Abstract
The influence of hypoxanthine (HX)/xanthine oxidase (XO) on short-term [electrical field stimulation (EFS; 4 Hz) for 10 s and 3 min; bolus of exogenous NO (10−5 M)] and long-term [EFS (4 Hz) and continuous NO-infusion for 20 min] nitrergic relaxations was investigated in circular muscle strips of the pig gastric fundus.
HX (3×10−4 M) / XO (64 mu ml−1) did not affect EFS for 10 s and 3 min; the short-lasting relaxation in response to a bolus of exogenous NO (10−5 M) was changed into a biphasic relaxation with a small and short first phase followed by a larger and prolonged second phase. Cu/Zn superoxide dismutase (Cu/Zn SOD; 1000 u ml−1) and uricase (100 mu ml−1) respectively enhanced the amplitude of the first phase and diminished the amplitude of the second phase. Ascorbate (5×10−4 M) and bilirubin (2×10−4 M) prevented the prolonged component.
Exposure to HX/XO during long-term EFS elicited a complete, stable reversal of relaxation starting after a delay. During continuous NO-infusion, HX/XO induced an immediate, complete but transient reversal.
The antioxidants bilirubin, ascorbate, α-tocopherol, urate, glutathione and Cu/Zn SOD, the hydrogen peroxide degrading enzyme catalase, the hydroxyl radical scavengers dimethylsulphoxide and mannitol, and the cofactor flavin adenine dinucleotide did not influence the reversal induced by HX/XO during either EFS or NO-infusion. The cell-permeable manganese SOD mimetic EUK-8 modified the stable reversal during long-term EFS into a transient one.
The results suggest that a nitrated uric acid derivative is responsible for the prolonged second phase in the relaxation to a bolus of exogenous NO in the presence of HX/XO. The exact underlying mechanism of the reversal induced by HX/XO during sustained relaxation remains unclear.
Keywords: Porcine gastric fundus, hypoxanthine/xanthine oxidase, ascorbic acid, bilirubin, uric acid, superoxide dismutase, nitric oxide, biphasic relaxation, reactive oxygen species
Introduction
There is general consensus that nitrergic motor neurons constitute a major component of the peripheral inhibitory non-adrenergic non-cholinergic (NANC) neurotransmission eliciting smooth muscle relaxation in the digestive tract (Sanders & Ward, 1992; Stark & Szurszewski, 1992; Rand & Li, 1995). Although it is unequivocally proven that neuronal nitric oxide synthase (nNOS) is the key enzyme in the biosynthesis of the nitrergic neurotransmitter (Gross & Wolin, 1995; Stuehr, 1997), no definite conclusion about the exact biochemical identity of this neurotransmitter has so far been reached. The existing controversy originates from the observation that several pharmacological compounds clearly differentiate between the actions of authentic NO and nitrergic nerve stimulation: they block or greatly attenuate the relaxation induced by exogenous NO, while having little or no effect on the relaxant responses to the nitrergic transmitter in the same tissue. Pyrogallol, duroquinone, 6-anilino-5,8-quinolinedione (LY83583) and the combination of hypoxanthine and xanthine oxidase are superoxide generators, documented to show this differentiating effect between the endogenous neurotransmitter and exogenously added NO at various nitrergic neuroeffector junctions throughout the gastrointestinal motor system (Barbier & Lefebvre, 1992; Gibson et al., 1994; De Man et al., 1996; Lefebvre, 1996; La & Rand, 1999).
Several hypotheses have been formulated to explain the differential effect of the superoxide anion generators: (1) the transmitter released into the synaptic cleft upon stimulation of nitrergic nerves is not NO but a superoxide resistant NO-adduct, the carrying molecule most likely being a thiol (Gibson et al., 1992; Barbier & Lefebvre, 1994); (2) the endogenous nitrergic transmitter is nitrogen monoxide but it is synthesized as or converted to another superoxide insensitive redox form (i.e. NO+ or NO−) before leaving its neuronal origin (Gibson et al., 1995); (3) the actual transmitter is free radical NO, but is protected from superoxide mediated destruction by the presence of antioxidant systems integrated in the neuro-effector junction (Gibson & Lilley, 1997).
Recent interest has focused on the latter hypothesis. Indeed, superoxide dismutase (SOD), immunohistochemically shown to be colocalized with nNOS in neurones of the rat anococcygeus muscle (Liu et al., 1997), might participate in the protection of the nitrergic neurotransmitter as Martin et al. (1994) demonstrated that upon pretreatment of the tissue with diethyldithiocarbamate (DETCA), a Cu-sequestering agent which irreversibly inhibits the cytosolic and extracellular Cu-dependent SOD isozymes (Kelner et al., 1989), nitrergic neurotransmission in the bovine retractor penis muscle became sensitive to the superoxide generators hypoxanthine/xanthine oxidase and pyrogallol. Although similar results were obtained in the mouse anococcygeus with duroquinone (Lilley & Gibson, 1995) and in the rat gastric fundus with LY83583 (Lefebvre, 1996), there was no alteration in the differential blocking capacity of hypoxanthine/xanthine oxidase in both those tissues after DETCA treatment (Lefebvre, 1996; Lilley & Gibson, 1996). It was therefore suggested that additional antioxidants to Cu/Zn SOD might contribute to the protection of the nitrergic neurotransmitter. The non-enzymatic antioxidants ascorbate, reduced glutathione and α-tocopherol were identified as eligible candidates as they provided protection against some or all of a battery of NO-inactivators in the mouse anococcygeus (Lilley & Gibson, 1996); moreover, ascorbate and the purine catabolic end product urate were released upon nerve depolarization in the rat anococcygeus (Lilley & Gibson, 1997).
During recent experiments in the pig gastric fundus, we observed that also bilirubin is able to protect NO from superoxide generators (Colpaert & Lefebvre, 2000). In this project we also noticed that the interaction of hypoxanthine/xanthine oxidase is more complex than can be explained by superoxide generation. When interacting with hypoxanthine, xanthine oxidase indeed also produces the antioxidant urate. Furthermore, evidence for xanthine oxidase-mediated decomposition of S-nitrosothiols, a putative candidate for the role of the nitrergic neurotransmitter, was recently provided by Trujillo et al. (1998). The aim of this study was therefore to investigate in detail the influence of hypoxanthine/xanthine oxidase on short-term as well as on long-term nitrergic relaxations in the pig gastric fundus with special attention for the role of urate.
