Abstract
Kinin receptor agonists and antagonists at the B1 and B2 receptors were injected intrathecally (i.t., at T-9 spinal cord level) to conscious unrestrained rats and their effects on mean arterial pressure (MAP) and heart rate (HR) were compared in streptozotocin (STZ)-diabetic rats (65 mg kg−1 STZ, i.p. 3 weeks earlier) and aged-matched control rats.
The B1 receptor agonist, des-Arg9-Bradykinin (BK) (3.2–32.5 nmol), evoked dose-dependent increases in MAP and tachycardia during the first 10 min post-injection in STZ-diabetic rats only. The cardiovascular response to 6.5 nmol des-Arg9-BK was reversibly blocked by the prior i.t. injection of antagonists for the B1 receptor ([des-Arg10]-Hoe 140, 650 pmol or [Leu8]-des-Arg9-BK, 65 nmol) and B2 receptor (Hoe 140, 81 pmol or FR173657, 81 pmol) or by indomethacin (5 mg kg−1, i.a.).
The i.t. injection of BK (8.1–810 pmol) induced dose-dependent increases in MAP which were accompanied either by tachycardiac (STZ-diabetic rats) or bradycardiac (control rats) responses. The pressor response to BK was significantly greater in STZ-diabetic rats. The cardiovascular response to 81 pmol BK was reversibly blocked by 81 pmol Hoe 140 or 81 pmol FR173657 but not by B1 receptor antagonists nor by indomethacin in STZ-diabetic rats.
The data suggest that the activation of kinin B1 receptor in the spinal cord of STZ-diabetic rats leads to cardiovascular changes through a prostaglandin mediated mechanism. Thus, this study affords an accessible model for studying the expression, the pharmacology and physiopathology of the B1 receptor in the central nervous system.
Keywords: Bradykinin, kinin B1 receptor, spinal cord, streptozotocin diabetes, prostaglandins, kinin receptor antagonists, blood pressure
Introduction
Bradykinin (BK) and its active kininase I metabolite (des-Arg9-BK) are pro-inflammatory mediators acting through the activation of B2 and B1 receptor, respectively (Regoli & Barabé, 1980; Marceau et al., 1998). Compelling evidence suggests a role for these peptides as neuromodulator and/or neurotransmitter in the central nervous system (Couture & Lindsey, 2000), particularly in the control of nociceptive information (Laneuville et al., 1989; Germany et al., 1996; Pelá et al., 1996; Dray, 1997; Couto et al., 1998), blood pressure (Madeddu et al., 1990; Lopes & Couture 1992; Privitera et al., 1994; Lindsey et al., 1997) and of core temperature (Walker et al., 1996; Coelho et al., 1997). Whereas the B2 receptor is constitutive, the B1 receptor is generally absent in normal tissues and healthy animals. The B1 receptor is induced and overexpressed during tissue injury, by bacterial endotoxins and cytokines such as interleukin-1β (IL-1β) and tumor necrosis factor alpha (TNF-α) (Marceau et al., 1998). The induction of B1 receptor by cytokines is mediated in part by the transcriptional nuclear factor κB (NF-κB) (Ni et al., 1998a; Schanstra et al., 1998; Zhou et al., 1998; Campos et al., 1999; Sardi et al., 1999). Moreover, sequence analysis of human and rat B1 receptor gene has revealed the presence of a transcriptional regulatory site for NF-κB (Bachvarov et al., 1996; Ni et al., 1998a, 1998b).
Current evidence indicates that insulin-dependent diabetes mellitus is due to an autoimmune response associated to an overproduction of cytokines including IL-1β and TNF-α that leads to the destruction of pancreatic islet β-cells (Hussain et al., 1996; Rabinovitch & Suarez-Pinzon, 1998; Rabinovitch, 1998). Hyperglycemia can activate NF-κB (Yerneni et al., 1999) and this factor is thought to play a major role in the vascular complications in diabetes mellitus (Bierhaus et al., 1998). Therefore, both the overproduction of cytokines and hyperglycemia could trigger the expression of B1 receptor through NF-κB in diabetes.
Recent evidence suggests that the B1 receptor intervenes in the pathogenesis of experimentally induced diabetes in the mouse as B1 receptor antagonist completely normalizes glycemia, diuresis, protein, nitrite and kallikrein excretion (Zuccolo et al., 1996). Also, the B1 receptor is overexpressed in the stomach fundus of streptozotocin (STZ)-induced diabetes in the mouse (Pheng et al., 1997). Although the B1 receptor is scarcely represented in the CNS of healthy animals and human (Couture & Lindsey, 2000), the possibility that B1 receptor can exert a regulatory influence in CNS kinin function of diabetic animals is appealing and worth investigation. With regard to the central control of blood pressure by BK, we reported that B2 receptors are exclusively involved in the spinal cord of control Wistar rats (Lopes & Couture, 1992; Lopes et al., 1993).
The present study was undertaken to test the hypothesis that the B1 receptor partakes in the cardiovascular effects of kinins in the spinal cord of diabetic rats. This was achieved with a pharmacological approach following the intrathecal injection of selective B1 and B2 receptor agonists and antagonists in the established animal model of diabetes induced with STZ (Tomlinson et al., 1992). Part of this work has been presented elsewhere (Couture et al., 1998).
Methods
Animal source and care
Male Wistar rats (225–250 g) were purchased 3–5 days prior to experiments from Charles River, St-Constant, Québec, Canada and housed 4–5 per cage under a 12 h light-dark cycle in a room with controlled temperature (20°C), humidity (53%) with food (Charles River Rodent) and tap water available ad libitum. The care of animals and research protocols conformed to the guiding principles for animal experimentation as enunciated by the Canadian Council on Animal Care and approved by the Animal Care Committee of our University. In this study, 60 diabetic and 16 control rats were used.
STZ-induced diabetes and surgery
Rats were made diabetic with a single dose of STZ (65 mg kg−1, i.p.) freshly dissolved in 0.05 M sodium citrate buffer, pH 4.5. Age-matched control rats received the vehicle only. Fifteen days later, glucose concentration from nonfasting animals was measured, in a blood sample obtained from the tail by pinprick, with a glucose oxidase-impregnated test strip and reflectance meter (Accu-Check III, Boehringer Mannheim, Germany). Only STZ-treated rats with a blood glucose concentration higher than 20 mM were considered as diabetic. Diabetic and control rats were anaesthetized with an i.p. injection of 65 mg kg−1 sodium pentobarbitone (Somnotol; M.T.C. Pharmaceuticals, Cambridge, Ontario, Canada) and a polyethylene catheter (PE-10; Intramedics, Clay Adams, NJ, U.S.A.) was inserted into the spinal subarachnoid space via an incision made in the dura at the atlanto-occipital junction and pushed to the 9th thoracic segment (T-9) as described previously (Lopes & Couture, 1992). About 20% of rats were excluded from the study because they presented motor deficit such as partial paralysis of one posterior or anterior leg. These rats were immediately humanely killed with an overdose of pentobarbitone. Thereafter, the rats were allowed to recover in individual plastic cages (40×23×20 cm) and housed in the same controlled conditions. The correct positioning of the intrathecal (i.t.) catheter was verified by post-mortem examination at the end of the experiment and the catheter was found either dorsally or laterally to the spinal cord.
