Abstract
Prostanoids, generated from cyclooxygenase (COX) isoenzymes, play a role in the physiological function of the lower urinary tract and are important mediators of inflammatory hyperalgesia. The present work evaluates the effects of the COX-1/COX-2 inhibitor dexketoprofen as well as of a selective COX-2 inhibitor, NS-398, on urodynamic function following endotoxin (LPS) or cyclophosphamide (CYP)-induced inflammation of the urinary bladder.
The application of arachidonic acid (330 μg rat−1) onto the serosal surface of the urinary bladder in control rats elicited bladder contractions which could be blocked in a dose-dependent manner by dexketoprofen (0.1–3 mg kg−1, i.v.) but not by NS-398 (0.2–6 mg kg−1, i.v.).
Dexketoprofen (3 mg kg−1, i.v.) decreased the micturition frequency and increased the pressure threshold for triggering the micturition either when administered within 15 min or 3 h following surgery in control animals. NS-398 (6 mg kg−1, i.v.) decreased the micturition frequency and increased the pressure threshold when administered 3 h but not 15 min following surgery.
Administration of LPS (2 mg kg−1, i.v., 90–120 min) increased both the micturition frequency and the pressure threshold for triggering the micturition reflex. Changes in urodynamic parameters induced by LPS were prevented by doses of either dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) which were ineffective in control animals.
Pretreatment with CYP (150 mg kg−1, i.p., 48 h) increased the micturition frequency, pressure threshold, and the minimal intravesical pressure but decreased the mean amplitude of micturition contractions. In CYP-treated rats, dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) blocked the CYP-induced urodynamic changes with exception of the micturition contraction amplitude.
These results indicate that COX-1 may be involved in modulating the threshold for activating the micturition reflex in the normal rats and also demonstrates that inhibition of COX-2 prevents or reverses the urodynamic changes associated with bladder inflammation induced either by surgery, LPS or CYP treatments.
Keywords: Cyclophosphamide, cystitis, dexketoprofen, endotoxin, hyperreflexia, NS-398, rat urinary bladder
Introduction
Prostanoids are products of enzymatic transformation of arachidonic acid by cyclooxygenase (COX) isoenzymes. Two distinct forms of COX enzymes have been identified: COX-1 is constitutively expressed in most cells and results in the production of prostanoids involved in physiological processes, whereas COX-2 is upregulated in response to inflammatory stimuli such as cytokines and lipopolysaccharide (LPS) (Mitchell & Warner, 1999). Although most non-steroidal anti-inflammatory drugs are nonselective inhibitors of COX enzymes (Mitchell et al., 1993), selective COX-2 inhibitors such as NS-398 have recently become available (Futaki et al., 1993; 1994; Vane & Botting, 1995). These selective COX-2 inhibitors are thought to exhibit less gastrointestinal or renal toxicity while retaining analgesic, anti-inflammatory and antipyretic activity (Futaki et al., 1993; 1994).
In the urinary bladder both the urothelium and the smooth muscle retain COX activity (Brown et al., 1980). Prostanoids, generated following bladder stretch (Gilmore & Vane, 1971; Poggesi et al., 1980) are thought to play a physiological role in lower urinary tract function. It has been demonstrated that these mediators contribute to the basal tone of the detrusor and/or modulate the activity of efferent and/or afferent bladder nerves (Maggi, 1992; Andersson, 1993 for reviews). In addition, systemic or topical administration of prostanoids on the mucosal or serosal surface of the urinary bladder has been shown to stimulate the micturition reflex (Bultitude et al., 1976; Khalaf et al., 1980; 1981; Maggi et al., 1984; Ishizuka et al., 1995). This effect may be due to activation of capsaicin-sensitive bladder afferents, since prostanoid-induced bladder hyperreflexia (following topical administration of prostanoids) can be blocked by pretreatment with capsaicin or with tachykinin receptor antagonists (Maggi et al., 1988; Ishizuka et al., 1995). Thus, prostanoids (released during smooth muscle stretch during filling) may regulate the threshold for activating the micturition reflex by stimulating or sensitizing capsaicin-sensitive bladder mechanoreceptors (Maggi et al., 1988; Maggi, 1992).
Inflammation or damage of the urinary bladder or bladder epithelium (Mikhailidis et al., 1987; Andersson, 1993; Meini et al., 1998; Downie & Karmazyn, 1984), exogenous application of pro-inflammatory neuropeptides (Saban et al., 1997; Meini et al., 1998) or activation of bladder nerves (Alkondon & Ganguly, 1980) can all promote prostanoid production in the urinary bladder. An increase in prostanoid production following injury or inflammation may lead to an increase in neural activity resulting in a bladder hyperactivity. These findings, together with the observations that the urinary content of prostaglandin E correlates with the onset and the duration of clinical symptoms of bacterial cystitis (Farkas et al., 1980) and that COX inhibitors ameliorate detrusor instability in humans (Cardozo & Stanton, 1980; Cardozo et al., 1980) suggest that prostanoids are important mediators following induction of cystitis or inflammation of the urinary bladder. However, the effectiveness of selective and non-selective COX inhibitors in these experimental paradigms have not been investigated.
This report evaluates the effect of NS-398, a selective COX-2 inhibitor (Futaki et al., 1993) and dexketoprofen, the active enantiomer of the racemic non-selective COX inhibitor ketoprofen (Mauleon et al., 1996) on the micturition reflex in the normal rat and following inflammation of the lower urinary tract using cystometrogram recording to evaluate changes in bladder intravesical pressure. Two distinct models were used to induce inflammation: (1) induction of a bacterial cystitis following systemic administration of bacterial LPS or (2) induction of chronic haemorrhagic cystitis following systemic administration of the chemotherapeutic agent cyclophosphamide (CYP). In addition, we also evaluated whether a time dependent (15 min–3 h) surgical placement of a bladder catheter could stimulate COX-2 and alter bladder reflexes in the absence of LPS or CYP induced inflammation. The effect of dexketoprofen and NS-398 on the activity of constitutive COX isoform(s) in the urinary bladder was determined on arachidonic acid-induced bladder motor responses administered 30 min after the preparation set-up. The present experiments revealed that COX-1 enzymes may play an important physiological role in bladder function and inhibition of COX-2 normalizes bladder reflexes following an experimentally-induced inflammation of the lower urinary tract.
