation with BTS 67 582 on actions of nutrient insulin secretagogues and KCl
Insulinotropic responses to stimulatory (16.7 mM) glucose, 10 mM 2-ketoisocaproic acid (KIC), 10 mM alanine, 10 mM arginine or 30 mM KCl were examined after 18 h culture in the absence or presence of 100 μM BTS 67 582. As shown in Figure 5, raising the glucose concentration from 1.1 to 16.7 mM glucose stimulated a 1.8 fold increase in insulin secretion, which remained unaffected after BTS 67 582 culture. The 2-keto acid, KIC stimulated a 2.2 fold (P<0.001) insulin secretory response which was inhibited (by 43%, P<0.001) by BTS 67 582 culture (Figure 5). Likewise, alanine stimulated a 7.1 fold (P<0.001) insulin secretory response which was impaired (41% decrease, P<0.001) following culture with BTS 67 582 (Figure 5). Interestingly, the respective 3.3 and 9.9 fold responses to 10 mM arginine and 30 mM KCl remained unaffected (Figure 5), indicating that BRIN-BD11 cells were still able to exhibit appropriate responses to membrane depolarization after BTS 67 582 culture. Other studies have confirmed 18 h culture with 100 μM tolbutamide did not affect subsequent secretory responses to equimolar glucose, KIC, alanine or KCl (unpublished observations), indicating the complexity of the mechanisms underlying desensitization by drugs.
Figure 5.
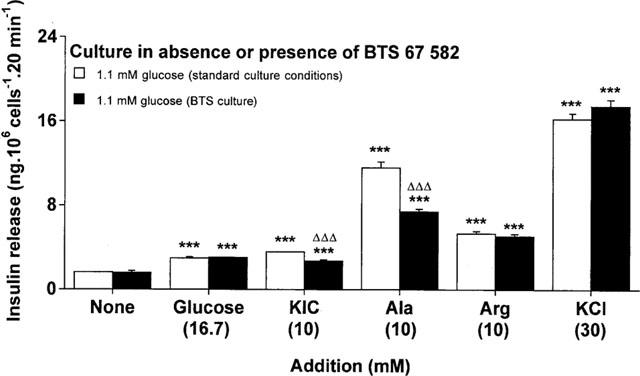
Effects of culture with BTS 67 582 on insulin secretory responses to nutrient secretagogues and KCl. After 18 h culture in the absence or presence of 100 μM BTS 67 582 (BTS culture), cells were preincubated for 40 min before 20 min acute incubation with a buffer containing 1.1 mM glucose in absence or presence of 16.7 mM glucose, 10 mM 2-ketoisocaproic acid (KIC), 10 mM L-alanine (Ala), 10 mM L-arginine (Arg) or 30 mM KCl. Values are mean±s.e.mean for six separate observations. ***P<0.001 compared with respective effects in the absence of addition. ΔΔΔP<0.001 compared with respective effects after standard culture conditions.
Effects of downregulation with BTS 67 582 culture on secretory pathways activated by carbachol, forskolin or PMA
Insulin secretory responsiveness to carbachol (100 μM), forskolin (25 μM) or phorbol 12-myristate 13-acetate (PMA; 10 nM) were assessed after 18 h culture in the absence or presence of 100 μM BTS 67 582. As shown in Figure 6, the 1.3, 3.7 and 7.4 fold insulin secretory responses to carbachol, forskolin and PMA were totally unaffected following BTS 67 582 culture, indicating that BRIN-BD11 cells retained intact phospholipase C (PLC), adenylate cyclase, protein kinase A (PKA) and protein kinase C (PKC) mediated signalling pathways.
Figure 6.
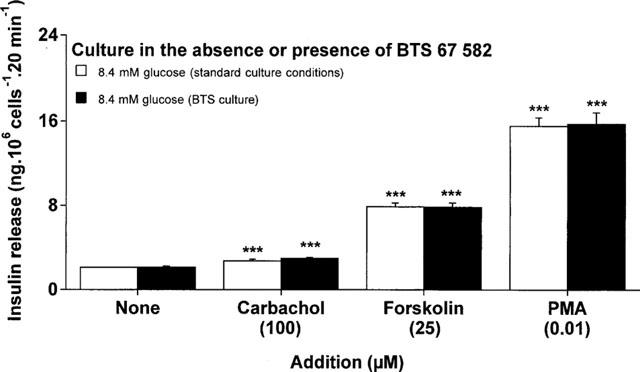
Effects of culture with BTS 67 582 on insulin secretory responses to carbachol, forskolin or phorbol-12-myristate 13-acetate (PMA). After 18 h culture in the absence or presence of 100 μM BTS 67 582 (BTS culture), cells were preincubated for 40 min before 20 min acute incubation with a buffer containing 8.4 mM glucose in absence or presence of 100 μM carbachol, 25 μM forskolin or 10 nM PMA. Values are mean±s.e.mean for six separate observations. ***P<0.001 compared with respective effects in the absence of addition.