Two days later, rats were re-anaesthetized with sodium pentobarbitone (65 mg kg−1, i.p.) and an intravascular siliconized (Sigmacote, Sigma, St-Louis, MO, U.S.A.) PE-50 catheter, filled with physiological saline containing 100 i.u. ml−1 heparin sodium salt (Sigma, St-Louis, MO, U.S.A.), was inserted into the abdominal aorta through the femoral artery for direct blood pressure recording and exteriorized at the back of the neck. In a group of 6 STZ-diabetic rats, two additional catheters (PE-50), filled with physiological saline containing 100 iu ml−1 heparin, were inserted into one jugular vein and one carotid artery to allow i.v. and i.a. injections of des-Arg9-BK. Recovery from anaesthesia was monitored closely under a warming lamp to maintain the body temperature of animals. Thereafter, rats were housed individually in a polyethylene cage with a top grid and returned to their resident room. Experimental protocols were initiated 24 h later, in conscious and unrestrained rats (18–21 days after the injection of STZ). At this time, the STZ-diabetic rats show no signs of hypovolemia and are in fluid and electrolyte balance (Hebden et al., 1986). None of the rats receiving STZ died within the 3 weeks post-injection although they exhibited excessive daily food and water consumption and a large increase in urine production. The STZ-diabetic rats had a significantly lower body weight comparatively to age-matched control rats (STZ-diabetic rats: 282.9±5.3 g, n=60; control: 349.4±4.3 g, n=16; P<0.05). This is consistent with previous findings with this model (Bennett et al., 1998).
Measurement of cardiovascular parameters
Blood pressure and heart rate were measured respectively with a Statham pressure Transducer (P23ID) and a cardiac tachometer (model 7P4) (triggered by the arterial blood pressure pulse) coupled to a Grass polygraph (model 79; Grass Instruments Co., Quincy, MA, U.S.A.). The cardiovascular response was measured 1 h after the rats were transported to the testing room. They remained in their resident cage but the top grid was removed and had no more access to the food and water for the duration of the experiment. When resting blood pressure and heart rate were stable, rats received an i.t. injection of 20 μl artificial cerebrospinal fluid (aCSF). Only rats (99%) which did not show cardiovascular changes to aCSF for 30 min period were selected in the study.
Experimental protocols
Dose-response curves to i.t. BK and des-Arg9-BK
Both STZ-diabetic (n=6–7) and control (n=7–9) rats initially received an i.t. injection of aCSF (20 μl) followed 15 min later by a single dose of either BK (0.81, 8,1, 81 and 810 pmol) or des-Arg9-BK (0.65, 3.2, 6.5 and 32.5 nmol) to construct a complete dose-response curve. Each rat was selected randomly and injected with only one of the two agonists for the remainder of the protocol. Increasing doses of BK or des-Arg9-BK were given at 40–60 min intervals. Peptides were administered in a volume of 10 μl of vehicle followed by 10 μl volume of aCSF which corresponds to the void volume of the catheter. Each dose was calculated per rat in 10 μl solution.
Effects of i.t. kinin receptor antagonists
In preliminary experiments, various B1 and B2 receptor antagonists (Table 1) were administered intrathecally between 0.65 and 65 nmol to determine the most effective and to verify whether or not they exert direct cardiovascular effects and/or other side effects (e.g. barrel rotation, flaccid paralysis of the hind limbs). Only the effective doses of antagonists which produced reliable and unequivocal inhibition were used in the further experiments.
Table 1.
Code, structure and molecular weight of kinin receptor antagonists used in this study
STZ-diabetic rats that had 24 h previously received randomly 6.5 nmol des-Arg9-BK and 81 pmol BK 1 h apart were given i.t one of the following antagonist: 65 nmol [Leu8]-des-Arg9-BK (n=7–8), 0.65 nmol [des-Arg10]-Hoe 140 (n=6–8), 81 pmol Hoe 140 (n=5–8), 81 pmol FR173657 (n=6) and then 3 min later (or 60 min for FR173657 based on preliminary experiments) were given randomly 6.5 nmol des-Arg9-BK or 81 pmol BK 20 min apart. To take into account the rapid degradation of [Leu8]-des-Arg9-BK, a second dose of this B1 antagonist was given 3 min prior to the injection of the second agonist. Agonists were re-injected 24 h later to assess the reversibility of any blockade observed with the antagonist on the preceding day. Only one antagonist was given to a rat.
Effect of a systemic treatment with a prostaglandin synthesis inhibitor
STZ-diabetic rats that had 24 h previously received 6.5 nmol des-Arg9-BK and 81 pmol BK at 1 h apart were given indomethacin (5 mg kg−1, i.a.; n=11) and then 1 h later were given randomly 6.5 nmol des-Arg9-BK and 81 pmol BK at 30 min intervals. The reversibility of inhibition was assessed by measuring the effect of the agonist alone 24 h later.
Drugs and solutions
The composition of aCSF was, (in nM):NaCl 128.6, KCl 2.6, MgCl2 2.0 and CaCl2 1.4; pH adjusted to 7.2. BK (MW: 1060.3) was purchased from Peninsula laboratories (San Carlos, CA, U.S.A.) and des-Arg9-BK (MW: 964.1) from Hukabel Scientific Ltd. (Longueuil, Canada). The structure, molecular weight and source of kinin receptor antagonists used in this study are indicated in Table 1. Streptozotocin (N-[Methylnitrosocarbamoyl]-D-glucosamine), indomethacin, heparin sodium salt (porcine, grade 1-A) were purchased from Sigma company (St-Louis, MS, U.S.A.). FR173657 was solubilized in dimethyl sulphoxide (DMSO; Fisher Scientific, Montreal, Quebec, Canada) and the solution was completed with aCSF (final concentration of DMSO <10%). Other antagonists and agonists were dissolved directly in aCSF. Indomethacin was freshly prepared in tetramethylene sulfone (Sigma) and the solution was completed with trizma base buffer (0.2 M, Sigma) (final concentration contains 10% tetramethylene sulfone). The stock solutions (10 mg ml−1) of agonists and antagonists were stored in aliquots of 100 μl at −20°C until use.
Statistical analysis of data
Results are expressed as means±s.e.mean. Statistical differences of baseline values (MAP, HR and weight) and maximal cardiovascular effects between STZ-diabetic and age-matched control rats were evaluated with unpaired Student's t-test. Single comparison of maximal cardiovascular effects within the same group was analysed with paired Student's t-test. The time course effects were analysed with a two-way analysis of variance (ANOVA) in conjunction with Bonferroni confidence intervals. Only probability values (P) less than 0.05 were considered to be statistically significant.
