Abstract
Isolated segments of porcine tracheal epithelium were mounted in Ussing chambers, current required to maintain transepithelial potential difference at 0 mV (short circuit current, ISC) was monitored and effects of nucleotides upon ISC were studied.
Mucosal UTP (100 μM) evoked a transient rise in ISC that was followed by a sustained fall below basal ISC maintained for 30 min. Mucosal ATP (100 μM) also stimulated a transient rise in ISC but in contrast to UTP did not inhibit basal ISC. Submucosal UTP and ATP both transiently increased ISC.
UTP-prestimulated epithelia were refractory to ATP but prestimulation with ATP did not abolish the response to UTP. The epithelia thus appear to express two populations of apical receptors allowing nucleotides to modulate ISC.
The UTP-induced rise was reduced by pretreatment with either bumetanide (100 μM), diphenylamin-2-carboxylic acid (DPC, 1 mM), or Cl− and HCO3−-free solution whilst the fall was abolished by amiloride pretreatment.
Thapsigargin (0.3 μM) abolished the UTP-induced increase in ISC but not the subsequent decrease. Staurosporine (0.1 μM) inhibited basal ISC and blocked UTP-induced inhibition of ISC. Inhibitors of either protein kinase C (PKC) (D-erythro sphingosine) or PKA (H89) had no effect.
This study suggests that UTP stimulates Cl− secretion and inhibits basal Na+ absorption. ATP has a similar stimulatory effect, which may be mediated by activation of P2Y2 receptors and an increase in [Ca2+]in, but no inhibitory effect, which is likely mediated by activation of a pyrimidine receptor and possible inhibition of a protein kinase other than PKC or PKA.
Keywords: Airway, ATP, cystic fibrosis, epithelium, ion transport, P2Y receptor, pyrimidine receptor, UTP
Introduction
CF is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), a protein that normally forms a cyclic AMP-dependent anion channel (Anderson et al., 1991) and exerts inhibitory control over epithelial Na+ channels (Stutts et al., 1995; Ismailov et al., 1996). As a consequence, the airway epithelia of patients with CF are characterized by reduced anion secretion and enhanced Na+ absorption (Boucher et al., 1986). These defects disturb the volume and/or ionic composition of the liquid lining the epithelial surface and, although the mechanism has not been determined (see Boucher, 1999), this precipitates chronic chest disease that can progressively destroy the lungs.
Luminal nucleotides stimulate anion secretion across airway epithelia by acting on P2Y receptors present in the apical membrane. The response is due to the activation of anion channels other than CFTR, and so it persists in CF cells (Mason et al., 1991; Stutts et al., 1992; 1994). Moreover, it has recently become clear that these receptors also mediate sustained inhibition of Na+ absorption (Iwase et al., 1997; Devor & Pilewski, 1999; Inglis et al., 1999; Ramminger et al., 1999). P2Y receptor agonists can thus oppose the defects in ion transport that cause chest disease in CF patients and this has led to the suggestion that such drugs may become useful in the treatment of this disease (Knowles et al., 1996). Such responses to apical nucleotides are often attributed to the activation of receptors belonging to the P2Y2 subclass (Parr et al., 1994; Knowles et al., 1996), which are equally sensitive to ATP and UTP. However, other P2Y receptor subtypes are present in the apical membranes of at least some cultured epithelia. These receptors are activated by pyrimidine nucleotides but essentially insensitive to ATP (Inoue et al., 1997; Lazarowski et al., 1997; Wilson et al., 1998; Ko et al., 1999), and could become important therapeutic targets if they were also expressed by native tissues. In the present study, we have therefore sought to establish whether such selective ‘pyrimidinoceptors' are present in the apical membranes of acutely isolated airway epithelia.
Methods
Measurement of transepithelial ion transport
Cotswold pigs (∼15 kg) were obtained from a local supplier. The animals were sedated with inhaled Halothane and then killed by an intravenous overdose of sodium pentobarbitone. The trachea was removed and divided into three approximately equal segments, each of which was opened longitudinally and pinned onto a Sylgard support with the mucosa uppermost. Epithelial sheets were then carefully dissected from each segment and mounted in Ussing chambers (area of exposed tissue 1.77 cm2) where they were bathed with Krebs–Ringer solution (37°C) that was continually circulated by gas lifts (5% CO2 in O2). All preparations were first maintained under open circuit conditions until the transepithelial potential difference (PD) had stablized (30–90 min). The transepithelial PD was then clamped at 0 mV, and the current required to maintain this PD (short circuit current, ISC) continually monitored and displayed using standard techniques. Data were recorded directly to disc using a MacLab computer interface (ADInstruments Ltd., Hastings, U.K.).
Data analysis
Values of PD are reported with respect to the submucosal compartment. Positive currents are defined as those that would be carried by anions moving from the basolateral to the apical compartments and are shown as upward deflections of the traces. Experimentally-induced changes in ISC were quantified by subtracting the current flowing at the peak of a response from basal ISC, where basal ISC is the current flowing immediately prior to addition of agonist. In some instances, responses were further characterized by integrating the current records with respect to time using commercially available software (AreaCalc 1.0, Cyber Solutions, Newport-on Tay, Fife, U.K.). The basal ISC was assigned a value of zero so that all responses reflect deviations from the charge transfer occurring under unstimulated conditions. The dose response relationship was fitted using Grafit (Erithacus Software, Staines, U.K.).
