Abstract
The effect of thyrotropin (TSH), on adenosine A1 receptor expression in thyroid FRTL-5 cells was examined by [3H]-1,3-dipropyl-,8-cyclopentyl xanthine ([3H]-DPCPX) binding on cells in suspension and on membrane preparation, and by in situ mRNA labelling.
The estimated KD for intact cells was 0.19 nM and about 47,000 binding sites per cell were found in cells constantly grown in the presence of TSH.
Three days deprivation of TSH decreased the number of [3H]-DPCPX binding sites without any significant effect of KD. Reintroduction of TSH to the cells returned the higher level of A1 receptors both in suspension binding studies on whole cells and on membrane preparations.
In situ hybridization revealed that TSH evoked an increase in the number of cells densely labelled with a probe against A1 receptor mRNA.
The potency of the A1 receptor agonist N6-cyclohexyladenosine (CHA) as an inhibitor of cyclic AMP formation induced by forskolin was increased in TSH-treated cells, with a shift in the IC50 from 2.05 nM in TSH-deprived cells to 0.14 nM in TSH-treated cells.
Since the activation of A1 receptors inhibits TSH-mediated cyclic AMP signalling, our results suggest a regulatory feedback mechanism between signalling via adenosine A1 receptors and TSH receptors.
Keywords: Adenosine A1 receptor, FRTL-5 cells, thyroid, thyrotropin
Introduction
Thyroid FRTL-5 cells, derived from the rat thyroid, grow continuously in the presence of thyrotropin (TSH) and maintain many functional properties of thyroid cells in situ, such as iodide uptake and thyroglobulin synthesis (Ambesi-Impiombato et al., 1980). FRTL-5 cells are therefore widely used to study the mechanisms of action of hormones and neuronal transmitters. Thyrotropin is the main regulator of thyroid cells, and the cyclic AMP signalling pathway has been related to most of the TSH-induced thyroid cell functions (Dere & Rapaport, 1986; Jin et al., 1986; Tramontano et al., 1988). Previously, the cyclic AMP-mediated TSH signalling has been shown to increase the expression of α1B-adrenergic receptors in FRTL-5 thyroid cells (Kanasaki et al., 1994).
Adenosine is released from thyroid cells as an autocrine factor (Okajima et al., 1994; Sho et al., 1991). Activation of adenosine A1 receptor by adenosine regulates TSH-mediated cyclic AMP signals via inhibition of adenylyl cyclase, thus modifying the physiological responses to TSH (Moses et al., 1989; Sho et al., 1991; 1999; Tramontano et al., 1988). The A1 adenosine receptor was first cloned from dog thyroid (Libert et al., 1989; 1991) and the FRTL-5 cell line has also been shown to express functional A1 receptors. Adenosine A1 receptors have previously been characterized in FRTL-5 cells by binding assays using cell membrane preparations (Okajima et al., 1994) or in intact cells grown in monolayer cultures (Frauman & Moses, 1989; Kosugi et al., 1989; Nayfeh et al, 1989; Okajima et al., 1989). In the present study a receptor binding assay utilizing trypsinized FRTL-5 cells in suspension was validated. Although the effects of adenosine on FRTL-5 cells and especially on the regulation of TSH function is well documented, the control mechanisms of A1 receptor expression in thyroid cells have not been studied before. Therefore we have investigated the possible regulatory effect of TSH on adenosine A1 receptors in rat thyroid FRTL-5 cells.
Methods
Materials
Culture medium and hormones needed for the cell culture were purchased from Sigma (St Louis, MO, U.S.A.). Serum was from Biological Industries (Beth Haemek, Israel). Culture dishes were from Falcon Plastics (Oxnard, CA, U.S.A.), Thyrotropin (TSH) was a generous gift from the National Hormone and Pituitary Program (Bethesda, MD, U.S.A.). Tritium labelled 1,3-dipropyl-,8-cyclopentyl xanthine ([3H]-DPCPX, 120 Ci mmol−1) and adenosine 3′,5′-cyclic phosphate ([3H]-cyclic AMP, 25 Ci mmol−1) were from New England Nuclear Corp. (Boston, MA, U.S.A.). N6-cyclohexyladenosine (CHA) was from Research Biochemicals Inc. (Natick, MA, U.S.A.). Adenosine deaminase was from Boehringer Mannheim (Germany). 4-(3-Butoxy-4-methoxybenzyl)-2-imidazolidinone (Ro 20-1724) was purchased from Calbiochem (La Jolla, CA, U.S.A.). Bio Rad protein assay was from Bio Rad Laboratories (Richmond, CA, U.S.A.). MultiScreen®-FB plates were from Millipore (Espoo, Finland). OptiPhase HiSafe III and SuperMix scintillation fluids were from Wallac (Turku, Finland).
Cell culture
FRTL-5 cells were initially obtained from the Interthyr Research Foundation. The cells were cultured in Coon's modified Ham's F-12 medium supplemented with 5% newborn calf serum and six hormones including TSH (TSH 0.3 mu ml−1, insulin 1 μg ml−1, transferrin 5 μg ml−1, somatostatin 10 ng ml−1, hydrocortisone 10 nM, the tripeptide Gly-L-His-L-Lys 10 ng ml−1). In TSH-depletion studies cells were grown for 3 days in medium without TSH prior to the stimulation with 1 mu ml−1 TSH for 24 h. Fresh medium was always added in the beginning of each stimulation.