Methods
General
Male Wistar rats (350–400 g, Charles River, Calco, Italy) were used for this study. In order to conduct the physiological experiments, rats were deeply anaesthetized with urethane (1.2 g kg−1, s.c.). An intravenous cannula (i.v., PE 50 tubing) was inserted in the right jugular vein for the purpose of drug administration. Two different preparations were used for physiological studies: (1) animals in which the urethral outlet remained open (continuous infusion) or (2) the urethra was closed via a ligature placed around the urethral orifice (isovolumetric recording).
For continuous infusion, the urinary bladder was exposed via a midline abdominal incision and cannulated using a double lumen polyethylene catheter (I.D. 0.71 mm., O.D. 1.5 mm.) implanted into the bladder dome and secured with a ligature: the urethra remained open to allow voiding. One end of the catheter was attached to a pressure transducer (Statham Spectramed P23 XL, Valley View, OH, U.S.A.) in order to record and measure changes in intravesical pressure (Windograph Gould, Valley View, OH, U.S.A.; MacLab, ADInstruments Ltd, Hastings, U.K.). The other end of the catheter was connected to a peristaltic pump (Ismatec IPN, PBI, Italy) to continuously infuse saline (rate 0.05 ml min−1).
For transurethral recordings, a polyethylene catheter (0.86 mm I.D., 1.27 mm O.D.) was inserted in and secured to the bladder neck. The urinary bladder was filled with a constant volume of saline (1 ml kg−1 body weight) which was subthreshold (in 84% of vehicle treated animals) for the activation of the micturition reflex.
Control animals: arachidonic acid challenge
Urinary bladder reflex contractions (isovolumetric recording) were elicited by the serosal application of arachidonic acid (sodium salt, Sigma-Aldrich, Gallarate, Italy, 330 μg rat−1 in 100 μl of saline). For topical application onto the bladder serosa, the bladder was exposed, separated from the adjoining viscera by a piece of parafilm and covered with a saline-moistened cotton gauze. The urinary bladder was filled with a subthreshold volume (1 ml kg−1) for triggering the micturition reflex. Arachidonic acid was administered 35 min following surgical implantation of a bladder catheter used to measure intravesical pressure. Dexketoprofen (tromethamine salt, Menarini Laboratorios, Barcelona, Spain, 0.1–3 mg kg−1, i.v.), NS-398 (RBI, Natick, MA, U.S.A., 0.2–6 mg kg−1, i.v.) or vehicle (dimethylsulphoxide, 100 μl kg−1, i.v.) was administered 30 min prior to application of arachidonic acid. Previous studies using this model determined that high amplitude (>15 mmHg) phasic or reflex bladder contractions induced by topical application of arachidonic acid were due to the activation of a tetrodotoxin-sensitive bladder-to-bladder reflex (Maggi et al., 1984). Thus, in these experiments, we will measure the occurrence, frequency and the mean amplitude of the phasic or reflex bladder contractions (> than 15 mmHg) as well as the difference in area under the curve both (15 min) prior to and after arachidonic acid challenge.
Control animals: cystometrogram measurements
The effect of dexketoprofen and NS-398 on bladder reflexes evoked by distension was evaluated by means of transvesical cystometries. Two different kinds of experiments were performed. In the first series of experiments, animals were treated with dexketoprofen, NS-398, or vehicle (10 min) prior to saline (3 h) infusion. The following urodynamic parameters were evaluated (6×30 min recording periods): (1) micturition frequency (the number of micturition cycles, or bladder contractions >5 mmHg, see Figure 1); (2) mean pressure threshold (threshold intravesical pressure, mmHg, for activating a bladder contraction); (3) minimal intravesical pressure (pressure, in mmHg, at the end of each bladder contraction) and (4) mean amplitude of the micturition contraction (measured in mmHg). In addition, the bladder capacity, pressure threshold and the amplitude of micturition reflex contractions were also measured. Because the urinary bladder myogenic activity did not exceed an intravesical pressure of 5 mmHg, the minimum amplitude of reflex bladder contraction was always ⩾5 mmHg during transvesical cystometry.
Figure 1.
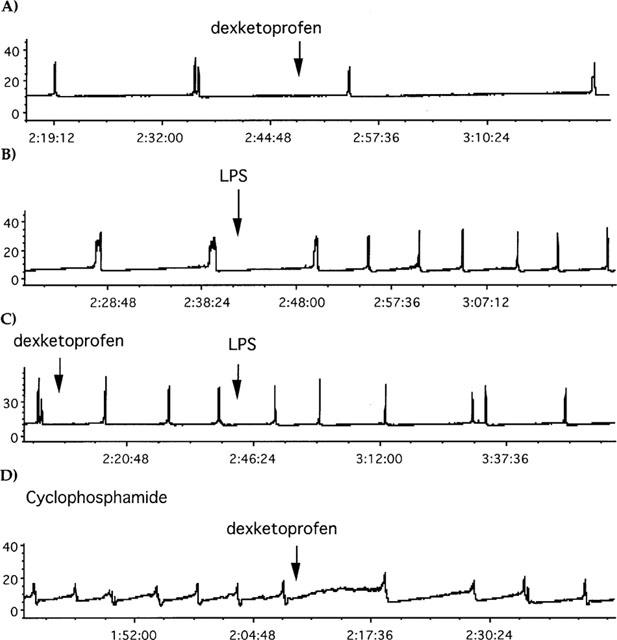
Traces showing the effect of dexketoprofen (1 mg kg−1, i.v.), LPS (2 mg kg−1, i.v.) or CYP (150 mg kg−1, i.p. 48 h before) in cystometries. The administration of dexketoprofen or LPS is indicated by the arrow. In X axis is represented the time (h:mins:s) after having started cystometries, in Y axis the intravesical pressure (mmHg). (A) Represents the effect of dexketoprofen in control rats. (B) The effect of LPS in control rats. (C) The effect of LPS in dexketoprofen-pretreated rats. (D) The effect of dexketoprofen in CYP-pretreated rats.
