Abstract
Angiogenesis is reportedly enhanced by prostaglandins (PGs). In the present study, we investigated whether or not cyclo-oxygenase (COX)-2 mediated angiogenesis in chronic and proliferate granuloma.
In rat sponge implants, angiogenesis was gradually developed over a 14-day experimental period as granuloma formed. This angiogenesis was enhanced by topical injections of human recombinant basic fibroblast growth factor (bFGF).
In sponge granuloma, mRNA of COX-1 was constitutively expressed, whereas that of COX-2 was increased with neovascularization in parallel with that of vascular endothelial growth factor (VEGF).
Topical injections of bFGF increased the expression of COX-2 mRNA. bFGF-stimulated angiogenesis was inhibited by indomethacin or selective COX-2 inhibitors, NS-398, nimesulide, and JTE-522.
The levels of PGE2 and 6-keto-PGF1α in the sponge granuloma were increased with bFGF 13 fold and 9 fold, respectively, and these levels were markedly reduced by NS-398.
The expression of VEGF mRNA in the granuloma was also enhanced by bFGF, and was reduced by NS-398.
Topical injections of PGE2 and beraprost sodium, a PGI2 analogue, increased the expression of VEGF mRNA, with angiogenesis enhancement.
The enhanced angiogenesis by bFGF was significantly inhibited by topical injections of VEGF anti-sense oligonucleotide.
These results suggested that COX-2 may enhance bFGF-induced neovascularization in sponge granuloma by PG-mediated expression of VEGF, and that a COX-2 inhibitor would facilitate the management of conditions involving angiogenesis.
Keywords: Angiogenesis, COX-2, prostaglandin, vascular endothelial growth factor, antisense oligonuculeotide
Introduction
Angiogenesis is a process involved in several physiological events including embryonic development, female reproductive cycle placentation and wound repair. It also plays a part in various pathological conditions such as tumour growth, diabetic retinopathy and rheumatoid arthritis (Colville-Nash & Scott, 1992; Folkman, 1971; Michaelson, 1948; Peacock et al., 1992; Wise, 1956). Angiogenesis is a very complex multistep process involving a variety of biologically active substances. Of them, the prostaglandins (PGs), which can induce several growth factors and proliferation of endothelial cells in vitro and in vivo (Form & Auerbach, 1983; Ziche et al., 1982), may be crucial for enhancing neovascularization.
PGs are formed from cell membrane phospholipids through the action of several enzymes. Recent findings concerning the inducible cyclo-oxygenase (COX), COX-2, in acute and exudative inflammatory sites (Seibert et al., 1994), but not in the non-inflamed sites, are facilitating the study of inhibitors for this isozyme in many pharmaceutical companies (Wallance, 1999). In such companies, it is hoped that COX-2 selective inhibitors will be non-steroidal anti-inflammatory drugs (NSAIDs) without the side effects of classical COX inhibitors, such as gastrointestinal or renal injury. However, COX-2 was reported to be induced in chronic and proliferative inflammatory sites (Crofford et al., 1994; Masferrer et al., 1994), in the repair stage of gastric ulcer (Mizuno et al., 1997), and in kidneys with renovascular hypertension (Hartner et al., 1998). Thus, the idea that inflammation can be treated with a selective COX-2 inhibitor without adverse effects is simplistic.
Recently, Tujii et al. (1998) reported that COX-2 overexpressing cells, produced PGs, proangiogenic factors, and stimulated both endothelial migration and tube formation in vitro. They further studied the regulation of angiogenesis induced by colon cancer cells. They concluded that cyclo-oxygenase regulated colon carcinoma-induced angiogenesis, and that COX-2 modulated the production of angiogenic factors by colon cancer cells. In another experiment, the angiogenesis observed around the angiosarcoma-like tumours, which was derived from COX-1-overexpressed immortalized ECV endothelial cells, was not inhibited by indomethacin, suggesting little contribution of COX-1 to tumour-induced angiogenesis (Narko et al., 1997). These results strongly suggested the involvement of COX-2 to the tumour-related angiogenesis. In chronic and proliferative inflammation and during the repair stage of an ulcer, angiogenesis is always observable. However, the direct relationship between COX-2 induction and angiogenesis in these pathological conditions has not been fully investigated especially in vivo.