Data are presented as mean±standard error (s.e.mean) and values of n refer to the number of experiments undertaken using tissues from different animals. The statistical significance of any difference between mean values was assessed using either Student's paired t-test or analysis of variance (ANOVA) followed by Dunnett's test.
Solutions and chemicals
Krebs–Ringer solution (in mM): NaCl 112; KCl 4.7; CaCl2 2.5; MgSO4 2.4; KH2PO4 1.2; NaHCO3 25; D-glucose 11.6. Solution was continuously bubbled with 5% CO2 in O2 to maintain pH 7.4. Cl− and HCO3−-free solution was similar to KRB but NaHCO3−, NaCl and KCl were replaced with equimolar N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES), Na gluconate and K gluconate respectively. CaCl2 was replaced with 11 mM Ca gluconate2 to counteract the chelation effect of gluconate (Christoffersen & Skibsted, 1975). Solution was titrated to pH 7.4 with HCl and gassed with 100% O2. All drugs and nucleotides were obtained from either Sigma Chemical Co. (Poole, Dorset, U.K.) or Calbiochem (Nottingham, U.K.). Stock solutions of nucleotides and amiloride were prepared in distilled water. Stock solutions of bumetanide, diphenylamine-2-carboxylic acid (DPC), KN-93, staurosporine, phorbol-12-myristate-13-acetate (PMA), D-erythrosphingosine, H89 and forskolin were in dimethylsulphoxide whilst thapsigargin was in ethanol. Appropriate experiments showed that the solvent vehicles did not affect the electrical properties of the experimental preparations.
Results
Under unstimulated conditions the bioelectrical properties of the tracheal segments used in this study were: transepithelial resistance (Rt) 168±6 Ω cm2; PD−23.3±1.1 mV; and ISC 132±3 μA cm−2 (n=190).
The effects of mucosal UTP and ATP
Both ATP and UTP (both 100 μM mucosal) evoked transient increases in ISC that reached clearly defined peaks of a similar magnitude after ∼1 min (Figure 1A, Table 1). Thereafter, the responses to ATP and UTP differed markedly. In ATP-stimulated tissues ISC decayed back towards its basal level until, after 10 min, it did not differ significantly from basal ISC (basal ISC=121±5 μA cm−2, 10 min after ATP addition ISC=120±6 μA cm−2, P>0.05, Figure 1A). In contrast, ISC fell much more sharply in response to UTP. Indeed, ISC remained above the basal level for only 3 min and, after 10 min, had fallen to a level significantly below the basal ISC (basal: 134±8 μA cm−2, after 10 min with UTP: 108±6 μA cm−2, P<0.05, Figure 1A). Although some recovery occurred ISC remained depressed for at least 30 min. For the purpose of clarity, we termed the period during which ISC is elevated the stimulatory phase and the period during which ISC is depressed the inhibitory phase of the response. To further quantify the difference in the responses to ATP and UTP, the transepithelial charge movement occurring during the two phases of the response was quantified. During the stimulatory phase, ATP-evoked charge transfer exceeded that seen during stimulation with UTP, while the reverse was true during the inhibitory phase (Figure 1B and Table 1). Indeed, over a 16 min period, ATP caused a net stimulation of charge transfer whilst the overall action of UTP was inhibitory (Figure 1B and Table 1).
Figure 1.
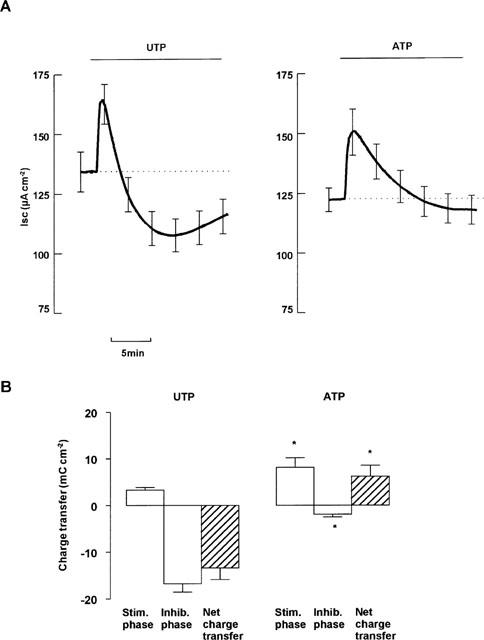
Effect of ATP and UTP on ISC across tracheal epithelium. Paired tissues were treated with either UTP (100 μM mucosal) or ATP (100 μM mucosal). (A) Shows mean ISC±s.e.mean of 11 pairs. (B) Shows charge transfer, calculated as area between UTP-induced ISC and basal ISC (dotted line in A). Positive charge transfer represents increased mucosal negativity. *Indicates significant difference from effect of UTP (Paired Student's t-test, P<0.05).
Table 1.