Discussion
The present study examines the long-term effects and modes of action of the novel antidiabetic drug BTS 67 582, using cultured clonal glucose-responsive insulin-secreting cells. The BRIN-BD11 cell line has been thoroughly characterized and displays appropriate secretory responses to a wide range of physiological and pharmacological regulators of insulin release (see McClenaghan et al., 1996a,1996b,1996c; McClenaghan & Flatt, 1999a,1999b). These attributes combined with a high degree of functional stability, make such cells particularly useful for evaluation of longer-term effects of drugs on pancreatic beta cell function in culture.
Consistent with previous observations, BTS 67 582 acted as both an initiator and potentiator of insulin release from BRIN-BD11 cells (McClenaghan et al., 1998c). In the presence of both non-stimulatory (1.1 mM) or stimulatory (8.4 mM) glucose concentrations, BTS 67 582 elicited insulin-secretory responses in a concentration-dependent manner over the range 100–400 μM. These data support studies using perifused rat islets which demonstrated notable insulin-releasing effects in the presence of physiological concentrations of glucose (5–8 mM) (Dickinson et al., 1997). BRIN-BD11 cells also responded to two other important classes of antidiabetic agent, the imidazoline drug, efaroxan, and the sulphonylurea, tolbutamide; and it is notable that equimolar amounts of each of these structurally diverse compounds had similar insulin-releasing potencies.
Prolonged use of sulphonyureas in type 2 diabetes is often associated with a progressive decline in their glucose-lowering ability (Grunberger, 1993; Harrower, 1994; Pontiroli et al., 1994). This phenomenon has been attributed to a progressive desensitization of the pancreatic beta cells to sulphonylurea action (Dunbar & Foa, 1974; Filiponni et al., 1983; Karam et al., 1986; Grunberger, 1993). The present study evaluated whether this phenomenon could be induced in vitro and exploited to study the site(s) of action of BTS 67 582 and other important antidiabetic drugs. New evidence is provided indicating desensitization to BTS 67 582 by 18 h exposure to this drug. Furthermore, culture with BTS 67 582 culture also induced desensitization to insulinotropic effects tolbutamide, but not efaroxan. Sulphonylurea desensitization could also be induced by 18 h culture of BRIN-BD11 cells with tolbutamide, indicating no particular advantage or disadvantage with either class of drug. The insulinotropic action of BTS 67 582 was also desensitized by sulphonylurea exposure, suggestive of common sites of action. The inability of either drug in culture to affect efaroxan-induced insulin secretion clearly indicates distinct and novel actions of imidazolines (Morgan et al., 1994; Efanov et al., 1998; Chapman et al., 1999).
Taken together, these observations support the notion (McClenaghan et al., 1998c) that BTS 67 582 exerts its primary effects through similar signalling pathways to tolbutamide, extending this view to suggest diverse sites of interaction from efaroxan. Notably, the induction of desensitization by BTS 67 582 followed a time-dependent pattern becoming significant after 12 h exposure in culture, with a maximum inhibition of both BTS 67 582- and tolbutamide-induced insulin release by 18 h. Similarly, exposure of BRIN-BD11 cells to 12 h tolbutamide culture significantly reduced the insulinotropic response to BTS 67 582, with an abolition of BTS 67 582-induced insulin secretion after 18 h tolbutamide culture. Reversibility of drug desensitization was inducible by exposing desensitized cells to culture in the absence of BTS 67 582 or tolbutamide. In each case, BTS 67 582 or tolbutamide desensitization of insulin secretion was significantly reversed after 6 h, with a full restoration of drug-induced secretory responsiveness by 12 h. These observations together with lack of effect of 18 h culture on basal insulin secretion or cellular insulin content argue against a simple toxic action of these drugs.