Methods
Tissue preparation and general methodology
Experiments were carried out on isolated circular smooth muscle strips of the porcine gastric fundus. The stomach was removed from healthy 6-month-old male castrated pigs, slaughtered at a local abattoir, and transported to the laboratory in ice-chilled physiological salt solution. After the mucosa was removed, strips (15×3 mm) were cut from the fundus in the direction of the circular muscle layer. All tissues were used immediately. Strips were mounted vertically between two platinum plate electrodes under a load of 2 g in 5 or 20 ml organ baths, containing physiological salt solution at 37°C and gassed with 95% O2/5% CO2. The composition of the physiological salt solution was (mM): Na+ 137, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, Cl− 124.1, HCO3− 25, H2PO4− 1.2 and glucose 11.5 (Mandrek & Milenov, 1991). To obtain NANC conditions, atropine (10−6 M) and guanethidine (4×10−6 M) were continuously present in the medium. Changes in length were recorded isotonically via Hugo Sachs B40 Lever transducers type 373 on a Graphtec Linearcorder 8 WR 3500 in the 5 ml baths and via Palmer Bioscience T3 transducers on a Graphtec Linearcorder WR 3701 F in the 20 ml organ baths. Electrical field stimulation (EFS; 40 V, 0.1 ms, 4 Hz) was applied by means of a Hugo Sachs Stimulator I type 215/I in the 5 ml baths and by a Grass S88 stimulator in the 20 ml baths. The tissues were equilibrated for 90 min with rinsing every 15 min before starting the experiment.
Protocols
After the equilibration period, all strips were first contracted with 3×10−7 M 5-hydroxytryptamine (5-HT) and subsequently relaxed by 10−5 M sodium nitroprusside (SNP). After an interval of 1 h with regular rinsing, the experiment was then continued. A first series of experiments was performed to study the effect of the combination of hypoxanthine (HX) and xanthine oxidase (XO) on relaxant responses induced by either electrical field stimulation (EFS; 40 V, 0.1 ms, 4 Hz; 10 s, 3 min and 20 min) or exogenous NO (10−5 M) administered in bolus or via continuous infusion for 20 min. Only one type of relaxant stimulus was studied per tissue. To study the influence of HX/XO on short-term nitrergic relaxation (EFS, 10 s or 3 min; NO-bolus), tone was raised with 3×10−7 M 5-HT and when a stable plateau contraction was obtained, the relaxant stimulus was applied and repeated a second time (only for EFS 10 s and NO-bolus) 8 min later. After another hour of repetitive rinsing, XO (64 mu ml−1) was incubated for 15 min and tone was again raised with 3×10−7 M 5-HT. When a stable contraction was obtained, HX (3×10−4 M) was administered at 2 and 10 min (only for EFS 10 s and NO-bolus) later the relaxant stimulus was repeated. In a similar protocol, NO (10−5 M) was administered five times consecutively at 5 min intervals. To study the influence of HX/XO on long-term nitrergic relaxation, tone was raised with 3×10−7 M 5-HT and a NO-infusion or EFS was started. After 5 min, HX (3×10−4 M) plus XO (64 mu ml−1) were added and the infusion or stimulation was continued for another 15 min. In parallel control tissues, the solvents of HX and XO were applied.
To investigate the influence of the antioxidants (ascorbic acid, 5×10−4 M; α-tocopherol, 4×10−4 M; glutathione, 5×10−4 M; uric acid, 4×10−4 M; bilirubin, 2×10−4 M; EUK-8, 3×10−4 M; Cu/Zn SOD, 1000 u ml−1) on the effect of HX/XO versus the relaxant stimuli, these substances were administered just before the addition of HX (strips submitted to NO-boli) or 2 min before the combination of HX/XO (protocols with NO-infusion and EFS for 20 min). For the long-term nitrergic relaxations, the influence of catalase (2000 and 4000 u ml−1), the radical scavengers mannitol (2×10−3 M) and dimethylsulphoxide (DMSO, 2×10−4 M) and the cofactor FAD (5×10−4 M) were also tested versus HX/XO.
The experimental protocol with the NO-boli was used to determine the influence of uricase, that oxidizes uric acid to allantoin, on the effect of HX/XO; uricase (100 mu ml−1) was added just before the addition of HX. The effect of uricase versus uric acid was studied in a slightly different way. Upon contraction with 3×10−7 M 5-HT, NO (10−5 M) was administered twice at 8 min intervals. Uric acid (4×10−4 M) was given 2 min before the second bolus. After 1 h of repetitive rinsing, contraction was again induced and uricase (100 or 200 mu ml−1) was administered 2 min before the first NO-bolus and uric acid (4×10−4 M) 2 min before the second.
Drugs
The following drugs were used (supplied by Sigma unless stated otherwise): L-ascorbic acid, atropine sulphate, bilirubin ditaurate (Calbiochem), catalase (from bovine liver), dimethylsulphoxide, EUK-8 (Calbiochem), flavin adenine dinucleotide, glutathione, guanethidine sulphate, 5-hydroxytryptamine creatinine monosulphate (Janssen Chimica), hypoxanthine, D-mannitol, sodium nitroprusside, Cu/Zn superoxide dismutase (from bovine erythrocytes), α-tocopherol, uric acid, uricase (from porcine liver), xanthine, xanthine oxidase (from buttermilk). Drugs were dissolved in deionized water except α-tocopherol that was dissolved in dimethylsulphoxide, and hypoxanthine, xanthine and uric acid they were dissolved in 0.1 M NaOH. Solvents themselves were without significant effect at the concentrations used in the experiments unless otherwise indicated. Stock solutions were made of SOD (100,000 u ml−1); other solutions were prepared on the day of the experiment. A saturated NO solution was prepared as described by Kelm & Schrader (1990), yielding a vial containing NO in a concentration taken to be 2×10−3 M. The vials which were used for the NO-infusion experiments also contained 3×10−7 M 5-HT. The amount yielding 10−5 M NO, when given in bolus, was infused into the organ bath per 10 s via a Braun infusion pump.