Contrasting effects of mucosal ATP and UTP
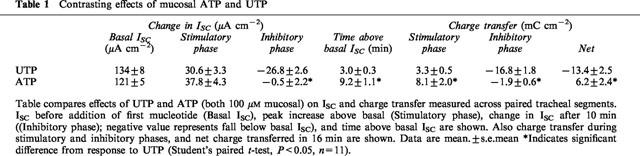
To establish the extent to which the responses were concentration dependent, epithelial segments were stimulated with one of six concentrations of UTP (0.1–500 μM, all mucosal). Each response was compared with that evoked by 100 μM UTP in tissues isolated from the same animal. Analysis showed that both the peak and inhibitory phases of the response to UTP were dose dependent (Figure 2) and that the stimulatory phase had a slightly lower EC50 (∼5 μM) than the inhibitory phase (∼20 μM). Analogous experiments (n=3) confirmed that 100 μM ATP elicits a monophasic response but also showed that this nucleotide did not evoke a discernible change in ISC at 1 μM (Basal ISC=97.4±12.4 μA cm−2, after 1 μM ATP=98.6±11.7 μA cm−2). At 10 μM, however, ATP evoked a clear increase in ISC (14.6±3 μA cm−2) that was significantly smaller than that seen during stimulation with 100 μM ATP (32.9±8.3 μA cm−2, P<0.05).
Figure 2.
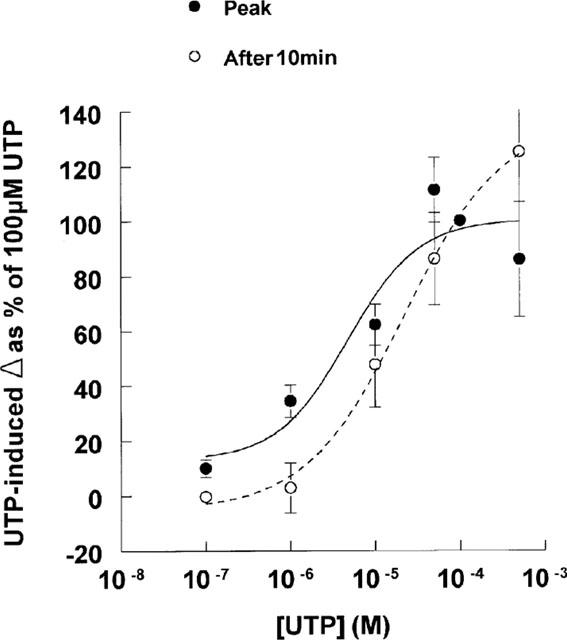
Dose dependency of the increase (Peak) and decrease below basal after 10 min (after 10 min) induced by UTP. Trachea were treated with either 10−7, 10−6, 10−5, 5×10−5, 10−4 or 5×10−4 M mucosal UTP. Responses are represented as a fraction of the response of paired tissues to 100 μM UTP. Data are mean±s.e.mean (n=6 (10−6, 10−5), 7 (10−7, 5×10−5) or 3 (5×10−4 M)).
Cross desensitization experiments
To test whether mucosal ATP and UTP act upon a common receptor population, epithelia were treated with UTP (100 μM mucosal) and a second aliquot of this nucleotide (also 100 μM) added once ISC had stabilized. The initial stimulus elicited a response essentially identical to that described above, whilst the second simply evoked a small decrease in ISC (Figure 3A, Table 2). The response to UTP is thus subject to essentially complete autologous desensitization and analogous experiments showed that this was also true of the response to ATP (Table 2).
Figure 3.
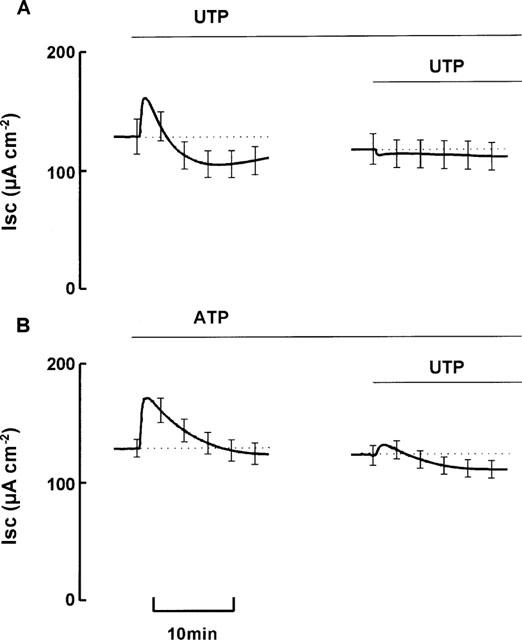
Cross desensitization of ATP and UTP. Paired tissues were pretreated with either UTP (100 μM mucosal; (A), or ATP (100 μM mucosal; (B), before addition of UTP (100 μM mucosal). Data are mean±s.e.mean (n=5).
Table 2.
Cross-desensitization of UTP and ATP
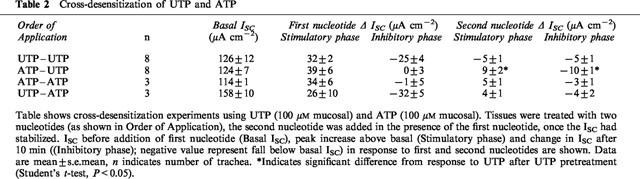
Cross desensitization experiments showed that ATP-stimulated tissues subsequently responded to UTP (Figure 3B, Table 2). Although it was smaller than the control response, this response to UTP was clearly biphasic. Moreover, both of its phases were significantly greater than those induced by UTP in tissues pretreated with UTP (P<0.05, n=8) (Table 2). Further experiments revealed that the response of UTP-pretreated tissues to ATP was not significantly different from the response of tissues pretreated with ATP (Table 2). These results suggest that the apical membrane contains at least two P2Y receptor subtypes, one desensitized by both ATP and UTP and one desensitized by UTP but not by ATP.