Binding of [3H]-DPCPX to intact cells in suspension
Cells grown on 10 cm culture dishes were harvested with a 0.2% trypsin solution and washed three times with phosphate buffered saline (PBS). The cells were gently resuspended in serum- and hormone-free Coon's F-12 medium buffered with 20 mM HEPES (pH 7.4) and supplemented with 0.1% bovine serum albumin (BSA), at a density of 3×106 cells ml−1. In some experiments adenosine deaminase (1 u ml−1) was added into the incubation buffer. The viability of cells after trypsinization was 94±2% and was not significantly decreased after a 2 h incubation period. The total cell number and the viability were determined by Trypan blue staining and cell counting. Aliquots (0.1 ml, 0.3×106 cells) were added to test tubes containing [3H]-DPCPX with or without cold ligand. In the saturation experiments the range of [3H]-DPCPX concentrations was 0.03 nM–6 nM. The incubations were carried out in a final volume of 1 ml. Non-specific binding was defined as that occurring in the presence of 40 μM CHA. Except in the association and dissociation experiments, the incubation time was 2 h at room temperature. In TSH-depletion studies the cells were grown to confluence in the presence of TSH and then further incubated without TSH for 3 days. In some experiments adenosine deaminase (3 u ml−1) was added to the medium 72 h prior to the withdrawal of TSH. Assays were terminated by rapid filtration through glass fibre C filters (Whatman) on a 12-well harvester system (Millipore, Espoo, Finland), and the filters were rapidly washed three times with 3 ml of ice-cold PBS. The filters were then dried and transferred to scintillation vials and, after the addition of 4 ml HiSafe III scintillation fluid, counted in a β-counter. The results are expressed as the amount of bound ligand per 106 cells.
Binding of [3H]-DPCPX to membranes
The cells were grown on 10 cm culture dishes to confluence and then grown without TSH for 3 days prior to incubation with TSH. After 24 h of incubation with or without TSH (1 mu ml−1) the cells were washed twice with cold PBS and scraped from the plates with a cell scraper, and washed once more with PBS. The cells were suspended in 1 ml of homogenization buffer (50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 5 mM EDTA) and disrupted by sonication (B. Braun, Labsonic U). Unbroken cells and nuclei were sedimented by centrifugation at 1000×g for 10 min at 4°C and discarded. Plasma membranes and the cytosolic fraction were then separated by centrifugation at 30,000× g for 60 min at 4°C. The membrane pellet was resuspended in assay buffer (50 mM Tris-HCl, pH 7.4, 2 mM MgCl2) at 0.5 mg protein ml−1 and preincubated with 5 u ml−1 adenosine deaminase for 60 min at room temperature to remove endogenous adenosine. Aliquots (0.1 ml, 50 μg of protein) were added to test tubes containing 0.03–6 nM of [3H]-DPCPX with or without CHA (40 μM) in a final volume of 1 ml. Membranes were incubated for 90 min at room temperature before filtration through Whatman glass fibre C filters. The filters were washed three times with 3 ml cold assay buffer and counted for radioactivity. Protein determinations were performed with Bio-Rad protein assay for microtiter plate.
Analysis of adenosine
The culture medium from cells incubated with or without 1 mu ml−1 TSH for 24 h was collected and proteins were precipitated by 0.4 M perchloric acid. The samples were neutralized by 4 M KOH, concentration by lyophilization, and the amount of adenosine was determined by HPLC as described previously (Fredholm & Sollevi, 1981). Blank values were obtained by treating medium not incubated with cells in the same way as samples.
In situ hybridization
Cells were plated in Lab-Tech glass chamber slides (Nunc) and were allowed to grow to confluence in TSH-containing culture medium. Thereafter, the cells were washed and grown for an additional 3 days in TSH-deprived medium. After treatment with TSH for 24 h the glasses were fixed for 30 min with 4% paraformaldehyde in PBS. Hybridization was performed in a cocktail containing 50% deionized formamide (Fluka, Buchs, Switzerland), 4 × salinic sodium citrate (SSC; NaCl 3 M, trisodium citrate 0.3 M), 1 × Denhart's solution, 1% sarcosyl, 0.02 M Na2HPO4 (pH 7.0), 10% dextran sulphate, 0.5 mg ml−1 yeast tRNA (Sigma, Labkemi, Stockholm, Sweden), 0.06 M dithiotreitol, 0.1 mg ml−1 sheared salmon sperm DNA and 107 c.p.m. ml−1 of the oligodeoxynucleotide probe. After hybridization for 16 h at 42°C, the glasses were washed four times, for 15 min each, in 1×SSC at 56°C, dipped briefly in water, 70, 95 and finally in 99.5% ethanol and air-dried. The glasses were then dipped in NTB-3 emulsion (Kodak, Järfälla, Sweden) and exposed for 4–10 weeks. After the emulsion had been developed, cells were lightly counter-stained in Cresyl Violet (0.5%). Grain counting was performed using a Microcomputer Imaging Device (Imaging Research) and the results were divided by the number of nuclei seen in a counted area. Non-specific background was subtracted from the results.