In a second series of experiments, two separate doses of dexketoprofen (0.1 and 3 mg kg−1, i.v.) or NS-398 (0.2 and 6 mg kg−1, i.v.) were administered (2 h) following cystometrogram recordings (1–1.5 h after bladder surgery). The effectiveness of COX inhibitors was reported as the difference between mean baseline measurements (30 min-period prior) and mean values of three recording periods (30 min) following drug administration. There were no significant differences in basal urodynamic values among experimental groups.
Induction of cystitis
Prior to drug administration, baseline cystometrogram measurements (2 h) were recorded. Irritation of the lower urinary tract was induced by pretreatment with either LPS (E.Coli, serotype 0.111:B4, Difco, Detroit, U.S.A., 2 mg kg−1 in saline, i.v., 150 min after saline infusion) or CYP monohydrate, (Sigma-Aldrich, Gallarate, Italy, 150 mg kg−1, dissolved in saline and given i.p. 48 h prior to experimentation).
Dexketoprofen (1 mg kg−1, i.v.), NS-398 (2 mg kg−1, i.v.) or vehicle (dimethylsulphoxide, 100 μg kg−1, i.v.) were administered (30 min prior) to administration of LPS. In a separate experimental group, rats were treated with either the COX inhibitor (dexketoprofen, or NS-398) or vehicle (dimethylsulphoxide) following CYP induced inflammation. These experiments were evaluated as previously described. The effectiveness of COX inhibitors was reported as the difference between mean baseline values (30 min-period prior) and mean values of 4–5 periods (30 min each) following administration. Basal urodynamic values did not differ significantly among experimental groups.
Statistics
Data are reported as mean±s.e.mean. Statistical analysis was performed using a randomized one-way or a two-way analysis of variance for repeated measures. Post hoc test (Fisher's least significant difference, LSD) was performed when the analysis of variance was significant (P<0.05).
Results
Control animals: arachidonic acid challenge
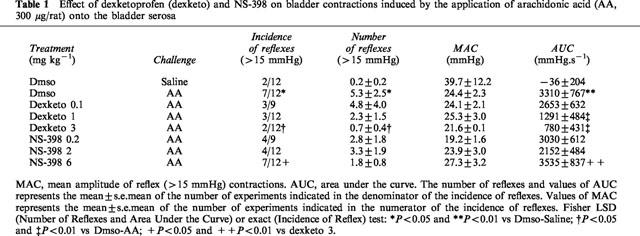
Reflex bladder contractions (>15 mmHg) could be elicited following saline infusion (1 ml kg−1 body weight) via a urethral catheter in a small number of either vehicle (1 out of 24), dexketoprofen- (two out of 33) or NS-398-treated (four out of 33) preparations. The application of arachidonic acid (330 μg rat−1) onto the urinary bladder serosa triggered a bladder motor response consisting of a tonic, low amplitude (<15 mmHg) bladder contraction (100% of preparations). In a large percentage of preparations (58%), the response was also accompanied by a series of high amplitude (>15 mmHg) reflex contractions. The topical application of saline (100 μl rat−1) elicited the high amplitude motor response in a small number (17%) of animals (Table 1). Dexketoprofen (0.1–3 mg kg−1, i.v.) reduced in a dose-dependent manner the number of bladder contractions as well as the area under the curve in response to arachidonic acid challenge. NS-398 (0.2–6 mg kg−1, i.v.) produced no consistent effect on the bladder motor responses elicited by arachidonic acid. Dexketoprofen treatment resulted in a significant reduction in both the incidence of reflex contractions and the area under the curve as compared to animals treated with NS-398. Neither dexketoprofen nor NS-398 altered the amplitude of reflex contractions after arachidonic acid challenge (Table 1).
Table 1.
Effect of dexketoprofen (dexketo) and NS-398 on bladder contractions induced by the application of arachidonic acid (AA, 300 μg/rat) onto the bladder serosa
Effect of COX inhibitors: control conditions
Dexketoprofen (3 mg kg−1, i.v.) but not NS-398 (6 mg kg−1, i.v.) increased both the bladder capacity and pressure threshold for the activation of bladder reflexes in the first micturition cycle (25 min following surgery and drug administration, Table 2). Dexketoprofen reduced the micturition frequency and increased the pressure threshold (60 min) following cystometries, whereas NS-398 had no effect. The pressure threshold and the micturition frequency recorded during the first 30 min in the dexketoprofen-treated animals were significantly different from corresponding values in the NS-398-treated group (Figure 2). Neither dexketoprofen or NS-398 affected the mean amplitude of micturition contractions on the minimal intravesical pressure (data not shown).
Table 2.
Effect of dexketoprofen (3 mg kg−1, i.v.) and NS-398 (6 mg kg−1, i.v.) on urodynamic parameters during the first micturition cycle of cytometry performed 15 min after bladder surgery in control rats.
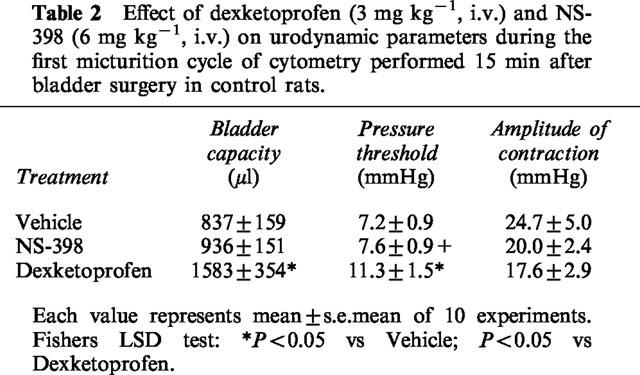
Figure 2.
