Abstract
We used YC-1 as a pharmacological tool to investigate the short-term blood pressure effects of NO-independent activation of sGC in normotensive and hypertensive rats. Four groups of normotensive Wistar-Kyoto rats were treated by i.v. injection with vehicle (V), YC-1 (YC-1), sodium nitroprusside (SNP), or YC-1 and SNP (YC-1+SNP). Hypertension was induced in four additional groups of WKY rats by 3 weeks of oral treatment with L-NAME. These animals were investigated with the same protocol as the normotensive animals: L-NAME/V, L-NAME/YC-1, L-NAME/SNP, L-NAME/YC-1+SNP. YC-1 lowered mean arterial blood pressure (MAP) in normotensive and hypertensive animals similarly to SNP alone (P<0.05, respectively). The combination of YC-1 with SNP caused a strong decrease of MAP in both the hypertensive and normotensive animals (P<0.05, respectively). SNP with YC-1 also induced a pronounced cyclic GMP increase in the aorta. This study shows for the first time the blood pressure lowering potential of bimodal targeting of the NO-sGC-system.
Keywords: Nitric oxide, blood pressure, hypertension, soluble guanylyl cyclase, cyclic GMP, YC-1, L-NAME
Introduction
Different substances such as fatty acid endoperoxides (Graff et al., 1978), protoporphyrin IX (Ignarro et al., 1982), polyunsaturated fatty acids (Gerzer et al., 1983), and carbon monoxide (Brune et al., 1990) are known to stimulate soluble guanylyl cyclase (sGC) independently from nitric oxide (NO). Poor sGC-activating effects, chemical instability, and toxicity made these substances useless for both clinical application and experimental in vivo studies. YC-1 [1-benzyl-3-(5′-hydroxymethyl-2′-furyl-)-indazol] was recently described as a novel activator of sGC, which exhibits also inhibitory effects on platelet aggregation (Ko et al., 1994). Interestingly, a direct activation of the prosthetic heme group of sGC by YC-1 could be ruled out (Friebe & Koesling, 1998). YC-1 rather increases the maximal catalytic rate and sensitizes the enzyme toward its gaseous activators by binding to an allosteric site on sGC molecules, thereby reducing the ligand dissociation rate from the haeme group (Friebe & Koesling, 1998). Further studies demonstrated an effect of YC-1 on prevention of venous thrombosis in vivo (Teng et al., 1997), and vasodilation of aortic rings in vitro (Mülsch et al., 1997; Galle et al., 1999). Recently, our in vitro studies on the purified sGC enzyme demonstrated that incubation with YC-1 leads to a 10 fold increase of sGC enzyme activity. Furthermore, the effect of NO on cyclic GMP production by sGC was potentiated by YC-1 both on purified sGC in vitro and in isolated human platelets (Friebe et al., 1996; 1998).
In vivo effects of YC-1 on blood pressure are currently unknown. Therefore, the aim of our study was to investigate the blood pressure effects of YC-1 in normotensive and hypertensive rats. Furthermore, we investigated whether in vivo application of YC-1 potentiates NO induced stimulation of sGC also in the arterial wall.
Methods
Animals
Four groups of male Wistar Kyoto (WKY) rats (n=6, each, 8 weeks of age) were treated for 3 weeks with normal tap water (normotensive control). In addition, hypertension was induced in four groups of WKY rats by 3 weeks of treatment with the NO-inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) in drinking water (20 mg kg−1 d−1).
Haemodynamic measurements
The rats were anaesthetized with an intraperitoneal injection of pentobarbiturate in saline (60 mg kg−1 body weight) and placed on a heating pad to maintain rectal temperature between 36.5 and 37.5°C. The trachea was cannulated to facilitate spontaneous respiration and a patent airway. Two polyethylene catheters (PE-50, Neolab) were implanted, one in the left jugular vein (for i.v. administration of drugs), and one into the right carotid artery (for arterial pressure and heart rate measurement). Arterial pressure and heart rate were monitored using a pressure transducer system (9100 series, TSE Bad Homburg, Germany) and continuously recorded on a computer-based registration system (BioSys, TSE, Bad Homburg, Germany). The cardiovascular parameters were allowed to stabilize for 20 min. Preliminary experiments had shown that blood pressure and heart rate are stable for more than 40 min using this experimental procedure. After stabilization, drugs were injected intravenously via the left jugular vein. The effects on arterial pressure and heart rate were recorded during an observation period of 10 min with continuous registration of both parameters.