Oligonucleotide probe
The 48-mer A1 receptor probe (Scandinavian Gene Synthesis AB, Köping, Sweden) was complementary to the nucleotides 985-1032 of the rat A1 receptor (Mahan et al., 1991). The oligodeoxynucleotide probe was radiolabelled using terminal deoxynucleotidyl transferase (LKB/Pharmacia) and α-[35S]-dATP (DuPont New England Nuclear, Stockholm, Sweden) to a specific activity of approximately 109 c.p.m. μg−1.
Measurement of total cyclic AMP
Cells, grown 3 days in TSH-deprived medium or in normal culture medium containing 0.3 mu ml−1 TSH, were harvested with a 0.2% trypsin solution and washed three times with HEPES-buffered salt solution (in millimolar concentrations: NaCl 118; KCl, 4.6; glucose, 10; CaCl2, 0.4; HEPES, 20; pH 7.2). The cells were gently resuspended in HEPES-buffered salt solution containing 1 u ml−1 adenosine deaminase and 0.1% BSA. Aliquots (0.35 ml, 0.5×106 cells) were added to test tubes and incubated 20 min at 37°C. Forskolin (final concentration 1 μM) with or without CHA (final concentration 0.01–1000 nM) was added to the test tubes together with Ro 20-1724 (final concentration of 100 μM). The cells were incubated with test reagents for 20 min. The incubation was stopped with 50 μl perchloric acid to a final concentration of 0.1 M. The samples were neutralized by KOH and the cyclic AMP content in the supernatants was determined by a protein binding method (Nordstedt & Fredholm, 1990). Briefly, the samples and standards (0–160 nM cyclic AMP) were incubated with protein extract (40 μg) from bovine adrenal cortex containing cyclic AMP binding protein and with [3H]-cyclic AMP (10,000 c.p.m. per tube) for 2.5 h at 4°C. The bound cyclic AMP was separated from free cyclic AMP by rapid filtration on MAFB Millipore MultiScreen filtration plates and the wells were washed three times with 250 μl HEPES-buffered salt solution. The plates were dried and 50 μl SuperMix scintillation fluid was added to the wells. The plate was counted using a MicroBeta scintillation counter (Wallac, Finland).
Statistics
Data from receptor binding studies were analysed by non-linear regression for receptor binding, using GraphPad Prism ligand binding software for MacIntosh computer (GraphPad Software, Inc., San Diego, CA, U.S.A.). Data are given as the means±s.e.mean from at least three experiments. Association and dissociation rate constants were calculated as previously described (Jenkinson, 1996). Values of grains per cell for in situ hybridization experiments were calculated from seven different 4-chamber glass slides having control and treated cells on the same slide. At least 100 cells were calculated per treatment. The results are pooled from two different sets of experiments. Student's t-test was used for obtaining the P-values. Values were considered significant if P<0.05. The difference in IC50- and KD- values was compared using logarithmic values.
Results
Characterization of [3H]-DPCPX binding on whole cells
The characterization of [3H]-DPCPX binding was performed on cells constantly grown in normal growing medium containing 0.3 mu ml−1 TSH. In these experiments adenosine deaminase was not added into the assay buffer. Incubation of trypsinized FRTL-5 cells in suspension with increasing concentrations of [3H]-DPCPX revealed a saturable specific binding (Figure 1). The calculated KD from saturation experiments was 0.19±0.04 nM with a maximal binding (Bmax) of 77±1 fmol per 106 cells (n=6), corresponding to approximately 47,000 binding sites per cell. Non-specific binding was less than 10% of the total binding up to a concentration of 1 nM, i.e. a concentration five times the estimated KD. It was important to determine whether the [3H]-DPCPX associated with the cells could be displaced by a competing receptor ligand. As seen in Figure 2a, addition of 10 μM CHA displaced almost all of the specifically bound radioligand over a 2 h period. Dissociation occurred with a half-time of 20 min. Half-maximal binding in association studies (Figure 2b) occurred after 4 min. The KD estimated from these kinetic experiments (approximately 0.27 nM) is in good agreement with the equilibrium binding data.
Figure 1.
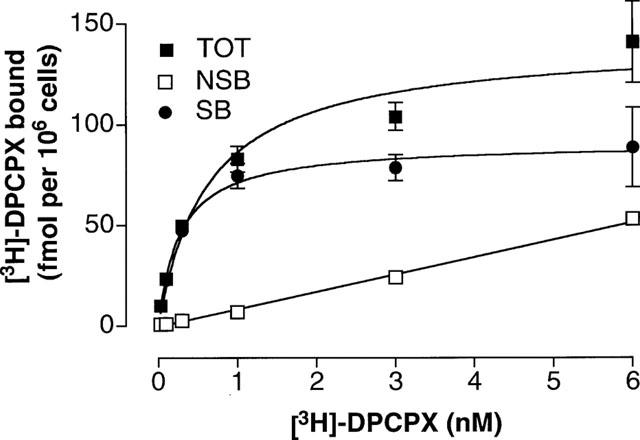
Saturation analysis of [3H]-DPCPX binding to intact FRTL-5 cells in suspension. The experiments were carried out at room temperature for 2 h as described in Methods. The presence and absence of 40 μM CHA was used to determined non-specific (NSB) and total binding (TOT), respectively. Specific binding (SB) was the difference between total and non-specific binding. Data are mean±s.e.mean from six separate experiments.
Figure 2.