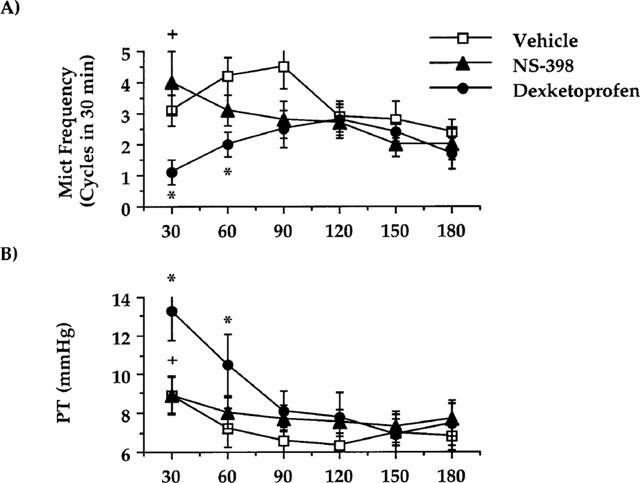
Effect of dexketoprofen (3 mg kg−1, i.v.) or NS-398 (6 mg kg−1, i.v.) on cystometries performed in control rats 15 min after bladder catheter insertion. (A) Represents the effect on the micturition frequency. (B) The effect on the pressure threshold for triggering micturition contractions. Fisher's LSD test: *P<0.05 vs time-matched vehicle-treated group; NS-398+P<0.05 vs time-matched dexketoprofen-treated group. Each point and bar represents mean±s.e.mean of 10 experiments.
Dexketoprofen (3 mg kg−1, i.v.) as well as NS-398 (6 mg kg−1, i.v.) decreased the micturition frequency and increased the pressure threshold for the activation of micturition reflex when administered (1.5–2 h) after initial experimental setup and establishment of baseline cystometry recordings (Figure 3). There were no significant differences in the effectiveness of either dexketoprofen or NS-398. The administration of 30 fold lower doses of either dexketoprofen (0.1 mg kg−1, i.v.) or NS-398 (0.2 mg kg−1, i.v.) had no effect on cystometries (n=12 for each group, data not shown).
Figure 3.
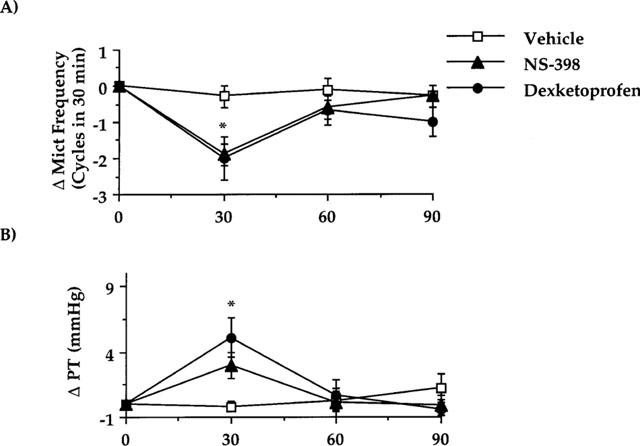
Effect of dexketoprofen (3 mg kg−1, i.v.) or NS-398 (6 mg kg−1, i.v.) on cystometries performed in control rats 3 h after bladder catheter insertion and 2 h after having started cystometries. (A) Represents the effect on the micturition frequency. (B) The effect on the pressure threshold for triggering micturition contractions. Fischer's LSD test: dexketoprofen and NS-398 *P<0.05 vs time-matched vehicle-treated group and vs their own basal values (time 0). Each point and bar represents mean±s.e.mean of eight experiments.
Effect of COX inhibitors: induction of cystitis
The effect of dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) was tested using ongoing cystometries in two different models of cystitis or in control conditions. All antiinflammatory drugs were administered 2 h following initial recording and 3–3.5 h following surgery (Figure 1).
In control animals, dexketoprofen (1 mg kg−1, i.v.) did not significantly reduce the frequency of micturition (Figures 1A and 4A). There was a significant increase in the pressure threshold as early as 30 min after drug administration (Figure 4B). Administration of NS-398 (2 mg kg−1, i.v.) did not significantly alter either the micturition frequency or pressure threshold as compared to vehicle-treated rats (Figure 4A, B). Neither dexketoprofen nor NS-398 changed the minimal pressure or the amplitude of micturition contractions in control animals (data not shown).
Figure 4.
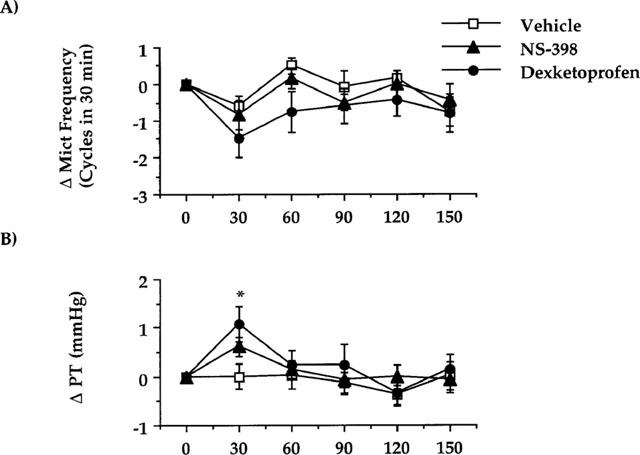
Effect of dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) on cystometries performed in control rats 3 h after bladder catheter insertion and 2 h after having started cystometries. (A) Represents the effect on the micturition frequency. (B) The effect on the pressure threshold for triggering micturition contractions. Fisher's LSD test: dexketoprofen *P<0.05 vs time-matched vehicle-treated group and vs their own basal values (time 0). Each point and bar represents mean±s.e.mean of 12 experiments.
Pretreatment with LPS (2 mg kg−1, i.v.) resulted in a bladder hyperactivity as compared to time-matched controls which peaked 120 min from the time of LPS administration (Figures 1B and 5A). This hyperactivity was also associated with an increased pressure threshold (Figure 5B). Neither the minimal intravesical pressure nor the amplitude of contractions significantly changed over the time-course of the experiment (data not shown). Either dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.), administered prior to induction of inflammation with LPS, prevented the increase in micturition frequency (Figures 1C and 5A). The pressure threshold was significantly reduced by NS-398 but not by dexketoprofen (Figure 5B). Neither dexketoprofen nor NS-398 significantly changed the amplitude of micturition contractions or the minimal pressure in LPS-treated animals (data not shown).