Results
Spinal effect of BK on the cardiovascular system
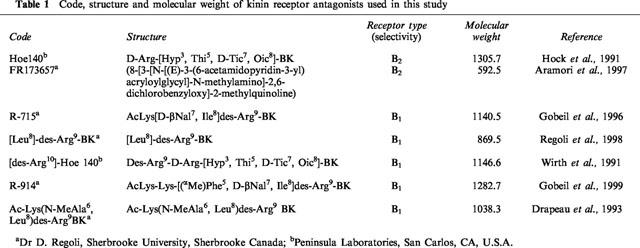
The effects of four increasing doses of BK on MAP and HR in STZ-diabetic and age-matched control rats are shown in Figure 1. Whereas the lowest dose of 0.81 pmol BK failed to alter MAP and HR when compared to aCSF values, BK (8.1–810 pmol) evoked transient increases in MAP and HR that peaked at 30 s post-injection in STZ-diabetic rats (Figure 1a). The pressor response to BK was statistically significant when compared to aCSF values at 8.1 pmol (0.5–2 min, P<0.05), 81 pmol (0.5–3 min, P<0.05) and 810 pmol (0.5–8 min, P<0.05). The tachycardia was also statistically significant at 8.1 pmol (0.5–4 min, P<0.05), 81 pmol (0.5–2 min, P<0.05) and 810 pmol (0.5–5 min, P<0.05).
Figure 1.
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) for a period of 15 min induced by increasing doses of BK injected to the T-9 spinal cord level of conscious STZ-diabetic rats (a) and age-matched control rats (b). Each point represents the means±s.e.mean of (n) rats.
In age-matched control rats, the pressor response to BK was statistically significant when compared to aCSF values at 8.1 pmol (0.5–2 min, P<0.05), 81 pmol (0.5–3 min, P<0.05) and 810 pmol (0.5–3 min, P<0.05) (Figure 1b). Contrary to STZ-diabetic rats which responded to BK by a tachycardia, HR was dose-dependently decreased by BK in control rats at 8.1 pmol (2–7 min, P<0.05), 81 pmol (2–8 min, P<0.05) and 810 pmol (2–15 min, P<0.05). The i.t. injection of 0.81 pmol BK had no significant effect on MAP and HR when compared to aCSF values.
The pressor responses evoked by BK in control rats were significantly less in intensity than those evoked by similar doses of BK in STZ-diabetic rats (control rats (n=9): 8.1 pmol =5.7±1.6 mmHg; 81 pmol=13.0±2.0 mmHg; 810 pmol =13.1±2.4 mmHg; STZ-diabetic rats (n=6): 8.1 pmol =17.7±4.5 mmHg, P<0.05; 81 pmol=22.8±3.8 mmHg, P<0.05; 810 pmol=35.0±5.2 mmHg, P<0.001). Baseline MAP and HR values were significantly lower in STZ-diabetic rats than in age-matched control rats (control rats (n=9): 110.9±1.8 mmHg; 352±7 beats min−1; STZ-diabetic rats (n=6): 96.2±7.1 mmHg, P<0.05; 245±16 beats min−1, P<0.001).
Spinal effect of des-Arg9-BK on the cardiovascular system
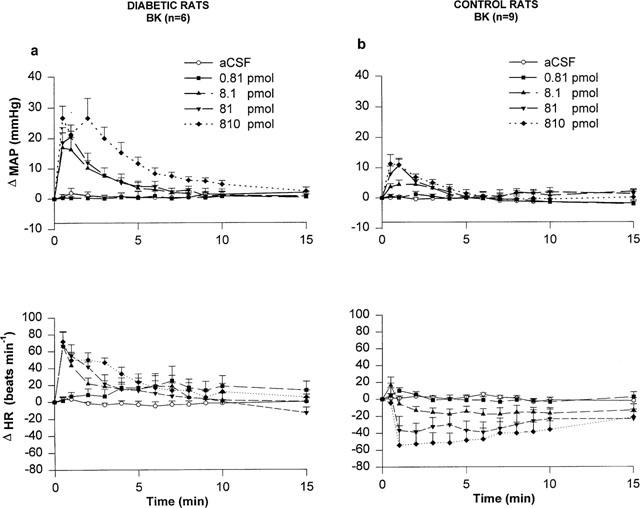
Whereas des-Arg9-BK produced a mild pressor effect at 6.5 and 32.5 nmol (1–3 min, P<0.05) and a slight bradycardia at 32.5 nmol (15 min, P<0.01) when compared to aCSF values in age-matched control rats, the B1 receptor agonist (3.2–32.5 nmol) induced dose- and time-dependent increases in MAP in STZ-diabetic rats (Figure 2). The pressor responses peaked at 1–2 min post-injection and returned gradually to baseline levels during the following 10 min. In STZ-diabetic rats, the pressor response to des-Arg9-BK was statistically significant when compared to aCSF values at 3.2 nmol (2–4 min, P<0.05), 6.5 nmol (0.5–9 min, P<0.05) and 32.5 nmol (0.5–15 min, P<0.05). Des-Arg9-BK (3.2, 6.5 and 32.5 nmol) produced tachycardiac responses of similar intensity which were maximum at 1 min post-injection (3.2 nmol (1–3 min, P<0.05), 6.5 nmol (0.5–2 min, P<0.05) and 32.5 nmol (0.5–2 min, P<0.05)). The lowest dose of 0.65 nmol des-Arg9-BK failed to alter MAP and HR when compared to aCSF values. Baseline HR values of STZ-diabetic rats were significantly lower compared to age-matched control rats (control rats (n=7): 361±11 beats min−1; STZ-diabetic rats (n=7): 292±20 beats min−1, P<0.001). However, no significant differences were found for baseline MAP between STZ-diabetic (90.8±7 mmHg) and age-matched control rats (103±2 mmHg).
Figure 2.
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) for a period of 15 min induced by increasing doses of des-Arg9-BK injected to the T-9 spinal cord level of conscious STZ-diabetic rats (a) and age-matched control rats (b). Each point represents the means±s.e.mean of (n) rats.