In the present report, we used the quantitative in vivo angiogenesis model, and we show that inducible COX-2-mediated PG formation takes part in the augmentation of angiogenesis through the induction of a potent angiogenesis inducer, vascular endothelial growth factor (VEGF), in granuloma tissues in a sponge implantation model.
Methods
The rat sponge model
Circular sponge discs (5 mm thick, 1.3 cm in diameter, and weighting 14.1+0.1 mg, n=6) were prepared from a sheet of polyether polyurethane foam by use of a wad pouch. The discs were soaked in 70% ethanol for 3 h and then rinsed in sterile distilled water. After drying at reduced pressure, all discs were exposed with ethylene oxide gas. Under light ether anaesthesia, they were then implanted into the subcutaneous tissue of the backs of male Sprague-Dawley strain rats (specific pathogen free, 8 weeks old, weighing 280–315 g), purchased from SLC (Hamamatsu, Japan). All rats were kept in the room which had constant temperature (25+1°C) and humidity (60+5%) throughout the experimental periods, and were allowed to access to normal chow and water freely. The present study was performed in accordance with the guidelines for animal experiments of Kitasato University School of Medicine.
Neovascularization was assessed by measuring the concentration of haemoglobin in the granulation tissues together with the enclosed sponge implants (Majima et al., 1997). Briefly, the granulation tissues with sponge were taken immediately after the death induced by the intraperitoneal administration of excess doses of sodium pentobarbital. After the granulation tissues including sponge were weighted, they were cut into several pieces with scissors. Distilled water four times the weight of the sample granulation tissues was added to each sample, which was then homogenized with a Polytrone homogenizer (Kinematica GMBH, Luzern, Switzerland). The haemoglobin concentration in the supernatant after centrifugation at 5000×g was determined using a haemoglobin assay kit (Hemoglobin B Testwako, Wako Pure Chemical Industries Ltd, Osaka, Japan). The concentrations of haemoglobin in the granulation tissues were expressed as mg g wet tissue−1. The detection limit of this assay was 0.1 mg g wet tissue−1. The coefficient of variation, when determined the sample with haemoglobin concentration of 1 mg g wet tissue−1, was less than 5% (n=10).
To enhance the neovascularization of the sponge implants, basic fibroblast growth factor (bFGF, 100 or 1000 ng sponge−1 day−1), epidermal growth factor (EGF, 1000 ng sponge−1 day−1), or their vehicle solution (physiological saline) were injected daily (once, 0.05 ml sponge−1) directly into the sponge implants using a 26G needle under light ether anaesthesia. Prostaglandin (PG) E2 or a PGI2 derivative, beraprost sodium was also topically administered (15, 50 ml sponge−1 day−1, once a day). Since the stock solution of PGs was prepared with 100% ethanol, and PG solution for topical administration was made immediately before the injection by 100 fold dilution of stock solution with physiological saline, we administered (0.05 ml sponge−1) 1% v v−1 ethanol in physiological saline as vehicle control.
RT–PCR analysis of cyclo-oxygenase isozymes and VEGF
The granulation tissues were removed from the rats, and were put into the liquid nitrogen. The freezed tissues were pulverized within a stainless cylinder, cooled with liquid nitrogen. Total RNA was extracted with 2 ml ISOGEN solution (Nippon Gene, Tokyo, Japan), and cDNA was synthesized from 1 mg of the extracted mRNA using 1.6 mg of oligop(dT) 15 primer and 20 units of AMV reverse transcriptase (Boehringer Mannheim, Mannheim, Germany). PCR was performed in 20 ml of 20 mM Tris-HCl, 50 mM KCl-1.5 mM MgCl2 buffer (pH 8.0) containing cDNA from 50 ng of total RNA, 5 mM tryptophan, 12.5 pmol Taq DNA polymerase (Gibco BRL, Gaithersburg, MD, U.S.A.), 5 nmol of dNTP mix, and 12.5 pmol of forward and reverse primers for COX-1, COX-2, and VEGF. RT–PCR for glyceral-dehydrogenase-3-phosphate dehydrogenase (G3PDH) was also performed using its primer pair. The resulted reactants were applied to agarose electrophoresis, and the products were stained with ethidium bromide. The placenta as a positive control for VEGF RT–PCR was isolated from the female pregnant Sprague-Dawley strain rats.