Acute treatment
YC-1 is a highly hydrophobic compound. Preliminary experiments using dimethyl sulphoxide (DMSO) as a vehicle revealed a significant effect of this compound on arterial blood pressure. Therefore, we used Chremophor® instead as vehicle for YC-1 injection. The normotensive rats were treated by i.v. injection with 10% Chremophor® in 0.9% saline vehicle (V), 5 mg kg−1 YC-1 (YC-1), 50 μg kg−1 sodium nitroprusside (SNP), 5 mg kg−1 YC-1 and 50 μg kg−1 sodium nitroprusside in saline (YC-1+SNP). The L-NAME hypertensive animals were subjected to the same pharmacological intervention as the normotensive animals: L-NAME/V, L-NAME/YC-1, L-NAME/SNP, L-NAME/YC-1+SNP. After termination of haemodynamic measurements, drug injections were repeated to investigate the effect on aortic cyclic GMP concentrations in both normotensive and L-NAME hypertensive animals. Animals were killed 2 min after this second injection, which corresponds to the peak of the observed haemodynamic effect.
Determination of cyclic GMP in aorta
The thoracic aorta was rapidly dissected and immediately frozen in liquid nitrogen using a pre-cooled metal forceps. The thoracic aorta was used for measurement of vascular cyclic GMP levels, since for reliable determination of cyclic GMP in tissues, quick removal and immediate cooling of a significant amount of arterial tissue are crucial prerequisites.
Frozen aortic strips were pulverized with a mortar and pestle cooled with liquid nitrogen. Subsequently, the samples were homogenized in 66% frozen ethanol with a glass/glass homogenizer and then centrifuged for 15 min at 4°C at 14,000×g. The supernatants were dried in a Speed-Vac centrifuge and used for measurements of cyclic GMP in a radioimmunoassay. The weights of the dried pellets were used in order to standardize the different samples. Preparation of tracer, acetylation of samples and standards, and incubation with antibody was performed as described in Brooker et al. (1979) and Friebe et al. (1998). The assay was performed in duplicates using different dilutions of samples.
Chemicals
Chremophor® and L-NAME were obtained from Sigma-Aldrich, Deisenhofen, Germany, YC-1 from Alexis, San Diego, U.S.A., sodium nitroprusside from Schwarz Pharma, Monheim, Germany, and pentobarbiturate (Narcoren®) from Rhone Merieux, Laupheim, Germany.
Statistical analysis
The results shown represent mean±standard error of the mean. Statistical analysis was performed by unpaired Student's t-test (two-tailed), by analysis of variance (ANOVA) followed by post hoc Bonferroni adjustment, and by ANOVA for repetitive measurements. A P-value<0.05 was considered significant.
Results
Normotensive WKY rats
The basal values of mean arterial pressure (MAP) and heart rate before treatment were 85±3 mmHg and 273±10 beats min−1. MAP changes in comparison to baseline values (0 min) are presented as ΔMAP. Development of ΔMAP after treatment with V, YC-1, SNP, or the combination of YC-1+SNP is shown in Figure 1. ANOVA for repetitive measurements revealed no significant changes in ΔMAP over time after treatment with V. Treatment with YC-1, SNP, and YC+SNP led to significant changes of ΔMAP over time. Two minutes after i.v. drug application MAP was significantly lowered in YC-1, SNP, and YC-1+SNP treated animals compared to administration of V alone (V +0.4±2.2 vs YC-1 −11.3±2.2, SNP −12.8±2.1, and YC-1+SNP −44.8±9.1 ΔMAP mmHg; P<0.05, respectively). Four minutes after i.v. drug application of either YC-1 or SNP ΔMAP was not significantly different from control (V). In animals with YC-1+SNP treatment MAP was still significantly reduced compared to the V group even 8 min after drug application (V −1.2±2.4 vs YC-1+SNP −21.0±6.4 ΔMAP mmHg; P<0.05, respectively).
Figure 1.