Data analysis
Relaxations elicited by EFS and NO are expressed as percentage of the relaxation induced by 10−5 M sodium nitroprusside at the beginning of the experiment. Responses in the presence of interfering drugs are related to those obtained before administration of these drugs. Experimental data are expressed as means±s.e.mean and n refers to the number of strips from different animals. Results within tissues are compared by a paired t-test and results between tissues with an unpaired t-test; a difference is considered statistically significant at P<0.05.
Results
Effect of HX/XO on nitrergic relaxations
Incubation with XO (64 mu ml−1) resulted in a moderate increase in basal tone in most strips, but did not alter the amplitude of contraction induced by 3×10−7 M 5-HT. When the substrate HX (3×10−4 M) was administered on top of this 5-HT-elicited contraction, in the presence of XO, some tissues responded with a small rise in tone.
The combination HX/XO did not affect the relaxant responses to short-term EFS for 10 s [respectively 91.1±12.5% (first EFS for 10 s) and 96.6±10.1% (second EFS for 10 s) of the value before administration of the drugs; n=6]. In contrast, when two NO-boli (10−5 M) were applied as short-lasting relaxant triggers, HX/XO clearly changed the shape of the NO-induced relaxations: they became biphasic with a small and short first phase, immediately followed by a larger and prolonged second phase. As both phases of the second NO-induced relaxation were enhanced in comparison to the corresponding phases of the first (Figure 1), we adapted the protocol by increasing the number of NO-boli to five. In the control tissues receiving only XO, the response to the five consecutive NO-boli was reproducible (Figure 2a). In a parallel set of tissues in the presence of XO, the substrate HX was added either before the first and fifth NO-bolus (Figure 2b) or before each application of NO (Figure 2c). Upon repetitive administration of NO after a single administration of HX, the first and second phase of the NO-induced relaxation progressively increased but replenishment of hypoxanthine blocked the enhancement of the first phase (Figure 2b). The latter was confirmed upon repetitive administration of the substrate, but this procedure did not prevent the second phase increasing to a constant value.
Figure 1.
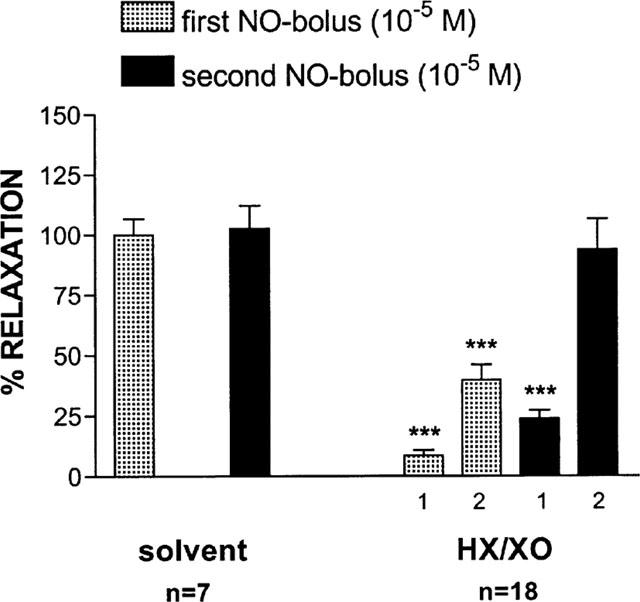
Effect of HX (3×10−4 M)/XO (64 mu ml−1) on the relaxant responses to 2 NO-boli (10−5 M) consecutively given with an interval of 8 min. On the left, the control situation is depicted where the strips receive the solvent of either HX or XO. In the presence of HX/XO (on the right), the relaxant response to NO became biphasic. Both phases are shown (1 and 2). The relaxations are expressed as a percentage of the response to the same stimulus before addition of interfering drugs. ***P<0.001: significantly different from the response before (paired t-test). HX=hypoxanthine; XO=xanthine oxidase.
Figure 2.
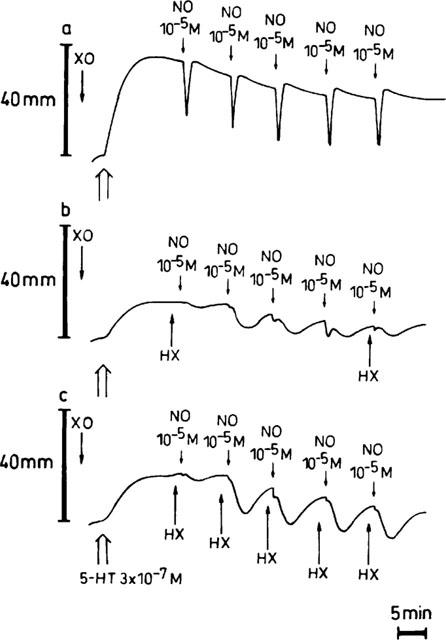
Representative traces showing the relaxant responses to 5 NO-boli (10−5 M) in the presence of XO (64 mu ml−1) alone (a), and when the substrate HX (3×10−4 M) is added before the first and fifth bolus (b) or before each bolus (c). HX=hypoxanthine; XO=xanthine oxidase.
HX/XO had no influence on EFS for 3 min (100.5±3.9% of the response before addition; n=5); however, when HX/XO were injected into the organ bath 5 min after the start of continuous EFS or NO-infusion for 20 min, a complete reversal of relaxation occurred (99.5±29.9 and 109.6±8.4% respectively; n=5). Neither HX nor XO alone (Figures 3a,c) was able to induce this reversal seen with the combination of these agents. The HX/XO-mediated reversal during NO-infusion (Figure 3b) was initiated immediately after addition of the substrate HX; it developed very fast, reaching its maximum within 1 min, and then waned so that the NO-induced relaxation returned to its original level. The reversal observed with HX/XO during long-term EFS (Figure 3d) differed from the one obtained during NO-infusion as it started after a delay of at least 2 min and progressed slowly to a stable level.