Additional experiments were perfomed to examine the nature of BTS 67 582 desensitization, and its impact on the actions of established secretagogues. Consistent with previous observations (McClenaghan et al., 1996a,1996b; 1998a; McClenaghan & Flatt, 1999a,1999b), BRIN-BD11 cells exposed to standard culture conditions showed notable insulin secretory responses to glucose, KIC, alanine, arginine and 30 mM KCl. Interestingly, BTS 67 582 desensitization was associated with a reduction in the secretory activity of the metabolizable nutrients KIC and alanine but not that of glucose or the depolarizing actions of either arginine or KCl. However, BTS 67 582 desensitization did not alter the actions of carbachol, forskolin or PMA indicating intact PLC-, PKA- and PKC-mediated signal function, respectively. Taken together these data indicate that BTS 67 582 desensitization, while not generally affecting late steps in stimulus secretion coupling, may share and hence alter common signalling pathways to KIC and alanine.
These actions may reflect a change in beta cell function relating to the regulation of KATP channel activity perhaps, in the case of metabolizable amino acids, mediated at a mitochondrial level, resulting in the alteration of metabolite generation and/or action on the channel. When considering the present data it is important to note that in addition to affecting the beta cell stimulus secretion pathway through ATP generation, KIC can also directly inhibit the KATP channel (Branstrom et al., 1998). While being cautious not to overinterpret these findings, is interesting to speculate that BTS 67 582 desensitization, in addition to influencing its own binding and hence secretory function, could also possibly interfere with direct actions of KIC and other compounds on the KATP channel.
In conclusion, while the exact site(s) of action of BTS 67 582 remains to be established, the present data support the view that BTS 67 582 and tolbutamide share common beta cell signalling pathways. This study also demonstrates for the first time the induction of BTS 67 582 densensitization in a clonal beta cell line and, as indicated by Chapman et al. (1999), elucidation of the mechanisms underlying desensitization by drugs is not straightforward. In addition to providing a unique model system in which to study the mechanism and sites of action of BTS 67 582, future studies utilizing this novel approach should help unravel the complex signalling pathways utilized by a spectrum of clinically relevant insulinotropic drugs.
Abbreviations
- BTS 67 582
1,1-dimethyl-2-(2-morpholinophenyl)guanidine fumarate
- cyclic AMP
3′: 5′-cyclic monophosphate
- KATP channel
ATP-sensitive-K+ channel
- KIC
2-ketoisocaproic acid
- PLC
phospholipase C
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- VDCC
voltage-dependent calcium channel
References
- AGUILAR-BRYAN L., BRYAN J. Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 1999;20:101–135. doi: 10.1210/edrv.20.2.0361. [DOI] [PubMed] [Google Scholar]
- AKIYOSHI M., KAKEI M., NAKAZAKI M., TANAKA H. A new hypoglycemic agent, A-4166, inhibits ATP-sensitive potassium channels in rat pancreatic beta-cells. Am. J. Physiol. 1995;268:E185–E193. doi: 10.1152/ajpendo.1995.268.2.E185. [DOI] [PubMed] [Google Scholar]
- AMMON H.P. Hyper- and hypoinsulinemia in type-2 diabetes: what may be wrong in the secretory mechanism of the B-cell. Exp. Clin Endocrinol. Diabetes. 1997;105 Suppl. 2:43–47. doi: 10.1055/s-0029-1211796. [DOI] [PubMed] [Google Scholar]
- ASHCROFT F.M., GRIBBLE F.M. Correlating structure and function in ATP- sensitive K+ channels. Trends Neurosci. 1998;21:288–294. doi: 10.1016/s0166-2236(98)01225-9. [DOI] [PubMed] [Google Scholar]