Figure 5.

Effect of LPS (2 mg kg−1, i.v.) on cystometries performed 3 h after bladder catheter insertion and 2 h after having started cystometries in vehicle- dexketoprofen (1 mg kg−1, i.v.)- or NS-398 (2 mg kg−1, i.v.)-pretreated rats. The arrow indicates LPS administration. (A) Represents the effect on the micturition frequency. (B) The effect on the pressure threshold for triggering micturition contractions. Fisher's LSD test: +P<0.05 vs time 0, *P<0.05 vs time-matched vehicle-treated group. Each point and bar represents mean±s.e.mean of 12 experiments.
Pretreatment with CYP (150 mg kg−1, i.p., 48 h) increased the number of micturition contractions. The hyperactivity induced by CYP pretreatment was also associated with an increase in the pressure threshold for activating the micturition reflex, and with a quantitatively similar increase in the minimal intravesical pressure. In contrast, the mean amplitude of micturition contractions was decreased (Figure 1D and Table 3). In CYP-pretreated animals, either dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) reduced the micturition frequency (Figures 1D and 6A). The effect of both dexketoprofen and NS-398 was significantly greater (P>0.01) in CYP-treated animals than in controls. Following administration (60 min) of either dexketoprofen or NS-398, the pressure threshold was significantly reduced (Figures 6B). In addition, both dexketoprofen and NS-398 significantly decreased the minimal pressure (60 min after administration) as compared to vehicle-treated animals or to their basal values (Figure 6C). The mean amplitude of reflex bladder contractions was reduced by either compound, but this effect was not statistically significant (Figure 6D).
Table 3.
Effect of cyclophosphamide pretreatment (150 mg kg−1 i.p., 48 h before) on urodynamic parameters during ongoing cytometries in urethane anaesthetized rats
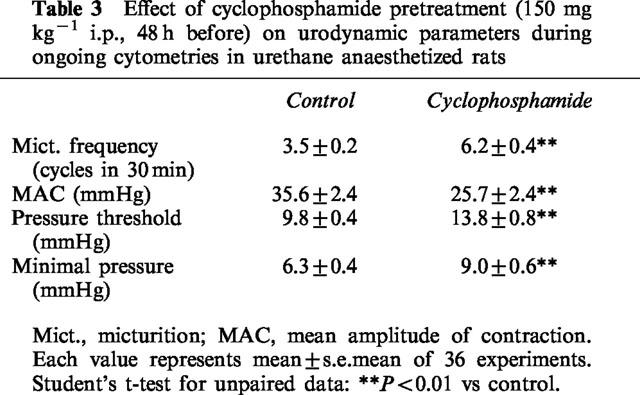
Figure 6.
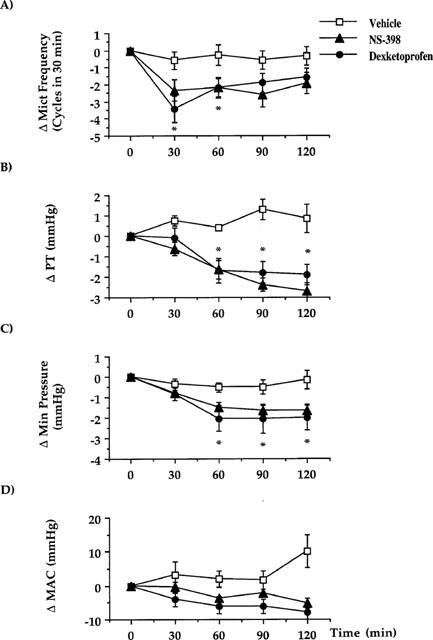
Effect of dexketoprofen (1 mg kg−1, i.v.) or NS-398 (2 mg kg−1, i.v.) on cystometries performed in CYP (150 mg kg−1, i.p., 48 h before)-treated rats 3 h after bladder catheter insertion and 2 h after having started cystometries. (A) Represents the effect on the micturition frequency. (B) The effect on the pressure threshold for triggering micturition contractions. (C) The effect on the minimal pressure. (D) The effect on the mean amplitude of micturition contractions. Fisher's LSD test: dexketoprofen *P<0.05 vs time-matched vehicle-treated group and vs their own basal values (time 0). Each point and bar represents mean±s.e.mean of 12 experiments.
Discussion
COX-1 and COX-2 isoenzymes, both expressed in the urinary bladder of adult rodents, are differentially regulated. For example, the levels of COX-2 are subject to both physiological (e.g., foetal development) or pathophysiological (e.g., bladder obstruction) variations in contrast to levels of COX-1 expression, which is constitutively expressed and is not altered under similar conditions (Park et al., 1997). In the lower urinary tract, it is well-established that non-selective COX inhibitors increase both bladder capacity and pressure threshold for activating the micturition reflex (Khalaf et al., 1981; Maggi et al., 1984; 1985; Morikawa et al., 1989), although the COX isoenzyme responsible for these effects is unknown. The present study suggests that COX-1 isoenzymes have a functional role in the micturition reflex elicited in anaesthetized rats. While the non-selective COX inhibitor dexketoprofen increased the bladder capacity and the pressure threshold for triggering the micturition reflex (and consequently decreased the micturition frequency), the selective COX-2 inhibitor had no effect on these urodynamic measurements (see Table 2 and Figure 1). These data suggest that a constitutive COX-1 is involved in the urodynamic changes induced by dexketoprofen. Alternatively, since both COX isoforms appear to be constitutively expressed in the urinary bladder (Park et al., 1997), it could be speculated that only the blockade of both COX-1 and COX-2 isoenzymes leads to urodynamic changes in control conditions.