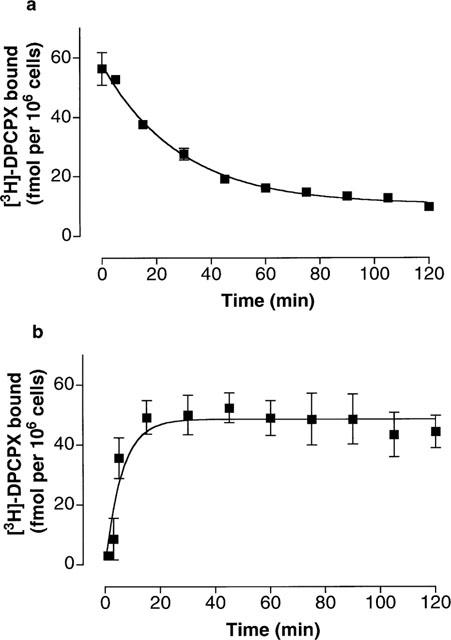
Kinetics of [3H]-DPCPX binding to FRTL-5 cells in suspension. (a) Shows the rate of dissociation of [3H]-DPCPX (1 nM) in FRTL-5 cells in suspension. Dissociation was initiated by addition of 10 μM CHA to cells that previously had been incubated with 1 nM [3H]-DPCPX for 2 h. The calculated koff from these experiments was 0.035 min−1. (b) Shows the rate of the specific binding of [3H]-DPCPX (1 nM) in FRTL-5 cells in suspension. The calculated kobs from these experiments was 0.164 min−1 with association constant rate (kon) of 0.129 nM−1 min−1. Using the ratio koff kon−1, the kinetic KD was calculated to be 0.27 nM. The cells in both experiments were grown to confluence in TSH-containing medium and harvested with trypsin solution. The experiments on the cells in suspension were carried out at room temperature.
Effects of TSH on [3H]-DPCPX binding
Depletion of TSH from the normal culture medium by 3 days of incubation without TSH decreased the maximal [3H]-DPCPX binding. Bmax decreased by approximately 63%, from 77±1 fmol per 106 cells to 28±4 fmol per 106 cells (n=4, P<0.05), as determined by saturation experiments (results not shown). The KD values were 0.11±0.02 nM in TSH-depleted cells and 0.19±0.04 nM in cells grown in TSH-containing medium.
To investigate the effect of TSH on TSH-deprived cells, i.e. cells grown without TSH for 3 days, the cells were treated with 1 mu ml−1 TSH for 24 h. After 24 h of incubation with 1 mu ml−1 TSH the Bmax value for intact cells was 62±9 fmol per 106 cells and the KD was 0.19±0.04 nM (n=4, Figure 3a). The stimulatory effect of TSH on A1 receptor expression was seen also when [3H]-DPCPX binding was studied in cell membrane preparations (data not shown). The values for Bmax in membranes prepared from TSH-depleted and TSH-treated cells (1 mu ml−1 for 24 h) were 330±41 fmol mg−1 and 450±76 fmol mg−1, respectively (n=9, P<0.05). In membranes prepared from TSH-depleted cells the KD value was 0.28±0.05 nM and in membranes prepared from TSH-treated cells 0.30±0.04 nM.
Figure 3.
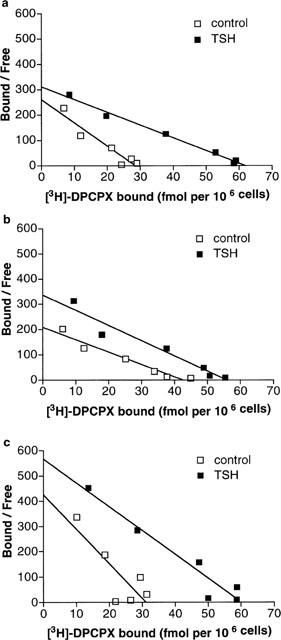
The effect of TSH on [3H]-DPCPX binding in TSH-deprived FRTL-5 cells. The cells were grown without TSH for 3 days and treated with or without 1 mu ml−1 TSH for 24 h. (a). Binding performed in suspension without adenosine deaminase. Saturation analysis for TSH-treated cells gave a Bmax value of 62±9 fmol per 106 cells compared with 28±4 fmol per 106 cells in TSH-depleted control cells (P<0.05). The KD values did not change (0.19 nM in TSH-treated cells and 0.11 nM in control cells). Data are means from four separate experiments. (b). Binding performed in the presence of 1 u ml−1 adenosine deaminase. The values for Bmax in TSH-depleted control cells and TSH-treated cells were 45±3 fmol per 106 cells and 56±4 fmol per 106 cells, respectively (P<0.05). The KD values were 0.26±0.02 nM for control cells and 0.18±0.02 nM for TSH-treated cells. Data are means from five separate experiments. (c). Binding performed on cells grown 7 days in the presence of 3 u ml−1 adenosine deaminase. For details, see Methods. The values for Bmax in TSH-depleted control cells and TSH-treated cells were 30±2 fmol per 106 cells and 58±4 fmol per 106 cells, respectively (P<0.05). The KD values were 0.05±0.02 nM for control cells and 0.09±0.03 nM for TSH-treated cells. Data are means from three separate experiments.