Effects of the kinin B1 receptor antagonists in STZ-diabetic rats
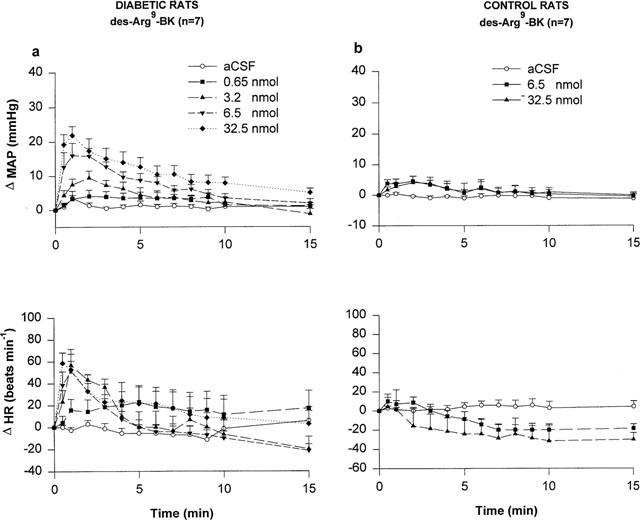
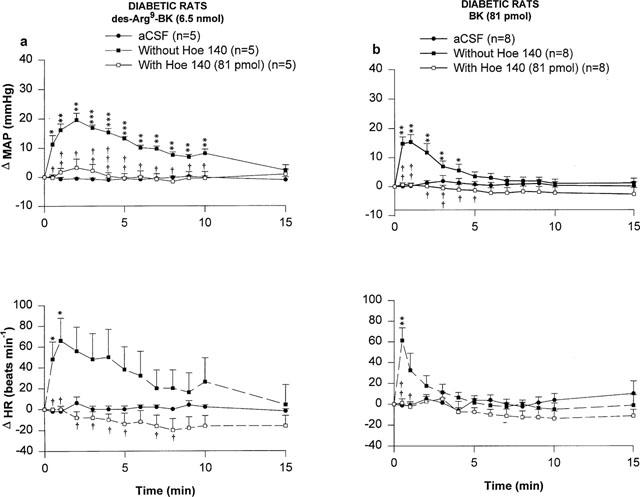
The effects of two kinin B1 receptor antagonists against the MAP and HR responses to 6.5 nmol des-Arg9-BK and 81 pmol BK injected i.t. to STZ-diabetic rats are shown in Figures 3 and 4. Both the MAP and HR effects of des-Arg9-BK were antagonized by [Leu8]-des-Arg9-BK (65 nmol i.t., 5 min beforehand) while the cardiovascular response to BK was not affected by this treatment in the same animals (Figure 3). However, the response to des-Arg9-BK was not affected by a lower dose (32.5 nmol) of this antagonist (data not shown). The cardiovascular response to des-Arg9-BK was completely back to pre-antagonist values when the agonist was re-injected alone 24 h later (Δ MAP=24.3±4.7 to 21.3±3.2 mmHg, P>0.05; and Δ HR=77±18 to 74±15 beats min−1, P>0.05, n=7). Likewise, the increases in MAP and HR induced by des-Arg9-BK were abolished by the prior i.t. injection of 650 pmol [des-Arg10]-Hoe 140 (Figure 4) The cardiovascular response to des-Arg9-BK was back to pre-antagonist values after 24 h (Δ MAP=26.0±5.9 to 21.4±6.2 mmHg, P>0.05 and Δ HR=90±26 to 75±29 beats min−1, P>0.05 (n=4)). While [des-Arg10]-Hoe 140 did not affect the intensity of the MAP and HR responses to BK, it slightly affected their time-course (Figure 4). The two latter B1 receptor antagonists had no direct effects on MAP and HR for a period of 30 min when injected alone (data not shown).
Figure 3.
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) induced by 6.5 nmol des-Arg9-BK (a) and 81 pmol BK (b) injected to the T-9 spinal cord level of conscious STZ-diabetic rats with or without [Leu8]-des-Arg9-BK. Basal values were: MAP: 91.5±8 mmHg; HR: 256±14 beats min−1 in (a) and MAP: 97±9 mmHg; HR: 244±17 beats min−1 in (b). Data are means±s.e.mean of (n) rats. Statistical comparison to vehicle values (*) or to the agonist without [Leu8]-des-Arg9-BK (†) is indicated by *,† P<0.05; **,†† P<0.01; ***,††† P<0.001.
Figure 4.
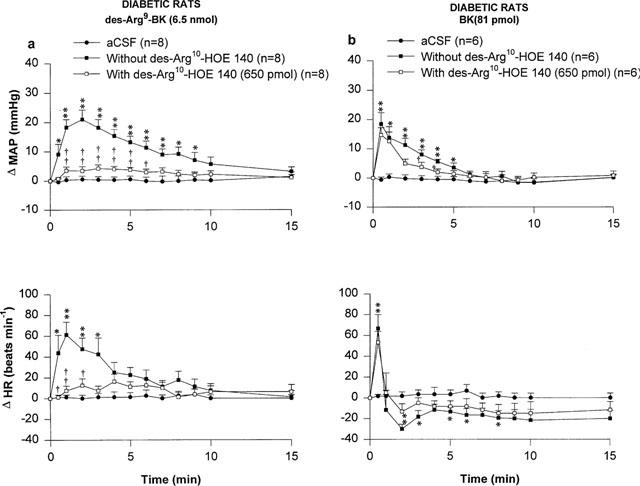
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) induced by 6.5 nmol des-Arg9-BK (a) and 81 pmol BK (b) injected to the T-9 spinal cord level of conscious STZ-diabetic rats with or without [des-Arg10]-Hoe 140. Basal values were: MAP: 93±10 mmHg; HR: 250±18 beats min−1 in (a) and MAP: 97±13 mmHg; HR: 283±24 beats min−1 in (b). Data are means±s.e.mean of (n) rats. Statistical comparison to vehicle values (*) or to the agonist without [des-Arg10]-Hoe 140 (†) is indicated by *,† P<0.05; **,†† P<0.01.
Three other B1 receptor antagonists were tested in STZ-diabetic rats (Table 1). In a first series of experiments, R-715 (65 nmol, i.t. 5 min earlier) prevented the cardiovascular response to des-Arg9-BK in six STZ-diabetic rats without affecting the cardiovascular response to BK in the same animals. However, this B1 antagonist was subsequently found toxic in many rats (three died within 5 min following marked increases of blood pressure and profound bradycardia accompanied by episodes of convulsions). The three others which survived after similar cardiovascular changes were immediately euthanized with an overdose of pentobarbitone. Lower doses of R-715 had either no (6.5 nmol) or less (32.5 nmol) toxic effects, yet these doses did not affect significantly the response to des-Arg9-BK. R-914 (32.5 nmol) produced similar toxic effects than R-715 and therefore this drug was abandoned. Finally, AcLys[N-meAla6,Leu8]des-Arg9-BK (65 nmol) reduced by about 60% the cardiovascular response to des-Arg9-BK without producing any apparent toxic effect.
Effects of the kinin B2 receptor antagonists in STZ-diabetic rats
Two kinin B2 receptor antagonists namely Hoe 140 and FR173657 were tested against the MAP and HR responses induced by 6.5 nmol des-Arg9-BK and 81 pmol BK in STZ-diabetic rats. The increases in MAP and HR evoked by des-Arg9-BK and BK were completely blocked by the prior i.t. injection of Hoe 140 (81 pmol, 5 min earlier) (Figure 5). FR173657 (81 pmol, 60 min earlier) also blocked significantly although not completely the MAP and HR responses to des-Arg9-BK and BK (Figure 6). The HR response to des-Arg9-BK was completely back to pre-antagonist (FR173657) values when the agonist was re-injected alone 24 h later while the MAP response was partially recovered (Δ MAP=29.6±3.1 to 18.3±1.6 mmHg, P<0.05; Δ HR=55±11 to 58±11 beats min−1, P>0.05, n=4). The cardiovascular response to BK was also back to pre-antagonist values (Δ MAP=22.4±4.0 to 13.9±2.1 mmHg, P>0.05 and Δ HR=88±7 to 65±12 beats min−1, P>0.05, n=6). Both B2 receptor antagonists were devoid of any direct effects on MAP and HR and they did not produce any apparent motor deficit (data not shown).
Figure 5.
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) induced by 6.5 nmol of des-Arg9-BK (a) and 81 pmol BK (b) injected to the T-9 spinal cord level of conscious STZ-diabetic rats with or without Hoe 140. Basal values were: MAP: 107±10 mmHg; HR: 216±14 beats min−1 in (a) and MAP: 105±8.3 mmHg; HR: 233±18 beats min−1 in (b). Data are means±s.e.mean of (n) rats. Statistical comparison to vehicle values (*) or to the agonist without Hoe 140 (†) is indicated by *,† P<0.05; **,†† P<0.01; ***,††† P<0.001.