It is likely that capsaicin-sensitive bladder nerves represent a target of prostanoids produced by urinary bladder COX isoforms. The impairment of these nerves obtained by pretreating adult rats with high doses of capsaicin (50–150 mg kg−1) produces urodynamic changes superimposable to those induced by dexketoprofen. In addition, the effect of COX inhibitors is not observed in capsaicin-pretreated animals (Maggi et al., 1985; 1988). These and other data suggest that capsaicin-sensitive afferent fibres might be activated by prostanoids released during urinary bladder stretch which could lead to an increased afferent discharge during the filling phase of the micturition reflex (Maggi 1992). COX inhibition has been demonstrated to decrease urinary bladder PGE2 content (2.5–0.4 pg mg−1, Morikawa et al., 1989). This type of inhibition might remove the facilitatory influence of endogenous prostanoids on capsaicin-sensitive bladder afferents (Maggi et al., 1988) increasing the pressure threshold for activating the micturition reflex and reducing the micturition frequency in control animals. Thus, COX isoenzymes present in the urinary bladder may play a role in modulating normal bladder function.
Injury to the urinary bladder has also been shown to induce a prompt, several-fold increase in COX-2 levels (Park et al., 1997). Tissue injury (such as implantation of bladder catheter or other surgical invasive procedure) may be a stimulus which increases COX-2 expression. It has been shown that implantation of a bladder catheter using sterile technique results in an increase in PGE2 bladder content (30 fold increase as compared to unoperated animals) and remains elevated for at least 1 week (Morikawa et al., 1989). In addition, it has been shown that racemic ketoprofen does not alter urodynamic parameters during a non-invasive cystometry in conscious healthy humans (Malinovsky et al., 1998), suggesting that the activity of bladder COX-2 isoenzyme can be upregulated following surgery. In the present study, the effect of NS-398 of dexketoprofen either in control animals (recording 3 h following surgery), LPS or CYP treated rats was similar (see Figures 3, 5 and 6). In control animals (recording 3 h following surgery), NS-398 (6 mg kg−1) as well as dexketoprofen (1 or 3 mg kg−1) reduced the micturition frequency and increased the pressure threshold for triggering the micturition reflex. In addition, both dexketoprofen and NS-398 prevent or reverse LPS-induced alterations in urodynamic parameters or CYP-induced bladder hyperreflexia. CYP has been demonstrated to induce a bladder cystitis, caused by the urinary excretion of acrolein, a metabolite of CYP (Cox, 1979), thought to stimulate capsaicin-sensitive bladder afferents (Maggi et al., 1992; Ahluwalia et al., 1994). CYP-induced inflammation alters the activity of bladder reflexes via stimulation of capsaicin-sensitive bladder afferents (Maggi et al., 1992). Following induction of CYP-cystitis, the increase in the micturition frequency and pressure threshold (also observed in the LPS model) was accompanied by an increase of the minimal intravesical pressure (see Table 2). This effect could be due to a reduction in bladder compliance (Lecci et al., 1999) caused by an increased expression of COX-2 in the smooth muscle layer (Park et al., 1997), since the CYP-induced increase in minimal pressure was reversed by both NS-398 and dexketoprofen. The switch of COX activity from COX-1 to the COX-2 isoenzyme could be due to the coexpression of an inducible phospholipase A2 (the enzyme responsible for the production of arachidonic acid, the COX substrate) which preferentially couples with COX-2 (Cirino, 1998). In addition, it has been shown that low concentrations of the COX substrate (arachidonic acid) could favour the activity of the COX-2 isoenyzmes (Murakami et al., 1999). These data might account for the lack of effect of NS-398 on bladder contractility following arachidonic acid challenge, despite the possibility of a constitutive expression of COX-2 in the urinary bladder (Park et al., 1997). Alternatively, as already suggested for the inhibitory effect of dexketoprofen on cystometries performed 15 min from bladder surgery, the blockade of both COX isoforms could be necessary to reduce bladder motor responses induced by arachidonic acid.
The intravesical instillation of lipopolysaccharide (LPS), the cell wall component of Gram negative bacteria (E. Coli), has been demonstrated to produce a localized inflammatory response which is accompanied by histological changes in the bladder wall, infiltration of neutrophils (Stein et al., 1996; Olsson et al., 1998) and a bladder hyperactivity (Lecci et al., 1998). The latter could be abolished by capsaicin pretreatment or administration of tachykinin NK2 receptor antagonists (Lecci et al., 1998). In the present study, systemic administration of LPS results in bladder hyperactivity accompanied by an increase in pressure threshold to induce a micturition contraction. This response could be due to the effect of prostanoids on bladder tone and/or smooth muscle of the bladder base and urethra. In addition, prostanoids produced following inflammation may also facilitate the release of tachykinins from bladder nerves located in close proximity to the urothelium, which could result in bladder hyperactivity (Ishizuka et al., 1995). Thus, prostanoids can directly stimulate sensory fibres and also may sensitize primary afferents to non-nociceptive stimuli such as stretch of the urinary bladder or changes in contractility (Juan & Lembeck 1974; Chahl & Iggo, 1977). The increase in bladder tone following LPS-induced inflammation was not altered by denervation of the urinary bladder (bilateral removal of major pelvic ganglia). These results suggest that the bacterial toxin affects bladder contractility independently from reflex activity of the urinary bladder (Lecci et al., 1999). The involvement of prostanoids in the LPS-induced increase in the bladder tone is also supported by data which demonstrates a lack of bladder motor responses following LPS administration to CYP treated animals (which exhibited an increase in both bladder tone and PGE2 levels) (Meini et al., 1998; Lecci et al., 1999). In addition, the observation that dexamethasone pretreatment prevents both expression of an inducible receptor (the kinin B1 receptor) as well as LPS-induced changes in the bladder tone, suggests that this effect could be mediated by an inducible protein, possibly COX-2 (Lecci et al., 1999).
Another interesting issue emerging from this study is relative to the potency of selective and non-selective COX inhibitors in the induction of urodynamic changes in inflamed and normal animals. There were significantly larger effects induced by both NS-398 and dexketoprofen in cystometries performed on LPS- or CYP-treated rats than in surgical controls. Similar results were obtained when studying the urodynamic effects of indomethacin in controls or in xylene-treated rats (Morikawa et al., 1990). These results show that the increased sensitivity to indomethacin or ketoprofen in models of cystitis does not depend on a preferential activity of these drugs on COX-2 over the COX-1 isoenzyme (Warner et al., 1999), since similar responses were detected with the selective COX-2 inhibitor NS-398. In this respect, a larger dose (6 mg kg−1) of NS-398 was necessary to produce urodynamic changes in controls, whereas a lower dose (2 mg kg−1) was found to induce similar effects in LPS- or CYP-treated rats.