The effects of submucosal UTP and ATP on ion transport
To test whether the biphasic effect of UTP was mediated by receptors present on the basolateral as well as the apical membrane, UTP (500 μM) was applied to the submucosal side of the epithelia. This high dose was chosen since we anticipated that the basolateral receptors may be less accessible than the apical ones. Submucosal UTP evoked a transient increase in ISC (8.1±1.5 μA cm−2) that was significantly smaller than the response to mucosal UTP seen in paired tissues (18.4±2.2, n=5, P<0.05). Submucosal UTP caused no inhibition of basal ISC (Figure 4). Net charge transfer was positive (5.4±1.1 mC cm−2), and significantly different from charge transfer induced by mucosal UTP (−14.1±4.4 mC cm−2, P<0.05). Addition of mucosal UTP after submucosal UTP induced a response (peak increase 29.8±5.6 μA cm−2, inhibition after 10 min −24.4±1.5 μA cm−2, net charge transfer −10.1±0.3 mC cm−2) indistinguishable from that induced by mucosal UTP alone (peak increase 18.4±2.2 μA cm−2, inhibition after 10 min −28.2±6.4 μA cm−2, net charge transfer −14.1±4.4 mC cm−2) (Figure 4). In contrast to the difference in the responses induced by mucosal UTP and ATP, the response to submucosal ATP (peak increase 11.2±2.4 μA cm−2, ISC after 10 min 8.8±3.4 μA cm−2 above basal, net charge transfer 7.2±2.1 mC cm−2) was indistinguishable from the response to submucosal UTP (Figure 4).
Figure 4.
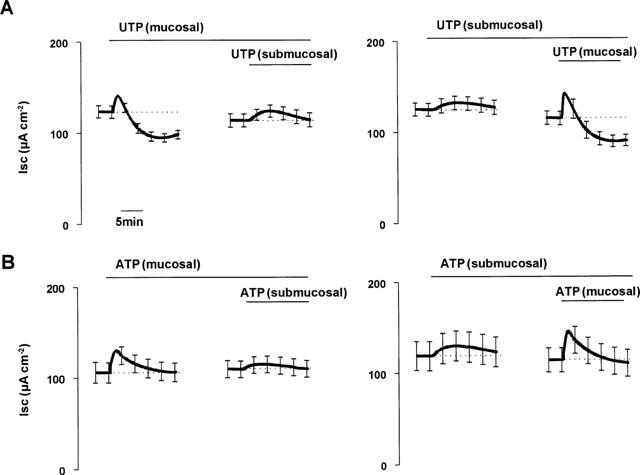
Effects of submucosal ATP and UTP. Paired tissues were treated with either mucosal or submucosal UTP (A) or ATP (B) before addition of the same nucleotide to the contralateral side. Data are mean±s.e.mean (n=5).
Ionic basis of the UTP-evoked changes in ISC
Treatment with bumetanide (100 μM submucosal) to inhibit Cl− secretion, blocked ∼33% of basal ISC (Table 3). Addition of UTP to these trachea induced an initial increase that was significantly smaller than the increase in paired control tissues (P<0.05, Figure 5, Table 4). The subsequent inhibition of ISC by UTP was not significantly different than control tissues. DPC (1 mM mucosal), a Cl− channel inhibitor, had a similar effect on both basal ISC (Table 3) and the response to UTP (Table 4). Replacement of Cl− and HCO3− with the impermeant anion gluconate inhibited ISC to 25% of control. The initial increase stimulated in ISC by UTP was significantly smaller than in control tissues (Table 4). The subsequent inhibition also tended to be attenuated compared with control, but this was not significant.
Table 3.
Effect of various agents on basal ISC
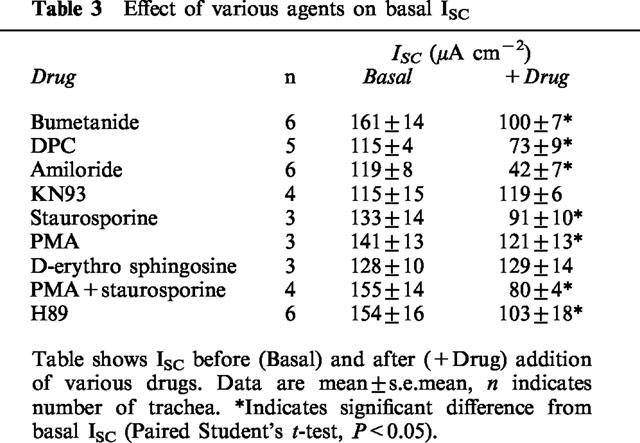
Figure 5.
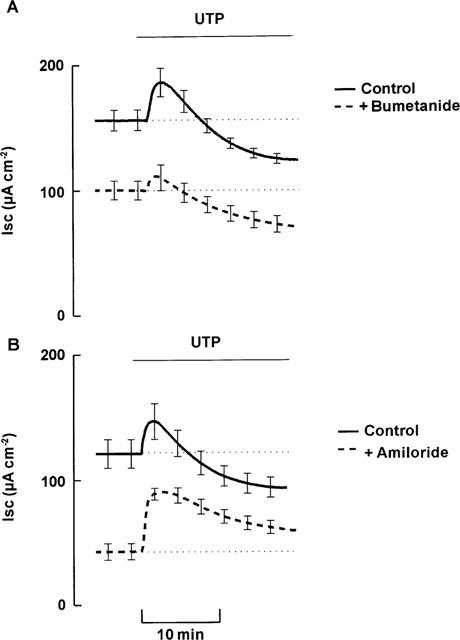
Effect of ion transport blockers on response to UTP (100 μM mucosal). (A) Shows effect on paired tissues either pretreated with bumetanide (100 μM submucosal; bumetanide) or left without pretreatment (control) (n=6). (B) Shows effect on paired tissues either pretreated with amiloride (10 μM mucosal; amiloride) or left without pretreatment (control) (n=6). Data are mean±s.e.mean.