The TSH-evoked increase in Bmax was smaller in cell membranes compared to intact cells. In membrane binding studies adenosine deaminase was constantly present in the assay to degrade adenosine generated from ATP released from broken cells. When whole cells were treated with adenosine deaminase (1 u ml−1) in the binding assay (Figure 3b), the Bmax of TSH-depleted cells increased from 28±4 fmol per 106 cells to 45±3 fmol per 106 cells (n=5, P<0.05). The Bmax for TSH-treated cells in the presence of adenosine deaminase (56±4 fmol per 106 cells, n=5) corresponds to that measured in the absence of adenosine deaminase. The TSH-induced increase in [3H]-DPCPX binding (from 45 to 56 fmol per 106 cells, P<0.05) in the presence of adenosine deaminase in the assay is of almost identical magnitude (24%) to that observed in the membrane preparation (36%). The KD-values in the presence of adenosine deaminase in the assay were 0.26±0.02 nM in control cells and 0.18±0.02 nM in TSH-treated cells.
If a high concentration of adenosine deaminase (3 u ml−1) was added to the growth medium 72 h before depletion of TSH, and maintained during the experiment (Figure 3c), Bmax-values (30±2 fmol per 106 cells for control and 58±4 fmol per 106 cells for TSH-treated cells, n=3) were almost identical with the Bmax-values obtained without adenosine deaminase. On the other hand, the KD-values were lower; 0.05±0.02 nM in control cells and 0.09±0.03 nM in TSH-treated cells.
The amount of adenosine in the culture medium
Adenosine was found in the culture medium of FRTL-5 cells. The medium obtained from TSH-deprived cells contained significantly higher amounts of adenosine (52±2 nM, n=3) compared to medium obtained from TSH-treated cells (16±2 nM, n=3). Adenosine deaminase (1 u ml−1 for 24 h) effectively removed adenosine from the medium (6±1 nM in adenosine deaminase-treated medium compared to 52±2 nM in control, TSH-depleted cells, n=4).
Effect of TSH on the A1-receptor mRNA level
To analyse if the increased [3H]-DPCPX binding after TSH-treatment correlate with the A1-receptor mRNA level we used in situ mRNA hybridization. Micrographs of control (TSH-depleted cells) and TSH-treated cells (1 mu ml−1 of TSH for 24 h) showed that TSH increased A1 receptor mRNA labelling (Figure 4a). This was also evident when the number of grains per cell was counted. The TSH-treatment decreased the percentage of cells with low labelling (<10 grains per cell) and increased the percentage of cells with higher grain density (Figure 4b). Background levels were measured and subtracted in each case.
Figure 4.
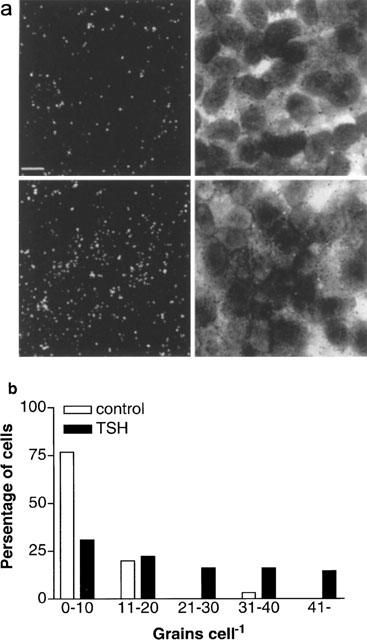
Treatment with TSH (1 mu ml−1) for 24 h increases the percentage of cells expressing A1 receptor mRNA. (a). Emulsion autoradiograms showing grains corresponding to A1 receptor mRNA. Dark field and light field micrographs of control cells (upper row) and TSH-treated cells (lower row). Scale bar corresponds to 10 μm. (b). The distribution of the number of grains present per cell in TSH-deprived control cells and in TSH-treated cells. The grains were counted in seven different 4-chamber glass slides having control and treated cells on the same slide. Background levels were measured and subtracted in each case. At least 100 cells were counted per treatment. The results are pooled from two different sets of experiments.
The effect of A1 receptor number on inhibition of adenylyl cyclase
Adenylyl cyclase was activated by 1 μM forskolin both in TSH deprived cells and cells constantly grown in TSH-containing medium. The adenosine A1 receptor agonist CHA (10 pM–1 μM) was used to inhibit the action of forskolin. In cells grown in TSH-containing medium the potency of CHA to inhibit the forskolin-induced cyclic AMP accumulation was increased (Figure 5). The IC50 for CHA changed from 2.05 nM (1.26–3.33 nM 95% confidence interval, n=5) in TSH-deprived cells to 0.14 nM (0.08–0.26 nM 95% confidence interval, n=5) in cells grown with TSH (P<0.05). The basal levels of cyclic AMP were; 7.7±1 pmol per 106 cells in cells grown in TSH-containing medium and 6.4±0.8 pmol per 106 cells in cells grown without TSH. In both preparations the maximal 1 μM forskolin-induced increase in total cyclic AMP was similar; 40.4±4 pmol per 106 cells in cells grown with TSH and 40.0±8 pmol per 106 cells in cells grown without TSH.
Figure 5.