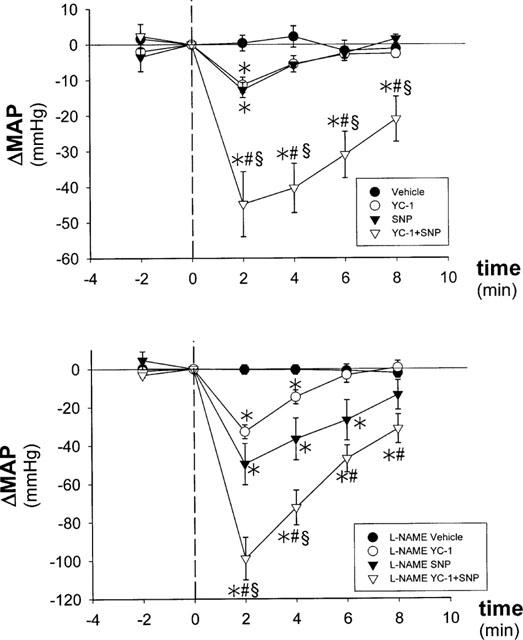
Effect of YC-1 on mean arterial pressure (MAP) in normotensive WKY control rats (top panel) and L-NAME hypertensive WKY rats (bottom panel). *vs Vehicle, # vs YC-1, § vs SNP, P<0.05, respectively.
No significant changes in heart rate over time occurred after treatment with V, YC-1, or SNP. ANOVA for repetitive measurements revealed a significant decrease in heart rate over time only after simultaneous treatment with YC-1+SNP (Δ −44 beats min−1; P=0.03).
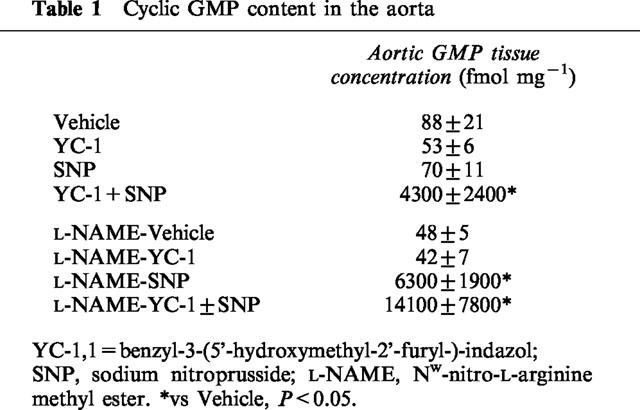
Data for aortic cyclic GMP concentrations are shown in Table 1. No significant changes in aortic cyclic GMP concentration were observed after YC-1 or SNP treatment compared to treatment with V alone. Combined treatment with YC-1+SNP led to a 49 fold increase in aortic cyclic GMP content compared to V (P<0.001).
Table 1.
Cyclic GMP content in the aorta
WKY-L-NAME hypertensive rats
The basal values of MAP and heart rate after 3 weeks of treatment with L-NAME were 142±4 mmHg and 307±10 beats min−1. Both parameters were significantly elevated compared to control WKY rats treated with normal tap water (MAP: +57 mmHg, P<0.0001, and heart rate: +34 beats min−1, P<0.05).
Development of ΔMAP after i.v. administration of V, YC-1, SNP, or the combination of YC-1+SNP is shown in Figure 1. ANOVA for repetitive measurements revealed no significant changes in ΔMAP over time after treatment with V. Administration of YC-1, SNP, or YC+SNP led to significant changes of ΔMAP over time. Two minutes after i.v. drug application MAP was significantly reduced in YC-1, SNP, and YC-1+SNP treated animals compared to V (L-NAME/V −0.3±2.5 vs L-NAME/YC-1 −32.8±3.6, L-NAME/SNP −49.7±10.8, and L-NAME/YC-1+SNP −99±11.1 ΔMAP mmHg; P<0.05, respectively).
Eight minutes after i.v. drug application of either YC-1 or SNP MAP was as high as in V treated animals. In animals with YC-1+SNP MAP was significantly lower compared to the V group even 8 min after drug application (L-NAME/V −2.3±2.5 vs L-NAME/YC-1+SNP −31.4±7.6 ΔMAP mmHg; P<0.05, respectively).
No significant changes in heart rate over time occurred after treatment with L-NAME/V, L-NAME/YC-1, or L-NAME/SNP. Only treatment with L-NAME/YC-1+SNP led to significant decrease of heart rate over time (Δ −45 beatsmin−1; P=0.012).