Histological and immunohistochemical studies
The granulating tissues were immediately fixed with 4% paraformaldehyde in 0.1 M phosphate buffer solution (pH 7.4). After fixation, the tissues were dehydrated with a graded series of ethanol, and embedded in paraffin. The sections (4 mm) from the paraffin-embedded tissues were mounted on glass slides, deparaffinized with xylene, and then placed in cold (4°C) acetone for immunostaining.
The procedure for staining dehydrated sections using Vectastain ABC Kit (Vector Lab., Burlingame, CA, U.S.A.) was as follows: (1) incubation with diluted normal horse serum; (2) incubation with diluted (×200) rabbit anti-murine-COX-2 anti-serum (Cayman Chemical, Ann Arbor, MI, U.S.A.) or diluted (×500) rabbit anti-rat VEGF anti-serum (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.); (3) incubation with biotinylated anti-rabbit IgG (7 mg ml−1); (4) incubation with avidin-biotin-peroxidase complex; (5) placement in 0.02% 3,3′-diaminobenzidine (DAB) and 0.3% nickel ammonium sulphate in 50 mM Tris-HCl buffer (pH 7.4); (6) colour development by immersion in the DAB solution containing 0.005% H2O2; (7) examination and photography with a light microscope. The cross reactivity of anti COX-2 anti-serum to COX-1 was less than 1%, and that of anti VEGF anti-serum to bFGF or EGF was also less than 1%.
Some sections were stained with van Gieson's methods (Majima et al., 1997).
Prostaglandin levels in sponge granulation tissues
Three minutes before exanguination of rats, indomethacin (10 mg kg−1) was intravenously administered to block the artificial formation of PGs under light ether anaesthesia. Indomethacin was first dissolved in absolute ethanol at concentration of 100 mg ml−1, and was diluted 10 fold with physiological saline just before injection. Diluted solution was administered (1 ml kg−1). The granulation tissues were removed from the back of rats with the skin, and were immediately put into the liquid nitrogen. After the cutting the extra skin around the granulation tissues, the freezed granulation tissues including the sponge were pulverized within stainless cylinder cooled with liquid nitrogen. The powder of the granulation tissues were immediately put into the 10 ml of 80% ethanol without thawing, and were mixed well with homogenizer. After the centrifugation at 1500×g for 30 min at 4°C, the supernatant was dried with reduced pressure. The residue was resolved with distilled water (5 ml), and was acidified to pH3 with addition of 1NHCl. Acidified samples were applied to the Sep-PakC18, and PG fractions were extracted with ethylacetate after washing the column with distilled water and methanol (Suzuki et al., 1990). The extract was dried under the reduced pressure, and the residue was resolved with absolute ethanol to apply to HPLC. The HPLC fraction containing PGE2 and that containing 6-keto-PGF1α were evaporated under the reduced pressure, and were dissolved with assay buffers. The levels of PGE2 and 6-keto-PGF1α were determined with respective ELISAs (Cayman Chemical). The detection limit of these PGs was 35 pg sponge−1. The coefficient of variation of each ELISA was less than 10%.
Administration of COX inhibitors
To the rats receiving bFGF (100 or 1000 ng sponge−1 day−1) or EGF (1000 ng sponge−1 day−1), or to the rats without growth factor treatment, COX inhibitors were administered orally. From the first day of the implantation, indomethacin in a suspension of 3 mg ml−1 in 5% gum arabic in distilled water was given three times a day (every 8 h) at dose of 3 mg kg−1. A selective COX-2 inhibitor, NS-398, was also administered, as a suspension of 3 or 30 mg ml−1 in 5% gum arabic in distilled water, three times (3 or 30 mg kg−1 8 h−1) a day during the experiments. Another COX-2 inhibitor, Nimesulide, suspended at the concentration of 3 mg ml−1 in 5% gum arabic in distilled water, was orally administered (3 mg kg−1 8 h−1, three times a day) to rats under bFGF treatment. Another COX-2 selective inhibitor, JTE-522 was administered three times (30 mg kg−1 8 h−1, suspended at the concentration of 30 mg ml−1 in 5% gum arabic in distilled water) a day to the rats treated with bFGF. For vehicle control rats, only vehicle solution (5% gum arabic in distilled water) was administered (1 ml kg−1) orally three times a day.