Figure 3.
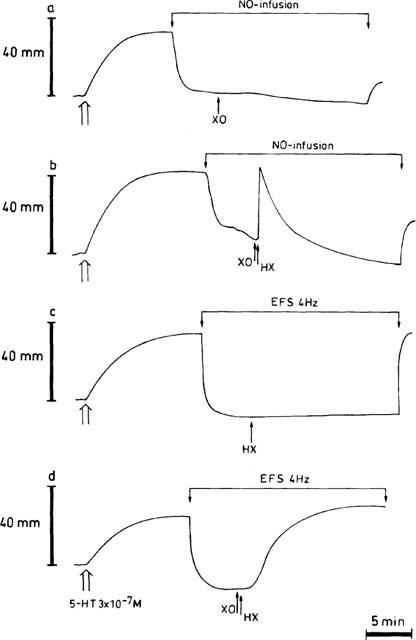
Typical tracings illustrating the influence of HX (3×10−4 M) / XO (64 mu ml−1) when added 5 min after starting a NO-infusion (b) or electrical field stimulation (EFS, 40 V, 0.1 ms, 4 Hz) (d). Time controls, which receive only the enzyme (a) or the substrate (c), are also shown. Both the NO-infusion and EFS were sustained for 20 min. HX=hypoxanthine; XO=xanthine oxidase.
Additional experiments with the relaxant agents VIP (10−5 M), isoprenaline (10−6 M) and SNP (10−5 M) were also included (data not shown). Only the relaxation induced by SNP was influenced by HX/XO. When HX/XO was administered during the relaxation by SNP, it was completely reversed in a stable manner after a delay of approximately 1 min. When HX/XO was added before SNP, the relaxation was reduced by approximately 50%.
Effect of uricase on relaxations induced by NO-boli in the presence of HX/XO or uric acid
There was no influence of uricase (100 and 200 mu ml−1) per se on the relaxant response to 10−5 M exogenous NO [respectively 101.4±14.5% (n=9) and 123.1±20.2% (n=6) of the control value before addition of uricase]. When uricase (100 mu ml−1) was tested versus HX/XO, it did not influence the first phase of the relaxant responses to the 2 NO-boli applied in the presence of HX/XO; however, there was a clear tendency to reduce the second phases, reaching statistical significance for the second NO-bolus (Figure 4). When uric acid (4×10−4 M) was added into the organ bath, the relaxation to an exogenous bolus of NO (10−5 M) showed a biphasic and prolonged time course (Figure 5); in strips from the same pig, the amplitude of the relaxant effect of NO (10−5 M) was either markedly potentiated (Figure 5a) or not (Figure 5b). Neither hypoxanthine (3×10−4 M) nor xanthine (3×10−4 M) exerted this effect. Uricase (100 mu ml−1) reduced the amplitude of the prolonged component of the NO-induced relaxation in the presence of uric acid to 34.8±7.1% of its control value (Figure 5c; P<0.01; n=6); 200 mu ml−1 of uricase even completely abolished this prolonged component (Figure 5d; P<0.01; n=4).
Figure 4.
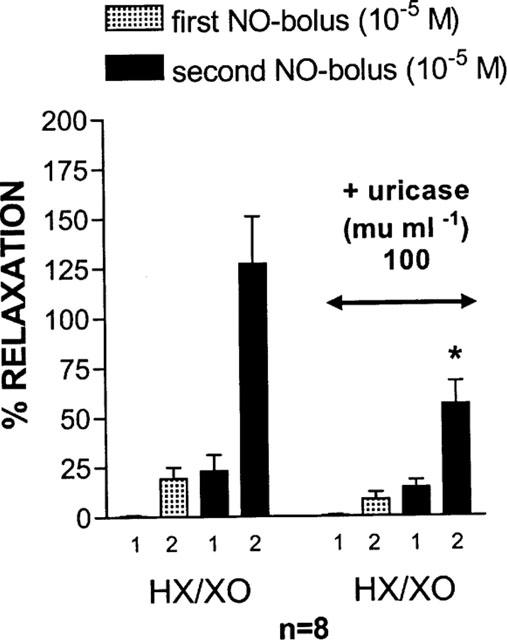
Influence of uricase (100 mu ml−1) on the relaxant responses to 2 NO-boli (10−5 M), administered with an interval of 8 min, in the presence of HX (3×10−4 M) / XO (64 mu ml−1). In the presence of HX/XO, the relaxant response to NO was biphasic and both phases (1 and 2) are shown. Results are expressed as a percentage of the same stimulus before addition of the interfering drugs. *P<0.05: significantly different from the response in the presence of HX/XO alone (unpaired t-test). HX=hypoxanthine; XO=xanthine oxidase.
Figure 5.
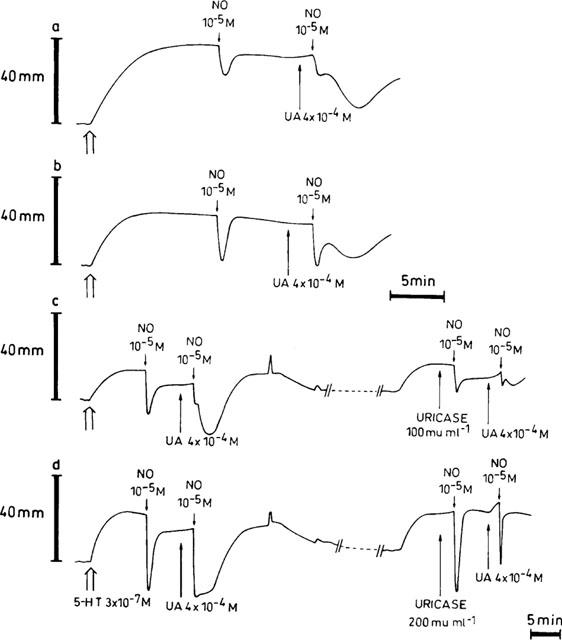
Representative traces demonstrating the effect of 4×10−4 M uric acid (UA) on relaxations induced by boli of exogenous NO (10−5 M) (a,b), and the influence of 100 mu ml−1 (c) and 200 mu ml−1 (d) of uricase on exogenous NO in the absence and presence of 4×10−4 M UA. During intervals (//- - - - - -//), the paper speed was reduced 5 fold.