Figure 6.
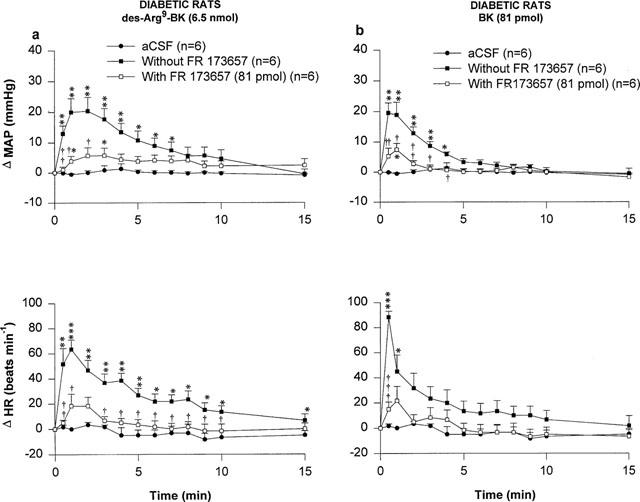
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) induced by 6.5 nmol of des-Arg9-BK (a) and 81 pmol BK (b) injected to the T-9 spinal cord level of conscious STZ-diabetic rats with or without FR173657. Basal values were: MAP: 102±5 mmHg; HR: 233±5.6 beats min−1 in (a) and MAP: 102±6 mmHg; HR: 228±7 beats min−1 in (b). Data are means±s.e.mean of (n) rats. Statistical comparison to vehicle values (*) or to the agonist without FR173657 (†) is indicated by *,† P<0.05; **,††P<0.01; ***,††† P<0.001.
Effect of indomethacin in STZ- diabetic rats
The cyclo-oxygenease inhibitor, indomethacin (5 mg kg−1, i.a. 1 h earlier), abolished the increases in MAP and HR evoked by 6.5 nmol des-Arg9-BK. However, increases in MAP and HR evoked by 81 pmol BK remained unaltered in STZ-diabetic rats pre-treated with indomethacin (Figure 7). The cardiovascular response to des-Arg9-BK was completely back to pre-indomethacin values 24 h later (Δ MAP=20.9±1.6 to 22.8±4.0 mmHg, P>0.05 and Δ HR=78±9 to 105±17 beats min−1, P>0.05, n=8). Indomethacin had no direct effects on MAP and HR baseline values and its vehicle failed to alter the cardiovascular response to both BK and des-Arg9-BK (data not shown).
Figure 7.
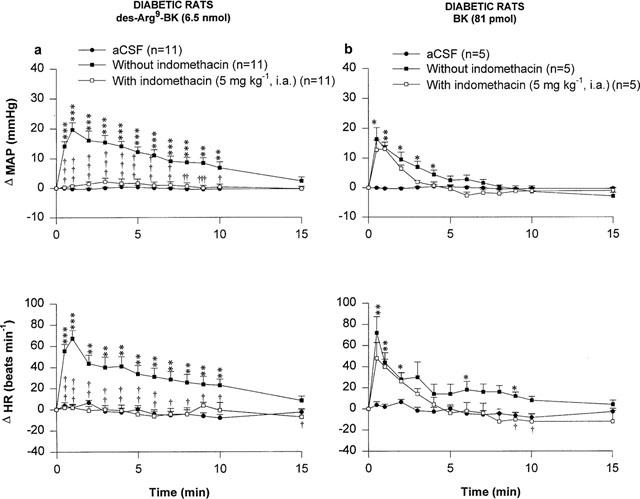
Time-course effects on changes in mean arterial pressure (Δ MAP) and heart rate (Δ HR) induced by 6.5 nmol des-Arg9-BK (a) and 81 pmol BK (b) injected to the T-9 spinal cord level of conscious STZ-diabetic rats with or without indomethacin. Basal values were: MAP: 96±5 mmHg; HR: 233±7 beats min−1 in (a) and MAP: 103±6 mmHg; HR: 224±12 beats min−1 in (b). Data are means±s.e.mean of (n) rats. Statistical comparison to vehicle values (*) or to the agonist without indomethacin (†) is indicated by *,† P<0.05; **,†† P<0.01; ***,††† P<0.001.
Peripheral effects of des-Arg9-BK
When des-Arg9-BK (10 and 1037 nmol kg−1, n=6) was administered i.v. and i.a. to STZ-diabetic rats, no significant changes of MAP and HR were seen when compared to saline values (data not shown).
Behavioural responses to kinins
Concomitant with the cardiovascular effects, i.t. injection of BK and des-Arg9-BK induced behavioural responses in control (BK only) and STZ-diabetic rats (both agonists). For a period that lasted up to 1 min after injection of agonists, the rat became restless and showed exploratory activity at all doses tested. A lateral rocking motion on the posterior limbs was also observed in most of the rats. The behavioural responses induced by des-Arg9-BK were less striking than those elicited by BK. The initial behavioural excitation induced by BK was followed by a period of sedation which lasted for more than 15 min. No differences were observed between age-matched control and STZ-diabetic rats regarding the behavioural responses to BK while only STZ-diabetic rats displayed a behavioural response to des-Arg9-BK. None of the animals in this study showed grooming or scratching. The behavioural responses induced by des-Arg9-BK and BK were both prevented by the B2 receptor antagonists and in the case of des-Arg9-BK by B1 receptor antagonists and indomethacin.
Discussion
In STZ-diabetic rats, the cardiovascular response to i.t. BK is altered. First the pressor response to the agonist is significantly enhanced and second, BK causes tachycardia instead of dose-dependent bradycardia as seen in control rats. The exact mechanism of these alterations is unknown. The BK-induced pressor response was due to the activation of the sympathoadrenal system while the decrease in HR was ascribable to a vagal reflex involving sensory C-fibres and a spinobulbar pathway in control rats (Lopes & Couture, 1992). It is therefore possible that sensory and autonomic neuropathies occuring in the STZ-diabetic rat (Tomlinson et al., 1992; Rittenhouse et al., 1996) are responsible for the changes in the cardiovascular response to BK. On the other hand, the reduced resting heart rate (with occasionally hypotension) has frequently been reported in STZ-treated rats and could be due to a reduction of cardiac β-adrenoceptor numbers and impaired coupling to second messenger systems and contractile function of the heart associated to STZ-induced hypothyroidism or alternatively to autonomic neuropathy and cardiomyopathy (Tomlinson et al., 1992; Hicks et al., 1998).