- ASHFIELD R., GRIBBLE F.M., ASHCROFT S.J., ASHCROFT F.M. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–1347. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- BAILEY C.J., WILLIAMS G., PICKUP J.C.New drugs in the management of diabetes and its complications Textbook of Diabetes 1997Oxford: Blackwell Science; 84.1–84.30.2nd edn. ed. Pickup, J.C. & Williams, G. pp [Google Scholar]
- BRANSTROM R., EFENDIC S., BERGGREN P.O., LARSSON O. Direct inhibition of the pancreatic beta-cell ATP-regulated potassium channel by α- ketoisocaproate. J. Biol. Chem. 1998;273:14113–14118. doi: 10.1074/jbc.273.23.14113. [DOI] [PubMed] [Google Scholar]
- BYROM W.D., ROTHERHAM N.E., BRATTY J.R. Relationship between hypoglycaemic response and plasma concentrations of BTS 67 582 in healthy volunteers. Br. J. Clin. Pharmacol. 1994;38:433–439. doi: 10.1111/j.1365-2125.1994.tb04379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYROM W.D., WEIL A., BROWN T.J., BRATTY J.R. BTS 67 582 improves 24 h plasma glucose control and post-prandial insulin secretion in NIDDM patients. Diabetologia. 1997;39 Suppl. 1:A44. [Google Scholar]
- CHAN S.L., DUNNE M.J., STILLINGS M.R., MORGAN N.G. The alpha 2- adrenoceptor antagonist efaroxan modulates K+ATP channels in insulin-secreting cells. Eur. J. Pharmacol. 1991;204:41–48. doi: 10.1016/0014-2999(91)90833-c. [DOI] [PubMed] [Google Scholar]
- CHAPMAN J.C., MCCLENAGHAN N.H., COSGROVE K.E., HASHMI M.N., SHEPHERD R.M., GIESBERTS A.N., WHITE S.J., AMMALA C., FLATT P.R., DUNNE M.J. ATP-sensitive potassium channels and efaroxan-induced insulin release in the electrofusion-derived BRIN-BD11 beta cell line. Diabetes. 1999;48:2349–2357. doi: 10.2337/diabetes.48.12.2349. [DOI] [PubMed] [Google Scholar]
- DeFRONZO R.A. Current Therapy of Diabetes Mellitus 1998St. Louis, MO: Mosby; (ed.)p 272 [Google Scholar]
- DICKINSON K., NORTH T.J., SILLS S., ANTHONY D.M., LOCK J.I., VOWLES D.T., JONES R.B. BTS 67 582 stimulates insulin secretion from perifused rat pancreatic islets. Eur. J. Pharmacol. 1997;339:69–76. doi: 10.1016/s0014-2999(97)01356-3. [DOI] [PubMed] [Google Scholar]
- DUNBAR J.C., FOA P.P. An inhibitory effect of tolbutamide and glibenclamide on the pancreatic islets of normal animals. Diabetologia. 1974;10:27–32. doi: 10.1007/BF00421411. [DOI] [PubMed] [Google Scholar]
- DUNNE M.J., HARDING E.A., JAGGAR J.H., AYTON B.J., SQUIRES P.E.Endogenous and chemical activators of ATP-regulated potassium channels in insulin-secreting cells: possible mechanisms and physiological significance Frontiers of Insulin Secretion and Pancreatic B-Cell Research 1994London: Smith Gordon; 153–159.ed. Flatt, P.R. & Lenzen, S. pp [Google Scholar]
- EFANOV A.M., ZAITSEV S.V., EFANOVA I.B., ZHU S., OSTENSON C.G., BERGGREN P.O., EFENDIC S. Signaling and sites of interaction for RX- 871024 and sulphonylurea in the stimulation of insulin release. Am. J. Physiol. 1998;274:E751–E757. doi: 10.1152/ajpendo.1998.274.4.E751. [DOI] [PubMed] [Google Scholar]
- FILIPONNI P., MARCELLI M., NICOLETTI I., PACIFICI R., SANEUSANIO F., BRUNETTI P. Suppressive effect of long-term sulphonylurea treatment on A, B, and D cells of normal rat pancreas. Endocrinology. 1983;113:1972–1979. doi: 10.1210/endo-113-6-1972. [DOI] [PubMed] [Google Scholar]
- FLATT P.R., BAILEY C.J. Abnormal plasma glucose and insulin responses in heterozygous (ob/+) mice. Diabetologia. 1981;20:573–577. doi: 10.1007/BF00252768. [DOI] [PubMed] [Google Scholar]
- FUHLENDORFF J., RORSMAN P., KOFOD H., BRAND C.L., ROLIN B., MACKAY P., SHYMKO R., CARR R.D. Stimulation of insulin release by repaglinide and glibenclamide involves both common and distinct processes. Diabetes. 1998;47:345–351. doi: 10.2337/diabetes.47.3.345. [DOI] [PubMed] [Google Scholar]
- GRUNBERGER G. Continuous versus intermittent sulphonylurea therapy in non-insulin-dependent diabetes mellitus. Drug Safety. 1993;9:249–253. doi: 10.2165/00002018-199309040-00002. [DOI] [PubMed] [Google Scholar]