Influence of antioxidants and oxyradical scavengers on the effect of HX/XO
Glutathione (5×10−4 M) and α-tocopherol (4×10−4 M) did not influence the effect of HX/XO on the response to the 2 NO-boli (Figure 6). Uric acid (4×10−4 M) decreased the first phase of the response to the second NO-bolus, while EUK-8 (3×10−4 M) increased it (Figure 6). Cu/Zn superoxide dismutase (1000 u ml−1) significantly augmented the first phases of the responses to both NO-boli (Figure 6). Bilirubin (2×10−4 M) and ascorbate (5×10−4 M) totally inhibited the generation of a prolonged component in the relaxant response to the NO-boli in the presence of HX/XO (Figure 6).
Figure 6.
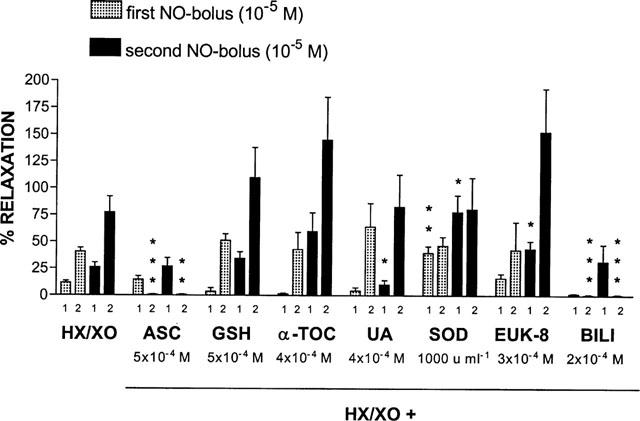
Relaxant responses to two boli of exogenous NO (10−5 M), applied with an interval of 8 min, in the presence of HX (3×10−4 M) / XO (64 mu ml−1) alone or plus one of the antioxidants indicated. The first (1) and second (2) phase of the relaxant response to NO in the presence of HX/XO is shown. The relaxations are expressed as a percentage of the response to the same stimulus before administration of the drugs under study. All results are the means±s.e.mean of 6–7 strips from different animals. *P<0.05; **P<0.01; ***P<0.001: significantly different from the response in the presence of HX/XO alone (unpaired t-test). ASC=ascorbic acid; GSH=glutathione; α-TOC=α-tocopherol; UA=uric acid; SOD=superoxide dismutase; BILI=bilirubin; HX=hypoxanthine; XO=xanthine oxidase.
The above cited antioxidants, the hydrogen peroxide degrading enzyme catalase (2000 and 4000 u ml−1), the hydroxyl radical scavengers DMSO (2×10−4 M) and mannitol (2×10−3 M), and the cofactor FAD (5×10−4 M) were also tested versus the capacity of HX/XO to reverse the stable relaxation evoked by NO-infusion or EFS for 20 min (n=at least four for each substance). None of these substances was able to counteract the HX/XO-mediated reversal; EUK-8, however, modified the stable reversal of the relaxation induced by long-term EFS, seen with HX/XO, into a transient one but had no influence on the delay (data not given).
Discussion
This manuscript deals with the effect of the substrate (hypoxanthine)/enzyme (xanthine oxidase) system on nitrergic relaxation in the pig gastric fundus. Xanthine oxidase (XO) catalyzes the sequential breakdown of hypoxanthine (HX) to xanthine and subsequently to uric acid; in this oxidation process, XO concomitantly generates several reactive oxygen species by reducing molecular oxygen (Kooij, 1994). Chemical interactions between reactive oxygen species (such as superoxide, a continuously formed free radical in aerobic cells) and NO are described as near-diffusion limited (Huie & Padmaja, 1993), and are recognized as being of critical importance for modulating the signal transduction actions of NO as well as for oxidative damage (Gross & Wolin, 1995).
HX/XO did not influence the short-term nitrergic relaxations induced by EFS for 10 s or 3 min; the short-lasting relaxant response to a bolus of exogenous NO, however, was converted to a biphasic relaxation. When added during a sustained relaxation, initiated either by continuous infusion of NO or by long-lasting EFS, the combination of HX/XO caused a complete reversal of relaxation in these two experimental conditions; nevertheless, clear differences in the onset and the duration of the reversal were noticed between the two relaxant situations. The above findings markedly deviate from previous results obtained with the superoxide generators LY83583 and hydroquinone in the pig gastric fundus (Colpaert & Lefebvre, 2000), as LY83583 and hydroquinone failed to reverse long-term EFS or to provoke a biphasic relaxation in response to a bolus of exogenous NO.
Influence of HX/XO on short-term nitrergic relaxation in the pig gastric fundus
In the presence of the combination HX/XO, the relaxant responses of the porcine gastric fundus strips to boli of exogenous NO exhibited a biphasic time course: a small and short-lasting first phase immediately passed into a larger and prolonged second phase. The first phase corresponds to the response to exogenous NO in the absence of HX/XO. The reduced amplitude is very probably due to interaction of NO with radicals generated by HX/XO. Indeed, upon repetitive administration of NO after a single administration of HX, the amplitude of the first phase progressively increased most likely due to substrate exhaustion and hence decreasing radical generation, while the amplitude of the first phase remained small when HX was repetitively replenished. The radical involved is at least partially extracellular superoxide as SOD increased the amplitude of the first phase in the presence of HX/XO.