In summary, the present findings provide evidence that COX-2-generated mediators play a role in the bladder hyperactivity following bladder inflammation induced by LPS, CYP or surgical procedures. Future studies may determine whether COX-1-derived prostanoids contribute to the sensitization of afferent nerves following bladder surgery in addition to a physiological role in modulating the micturition reflex.
Abbreviations
- COX
cyclooxygenase
- CYP
cyclophosphamide
- LPS
endotoxin
References
- AHLUWALIA A., MAGGI C.A., SANTICIOLI P., LECCI A., MAGGI C.A. Characterization of the capsaicin-sensitive component of cyclophosphamide-induced inflammation in the rat urinary bladder. Br. J. Pharmacol. 1994;111:1017–1022. doi: 10.1111/j.1476-5381.1994.tb14845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALKONDON M., GANGULY D.K. Release of prostaglandin E from the isolated urinary bladder of the guinea-pig. Br. J. Pharmacol. 1980;69:573–577. doi: 10.1111/j.1476-5381.1980.tb07906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSSON K.-E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- BROWN W.W., ZENSER T.V., DAVIS B.B. Prostaglandin production by rabbit urinary bladder. Am. J. Physiol. 1980;239:F452–F458. doi: 10.1152/ajprenal.1980.239.5.F452. [DOI] [PubMed] [Google Scholar]
- BULTITUDE M.I., HILLS N.H., SHUTTLEWORTH K.E.D. Clinical and experimental studies on the action of prostaglandins and their synthesis inhibitors on detrusor muscle in vitro and in vivo. Br. J. Urol. 1976;48:631–637. doi: 10.1111/j.1464-410x.1976.tb06711.x. [DOI] [PubMed] [Google Scholar]
- CARDOZO L., STANTON S.L. A comparison between bromocriptine and indomethacin in the treatment of detrusor instability. J. Urol. 1980;123:399–401. doi: 10.1016/s0022-5347(17)55955-8. [DOI] [PubMed] [Google Scholar]
- CARDOZO L., STANTON S.L., ROBINSON H., HOLE D. Evaluation of flurbiprofen in detrusor instability. Br. Med. J. 1980;280:281–282. doi: 10.1136/bmj.280.6210.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAHL L.A., IGGO A. The effects of bradykinin and prostaglandin E1 on rat cutaneous afferent nerve activity. Br. J. Pharmacol. 1977;59:343–347. doi: 10.1111/j.1476-5381.1977.tb07498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRINO G. Multiple controls of inflammation. Biochem. Pharmacol. 1998;55:105–111. doi: 10.1016/s0006-2952(97)00215-3. [DOI] [PubMed] [Google Scholar]
- COX P.J. Cyclophosphamide cystitis: identification of acrolein as the causative agent. Biochem. Pharmacol. 1979;28:2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- DOWNIE J.W., KARMAZYN M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in the rabbit detrusor. J. Pharmacol. Exp. Ther. 1984;230:445–449. [PubMed] [Google Scholar]
- FARKAS A., ALAJEM D., DEKEL S., BINDERMAN I. Urinary prostaglandin E2 in acute bacterial cystitis. J. Urol. 1980;124:455–457. doi: 10.1016/s0022-5347(17)55493-2. [DOI] [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., OTOMO S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- FUTAKI N., YOSHIKAWA K., HAMASAKA Y., ARAI I., HIGUCHI S., IIZUKA H., OTOMO S. NS-398, a novel non-steroidal anti-inflammatory drug with potent analgesic and antipyretic effects, which causes minimal stomach lesions. Gen. Pharmacol. 1993;24:105–110. doi: 10.1016/0306-3623(93)90018-s. [DOI] [PubMed] [Google Scholar]
- GILMORE N.J., VANE J.R. Hormones released into the circulation when the urinary bladder of anaesthetized dog is distended. Clin. Sci. 1971;41:69–83. doi: 10.1042/cs0410069. [DOI] [PubMed] [Google Scholar]
- ISHIZUKA O., MATTIASSON A., ANDERSSON K.E. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins. J. Urol. 1995;153:2034–2038. doi: 10.1016/s0022-5347(01)67397-x. [DOI] [PubMed] [Google Scholar]
- JUAN H., LEMBECK F. Action of peptides and other algesic agents on paravascular pain receptors of the isolated perfused rabbit ear. Naunyn-Schmiedeberg's Arch. Pharmacol. 1974;283:151–164. doi: 10.1007/BF00501142. [DOI] [PubMed] [Google Scholar]
- KHALAF I.M., GHONEIM M.A., ELHILALI M.M. The effect of exogenous prostaglandin F2alpha and E2 and indomethacin on micturition. Br. J. Urol. 1981;53:21–28. doi: 10.1111/j.1464-410x.1981.tb03123.x. [DOI] [PubMed] [Google Scholar]
- KHALAF I.M., RIOUX F., QUIRION R., ELHILALI M.M. Intravesical prostaglandin: release and effect of bladder instillation on some micturition parameters. Br. J. Urol. 1980;52:351–356. doi: 10.1111/j.1464-410x.1980.tb03059.x. [DOI] [PubMed] [Google Scholar]
- LECCI A., MEINI S., PATACCHINI R., TRAMONTANA M., GIULIANI S., CRISCUOLI M., MAGGI C.A. Effect of dexamethasone on cyclophosphamide-induced cystitis in rats: lack of relation with bradykinin B1 receptor-mediated motor responses. Eur. J. Pharmacol. 1999;369:99–106. doi: 10.1016/s0014-2999(99)00052-7. [DOI] [PubMed] [Google Scholar]
- LECCI A., TRAMONTANA M., GIULIANI S., CRISCUOLI M., MAGGI C.A. Effect of tachykinin NK2 receptor blockade on detrusor hyperreflexia induced by bacterial toxin in rats. J. Urol. 1998;160:206–209. [PubMed] [Google Scholar]