B1 receptor agonist response in the spinal cord of STZ-diabetic rats
The major finding in this study is the occurence of a cardiovascular response to i.t. injection of des-Arg9-BK in STZ-diabetic rats which is characterized by increases of blood pressure and HR. Consistent with a previous study, this B1 receptor agonist has little spinal effect except at very high doses in control rats (Lopes & Couture, 1992). Thus, the cardiovascular response to des-Arg9-BK, the most potent agonist at rat B1 receptor (Ni et al., 1998b), may be due to the induction and expression of de novo B1 receptors in the spinal cord of STZ-diabetic rats. This hypothesis is substantiated by the reversible blockade of the spinal action of des-Arg9-BK with two B1 receptor selective antagonists, [Leu8]-des-Arg9-BK and [des-Arg10]-Hoe 140. The latter compounds failed to alter the BK-induced cardiovascular response in STZ-diabetic rats and therefore they blocked in a specific and selective manner the B1 receptor. It is noteworthy that [des-Arg10]-Hoe 140 seems the most suitable antagonist in the rat spinal cord because it was more potent than the first developed and prototype B1 receptor antagonist, [Leu8]-des-Arg9-BK, and not toxic contrary to the recently developed B1 receptor antagonists, namely R-715 and R-914 (Gobeil et al., 1996; 1999; Regoli et al., 1998.
Treatment of STZ-diabetes rats with indomethacin has permitted to dissociate further the cardiovascular response induced by the activation of spinal B1 and B2 receptors. While the spinal effects of des-Arg9-BK were abolished by indomethacin, this treatment was without any effect against the spinal effects of BK, suggesting that prostaglandins are involved in the activation of B1 but not B2 receptors in the spinal cord of diabetic rats.
Site of action of intrathecal kinins
The cardiovascular responses induced by BK and des-Arg9-BK had a rapid onset, suggesting an action directly at the segment of injection in the spinal cord. Indeed, the biological half-life of BK after i.t. injection is thought to be as short as that reported (<30 s) after intracerebroventricular (i.c.v.) administration in conscious rats (Kariya et al., 1982). Although the biological half-life of des-Arg9-BK in the CSF remains unknown, this fragment of BK is also a substrate for kininase II (Décarie et al., 1996), the major metabolic pathway for kinins. Peripheral leakage of the injected peptide into systemic circulation cannot account for the cardiovascular responses to kinins because similar or higher doses of des-Arg9-BK injected into the systemic circulation (both i.v. and i.a.) had no effect on MAP and HR. Also BK produces vasodepressor responses in the periphery contrary to its spinal vasopressor effect (Regoli & Barabé, 1980). Previous studies using dyes and radiotracers have documented that drugs and peptides injected to rats by an i.t. catheter do not reach supraspinal structures within the time frame of the experiments (Yaksh & Rudy, 1976; Cridland et al., 1987). This is supported by the failure to induce cardiovascular changes upon i.c.v. injection of des-Arg9-BK (650 pmol) in STZ-diabetic rats (data not shown).
Factors involved in the development of cardiovascular response to intrathecal B1 agonist
Present data suggest that the B1 receptor is induced and up-regulated in the spinal cord of STZ-diabetic rats. Although this hypothesis remains to be supported by relevant molecular evidence, the initial production of cytokines during the onset of the disease induced by the inflammatory action of STZ on the pancreatic islet β-cells (Lukic et al., 1998) and the activation of NF-κB by hyperglycemia and the oxidative stress (Yerneni et al., 1999) represent putative factors known to induce the B1 receptor (Marceau et al., 1998).
Although one cannot exclude the direct involvement of STZ in the induction of B1 receptor after 3 weeks of STZ injection, this possibility is unlikely based on two arguments: (1) STZ is selectively toxic for pancreatic islet β-cells where it causes an inflammation associated to cytokines (Tomlinson et al., 1992; Lukic et al., 1998) and (2) STZ is subjected to rapid metabolic degradation in the liver. Its serum half-life is 15 min while its renal clearance is 70% within the first 6 h (Like & Rossini, 1976; Karunanayake et al., 1976).
Involvement of B2 receptors in the spinal action of kinins
Surprisingly, the B2 receptor selective antagonist, Hoe 140 (Hock et al., 1991), blocked at relatively low doses (81 pmol) the cardiovascular responses induced by des-Arg9-BK and BK in STZ-diabetic rats. This blockade cannot be due to the metabolic transformation of Hoe 140 into [des-Arg10]-Hoe 140 (B1 receptor antagonist, Wirth et al., 1991) because FR 173657, a non peptide B2 receptor antagonist (Aramori et al., 1997), was as effective as Hoe 140 to prevent the spinal effects of the B1 agonist. The significance of the inhibition of B1 receptor mediated effects by B2 receptor antagonists remains unclear at the present time. One possibility would be that the B1 receptor in the spinal cord is pharmacologically different from that found in the periphery. This contention is supported by the existence of two distinct B1 receptor mRNAs in the rat (Ni et al., 1998a; Jones et al., 1999). On the other hand, it is feasible that endogenous BK (or related B2 receptor ligand) is released in the spinal cord by the B1 receptor agonist via a prostanglandin-mediated mechanism. Indeed, our data with indomethacin suggest that the prostaglandin component is located upstream to B2 receptor activation. It is interesting to note that the hyperalgesic effect of B1 receptor agonists on primary sensory neurons is mediated by prostaglandins (Davis & Perkins, 1994). BK-like immunoreactivity was localized in dorsal horn interneurons and projection neurons of the rat spinal cord (Lopes & Couture, 1997). Thus, the precise cellular localization of B1 receptor in the spinal cord (neuronal elements, astrocytes or microvessels) will be instrumental to better define the putative role of this receptor in central cardiovascular regulation.
Data with B2 receptor antagonists confirm that the cardiovascular response to i.t. BK in STZ-diabetic rats is mediated by B2 receptors as previously reported in control rats (Lopes et al., 1993). Contrary to control rats, however, Hoe 140 was about 100 fold more potent in inhibiting BK intrathecally injected to STZ-diabetic rats. This could be due to the higher BK sensitivity observed in those animals. Evidence suggests that the cardiovascular response to i.t. kinins does not derive from increased behavioural activity because the pressor response to BK persisted and was even enhanced in spinal-transected rats (Lopes & Couture, 1992).
Because the non toxic B1 receptor ([Leu8]-des-Arg9-BK and [des-Arg10]-Hoe 140) and B2 receptor (Hoe 140 and FR173657) antagonists tested in this study had no direct effect on blood pressure and heart rate upon their i.t. administration, it is unlikely that these receptors are involved in the tonic control of blood pressure at the level of the spinal cord in STZ-diabetic rats. A similar conclusion was drawn in control rats (Lopes et al., 1993). However, it is feasible that kinins act as neuromodulators of spinal autonomic functions.
In conclusion, our results provide pharmacological evidence that the B1 receptor is upregulated in the spinal cord of rats treated 3 weeks earlier with STZ. The activation of this receptor in the conscious unrestrained rat leads to vasopressor and tachycardiac responses involving a prostaglandin mediated mechanism. Whereas the significance of this finding can only be speculative at this time, the present functional evidence gives an example of peptide receptor plasticity in the central control of the cardiovascular system. Also, this study provides a new model for studying the pharmacology, the expression and function of B1 receptors in the central nervous system.