Data for aortic cyclic GMP concentrations are shown in Table 1. No significant changes in aortic cyclic GMP concentration were observed after YC-1 treatment compared to treatment with V. In contrast to the normotensive group, treatment with SNP alone led already to a strong increase in aortic cyclic GMP content compared to V (P<0.001). Simultaneous injection of YC-1+SNP caused a significant further increase compared to SNP alone (P=0.002).
Discussion
NO is an important regulator of arterial blood pressure. Its direct effect on vascular tone is brought about by cyclic GMP formation in vascular smooth muscle cells. The cross-talk between the NO-system and other important systems such as the renin-angiotensin-system, the sympathetic nervous system, and the endothelin system, explains the central role of NO in acute blood pressure control.
Recently, we have shown a new mechanism of sGC stimulation by the substance YC-1 in vitro (Friebe & Koesling, 1998). In the current study we demonstrate that NO-independent stimulation of sGC has indeed, as suggested from those in vitro experiments, a strong blood pressure lowering potential in normotensive and L-NAME hypertensive rats. In both normotensive and L-NAME hypertensive animals NO-independent activation of sGC with the novel sGC activator YC-1 did significantly lower MAP after acute i.v. application.
It is well known that under in vitro conditions YC-1 increases cyclic GMP levels and directly stimulates purified sGC activity (Ko et al., 1994; Friebe et al., 1996; 1998). Our data did not reveal an increase of aortic cyclic GMP levels after treatment with YC-1 alone. This finding is consistent with data derived from in vitro experiments in rabbit aortic rings, in which YC-1 elicited a concentration-dependent relaxation, without a significant increase in aortic cyclic GMP levels (Mülsch et al., 1997). In contrast, one other study performed in aortic rings of rabbits reported a moderate increase of aortic cyclic GMP level after application of YC-1 (Galle et al., 1999). It could be that under in vivo conditions the experimental set-up in our study was not suited to detect relatively small changes of cyclic GMP as the increased amount in cyclic GMP may be degraded during the time required to dissect the aorta. Nevertheless, acute application of YC-1 induced a moderate blood pressure reduction in normotensive rats that was comparable to the decrease induced by SNP treatment. Similarly, no significant changes of aortic cyclic GMP content were detected after acute SNP treatment in normotensive rats.
Alternatively, the fall in MAP after YC-1 and SNP could be due in part to stimulation of sGC in other regions of the vascular system, i.e. arteriolar resistance vessels, or to a primary central venous stimulation of sGC with consecutive decrease of cardiac preload. Finally, the combination of both mechanisms could explain the fall in MAP despite the lack of an elevated cyclic GMP content in the aorta.
Interestingly, the blood pressure lowering effect of SNP and the effect on aortic cyclic GMP content was significantly more pronounced in L-NAME hypertensive rats. This finding is in agreement with a study revealing a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo (Moncada et al., 1991).
Previous experiments have shown that flow-induced release of factors such as NO contribute to blood pressure regulation under normal conditions (Rubanyi et al., 1986). Our finding that YC-1 induces a short hypotensive response even when administered in normotensive animals is in agreement with this results and demonstrates that the stimulation of sGC is important for blood pressure regulation in the normotensive state.
While YC-1 and SNP applied alone elicited no effect on aortic cyclic GMP levels in normotensive rats, co-administration of the two compounds led to a high increase of aortic cyclic GMP levels, thus revealing a strong synergistic effect between direct NO administration and NO-independent sGC stimulation in the aortic wall in vivo. This synergistic effect was observed in both normotensive and L-NAME hypertensive animals and was paralleled by a profound fall in blood pressure, which reached almost −100 mmHg in the L-NAME treated animals. These findings are in agreement with recent in vitro data showing that YC-1 potentiated the activation sGC by NO and other sGC stimulating substances in isolated human platelets (Friebe et al., 1996; 1998). A synergistic action between YC-1 and SNP was also reported for the relaxation and the cyclic GMP content in rabbit aortic rings in organ chamber experiments (Mülsch et al., 1997).
Unlike YC-1 application alone, SNP treatment alone induced already a strong increase in aortic cyclic GMP levels in L-NAME hypertensive rats. The increase observed was similar to the effect on aortic cyclic GMP levels after combined treatment of YC1 and SNP in the normotensive group. The blood pressure reduction after SNP application in the L-NAME hypertensive animals (−50 mmHg) and after YC1 and SNP co-administration in the normotensive WKY animals (−45 mmHg) was indeed similar.