Administration of anti-sense oligonucleotide against VEGF
To the rats receiving bFGF (1000 ng sponge−1 day−1) for 4 days, anti-sense oligonucleotide against VEGF, which consists 20 nucleotide residues of 5′-terminal of VEGF gene were administered (agagcagaaagttcatggtt, 25 nmol sponge−1) topically twice a day for 4 days. For control rats, a randomized control oligonucleotide (gtactgatagaatgagtagc), which has the same nucleotide components but has a randomized composition, was administered topically. Another control rats were given vehicle solution of oligonucleotide (physiological saline, 0.1 ml sponge−1, twice a day) for 4 days.
Chemicals and other materials
EGF and bFGF was purchased from Genzyme Corp. (Cambridge, MA, U.S.A.). Indomethacin was a gift from Merck (Rahway, NJ, U.S.A.), and NS-398 (N-[2-(cyclohexyloxy)-4-nitrophenyl]methanesulphonamide) was purchased from Cayman Chemical (Ann Arbor, MI, U.S.A.). Nimesulide (N-(4-nitro-2-phenoxyphenyl)-methane-sulphonilamide) was synthesized in our laboratory. JTE-522 (4-[4-cyclohexyl-2-methyloxazol-5-yl]2-fluorobenzenesulphonamide) was also a gift from Japan Tobacco Inc. (Osaka, Japan). The hemoglobin assay kit was obtained from Wako Pure Chemical Industries Ltd. (Osaka, Japan). All other substances used were of analytical grade, and were purchased from commercial sources.
Statistical analysis
All values were expressed as the means±s.e.mean. For the results from multiple groups, factorial ANOVA was used to evaluate the significance of difference, followed by Scheffe's test. A P value of less than 0.05 was considered to be significant.
Results
When circular sponge discs were implanted into the rat subcutaneous tissues, the granuloma tissues around the implants, which were constituted mainly of collagen fibres, developed, and appeared on van Gieson's staining as reddish fibres. Figure 1 shows a typical histological section of the granuloma formed around the sponge implants 4 days after implantation. A higher dose of basic fibroblast growth factor (bFGF, 1000 ng site−1) was injected topically for 4 days, the implanted sponge was encapsulated with thick fibrous connective tissue (Figure 1b). The capsules that formed around the sponge implants were certainly thicker than those that formed without the injection of growth factor. Newly developed capillaries and more extensive vascularization were evident in areas of bFGF-stimulated fibrous encapsulation (closed triangles). Proliferated granuloma around the sponge matrix was composed of infiltration of mononuclear cells accompanied by very few polymorphonuclear cells. The number of these infiltrated cells was also predominant in bFGF-treated sponges.
Figure 1.
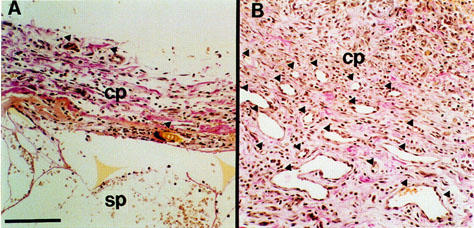
Histological sections of the sponge granuloma at day 4 with and without bFGF injections (1000 ng site−1 day−1). (a) without bFGF, (b) with bFGF. Sections were stained by the van Gieson method. Bar represents 50 μm. Closed triangles indicate newly developed blood vessels. cp, fibroblastic capsule; sp, sponge matrix.
In this model, angiogenesis was quantified by the determination of haemoglobin concentrations in the granuloma tissues. Angiogenesis occurred gradually in this sponge granuloma (Figure 2a), and direct topical injections of biologically active substances, such as bFGF, epidermal growth factor (EGF), PGE2, and beraprost sodium, an analogue of PGI2, significantly facilitated angiogenesis in a dose-dependent manner (Figure 2b,c), suggesting that the present method determining haemoglobin concentrations was enough quantitative.
Figure 2.