Opposite to the first phase, the amplitude of the second phase developed to a constant value irrespective of further substrate application. To test the hypothesis that uric acid, final reaction product of the interaction between HX and XO, participated in the genesis of the second phase, we evaluated the effect of uricase which oxidizes uric acid to allantoin (Wu et al., 1994). The amplitude of the second phase of the biphasic relaxation appeared to be strongly attenuated by uricase, while the amplitude of the initial phase was unaffected. Furthermore, when the influence of uric acid per se was tested, the relaxant response to a bolus of exogenous NO demonstrated an identical biphasic pattern as described above; this effect was not observed in the presence of hypoxanthine or xanthine alone, both precursor molecules of uric acid. The prolonged phase of the NO-induced biphasic relaxation in the presence of uric acid was also demonstrated to be uricase sensitive. The prolonged relaxation component to NO in the presence of HX/XO or urate might represent the biological action of a nitrated uric acid derivative. Our experimental data are indeed in accordance with the observations of Skinner et al. (1998), who described for the first time a nitrated uric acid derivative (identified as 2-nitrito-4-amino-5-hydroxyimidazoline) capable of inducing endothelium-independent vasorelaxation of rat thoracic aorta; the spontaneous and protracted release of NO was confirmed electrochemically. HX/XO gives rise to both uric acid and different reactive oxygen species (superoxide, hydrogen peroxide and possibly hydroxyl radicals; see further). Under conditions where radical species are formed, the uric acid molecule will be chemically modified upon oxyradical scavenging, resulting in a urate radical intermediate which is relatively stable due to marked delocalization of the unpaired electron over the heterocyclic rings (Simic & Jovanovic, 1989). This radical does not react with molecular oxygen (Simic & Jovanovic, 1989), but may combine with free radical NO to form a nitrated uric acid derivative. The antioxidants ascorbic acid and bilirubin totally prevented the development of the prolonged second phase in the relaxant response to exogenous NO in the presence of HX/XO. As the redox repair of the urate radical to uric acid by ascorbic acid has been reported in the literature (Maples & Mason, 1988), this might be explained as follows. Ascorbic acid will compete with NO for interaction with the urate radical; upon interaction with the urate radical, ascorbate will regenerate uric acid and thus decrease the formation of the nitrated uric acid derivative responsible for the prolonged relaxation. A similar mechanism apparently also occurs with bilirubin but not with the other antioxidants.
HX/XO did not influence the relaxations of the fundic strips induced by the release of the nitrergic neurotransmitter upon EFS for 10 s and 3 min. This might be related to the optimal antioxidant protection of the neurotransmitter fencing off radical attack; the defense molecules might represent compounds constitutively present in the neuroeffector junction such as uric acid which is thought of as a replenishable antioxidant in all hydrophilic tissue compartments (Becker, 1993), or alternatively, they may be released along with the neurotransmitter during nerve depolarization as recently suggested for ascorbic acid in the nitrergically innervated anococcygeus muscle of the rat (Lilley & Gibson, 1997). The non-appearance of a prolonged relaxation component in the response to EFS in the presence of HX/XO, receives a feasible explanation by this latter observation: ascorbate and/or bilirubin, released simultaneously with the nitrergic neurotransmitter or present in the synaptic cleft, could prevent the formation of the nitrated uric acid derivative via the mechanism described above.
Influence of HX/XO on long-term nitrergic relaxation in the pig gastric fundus
HX/XO provoked a complete but temporary reversal of relaxation when administered during a sustained relaxation elicited by continuous NO-infusion. A non-specific effect can be excluded as the relaxations induced by VIP and the β-agonist isoprenaline, thought to evoke relaxation via a cyclic AMP-dependent pathway, were not influenced by HX/XO. The instant onset upon substrate addition of the reversal during NO-infusion suggests that interaction of NO with radical species generated by the addition of HX/XO is involved. In contrast to what was seen versus a NO bolus, extracellular superoxide is not involved as SOD did not influence the HX/XO-induced reversal. The same applied for the cell-permeable manganese SOD mimetic EUK-8, so that the involvement of intracellular superoxide is unlikely. XO can also produce hydrogen peroxide by the divalent reduction of molecular oxygen (Porras et al., 1981), but this reactive oxygen species seems not involved as catalase, which degrades hydrogen peroxide to water and oxygen, did not influence the reversal induced by HX/XO. The presence of redox-active metal catalysts in isolated organ preparations and the adventitious iron contamination in experimental systems may induce transition metal-dependent hydroxyl radical production starting from hydrogen peroxide. The extracellularly acting hydroxyl radical scavenger mannitol (Pieper & Dondlinger, 1997) did not prevent the development of the reversal of the sustained relaxation induced by NO-infusion; therefore, hydroxyl radicals formed extracellularly are probably not involved. Since hydrogen peroxide readily crosses cell membranes and may react with intracellular transitional iron or copper in a Fenton-like reaction, the possibility of an intracellular generation of hydroxyl radicals accounting for the observed reversal was also investigated. However, the intracellular hydroxyl radical scavenger DMSO (Laight et al., 1997) also did not counteract the relaxation reversal induced by HX/XO. The identity of the radical responsible for this effect remains so far thus undefined or another mechanism is involved. The transient nature of the reversal might be related to a decreasing generation of reactive species due to depletion of the substrate HX and/or to the production of the proposed nitrated uric acid derivative which exerts a relaxant effect through the liberation of its NO. Why then the addition of bilirubin or ascorbate, shown to inhibit the formation of the nitrated uric acid derivative when HX/XO is tested versus a bolus of exogenous NO, did not alter the transient reversal during continuous NO-infusion in a more sustained one is as yet unknown. The differential effect exerted by HX/XO on sustained SNP- and NO-induced relaxations (stable reversal versus temporary reversal) might be explained by findings that the relaxant effect of SNP is due to its metabolism by membrane fractions of smooth muscle cells to release NO (Kowaluk et al., 1992; Kimura et al., 1998): potential interactions between the extracellular HX/XO-derived uric acid and NO, resulting in the nitrated uric acid derivative, are less likely to occur given the site of generation of the NO yielded by SNP.