Taken together, our findings demonstrate that NO-independent stimulation of sGC by YC-1 is clearly capable of reducing blood pressure in both normotensive and L-NAME hypertensive rats. Simultaneous application of YC-1 and SNP induced already a marked blood pressure reduction in normotensive rats, which was more then doubled in L-NAME hypertensive rats. Thus, a strong synergistic effect of bimodal targeting the NO-sGC-system in terms of blood pressure regulation was shown for the first time.
Acknowledgments
This study was supported by the Forschungsschwerpunkt Kardiovaskuläre Medizin of the Benjamin Franklin Medical Center.
Abbreviations
- Cyclic GMP
cyclic 3′:5′ guanosine monophosphate
- DMSO
dimethyl sulphoxide
- L-NAME
Nω-nitro-L-arginine methyl ester
- NO
nitric oxide
- sGC
soluble guanylyl cyclase
- MAP
mean arterial blood pressure
- SNP
sodium nitroprusside
- YC-1
1-benzyl-3-(5′-hydroxymethyl-2′-furyl-)-indazol
References
- BROOKER G., HARPER J.F., TERASAKI W.L., MOYLAN R.D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv. Cyclic. Nucleotide. Res. 1979;10:1–33. [PubMed] [Google Scholar]
- BRUNE B., SCHMIDT K.U., ULLRICH V. Activation of soluble guanylate cyclase by carbon monoxide and inhibition by superoxide anion. Eur. J. Biochem. 1990;192:683–688. doi: 10.1111/j.1432-1033.1990.tb19276.x. [DOI] [PubMed] [Google Scholar]
- FRIEBE A., KOESLING D. Mechanism of YC-1-induced activation of soluble guanylyl cyclase. Mol. Pharmacol. 1998;53:123–127. doi: 10.1124/mol.53.1.123. [DOI] [PubMed] [Google Scholar]
- FRIEBE A., MÜLLERSHAUSEN F., SMOLENSKI A., WALTER U., SCHULTZ G., KOESLING D. YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol. Pharmacol. 1998;54:962–967. doi: 10.1124/mol.54.6.962. [DOI] [PubMed] [Google Scholar]
- FRIEBE A., SCHULTZ G., KOESLING D. Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- GALLE J., ZABEL U., HUBNER U., HATZELMANN A., WAGNER B., WANNER C., SCHMIDT H.H. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERZER R., HAMET P., ROSS A.H., LAWSON J.A., HARDMAN J.G. Calcium-induced release from platelet membranes of fatty acids that modulate soluble guanylate cyclase. J. Pharmacol. Exp. Ther. 1983;226:180–186. [PubMed] [Google Scholar]
- GRAFF G., STEPHENSON J.H., GLASS D.B., HADDOX M.K., GOLDBERG N.D. Activation of soluble splenic cell guanylate cyclase by prostaglandin endoperoxides and fatty acid hydroperoxides. J. Biol. Chem. 1978;253:7662–7676. [PubMed] [Google Scholar]
- IGNARRO L.J., WOOD K.S., WOLIN M.S. Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc. Natl. Acad. Sci. U.S.A. 1982;79:2870–2873. doi: 10.1073/pnas.79.9.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KO F.N., WU C.C., KUO S.C., LEE F.Y., TENG C.M. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- MONCADA S., REES D.D., SCHULZ R., PALMER R.M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. U.S.A. 1991;88:2166–2170. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜLSCH A., BAUERSACHS J., SCHAFER A., STASCH J.P., KAST R., BUSSE R. Effect of YC-1, an NO-independent, superoxide-sensitive stimulator of soluble guanylyl cyclase, on smooth muscle responsiveness to nitrovasodilators. Br. J. Pharmacol. 1997;120:681–689. doi: 10.1038/sj.bjp.0700982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBANYI G.M., ROMERO J.C., VANHOUTTE P.M. Flow-induced release of endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- TENG C.M., WU C.C., KO F.N., LEE F.Y., KUO S.C. YC-1, a nitric oxide-independent activator of soluble guanylate cyclase, inhibits platelet-rich thrombosis in mice. Eur. J. Pharmacol. 1997;320:161–166. doi: 10.1016/s0014-2999(96)00911-9. [DOI] [PubMed] [Google Scholar]