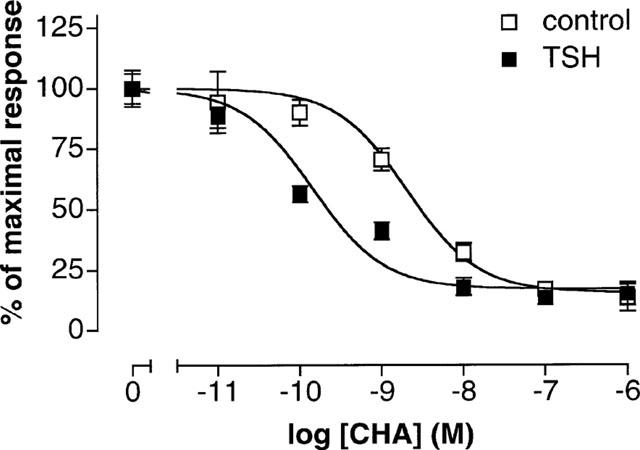
CHA-mediated inhibition of cyclic AMP formation. The figure shows the ability of CHA to inhibit the formation of cyclic AMP induced by 1 μM forskolin in TSH-deprived control cells with an IC50 of 2.05 nM and in cells constantly grown with 0.3 mu ml−1 TSH with an IC50 of 0.14 nM. Forskolin induced a 525% increase in cellular cyclic AMP in TSH-deprived cells and a 425% increase in cells constantly grown with TSH. The basal values were 6.4 pmol per 106 cells and 7.7 pmol per 106 cells, respectively. Data are mean±s.e.mean from five separate experiments.
Discussion and conclusions
In order to study the regulation of adenosine A1 receptors in intact thyroid FRTL-5 cells we have characterized and quantified the receptors using the selective A1 receptor ligand [3H]-DPCPX (Lohse et al., 1987). We have mostly studied [3H]-DPCPX binding to trypsinized FRTL-5 cells in suspension in the presence or absence of adenosine deaminase and confirmed the key results on membrane preparations. The binding of [3H]-DPCPX to cells in suspension was saturable, reversible and of high affinity. The Bmax value for cells grown in the presence of TSH corresponds to approximately 47,000 binding sites per cell. This number is lower than the number of A1 receptors obtained in the DDT1 MF-2 smooth muscle cell line (110,000 sites per cell) (Gerwins et al., 1990), but roughly of the same magnitude as that reported in cultured rat heart cells (14,000–40,000 sites per cell) (El-Ani et al., 1994; Martens et al., 1988). The previous studies on adenosine A1 receptors in FRTL-5 cells on monolayer cultures (Frauman & Moses, 1989; Kosugi et al., 1989; Nayfeh et al., 1989; Okajima et al., 1989) are not strictly comparable to our results due to differences in binding assays.
The present study showed that withdrawal of TSH from the culture medium decreased the number of [3H]-DPCPX binding sites in FRTL-5 cells. Acute stimulation with TSH (1 mu ml−1 for 24 h) after TSH-depletion increased the receptor number close to that detected in cells constantly grown in the presence of TSH. The effect of acute TSH stimulation was confirmed in membrane preparations isolated from TSH-deprived cells and cells treated with 1 mu ml−1 TSH for 24 h. The in situ labelling of A1 receptor mRNA showed that TSH was able to increase the level of A1 receptor mRNA in FRTL-5 cells. These results are in agreement with [3H]-DPCPX binding studies using cells in suspension and membrane preparations, suggesting that new adenosine A1 receptors were indeed synthetized after TSH stimulation.
Thyrotropin had a smaller effect on A1 receptor number in the membrane binding assay compared with that in the whole cell assay. When adenosine deaminase (present in membrane binding assay) was added to the whole cell assay buffer, the results were very similar in both assays. This finding, and the adenosine deaminase-evoked increase in Bmax in the control cells, suggests that part of the TSH-induced increase seen when intact cells were studied could be due to changes in the amount of adenosine competing with [3H]-DPCPX during the binding assay. Indeed, TSH treatment does reduce the levels of adenosine in the medium. However, changes in the levels of adenosine is not the entire explanation for the present findings, since part of the TSH-induced increase occurred also with adenosine deaminase present during the binding assay. Adenosine deaminase is not generally added into the A1 receptor binding assays on intact FRTL-5 cells (Frauman & Moses, 1989; Kosugi et al., 1989; Okajima et al., 1989). However, in the light of our results endogenous adenosine might interfere with the interpretation of binding data. Thus, the use of adenosine deaminase should be considered also in binding assays made on whole cell preparations.
It may be argued that the effect of TSH is related to a decreased down-regulation of A1 receptors. A prolonged agonist treatment is known to down-regulate A1 receptors (Ciruela et al., 1997; Green, 1987; Palmer & Stiles, 1997) and higher concentration of adenosine was found in the culture medium of TSH-depleted cells compared to that found in the culture medium of TSH-treated cells. To avoid the long-term effect of endogenous adenosine on receptor number we treated the cells with 3 u ml−1 adenosine deaminase starting 72 h prior to TSH depletion. Surprisingly, the Bmax-values were comparable with those obtained without adenosine deaminase. Hence, it can be concluded that changes in medium adenosine does not explain the adaptive changes in A1 receptor number that are caused by TSH treatment. Instead TSH treatment appears to regulate A1 receptor expression secondarily to changes in transcription. By contrast, the endogenous agonist can affect receptor number without any changes in receptor mRNA (Johansson et al., 1993).