The HX/XO-mediated reversal during long-term EFS differed from the one obtained during NO-infusion: it started after a delay and progressed slowly to a stable level as opposed to the immediate onset and transient nature of the reversal observed with HX/XO during NO-infusion. The delay indicates that a mechanism other than interaction of NO with radicals, as proposed for the immediate reversal during NO-infusion, is involved; it also explains why no inhibition was seen with HX/XO versus relaxations induced by EFS for 10 s or 3 min. XO and neuronal nitric oxide synthase (nNOS) both require FAD for their activity and cofactor competition and subsequent depletion resulting in inhibition of nNOS might explain the result; but FAD supplementation did not prevent the reversal. Still, the possibility that a byproduct of the HX/XO reaction might progressively inhibit nNOS cannot be excluded. If the reversal is not related to nNOS inhibition and the nitrergic neurotransmitter is continuously released in the presence of HX/XO, the formation of the nitrated uric acid derivative might be prevented by the concomitant release of ascorbate and/or bilirubin as explained above. This will then contribute to the stable character of the reversal. Of the antioxidants and oxyradical scavengers tested, only EUK-8, a synthetic salen-manganese complex with high SOD but also catalase and oxyradical scavenger properties, influenced the HX/XO-induced reversal of the relaxation by long-term EFS: it changed the stable reversal into a transient one. We have no explanation for this result.
Recently, Trujillo et al. (1998) showed that XO, in the presence of purine (hypoxanthine, xanthine) substrates, induced S-nitrosocysteine and S-nitrosoglutathione decomposition under aerobic conditions; both a pure enzymatic and a superoxide dependent pathway of nitrosothiol decomposition were proposed. Since this decomposition inevitably would imply an enhanced release of NO and thus potentiation of relaxation in our organ preparations, a role for nitrosothiols as nitrergic intermediaries in the pig gastric fundus seems not likely, as HX/XO exerted the opposite effect in our experimental conditions i.e. it reversed relaxation.
Conclusion
HX/XO, that generates both several reactive oxygen species and the antioxidant uric acid, changed the short-lasting relaxation by a bolus of exogenous NO into a biphasic one, with a very small first phase and a more pronounced and sustained second phase. The small amplitude of the first phase is probably related to the interaction of NO with superoxide anions, generated by HX/XO, while the second phase might be related to the formation of a nitrated uric acid derivative, that slowly releases NO. Exposure to HX/XO during long-term nitrergic relaxation led to an immediate, complete but temporary reversal during continuous NO-infusion but to, after some delay, a progressive, complete and stable reversal during long-term EFS; the underlying mechanisms of this differential effect await further elucidation.
Acknowledgments
E.E. Colpaert is a research assistant of the Fund for Scientific Research Flanders. The study was financially supported by grant No. 3G0031.96 from the Fund for Scientific Research Flanders, grant 011A1696 from the Special Investigation Fund of the Ghent University and by Interuniversity Pole of Attraction Programme P4/16 (Services to the Prime Minister – Federal Services for Scientific, Technical and Cultural Affairs).
Abbreviations
- DETCA
diethyldithiocarbamate
- DMSO
dimethylsulphoxide
- EFS
electrical field stimulation
- FAD
flavin adenine dinucleotide
- 5-HT
5-hydroxytryptamine
- HX
hypoxanthine
- LY83583
6-anilino-5,8-quinolinedione
- NANC
non-adrenergic non-cholinergic
- nNOS
neuronal nitric oxide synthase
- NO
nitric oxide
- NO+
nitrosonium cation
- NO−
nitroxyl anion
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- VIP
vasoactive intestinal polypeptide
- XO
xanthine oxidase
References
- BARBIER A.J.M., LEFEBVRE R.A. Effect of LY83583 on relaxation induced by non-adrenergic non-cholinergic nerve stimulation and exogenous nitric oxide in the rat gastric fundus. Eur. J. Pharmacol. 1992;219:331–334. doi: 10.1016/0014-2999(92)90315-u. [DOI] [PubMed] [Google Scholar]
- BARBIER A.J.M., LEFEBVRE R.A. Influence of S-nitrosothiols and nitrate tolerance in the rat gastric fundus. Br. J. Pharmacol. 1994;111:1280–1286. doi: 10.1111/j.1476-5381.1994.tb14884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER B.F. Towards the physiological function of uric acid. Free Rad. Biol. Med. 1993;14:615–631. doi: 10.1016/0891-5849(93)90143-i. [DOI] [PubMed] [Google Scholar]
- COLPAERT E.E., LEFEBVRE R.A. Influence of bilirubin and other antioxidants on nitrergic relaxation in the pig gastric fundus. Br. J. Pharmacol. 2000;129:1201–1211. doi: 10.1038/sj.bjp.0703176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAN J.G., DE WINTER B.Y., BOECKXSTAENS G.E., HERMAN A.G., PELCKMANS P.A. Effect of thiol modulators and Cu/Zn superoxide dismutase inhibition on nitrergic relaxations in the rat gastric fundus. Br. J. Pharmacol. 1996;119:1022–1028. doi: 10.1111/j.1476-5381.1996.tb15773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., BABBEDGE R., BRAVE S.R., HART S.L., HOBBS A.J., TUCKER J.F., WALLACE P., MOORE P.K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br. J. Pharmacol. 1992;107:715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., BRAVE S.R., MCFADZEAN I., TUCKER J.F., WAYMAN C. The nitrergic transmitter of the anococcygeus - NO or not. Arch. Int. Pharmacodyn. 1995;32:39–51. [PubMed] [Google Scholar]
- GIBSON A., BRAVE S.R., TUCKER J.F. Differential effect of xanthine/xanthine oxidase on NANC and NO-induced relaxations of the mouse anococcygeus. Can. J. Physiol. Pharmacol. 1994;72 Suppl. 1:475. [Google Scholar]