Table 4.
Effect of ion transport inhibitors and ion replacement on response to UTP
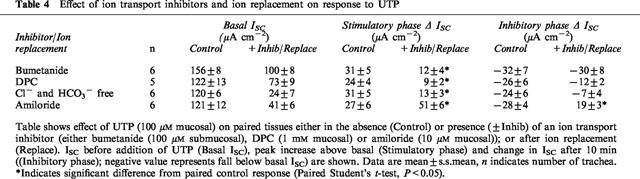
Amiloride (10 μM mucosal), a blocker of epithelial Na+ channels, inhibited ∼65% of basal ISC (Table 3). In the presence of amiloride the initial, stimulatory component of the response to UTP was augmented and the subsequent, inhibitory phase of this response was completely abolished (Figure 5, Table 4). In a separate series of experiments, amiloride inhibited significantly less ISC after UTP treatment (42±6 μA cm−2) than in paired trachea not treated with UTP (78±6 μA cm−2), confirming that UTP inhibits basal amiloride-sensitive ISC.
Effect of thapsigargin and Ca2+/calmodulin-dependent kinase inhibitor on response to UTP
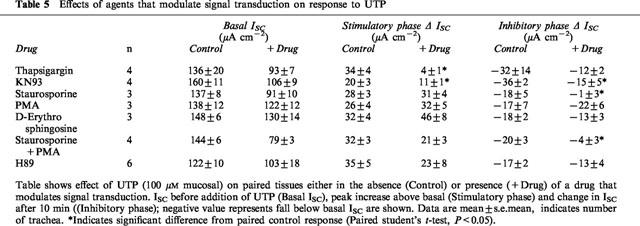
To investigate the role of changes in [Ca2+]in in the response to UTP, epithelia were pretreated with thapsigargin, to inhibit the uptake of Ca2+ into intracellular stores and thereby clamp [Ca2+]in at a high level. Thapsigargin alone (0.3 μM, mucosal and submucosal) induced a transient rise in ISC (from 108±15 μA cm−2 to 125±11 μA cm−2, n=4) followed by a gradual decline. The initial UTP-induced stimulation of ISC was abolished in the presence of thapsigargin. The subsequent UTP-induced inhibition of ISC was not significantly different from control tissues (Table 5). Pretreatment with the Ca2+/calmodulin-dependent kinase inhibitor, KN-93 (30 μM mucosal) significantly attenuated both the initial increase and secondary decrease in ISC (Table 5). KN-93 itself had no effect on basal ISC (Table 3).
Table 5.
Effects of agents that modulate signal transduction on response to UTP
Effect of protein kinase inhibitors on basal ISC and response to UTP
Staurosporine, a relatively non-specific kinase inhibitor (0.1 μM, mucosal and submucosal) inhibited ∼30% of basal ISC (Table 3). This effect was abolished by pretreatment with amiloride (67±16 μA cm−2 after amiloride alone; 61±15 μA cm−2 after subsequent treatment with staurosporine for 20 min, n=3). In the presence of staurosporine the UTP-induced increase in ISC was not significantly different from the response of paired control tissues (P>0.05, n=4), whilst the inhibitory effect of UTP was abolished (Table 5).
To investigate the role of PKC in regulation of basal Na+ absorption tissues were treated with PMA, which activates PKC. This substance (16.2 nM, mucosal and submucosal) inhibited ∼14% of basal ISC (P<0.05, n=3, Table 3) whilst D-erythro sphingosine, a relatively specific PKC inhibitor, (10 μM, mucosal and submucosal) had no effect on basal ISC (P>0.05, n=3, Table 3). The subsequent response to UTP was unaffected by pretreatment with either PMA or D-erythro sphingosine (Table 5). To investigate whether the effect of staurosporine was mediated by inhibition of PKC, epithelia were treated with both staurosporine and PMA because under these conditions any effects of staurosporine on PKC are likely to be opposed by PMA and residual effects of staurosporine are thus likely to be mediated by mechanisms other than inhibition of PKC. This pretreatment significantly inhibited ∼46% of basal ISC (P<0.05, n=4, Table 3) and the subsequent response to UTP resembled the response of epithelia pretreated with staurosporine (Table 5).
To investigate the role of PKA in regulation of basal ISC, tissues were treated with the PKA inhibitor, H89 (20 μM, mucosal). H89 inhibited basal ISC (P<0.05, n=6, Table 3) but had no effect on the subsequent response to UTP (Table 5).