Our results indicate that TSH up-regulated the number of adenosine A1 receptors. Activation of adenosine A1 receptors regulate TSH-mediated cyclic AMP signals via inhibition of adenylyl cyclase, thus modifying TSH-mediated responses (Dere & Rapaport, 1986; Moses et al., 1989; Tramontano et al., 1988). We showed that the potency of the A1 receptor agonist CHA to inhibit cyclic AMP formation induced by forskolin was increased in TSH-treated cells. The leftward shift of the dose response curve to an adenosine analogue as a consequence of an increase in A1 receptor number is similar to, but larger than, that reported earlier for DDT1 MF-2 cells treated with a glucocorticoid (Gerwins & Fredholm, 1991). Together these findings show that the functional consequences of the actions of endogenous adenosine acting at A1 receptors can be strongly affected by changes in receptor number following alterations in the hormonal status.
In summary, we have studied the regulation of A1 receptors by TSH in FRTL-5 rat thyroid cells. Thyrotropin increased [3H]-DPCPX binding in intact cells, and in membranes prepared from TSH-treated cells. In addition, TSH-treatment increased A1 receptor mRNA in these cells. As activation of A1 receptors inhibits TSH-mediated cyclic AMP signalling our results suggest a regulatory feedback mechanism between signalling via TSH receptors and adenosine A1 receptors.
Acknowledgments
We are grateful to Karin Lindström for her technical assistance. This study was supported by the Ella and Georg Ehrnrooth Foundation, the Foundation of Turku University, the Liv och Hälsa Foundation, the Magnus Ehrnrooth Foundation, the Nordic Academy for Advanced Study, the Sigrid Juselius Foundation, the Emil Aaltonen Foundation, and by the Swedish Medical Research Council (Project no 2553 and 12707) which are gratefully acknowledged.
Abbreviations
- CHA
N6-cyclohexyladenosine
- DPCPX
1,3-dipropyl-,8-cyclopentyl xanthine
- FRTL-5
Fisher rat thyroid cells
- Ro 20-1724
4-(3-Butoxy-4-methoxybenzyl)-2-imidazolidinone
References
- AMBESI-IMPIOMBATO F.S., PARKS L.A.M., COON H.G. Culture of hormone-dependent functional epithelial cells from rat thyroid. Proc. Natl. Acad. Sci. U.S.A. 1980;77:3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRUELA F., SAURA C., CANELA E.I., MALLOL J., LLUIS C., FRANCO R. Ligand-induced phosphorylation, clustering, and desensitization of A1 adenosine receptors. Mol. Pharmacol. 1997;52:788–797. doi: 10.1124/mol.52.5.788. [DOI] [PubMed] [Google Scholar]
- DERE W.H., RAPAPORT B. Control of growth in cultured rat thyroid cells. Mol. Cell. Endocrinol. 1986;44:195–199. doi: 10.1016/0303-7207(86)90124-3. [DOI] [PubMed] [Google Scholar]
- EL-ANI D., JACOBSON K., SHAINBERG A. Characterization of adenosine receptors in intact cultured heart cells. Biochem. Pharmacol. 1994;48:727–735. doi: 10.1016/0006-2952(94)90050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAUMAN A.G., MOSES A.C. The A1 adenosine receptor antagonist 1,3, dipropyl-8-cyclopentylxantine (DPCPX) displays adenosine agonist properties in the FRTL-5 thyroid cell line. Biochem. Biophys. Res. Commun. 1989;159:355–362. doi: 10.1016/0006-291x(89)92446-7. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., SOLLEVI A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J. Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERWINS P., FREDHOLM B.B. Glucocorticoid receptor activation leads to up-regulation of adenosine A1 receptors and down-regulation of adenosine A2 responses in DDT1 MF-2 smooth muscle cells. Mol. Pharmacol. 1991;40:149–155. [PubMed] [Google Scholar]
- GERWINS P., NORDSTEDT C., FREDHOLM B.B. Characterization of adenosine A1 receptors in intact DDT1 MF-2 smooth muscle cells. Mol. Pharmacol. 1990;38:660–666. [PubMed] [Google Scholar]
- GREEN A. Adenosine receptor down-regulation and insulin resistance following prolonged incubation of adipocytes with an A1 adenosine receptor agonist. J. Biol. Chem. 1987;262:15702–15707. [PubMed] [Google Scholar]
- JENKINSON D.H.Classical approaches to the study of drug-receptor interactions Textbook of receptor pharmacology 1996New York: CRC Press; 3–62.ed. Foreman, J.C. & Johansen, T. pp [Google Scholar]
- JIN S., HORNICEK F.J., NEYLAN D., AKARIJA M., MCKENZIE J.M. Evidence that adenosine 3′,5′-monophosphate mediates stimulation of thyroid growth in FRTL-5 cells. Endocrinology. 1986;119:802–810. doi: 10.1210/endo-119-2-802. [DOI] [PubMed] [Google Scholar]