- GIBSON A., LILLEY E. Superoxide anions, free-radical scavengers, and nitrergic neurotransmission. Gen. Pharmacol. 1997;28:489–493. doi: 10.1016/s0306-3623(96)00281-9. [DOI] [PubMed] [Google Scholar]
- GROSS S.S., WOLIN M.S. Nitric oxide: pathophysiological mechanisms. Annu. Rev. Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- HUIE R.E., PADMAJA S. The reaction of NO with superoxide. Free Rad. Res. Comm. 1993;18:195–199. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- KELM M., SCHRADER J. Control of coronary vascular tone by nitric oxide. Circ. Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- KELNER M.J., BAGNELL R., HALE B., ALEXANDER N.M. Inactivation of intracellular copper-zinc superoxide dismutase by copper chelating agents without glutathione depletion and methemoglobin formation. Free Rad. Biol. Med. 1989;6:355–360. doi: 10.1016/0891-5849(89)90079-8. [DOI] [PubMed] [Google Scholar]
- KIMURA M., WHITE R.P., WOLF E.W., ROBERTSON J.T. Responses of human basilar and other isolated arteries to novel nitric oxide donors. J. Cardiovasc. Pharmacol. 1998;32:695–701. doi: 10.1097/00005344-199811000-00003. [DOI] [PubMed] [Google Scholar]
- KOOIJ A. A re-evaluation of the tissue distribution and physiology of xanthine oxidoreductase. Histochem. J. 1994;26:889–915. [PubMed] [Google Scholar]
- KOWALUK E.A., SETH P., FUNG H.-L. Metabolic activation of sodium nitroprusside to nitric oxide in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1992;262:916–922. [PubMed] [Google Scholar]
- LA M., RAND M.J. Effects of pyrogallol, hydroquinone and duroquinone on responses to nitrergic nerve stimulation and NO in the rat anococcygeus muscle. Br. J. Pharmacol. 1999;126:342–348. doi: 10.1038/sj.bjp.0702277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAIGHT D.W., CARRIER M.J., ANGGARD E.E. Investigation of role for oxidant stress in vascular tolerance development to glyceryl trinitrate in vitro. Br. J. Pharmacol. 1997;120:1477–1482. doi: 10.1038/sj.bjp.0701078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Influence of superoxide dismutase inhibition on the discrimination between NO and the nitrergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 1996;118:2171–2177. doi: 10.1111/j.1476-5381.1996.tb15659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Inhibition of relaxations to nitrergic stimulation of the mouse anococcygeus by duroquinone. Br. J. Pharmacol. 1995;116:3231–3236. doi: 10.1111/j.1476-5381.1995.tb15129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Antioxidant protection of NO-induced relaxations of the mouse anococcygeus muscle against inhibition by superoxide anions, hydroquinone and carboxy-PTIO. Br. J. Pharmacol. 1996;119:432–438. doi: 10.1111/j.1476-5381.1996.tb16004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LILLEY E., GIBSON A. Release of the antioxidants ascorbate and urate from a nitrergically-innervated smooth muscle. Br. J. Pharmacol. 1997;122:1746–1752. doi: 10.1038/sj.bjp.0701571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X., MILLER S.M., SZURSZEWSKI J.H. Protection of nitrergic transmission by colocalization of neural nitric oxide synthase with copper zinc superoxide dismutase. J. Auton. Nerv. Syst. 1997;62:126–133. doi: 10.1016/s0165-1838(96)00113-0. [DOI] [PubMed] [Google Scholar]
- MANDREK K., MILENOV K. Responses of porcine gastric and duodenal smooth muscle to VIP. J. Autonom. Pharmacol. 1991;11:353–364. doi: 10.1111/j.1474-8673.1991.tb00259.x. [DOI] [PubMed] [Google Scholar]
- MAPLES K.R., MASON R.P. Free radical metabolite of uric acid. J. Biol. Chem. 1988;263:1709–1712. [PubMed] [Google Scholar]
- MARTIN W., MCALLISTER K.M.H., PAISLEY K. NANC neurotransmission in the bovine retractor penis muscle is blocked by superoxide anion following inhibition of superoxide dismutase with diethyldithiocarbamate. Neuropharmacology. 1994;33:1293–1301. doi: 10.1016/0028-3908(94)90029-9. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., DONDLINGER L. Glucose elevations alter bradykinin-stimulated intracellular calcium accumulation in cultured endothelial cells. Cardiovasc. Res. 1997;34:169–178. doi: 10.1016/s0008-6363(97)00007-2. [DOI] [PubMed] [Google Scholar]
- PORRAS A.G., OLSON J.S., PALMER G. The reaction of reduced xanthine oxidase with oxygen. J. Biol. Chem. 1981;256:9096–9103. [PubMed] [Google Scholar]
- RAND M.J., LI C.G. Nitric oxide as a neurotransmitter in peripheral nerves: nature of transmitter and mechanism of transmission. Annu. Rev. Physiol. 1995;57:659–682. doi: 10.1146/annurev.ph.57.030195.003303. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M., WARD S.M. Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am. J. Physiol. 1992;262:G379–G392. doi: 10.1152/ajpgi.1992.262.3.G379. [DOI] [PubMed] [Google Scholar]
- SIMIC M.G., JOVANOVIC S.V. Antioxidation mechanisms of uric acid. J. Am. Chem. Soc. 1989;111:5778–5782. [Google Scholar]
- SKINNER K.A., WHITE C.R., PATEL R., TAN S., BARNES S., KIRK M., DARLEY-USMAR V., PARKS D.A. Nitrosation of uric acid by peroxynitrite. J. Biol. Chem. 1998;273:24491–24497. doi: 10.1074/jbc.273.38.24491. [DOI] [PubMed] [Google Scholar]
- STARK M.E., SZURSZEWSKI J.H. Role of nitric oxide in gastrointestinal and hepatic function and disease. Gastroenterology. 1992;103:1928–1949. doi: 10.1016/0016-5085(92)91454-c. [DOI] [PubMed] [Google Scholar]
- STUEHR D.J. Structure-function aspects in the nitric oxide synthases. Annu. Rev. Pharmacol. Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- TRUJILLO M., ALVAREZ M.N., PELUFFO G., FREEMAN B.A., RADI R. Xanthine oxidase-mediated decomposition of S-nitrosothiols. J. Biol. Chem. 1998;273:7828–7834. doi: 10.1074/jbc.273.14.7828. [DOI] [PubMed] [Google Scholar]
- WU X., WAKAMIYA M., VAISHNAV S., GESKE R., MONTGOMERY C., JR, JONES P., BRADLEY A., CASKEY C.T. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc. Natl. Acad. Sci. U.S.A. 1994;91:742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]