- MAGGI C.A. Prostanoids as local modulators of reflex micturition. Pharmacol. Res. 1992;25:13–20. doi: 10.1016/s1043-6618(05)80059-3. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., EVANGELISTA S., GRIMALDI G., SANTICIOLI P., GIOLITTI A., MELI A. Evidence for the involvement of arachidonic acid metabolites in spontaneous and drug-induced contractions of rat urinary bladder. J. Pharmacol. Exp. Ther. 1984;230:500–513. [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., CONTE B., FURIO M., SANTICIOLI P., MELI P., GRAGNANI L., MELI A. Prostanoids modulate reflex micturition by acting through capsaicin-sensitive afferents. Eur. J. Pharmacol. 1988;145:105–112. doi: 10.1016/0014-2999(88)90221-x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., LECCI A., SANTICIOLI P., DEL BIANCO E., GIULIANI S. Cyclophosphamide cystitis in rats: involvement of capsaicin-sensitive primary afferents. J. Auton. Nerv. Syst. 1992;38:201–208. doi: 10.1016/0165-1838(92)90031-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., FURIO M., MELI A. The effect of cyclooxygenase inhibitors on the low filling rate cystometrogram in urethane anesthetized rats. J. Urol. 1985;134:800–803. doi: 10.1016/s0022-5347(17)47442-8. [DOI] [PubMed] [Google Scholar]
- MALINOVSKY J.M., LE NORMAND L., LEPAGE J.Y., MALINGE M., COZIAN A., PINAUD M., BUZELIN J.M. The urodynamic effects of intravenous opioids and ketoprofen in humans. Anesth. Analgesia. 1998;87:456–461. doi: 10.1097/00000539-199808000-00042. [DOI] [PubMed] [Google Scholar]
- MAULEON D., ARTIGAS R., GARCIA M.L., CARGANICO G. Preclinical and clinical development of dexketoprofen. Drugs. 1996;52 Suppl. 5:24–46. doi: 10.2165/00003495-199600525-00005. [DOI] [PubMed] [Google Scholar]
- MEINI S., LECCI A., CUCCHI P., CATALIOTO R.-M., CRISCUOLI M., MAGGI C.A. Inflammation modifies the role of cyclooxygenases in the contractile responses of the rat detrusor smooth muscle to kinin agonists. J. Pharmacol. Exp. Ther. 1998;287:137–143. [PubMed] [Google Scholar]
- MIKHAILIDIS D.P., JEREMY J.Y., DANDONA P. Urinary bladder prostanoids. Their synthesis, function and possible role in the pathogenesis and treatment of disease. J. Urol. 1987;137:577–582. doi: 10.1016/s0022-5347(17)44109-7. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.A., ARASEREENONT P.A., THIEMERMANN C., FLOWER R.G., VANE J.R. Selectivity of non-steroidal anti-inflammatory drugs as inhibitors of constitutive and inducible cyclooxygenases. Proc. Natl. Acad. Sci. 1993;90:11693–11697. doi: 10.1073/pnas.90.24.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL J.A., WARNER T.D. Cyclooxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br. J. Pharmacol. 1999;128:1121–1132. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORIKAWA K., FUKUOKA M., KAKIUCHI M., KATO H., ITO Y., GOMI Y. Detrusor hyperreflexia induced by intravesical instillation of xylene in conscious rats. Jpn. J. Pharmacol. 1990;52:587–595. doi: 10.1254/jjp.52.587. [DOI] [PubMed] [Google Scholar]
- MORIKAWA K., ICHIHASHI M., KAKIUCHI M., YAMAUCHI T., KATO H., ITO Y., GOMI Y. Effects of various drugs on bladder function in conscious rats. Jpn. J. Pharmacol. 1989;50:369–376. doi: 10.1254/jjp.50.369. [DOI] [PubMed] [Google Scholar]
- MURAKAMI M., KAMBE T., SHIMBARA S., KUDO I. Functional coupling between various phospholipase A2s and cyclooxygenases in immediate and delayed prostanoid biosynthetic pathways. J. Biol. Chem. 1999;274:3103–3115. doi: 10.1074/jbc.274.5.3103. [DOI] [PubMed] [Google Scholar]
- OLSSON L.E., WHEELER M.A., SESSA W.C., WEISS R.M. Bladder instillation and intraperitoneal injection of Escherichia coli lipopolysaccharide up-regulate cytokines and iNOS in rat urinary bladder. J. Pharmacol. Exp. Ther. 1998;284:1203–1208. [PubMed] [Google Scholar]
- PARK J.M., YANG T., AREND L.J., SMART A.M., SCHNERMANN J.B., BRIGGS J.P. Cyclooxygenase-2 is expressed in bladder during fetal development and stimulated by outlet obstruction. Am. J. Physiol. 1997;273:F538–F544. doi: 10.1152/ajprenal.1997.273.4.F538. [DOI] [PubMed] [Google Scholar]
- POGGESI L., NICITA G., CASTELLANI S., SELLI C., GALANTI G., TURINI D., MASOTTI G. The role of prostaglandins in the maintenance of the tone of the urinary bladder. Invest. Urol. 1980;17:454–458. [PubMed] [Google Scholar]
- SABAN M.R., SABAN R., BJORLING D.E. Kinetics of peptide-induced release of inflammatory mediators by the urinary bladder. Br. J. Urol. 1997;80:742–747. doi: 10.1046/j.1464-410x.1997.00415.x. [DOI] [PubMed] [Google Scholar]
- STEIN P.C., PHAM H., ITO T., PARSONS C.L. Bladder injury model induced by exposure of protamine sulfate followed by bacterial endotoxin. J. Urol. 1996;155:1133–1138. [PubMed] [Google Scholar]
- VANE J.R., BOTTING R.M. New insights into the mode of action of anti-inflammatory drugs. Inflamm. Res. 1995;44:1–10. doi: 10.1007/BF01630479. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., GIULIANO F., VOJNOVIC I., BUKASA A., MITCHELL J.A., WEIN J.R. Nonsteroid drug selectivity for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]