- JOHANSSON B., AHLBERG S., VAN DER PLOEG I., BRENÉ S., LINDEFORS N., PERSSON H., FREDHOLM B.B. Effect of long-term caffeine treatment on A1 and A2 adenosine receptor binding and on mRNA levels in rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1993;347:407–414. doi: 10.1007/BF00165391. [DOI] [PubMed] [Google Scholar]
- KANASAKI M., MATSUBARA H., MURASAWA S., MASAKI H., NIO Y., INADA M. cAMP responsive element-mediated regulation of the gene transcription of the alpha 1B adrenergic receptor by thyrotropin. J. Clin. Invest. 1994;94:2245–2254. doi: 10.1172/JCI117587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSUGI S., MORI T., IWAMORI M., NAGAI Y., IMURA H. α2- and β-adrenergic receptors and adenosine A1 receptor of FRTL-5 rat thyroid cells in relation to fucosyl GM1 ganglioside. Endocrinology. 1989;124:2707–2710. doi: 10.1210/endo-124-6-2707. [DOI] [PubMed] [Google Scholar]
- LIBERT F., PARMENTIER M., LEFORT A., DINSART C., VAN SANDE J., MAENHAUT C., SIMONS M., DUMONT J., VASSART G. Selective amplification and cloning of four new members of the G protein-coupled receptor family. Science. 1989;244:569–572. doi: 10.1126/science.2541503. [DOI] [PubMed] [Google Scholar]
- LIBERT F., SCHIFFMANN S., LEFORT A., PARMENTIER M., GERARD C., DUMONT J., VANDERHAEGEN J., VASSART G. The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991;10:1677–1682. doi: 10.1002/j.1460-2075.1991.tb07691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOHSE M., KLOTZ K.-N., LINDENBORN-FOTINOS J., REDDINGTON M., SCHWABE U., OLSSON R. 8-cyclopentyl-1,3-dipropylxanthine (DPCPX)- a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- MAHAN L., MCVITTIE L., SMYK-RANDALL E., NAKATA H., MONSMA J.F., GERFEN C., SIBLEY D. Cloning and expression of an A1 adenosine receptor from rat brain. Mol. Pharmacol. 1991;40:1–7. [PubMed] [Google Scholar]
- MARTENS D., LOHSE M., SCHWABE U. [3H]-8-Cyclopentyl-1,3-dipropylxanthine binding to A1 adenosine receptors of intact rat ventricular myocytes. Circ. Res. 1988;63:613–620. doi: 10.1161/01.res.63.3.613. [DOI] [PubMed] [Google Scholar]
- MOSES A.C., TRAMONTANO D., VENEZIANI B.M., FRAUMAN A.G. Adenosine has divergent effects on deoxyribonucleic acid synthesis in FRTL-5 cells: inhibition of thyrotropin-stimulated and potentiation if insulin-like growth factor-I-stimulated thymidine incorporation. Endocrinology. 1989;125:2758–2765. doi: 10.1210/endo-125-5-2758. [DOI] [PubMed] [Google Scholar]
- NAYFEH S., BERMAN M., THOMAS C., JrAttenuation of TSH-stimulated cAMP accumulation by adenosine and catecholamines FRTL-5 today 1989Amsterdam: Elsevier Science Publishers B.V; 113–116.ed. Ambesi-Impiombato, F. & Perrid, H. pp [Google Scholar]
- NORDSTEDT C., FREDHOLM B.B. A modification of a protein-binding method for rapid quantification of cAMP in cell-culture supernatants and body fluid. Anal. Biochem. 1990;189:231–234. doi: 10.1016/0003-2697(90)90113-n. [DOI] [PubMed] [Google Scholar]
- OKAJIMA F., AKBAR M., MAJID M.A., SHO K., TOMURA H., KONDO Y. Genistein, an inhibitor of protein tyrosine kinase, is also a competitive agonist for P1-purinergic (adenosine) receptor in FRTL-5 thyroid cells. Biochem. Biophys. Res. Commun. 1994;203:1488–1495. doi: 10.1006/bbrc.1994.2353. [DOI] [PubMed] [Google Scholar]
- OKAJIMA F., SATO K., NAZAREA M., SHO K., KONDO Y. A permissive role of pertussis toxin substrate G-protein in P2-purinergic stimulation of phosphoinositide turnover and arachidonate release in FRTL-5 thyroid cells. J. Biol. Chem. 1989;264:13029–13037. [PubMed] [Google Scholar]
- PALMER T.M., STILES G.L. Structure-function analysis of inhibitory adenosine receptor regulation. Neuropharmacology. 1997;36:1141–1147. doi: 10.1016/s0028-3908(97)00128-7. [DOI] [PubMed] [Google Scholar]
- SHO K., NARITA T., OKAJIMA F., KONDO Y. An adenosine receptor agonist-induced modulation of TSH-dependent cell growth in FRTL-5 thyroid cells mediated by inhibitory G protein Gi. Biochemie. 1999;81:341–346. doi: 10.1016/s0300-9084(99)80079-0. [DOI] [PubMed] [Google Scholar]
- SHO K., OKAJIMA F., MAJID M.A., KONDO Y. Reciprocal modulation of tyrotropin actions by P1-purinergic agonist in FRTL-5 cells. Inhibition of cAMP pathway and stimulation of phospholipase C-Ca2+ pathway. J. Biol. Chem. 1991;266:12180–12184. [PubMed] [Google Scholar]
- TRAMONTANO D., MOSES A.C., VENEZIANI B.M., INGBAR S.H. Adenosine 3′,5′-monophosphate mediates both the mitogenic effect of thyrotropin and its ability to amplify the response to insulin-like growth factor I in FRTL-5 cells. Endocrinology. 1988;122:127–132. doi: 10.1210/endo-122-1-127. [DOI] [PubMed] [Google Scholar]