Discussion
The results of this study indicate that mucosal UTP has a biphasic effect on ISC in porcine tracheal epithelium and similar effects have been noted in a number of other airway epithelia (Mason et al., 1991; Devor & Pilewski, 1999; Inglis et al., 1999; Ramminger et al., 1999). We now show, however, that mucosal ATP simply evokes a monophasic response in porcine tracheal epithelium. To our knowledge, this is the first demonstration that ATP and UTP have different effects upon airway ion transport, and it suggests that the effects of nucleotides upon this tissue may be mediated by at least two distinct populations of P2Y receptors. Since UTP and ATP induce initial increases in ISC of similar magnitudes, it is likely that this early response is mediated, at least in part, by P2Y2 receptors, which are equally sensitive to these nucleotides and have been widely implicated in nucleotide-induced Cl− secretion in airway epithelia (Knowles et al., 1991; Mason et al., 1991; Van Scott et al., 1995; Yamaya et al., 1996). After this initial peak, however, an inhibitory phase of the response to UTP becomes apparent. This, together with the cross desensitization experiments suggests that additional P2Y receptors sensitive to UTP but not to ATP are present in this tissue. Interestingly, submucosal UTP and ATP simply elicited a monophasic response. The inhibitory phase of the response to UTP thus appears to be mediated by ATP-insensitive receptors that are essentially confined to the apical membrane.
At least two such receptor subtypes have been described in airway epithelia. Lazarowski et al. (1997) showed that P2Y6 receptors, which are activated primarily by UDP, are expressed in cultured human nasal epithelia. Since UDP has no discernible effect on either porcine trachea or bronchi (Inglis et al., 1999) it is unlikely that these receptors regulate ion transport in these tissues. This may indicate that human and porcine airways express different P2Y receptor subtypes. Alternatively, it is possible that P2Y6 receptors are not normally expressed in native airways, and that their expression may have been induced during the isolation and culture of human nasal epithelia. It is clear that culture conditions can acutely alter the functional expression of P2Y receptors, possibly by controlling gene expression (Clunes et al., 1998; Wilson et al., 1998). A second pyrimidine receptor, P2Y4, which is sensitive to UTP with UDP acting as a weak partial agonist, has been reported in airway epithelia (Merten et al., 1998; Communi et al., 1999), and this may well be the receptor subtype that mediates UTP-induced inhibition of basal ISC in porcine trachea. We cannot, however, rule out the possibility that another, as yet unidentified receptor mediates these responses.
Experiments with ion transport blockers and ion substitution suggest that UTP initially stimulates anion secretion before inhibiting basal Na+ absorption. Nucleotide-induced stimulation of Cl− secretion has been described both in vivo (Knowles et al., 1991) and in vitro, in native (Iwase et al., 1997; Inglis et al., 1999) and in cultured (e.g. Mason et al., 1991; Van Scott et al., 1995; Yamaya et al., 1996) airways. Inhibition of Na+ absorption has also been reported in a number of airway epithelia (Mason et al., 1991; Devor & Pilewski, 1999; Inglis et al., 1999; Ramminger et al., 1999). The increased peak response seen in the presence of amiloride is likely to reflect an increased Cl− secretion, driven by amiloride-induced depolarization of the apical membrane. The maximal dose of UTP inhibited 40% of amiloride-sensitive ISC, indicating that a substantial portion of amiloride-sensitive ISC is not sensitive to inhibition by UTP. Similarly, in cultured human bronchial epithelia, 25% of amiloride-sensitive Na+ absorption remains after UTP-evoked inhibition (Devor & Pilewski, 1999).
Since P2Y2 and P2Y4 receptors allow external nucleotides to increase [Ca2+]in, we anticipated that UTP-induced regulation of Cl− secretion and Na+ absorption would be mediated by changes in [Ca2+]in. Certainly the stimulation of Cl− secretion appears to be almost completely dependent on [Ca2+]in. This is in contrast to our recent studies of porcine distal bronchi showing that their Cl− secretory response to UTP is [Ca2+]in-independent (Inglis et al., 1999), and it suggests that the mechanisms that regulate Cl− secretion are different in different regions of the airway. The mechanisms are clearly complex, since both Ca2+-dependent and Ca2+-independent components of nucleotide-induced Cl− secretion have been reported (Stutts et al., 1992; 1994; Hwang et al., 1996). Although we cannot rule out the involvement of Ca2+-independent mechanisms, our data suggest that in porcine trachea UTP-induced Cl− secretion is mediated primarily by changes in [Ca2+]in.
It is well known that increases in [Ca2+]in can inhibit epithelial Na+ channels (Ishikawa et al., 1998) and transepithelial Na+ absorption (e.g. Graham et al., 1992; Koster et al., 1996) so we may have expected this to be the main mechanism involved in the inhibitory phase of the response to UTP. However, it was clear that the effect of UTP upon Na+ absorption is not entirely dependent on [Ca2+]in, suggesting that the pyrimidine receptor expressed by this tissue is coupled to additional intracellular mechanisms that allow inhibition of basal ISC. Studies using inhibitors of different kinases suggested that basal ISC was reduced both by increased activity of PKC (as described in sheep trachea Graham et al., 1992) and by inhibition of PKA. These results suggest that the basal rate of ion transport is under complex control, and may be set by the relative activities of PKA and PKC within the cell. Surprisingly, however, the inhibitory effect of UTP on basal Na+ absorption does not seem to be mediated by either PKA or PKC, since neither PMA, H89, nor the PKC inhibitor D-erythro sphingosine had any effect on this response. This was unexpected since P2Y receptor-induced activation of phospholipase C is likely to activate PKC. However, a similar lack of involvement of PKC was found in the response of bronchial epithelia to UTP (Devor & Pilewski, 1999). The inhibitory effect of UTP was however blocked by staurosporine, a non specific protein kinase inhibitor. This suggests that another, as yet unidentified protein kinase is involved in this effect of UTP. Another possible mechanism by which UTP may inhibit Na+ absorption is through inhibition of basolateral K+ channels, as described in human bronchial epithelia (Devor & Pilewski, 1999). This mechanism is also involved in inhibition of Na+ absorption by other Ca2+-mobilizing agonists, e.g. acetylcholine (Venglarik & Dawson, 1986; Inglis et al., 1992).