- GULLO D., RABUAZZO A.M., VETRI M., GATTA C., VINCI C., BUSCEMA M., VIGNERI R., PURRELLO F. Chronic exposure to glibenclamide impairs insulin secretion in isolated rat pancreatic islets. J. Endocrinol. Invest. 1991;14:287–291. doi: 10.1007/BF03346813. [DOI] [PubMed] [Google Scholar]
- HARROWER A.D. Comparison of efficacy, secondary failure rate, and complications of sulphonylureas. J. Diabetes Complications. 1994;8:201–203. doi: 10.1016/1056-8727(94)90044-2. [DOI] [PubMed] [Google Scholar]
- JONES R.B., DICKINSON K., ANTHONY D.M., MARITA A.R., KAUL C.L., BUCKETT W.R. Evaluation of BTS 67 582, a novel antidiabetic agent, in normal and diabetic rats. Br. J. Pharmacol. 1997;120:1135–1143. doi: 10.1038/sj.bjp.0701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARAM J.H., SANZ N., SALAMON E., NOLTE M.S. Selective unresponsiveness of pancreatic beta-cells to acute sulfonylurea stimulation during sulfonylurea therapy in NIDDM. Diabetes. 1986;35:1314–1320. doi: 10.2337/diab.35.12.1314. [DOI] [PubMed] [Google Scholar]
- KAUL C.L., MARITA A.R., DICKINSON K., JONES R.B. BTS 67 582, a novel glucose-lowering agent. Br. J. Pharmacol. 1995;114:256P. doi: 10.1038/sj.bjp.0701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOUCHAMI K., JIJAKLI H., JONES R.B., MALAISSE W.J. Effect of 1,1- dimethyl-2-[2-morpholinophenyl]guanidine fumarate on pancreatic islet function. Eur. J. Pharmacol. 1998;352:289–297. doi: 10.1016/s0014-2999(98)00352-5. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BARNETT C.R., AH-SING E., ABDEL-WAHAB Y.H., O'HARTE F.P., YOON T.W., SWANSTON-FLATT S.K., FLATT P.R. Characterization of a novel glucose-responsive insulin-secreting cell line, BRIN-BD11, produced by electrofusion. Diabetes. 1996a;45:1132–1140. doi: 10.2337/diab.45.8.1132. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BARNETT C.R., FLATT P.R. Na+ cotransport by metabolizable and nonmetabolizable amino acids stimulates a glucose-regulated insulin- secretory response. Biochem. Biophys. Res. Commun. 1998a;249:299–303. doi: 10.1006/bbrc.1998.9136. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., BARNETT C.R., O'HARTE F.P., FLATT P.R. Mechanisms of amino acid-induced insulin secretion from the glucose-responsive BRIN- BD11 pancreatic B-cell line. J. Endocrinol. 1996b;151:349–357. doi: 10.1677/joe.0.1510349. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., ELSNER M., TIEDGE M., LENZEN S. Molecular characterization of the glucose-sensing mechanism in the clonal insulin-secreting BRIN-BD11 cell line. Biochem. Biophys. Res. Commun. 1998b;242:262–266. doi: 10.1006/bbrc.1997.7947. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., FLATT P.R. Physiological and pharmacological regulation of insulin release: insights offered through exploitation of insulin-secreting cell lines. Diabetes, Obesity Metabolism. 1999a;1:137–150. doi: 10.1046/j.1463-1326.1999.00017.x. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., FLATT P.R. Glucose and non-glucidic nutrients exert permissive effects on 2-keto acid regulation of pancreatic beta-cell function. Biochim. Biophys. Acta. 1999b;1426:110–118. doi: 10.1016/s0304-4165(98)00144-5. [DOI] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., FLATT P.R., BAILEY C.J. Insulin-releasing action of the novel antidiabetic agent BTS 67 582. Br. J. Pharmacol. 1998c;123:400–404. doi: 10.1038/sj.bjp.0701631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCLENAGHAN N.H., GRAY A.M., BARNETT C.R., FLATT P.R. Hexose recognition by insulin-secreting BRIN-BD11 cells. Biochem. Biophys. Res. Commun. 1996c;223:724–728. doi: 10.1006/bbrc.1996.0963. [DOI] [PubMed] [Google Scholar]