Acknowledgments
Authors acknowledge Dr D. Regoli (Sherbrooke Université, Sherbrooke, Canada) for the donation of kinin B1 and B2 receptor antagonists. This work was supported by the Medical Research Council of Canada.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- HR
heart rate
- i.t.
intrathecal
- MAP
mean arterial blood pressure
- NF-κB
transcriptional nuclear factor kappa B
- STZ
streptozotocin
References
- ARAMORI I., ZENKOH J., MORIKAWA N., O'DONNELL N., ASANO M., NAKAMURA K., IWAMI M., KOJO H., NOTSU Y. Novel subtype-selective nonpeptide bradykinin receptor antagonists FR167344 and FR173657. Mol. Pharmacol. 1997;51:171–176. doi: 10.1124/mol.51.2.171. [DOI] [PubMed] [Google Scholar]
- BACHVAROV D.R., HESS J.F., MENKE J.G., LARRIVEE J.F., MARCEAU F. Structure and genomic organization of the human B1 receptor gene for kinins (BDKRB1) Genomics. 1996;33:374–381. doi: 10.1006/geno.1996.0213. [DOI] [PubMed] [Google Scholar]
- BENNETT G.S., GARRETT N.E., DIEMEL L.T., BRAIN S.D., TOMLINSON D.R. Neurogenic cutaneous vasodilatation and plasma extravasation in diabetic rats: effect of insulin and nerve growth factor. Br. J. Pharmacol. 1998;124:1573–1579. doi: 10.1038/sj.bjp.0701986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIERHAUS A., ZIEGLER R., NAWROTH P.P. Molecular mechanisms of diabetic angiopathy-clues for innovative therapeutic interventions. Horm Res. 1998;50 Suppl 1:1–5. doi: 10.1159/000053094. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. In vivo B1 kinin-receptor upregulation. Evidence for involvement of protein kinases and nuclear factor κB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COELHO M.M., OLIVEIRA C.R., PAJOLLA G.P., CALIXTO J.B., PELÁ I.R. Central involvement of kinin B1 and B2 receptors in the febrile response induced by endotoxin in rats. Br. J. Pharmacol. 1997;121:296–302. doi: 10.1038/sj.bjp.0701110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTO L.B., CORRÊA F.M.A., PELÁ I.R. Brain sites involved in the antinociceptive effect of bradykinin in rats. Br. J. Pharmacol. 1998;125:1578–1584. doi: 10.1038/sj.bjp.0702209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUTURE R., BUCK H.S., PINHEIRO V.L., CLOUTIER F., REGOLI D., LINDSEY C.J. The Fifteenth International Conference on Kinins, October 19–24, 1998. Nara, Japan (Abstract S-III-4); 1998. Distribution of kinin receptors in the CNS and possible relevance to function. [Google Scholar]
- COUTURE R., LINDSEY C.J.Brain kallikrein-kinin system: from receptors to neuronal pathways and physiological functions Handbook of Chemical Neuroanatomy 200016241–300.ed. Quirion, R., Björklund, A., Hökfelt, TPeptide Receptors, Part I pp [Google Scholar]
- CRIDLAND R.A., YASHPAL K., ROMITA V.V., GAUTHIER S., HENRY J.L. Distribution of label after intrathecal administration of 125I-subtances P in the rat. Peptides. 1987;8:213–221. doi: 10.1016/0196-9781(87)90092-1. [DOI] [PubMed] [Google Scholar]
- DÉCARIE A., RAYMOND P., GERVAIS N., COUTURE R., ADAM A. Serum interspecies differences in metabolic pathways of bradykinin and [des-Arg9]BK: Influence of enalaprilat. Am. J. Physiol. 1996;270:H1340–H1347. doi: 10.1152/ajpheart.1996.271.4.H1340. [DOI] [PubMed] [Google Scholar]
- DAVIS A.J., PERKINS M.N. The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. Br. J. Pharmacol. 1994;113:63–68. doi: 10.1111/j.1476-5381.1994.tb16174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPEAU G., AUDET R., LÉVESQUE L., GODIN D., MARCEAU F. Development and in vivo evaluation of metabolically resistant antagonists of B1 receptors for kinins. J. Pharmacol. Exp. Ther. 1993;266:192–199. [PubMed] [Google Scholar]
- DRAY A. Kinins and their receptors in hyperalgesia. Can. J. Physiol. Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- GERMANY A., GONZÁLEZ P., CONTRERAS E. Possible role of nitric oxide in the antinociceptive action of intraventricular bradykinin in mice. Eur. J. Pharmacol. 1996;310:123–127. doi: 10.1016/0014-2999(96)00384-6. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., CHARLAND S., FILTEAU C., PERRON S.I., NEUGEBAUER W., REGOLI D. Kinin B1 receptor antagonists containing α-Methyl-L-Phenylalanine: In vitro and in vivo antagonistic activities. Hypertension. 1999;33:823–829. doi: 10.1161/01.hyp.33.3.823. [DOI] [PubMed] [Google Scholar]
- GOBEIL F., NEUGEBAUER W., FILTEAU C., JUKIC D., NSA ALLOGHO S., PHENG L.H., NGUYEN-LE X.K., BLOUIN D., REGOLI D. Structure-activity studies of B1 receptor-related peptides antagonists. Hypertension. 1996;28:833–839. doi: 10.1161/01.hyp.28.5.833. [DOI] [PubMed] [Google Scholar]
- HEBDEN R.A., GARDINER S.M., BENNETT T., MACDONALD I.A. The influence of streptozotocin-induced diabetes mellitus on fluid and electrolyte handling in rats. Clinical. Science. 1986;70:111–117. doi: 10.1042/cs0700111. [DOI] [PubMed] [Google Scholar]
- HICKS K.K., SEIFEN E., STIMERS J.R., KENNEDY R.H. Effects of streptozotocin-induced diabetes on heart rate, blood pressure and cardiac autonomic nervous control. J. Auton. Nerv. Syst. 1998;69:21–30. doi: 10.1016/s0165-1838(98)00004-6. [DOI] [PubMed] [Google Scholar]
- HOCK F.J., WIRTH K., ALBUS U., LINZ W., GERHARDS H.J., WIEMER G., HENKE St., BREIPOHL G., KÖNIG W., KNOLLE J., SCHÖLKENS B.A. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br. J. Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSSAIN M.J., PEAKMAN M., GALLATI H., LO S.S.S., HAWA M., VIBERTI G.C., WATKINS P.J., LESLIE R.D.G., VERGANI D. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia. 1996;39:60–69. doi: 10.1007/BF00400414. [DOI] [PubMed] [Google Scholar]
- JONES C., PHILLIPS E., DAVIS C., ARBUCKLE J., YAQOOB M., BURGESS G.M., DOCHERTY R.J., WEBB M., BEVAN S.J., MCINTYRE P. Molecular characterisation of cloned bradykin B1 receptors from rat and human. Eur. J. Pharmacol. 1999;374:423–433. doi: 10.1016/s0014-2999(99)00315-5. [DOI] [PubMed] [Google Scholar]
- KARUNANAYAKE E.H., HEARSE D.J., MELLOWS G. Streptozotocin: Its excretion and metabolism in the rat. Diabetologia. 1976;12:483–488. doi: 10.1007/BF01219512. [DOI] [PubMed] [Google Scholar]
- KARIYA K., YAMAUCHI A., HATTORI S., TSUDA Y., OKADA Y. The disappearance rate of intraventricular bradykinin in the brain of the conscious rat. Biochem. Biophys. Res. Commun. 1982;107:1461–1466. doi: 10.1016/s0006-291x(82)80163-0. [DOI] [PubMed] [Google Scholar]