In summary, since P2Y receptor agonists can both stimulate Cl− secretion in CF (Mason et al., 1991), and inhibit basal Na+ absorption, they provide a potential mechanism by which the ion transport defects in CF may be bypassed therapeutically. The results of this study suggest that the effects on Cl− secretion and Na+ absorption may be mediated by distinct P2Y receptor subtypes, which may potentially be activated separately by different therapeutic drugs.
Acknowledgments
The authors thank Elaine A. Waller for technical assistance. This work was supported by Wellcome (Project Grant 049986; Programme Grant 039124; Career Development Fellowship (S.K. Inglis)), Anonymous and Tenovus Trusts.
Abbreviations
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- DPC
diphenylamin-2-carboxylic acid
- ISC
short-circuit current
- PD
potential difference
- PKA
protein kinase A
- PKC
protein kinase C
- PMA
phorbol-12-myristate-13-acetate
- Rt
transepithelial resistance
References
- ANDERSON M.P., GREGORY R.J., THOMPSON S., SOUZA D.W., PAUL S., MULLIGAN R.C., SMITH A.E., WELSH M.J. Demonstration that CFTR is a chloride channel by alteration of its anion selectivity. Science. 1991;253:202–207. doi: 10.1126/science.1712984. [DOI] [PubMed] [Google Scholar]
- BOUCHER R.C. Molecular insights into the physiology of the 'thin film' of airway surface liquid. J. Physiol. Lond. 1999;516.3:631–638. doi: 10.1111/j.1469-7793.1999.0631u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOUCHER R.C., STUTTS M.J., KNOWLES M.R., GATZY J.T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J. Clin. Inv. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOFFERSEN G.R.J., SKIBSTED L.H. Calcium ion activity in physiological salt solutions: influence of anions substituted for chloride. Comp. Biochem. Physiol. 1975;A52:317–322. doi: 10.1016/s0300-9629(75)80094-6. [DOI] [PubMed] [Google Scholar]
- CLUNES M.T., COLLETT A., BAINES D.L., BOVELL D.L., MURPHIE H., INGLIS S.K., MCALROY H.L., OLVER R.E., WILSON S. Culture substrate-specific expression of P2Y2 receptors in distal lung epithelial cells isolated from foetal rats. Br. J. Pharm. 1998;124:845–847. doi: 10.1038/sj.bjp.0701942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., PAINDAVOINE P., PLACE G.A., PARMENTIER M., BOEYNAEMS J.-M. Expression of P2Y receptors in cell lines derived from the human lung. Br. J. Pharm. 1999;127:562–568. doi: 10.1038/sj.bjp.0702560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVOR D.C., PILEWSKI J.M. UTP inhibits Na absorption in wild-type and deltaF508 CFTR-expressing human bronchial epithelia. Am. J. Physiol. 1999;276:C827–C837. doi: 10.1152/ajpcell.1999.276.4.C827. [DOI] [PubMed] [Google Scholar]
- GRAHAM A., STEEL D.M., ALTON E.W.F.W., GEDDES D.M. Second-messenger regulation of sodium transport in mammalian airway epithelia. J. Physiol. Lond. 1992;453:475–491. doi: 10.1113/jphysiol.1992.sp019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG T.-H., SCHWIEBERT E.M., GUGGINO W.B. Apical and basolateral ATP stimulates tracheal epithelial chloride secretion via multiple purinergic receptors. Am. J. Physiol. 1996;270:C1611–C1623. doi: 10.1152/ajpcell.1996.270.6.C1611. [DOI] [PubMed] [Google Scholar]
- INGLIS S.K., COLLETT A., MCALROY H.L., WILSON S.M., OLVER R.E. Effect of luminal nucleotides on Cl− secretion and Na+ absorption in distal bronchi. Pflügers Arch. 1999;438:621–627. doi: 10.1007/s004249900096. [DOI] [PubMed] [Google Scholar]
- INGLIS S.K., WARD M.R., ACEVEDO M. Changes in the basolateral potassium conductance induced by acetylcholine (ACh) in isolated sheep tracheal epithelium. J. Physiol. Lond. 1992;452:144P. [Google Scholar]
- INOUE C.N., WOO J.S., SCHWIEBERT E.M., MORITA T., HANAOKA K., GUGGINO S.E., GUGGINO W.B. Role of purinergic receptors in chloride secretion in Caco-2 cells. Am. J. Physiol. 1997;272:C1862–C1870. doi: 10.1152/ajpcell.1997.272.6.C1862. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA T., MARUNAKA Y., ROTIN D. Electrophysiological characterisation of the rat epithelial Na+ channel (rENaC) expressed in MDCK cells. J. Gen. Physiol. 1998;111:825–846. doi: 10.1085/jgp.111.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISMAILOV I.I., AWAYDA M.S., JOVOV B., BERDIEV B.K., FULLER C.M., DEDMAN J.R., KAETZEL M.A., BENOS D.J. Regulation of epithelial sodium channels by the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 1996;271:4725–4732. doi: 10.1074/jbc.271.9.4725. [DOI] [PubMed] [Google Scholar]