- MORGAN N.G., CHAN S.L., LACEY R.J., BROWN C.A.Pharmacology and molecular biology of islet cell adrenoceptors Frontiers of Insulin Secretion and Pancreatic B-Cell Research 1994London: Smith Gordon; 359–368.ed. Flatt, P.R. & Lenzen, S. pp [Google Scholar]
- NELSON D.A., AGUILAR-BRYAN L., RAEF H., BOYD A.E.Molecular mechanisms of sulphonylurea action in the pancreatic B-cell Nutrient Regulation of Insulin Secretion 1992London: Portland Press; 319–339.ed. Flatt, P.R. pp [Google Scholar]
- OSTENSON C.G., GRILL V., NULEN A. Modulation by IBMX, fasting and experimental diabetes of glibenclamide-induced islet hormone release from the perfused rat pancreas. Diab. Metab. 1983;9:58–65. [PubMed] [Google Scholar]
- PAGE T., BAILEY C.J. Glucose-lowering effect of BTS 67 582. Br. J. Pharmacol. 1997;122:1464–1468. doi: 10.1038/sj.bjp.0701537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTIROLI A.E., CALDERARA A., POZZA G. Secondary failure of oral hypoglycaemic agents: frequency, possible causes, and management. Diabetes Metab Rev. 1994;10:31–43. doi: 10.1002/dmr.5610100104. [DOI] [PubMed] [Google Scholar]
- PORTHA B., GIROIX M.H., SERRADAS P., MORIN L., SAULNIER C., BAILBE D. Glucose refractoriness of pancreatic beta-cells in rat models of non-insulin dependent diabetes. Diabete Metab. 1994;20:108–115. [PubMed] [Google Scholar]
- PROKS P., ASHCROFT F.M. Phentolamine block of KATP channels is mediated by Kir6.2. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11716–11720. doi: 10.1073/pnas.94.21.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABUAZZO A.M., BUSCEMA M., VINCI C., CALTABIANO V., VETRI M., FORTE F., VIGNERI R., PURRELLO F. Glyburide and tolbutamide induce desensitization of insulin release in rat pancreatic islets by different mechanisms. Endocrinology. 1992;131:1815–1820. doi: 10.1210/endo.131.4.1396327. [DOI] [PubMed] [Google Scholar]
- RUSTENBECK I., KOWALEWSKI R., HERRMANN C., DICKEL C., RATZKA P., HASSELBLATT A. Effects of imidazoline compounds on cytoplasmic Ca2+ concentration and ATP-sensitive K+ channels in pancreatic B-cells. Exp Clin Endocrinol Diabetes. 1995;103 Suppl 2:42–45. doi: 10.1055/s-0029-1211393. [DOI] [PubMed] [Google Scholar]
- SAKO Y., WASADA T., UMEDA F., IBAYASTIE H. Effect of glibenclamide on pancreatic hormone release from isolated perifused islets of normal and cysteamine- treated rats. Metabolism. 1986;35:944–949. doi: 10.1016/0026-0495(86)90059-4. [DOI] [PubMed] [Google Scholar]
- SKILLMAN C.A., RASKIN P. A double-masked placebo-controlled trial assessing effects of various doses of BTS 67 582, a novel insulinotropic agent, on fasting hyperglycemia in NIDDM patients. Diabetes Care. 1997;20:591–596. doi: 10.2337/diacare.20.4.591. [DOI] [PubMed] [Google Scholar]
- STOREY D.A., BAILEY C.J. Biphasic insulin-releasing effect of BTS 67 582 in rats. J. Pharm. Pharmacol. 1998;50:1357–1360. doi: 10.1111/j.2042-7158.1998.tb03359.x. [DOI] [PubMed] [Google Scholar]
- WARD W.K., BEARD J.C., HALTER J.B., PFEIFER M.A., PORTE D., JR Pathophysiology of insulin secretion in non-insulin-dependent diabetes mellitus. Diabetes Care. 1984;7:491–502. doi: 10.2337/diacare.7.5.491. [DOI] [PubMed] [Google Scholar]
- YKI-JARVINEN H. Toxicity of hyperglycaemia in type 2 diabetes. Diabetes Metab. Rev. 1998;14 Suppl 1:S45–S50. [PubMed] [Google Scholar]
- ZAITSEV S.V., EFANOV A.M., EFANOVA I.B., LARSSON O., OSTENSON C.G., GOLD G., BERGGREN P.O., EFENDIC S. Imidazoline compounds stimulate insulin release by inhibition of KATP channels and interaction with the exocytotic machinery. Diabetes. 1996;45:1610–1618. doi: 10.2337/diab.45.11.1610. [DOI] [PubMed] [Google Scholar]
- ZAWALICH W.S., BONNET-EYMARD M., ZAWALICH K.C. Glucose- induced desensitization of the pancreatic beta-cell is species dependent. Am. J. Physiol. 1998;275:E917–E924. doi: 10.1152/ajpendo.1998.275.6.E917. [DOI] [PubMed] [Google Scholar]