- LANEUVILLE O., READER T.A., COUTURE R. Intrathecal bradykinin acts presynaptically on spinal noradrenergic terminals to produce antinociception in the rat. Eur. J. Pharmacol. 1989;159:273–283. doi: 10.1016/0014-2999(89)90158-1. [DOI] [PubMed] [Google Scholar]
- LIKE A.A., ROSSINI A.A. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science. 1976;193 (4251):415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- LINDSEY C.J., BUCK H.S., FIOR-CHADI D.R., LAPA R.C.R.S. Pressor effect mediated by bradykinin in the paratrigeminal nucleus of the rat. J. Physiol. 1997;502:119–129. doi: 10.1111/j.1469-7793.1997.119bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPES P., COUTURE R. Cardiovascular responses elicited by intrathecal kinins in the conscious rat. Eur. J. Pharmacol. 1992;210:137–147. doi: 10.1016/0014-2999(92)90664-p. [DOI] [PubMed] [Google Scholar]
- LOPES P., COUTURE R. Localization of bradykinin-like immunoreactivity in the rat spinal cord: effects of capsaicin, melletin, dorsal rhizotomy and peripheral axotomy. Neurosci. 1997;78:481–497. doi: 10.1016/s0306-4522(96)00554-4. [DOI] [PubMed] [Google Scholar]
- LOPES P., REGOLI D., COUTURE R. Cardiovascular effects of intrathecally administered bradykinin in the rat: characterization of receptors with antagonists. Br. J. Pharmacol. 1993;110:1369–1374. doi: 10.1111/j.1476-5381.1993.tb13971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUKIĆ M.L., STOŠIĆ-GRUJIČIĆ S., SHAHIN A. Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev. Immunol. 1988;6:119–128. doi: 10.1155/1998/92198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADEDDU P., GLORIOSO N., SORO A., TONOLO G., MANUNTA P., TROFFA C., DEMONTIS M.P., VARONI M.V., ANANIA V. Brain kinins are responsible for the pressor effect of intracerebroventricular captopril in spontaneously hypertensive rats. Hypertension. 1990;15:407–412. doi: 10.1161/01.hyp.15.4.407. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol. Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- NI A., CHAO L., CHAO J. Transcription factor nuclear factor NF-κB regulates the inducible expression of the human B1 receptor gene in inflammation. J. Biol. Chem. 1998a;273:2784–2791. doi: 10.1074/jbc.273.5.2784. [DOI] [PubMed] [Google Scholar]
- NI A., CHAI K.X., CHAO L., CHAO J. Molecular cloning and expression of rat bradykinin B1 receptor. Biochim. Biophys. Acta. 1998b;1442:177–185. doi: 10.1016/s0167-4781(98)00163-8. [DOI] [PubMed] [Google Scholar]
- PELÁ I.R., ROSA A.L., SILVA C.A.A., HUIDOBRO-TORO J.P. Central B2 receptor involvement in the antinociceptive effect of bradykinin in rats. Br. J. Pharmacol. 1996;118:1488–1492. doi: 10.1111/j.1476-5381.1996.tb15564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHENG L.H., NGUYEN-LE X.K., NSA ALLOGHO S., GOBEIL F., REGOLI D. Kinin receptors in the diabetic mouse. Can. J. Physiol. Pharmacol. 1997;75:609–611. [PubMed] [Google Scholar]
- PRIVITERA P.J., THIBODEAUX H., YATES P. Rostral ventrolateral medulla as a site for the central hypertensive action of kinins. Hypertension. 1994;23:52–58. doi: 10.1161/01.hyp.23.1.52. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes-Metabolism Reviews. 1998;14:129–151. doi: 10.1002/(sici)1099-0895(199806)14:2<129::aid-dmr208>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH A., SUAREZ-PINZON W.L. Cytokines and their roles in pancreatic islet β-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BARABÉ J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980;32:1–46. [PubMed] [Google Scholar]
- REGOLI D., NSA ALLOGHO S., RIZZI A., GOBEIL F., JR Bradykinin receptors and their antagonists. Eur. J. Pharmacol. 1998;348:1–10. doi: 10.1016/s0014-2999(98)00165-4. [DOI] [PubMed] [Google Scholar]
- RITTENHOUSE P.A., MARCHAND J.E., CHEN J., KREAM R.M., LEEMAN S.E. Streptozotocin-induced diabetes is associated with altered expression of peptide-encoding mRNAs in rat sensory neurons. Peptides. 1996;17:1017–1022. doi: 10.1016/0196-9781(96)00129-5. [DOI] [PubMed] [Google Scholar]
- SARDI S.P., DARAY F.M., ERRASTI A.E., PELOROSSO F.G., PUJOL-LEREIS V.A., REY-ARES V., ROGINES-VELO M.P., ROTHLIN R.P. Further pharmocological characterization of bradykinin B1 receptor up-regulation in human umbilical vein. J. Pharmacol. Exp. Ther. 1999;290:1019–1025. [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., CASTAÑO M.E.M., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.P., BASCANDS J.L. The B1-agonist [des-Arg10]-kallidin activates transcription factor NF-κB and induces homologous upregulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMLINSON K.C., GARDINER S.M., HEBDEN R.A., BENNETT T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol. Rev. 1992;44:103–150. [PubMed] [Google Scholar]
- WALKER K., DRAY A., PERKINS M. Development of hyperthermia following intracerebroventricular administration of endotoxin in the rat: effect of kinin B1 and B2 receptor antagonists. Br. J. Pharmacol. 1996;117:684–688. doi: 10.1111/j.1476-5381.1996.tb15244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIRTH K., BREIPOHL G., STECHL J., KNOLLE L., HENKE S., SCHÖLKENS B.A. Des-Arg9-D-Arg-[Hyp3,Thi5,D-Tic7,Oic8]-BK (des-Arg10-[Hoe140]) is a potent bradykinin B1 receptor antagonist. Eur. J. Pharmacol. 1991;205:217–218. doi: 10.1016/0014-2999(91)90824-a. [DOI] [PubMed] [Google Scholar]
- YAKSH T.L., RUDY T.A. Chronic catheterization of the spinal subarachnoid space. Physiol. Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- YERNENI K.K.V., BAI W., KHAN B.V., MEDFORD R.M., NATARAJAN R. Hyperglycemia-induced activation of nuclear transcription factor kappaB in vascular smooth muscle cells. Diabetes. 1999;48:855–864. doi: 10.2337/diabetes.48.4.855. [DOI] [PubMed] [Google Scholar]
- ZHOU X., POLGAR P., TAYLOR L. Role for interleukin-1β, phorbol ester and a post-transcriptional regulator in the control of bradykinin B1 receptor gene expression. Biochem. J. 1998;330:361–366. doi: 10.1042/bj3300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUCCOLLO A., NAVARRO M., CATANZARO O. Effects of B1 and B2 kinin receptor antagonists in diabetic mice. Can. J. Physiol. Pharmacol. 1996;74:586–589. [PubMed] [Google Scholar]