- IWASE N., SASAKI T., SHIMURA S., YAMAMOTO M., SUZUKI S., SHIRATO K. ATP-induced Cl secretion with supressed Na+ absorption in rabbit tracheal epithelium. Resp. Physiol. 1997;107:173–180. doi: 10.1016/s0034-5687(96)02516-9. [DOI] [PubMed] [Google Scholar]
- KNOWLES M.R., CLARKE L.L., BOUCHER R.C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N. Engl. J. Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- KNOWLES M.R., OLIVIER K., NOONE P., BOUCHER R.C. Pharmacological modulation of salt and water transport in the airway epithelium in cystic fibrosis. Am. J. Resp. Crit. Care Med. 1996;151:S65–S69. doi: 10.1164/ajrccm/151.3_Pt_2.S65. [DOI] [PubMed] [Google Scholar]
- KO W.H., LAW W.Y.K., WONG C.Y., WILSON S.M. The simultaneous measurement of ISC and intracellular free Ca2+ in equine cultured equine sweat gland secretory epithelia. J. Membrane Biol. 1999;170:205–211. doi: 10.1007/s002329900550. [DOI] [PubMed] [Google Scholar]
- KOSTER H.P.G., HARTOG A., VAN OS C.H., BINDELS R.J.M. Inhibition of Na+ and Ca2+ reabsorption by P2u purinoceptors requires PKC but not Ca2+ signalling. Am. J. Physiol. 1996;270:F53–F60. doi: 10.1152/ajprenal.1996.270.1.F53. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., PARADISO A.M., WATT W.C., HARDEN K., BOUCHER R.C. UDP activates a mucosal-restricted receptor on human nasal epithelial cells that is distinct from the P2Y2 receptor. Proc. Natl. Acad. Sci. 1997;94:2599–2603. doi: 10.1073/pnas.94.6.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON S.J., PARADISO A.M., BOUCHER R.C. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human and cystic fibrosis airway epithelium. Br. J. Pharm. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERTEN M.D., SALEH A., KAMMOUNI W., MARCHAND S., FIGARELLA C. Characterisation of two distinct P2Y receptors in human tracheal gland cells. Eur. J. Biochem. 1998;251:19–24. doi: 10.1046/j.1432-1327.1998.2510019.x. [DOI] [PubMed] [Google Scholar]
- PARR C.E., SULLIVAN D.M., PARADISO A.M., LAZAROWSKI E.R., BIRCH L.H., OLSEN J.C., ERB L., WEISMAN G.A., BOUCHER R.C., TURNER J.T. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMMINGER S.J., COLLETT A., BAINES D.L., MURPHIE H., MCALROY H.L., OLVER R.E., INGLIS S.K., WILSON S.M. P2Y2 receptor-mediated inhibition of ion transport in distal lung epithelial cells. Br. J. Pharm. 1999;128:293–300. doi: 10.1038/sj.bjp.0702767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUTTS M.J., CANESSA C.M., OLSEN J.C., HAMRICK M., COHN J.A., ROSSIER B.C., BOUCHER R.C. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- STUTTS M.J., CHINET T.C., MASON S.J., FULLTON J.M., CLARKE L.L., BOUCHER R.C. Regulation of Cl− channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUTTS M.J., FITZ J.G., PARADISO A.M., BOUCHER R.C. Multiple modes of regulation of airway epithelial chloride secretion by extracellular ATP. Am. J. Physiol. 1994;267:C1442–C1451. doi: 10.1152/ajpcell.1994.267.5.C1442. [DOI] [PubMed] [Google Scholar]
- VAN SCOTT M.R., CHINET T.C., BURNETTE A.D., PARADISO A.M. Purinergic regulation of ion transport across nonciliated bronchiolar epithelial (Clara) cells. Am. J. Physiol. 1995;269:L30–L37. doi: 10.1152/ajplung.1995.269.1.L30. [DOI] [PubMed] [Google Scholar]
- VENGLARIK C.J., DAWSON D.C. Cholinergic regulation of Na absorption by turtle colon: role of basolateral K conductance. Am. J. Physiol. 1986;251:C563–C570. doi: 10.1152/ajpcell.1986.251.4.C563. [DOI] [PubMed] [Google Scholar]
- WILSON S.M., LAW V.W.Y., PEDIANI J.D., ALLEN E.A., WILSON G., KHAN Z., KO W.H. Nucleotide-evoked calcium signals and anion secretion in equine cultured epithelia that express P2Y2 receptors and pyrimidine nucleotide receptors. Br. J. Pharm. 1998;124:832–838. doi: 10.1038/sj.bjp.0701888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAYA M., SEKIZAWA K., KAKUTA Y., OHRUI T., SAWAI T., SASAKI H. P2u-purinoceptor regulation of chloride secretion in cultured human tracheal submucosal glands. Am. J. Physiol. 1996;270:L979–L984. doi: 10.1152/ajplung.1996.270.6.L979. [DOI] [PubMed] [Google Scholar]