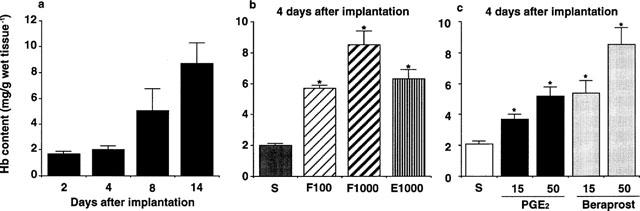
Angiogenesis in rat sponge granulomas. Two, 4, 8, and 14 days after implantation, the sponges with the surrounding granulation tissue were excised to measure the hemoglobin content as an index of angiogenesis. (a) Angiogenesis in the sponge granuloma without topical injections of growth factors or prostaglandins. (b,c) Angiogenesis after daily topical injections of saline, growth factors and prostaglandins for 4 days. (b) F100 & F1000: basic fibroblast growth factor at 100 and 1000 ng sponge−1 day−1. E1000: epidermal growth factor at 1000 ng sponge−1 day−1. (c) 15,50: doses of 15 and 50 nmole sponge−1 day−1. S: injection of 50 μl saline. Each column represents the mean±s.e.m. of eight sponges (a) and ten sponges (b,c). *P<0.05 (compared with saline-injected sponges or those from vehicle-treated rats, ANOVA).
In the granulation tissue, the expression of mRNA of COX-1, a constitutive COX, was apparently constant throughout the experimental period. By contrast, that of COX-2, an inducible COX, was gradually enhanced, and peaked at day 10 (Figure 3a). In the connective tissues in the backs of rats without sponge implantation, we could detect only COX-1 mRNA, not COX-2 mRNA (data not shown). Topical injections of bFGF or EGF increased the expression of COX-2 mRNA, but not of COX-1 mRNA (Figure 3b).
Figure 3.
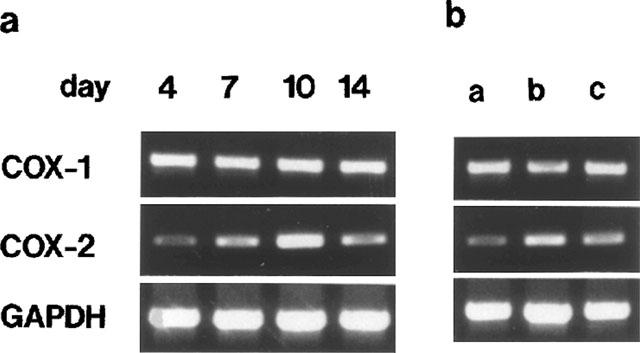
Expression of COXs in rat sponge granulomas. RT–PCR results of COX mRNA. Temporal changes were noted in COX-2 mRNA in sponge granulomas without growth factor (a). Enhanced expression of COX-2 mRNA with growth factors (b) (a, 0 ng sponge−1 day−1; b, bFGF1000 ng sponge−1 day−1; c, EGF1000 ng sponge−1 day−1).
Immunohistochemical studies revealed that the COX-2-positive cells were mainly neovascularized endothelial cells (as indicated by arrows) with a small number of spherical infiltrated inflammatory cells (Figure 4ab). The positive reaction to COX-2 was obvious in the bFGF-stimulated sponge granulomas (Figure 4b).
Figure 4.
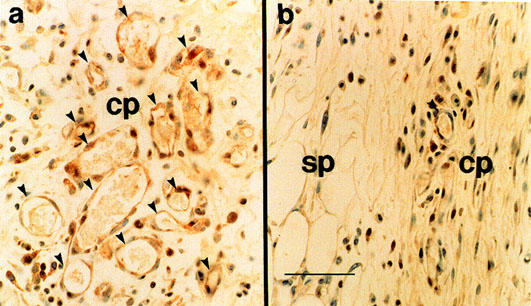
Immunohistochemical localization of COX-2 in the granulomas at day 4. (a) treated with bFGF at 1000 ng site−1 day−1; (b) vehicle-treated. Note the presence of COX-2 immunoreactivity (brown stain) in the endothelial cells in newly formed microvessels. cp: capsule of granulomas. sp: sponge matrix. Bar=25 μm.
Next, the contribution of these COX moieties to endogenous PG generation was investigated (Figure 5). Normal rat cutaneous tissue had less than 70 pg sponge−1 of PGE2 and 6-keto-PGF1α, a metabolite of PGI2. The implantation of a sponge increased these PGs by 5× and by 3×, respectively, at day 4. Topical injections of bFGF, which can selectively enhance the expression of COX-2, increased these levels markedly. Concurrent daily oral administration of NS-398, a selective COX-2 inhibitor, reduced the elevated PGE2 and 6-keto- PGF1α levels by 88% and 81%, respectively.
Figure 5.
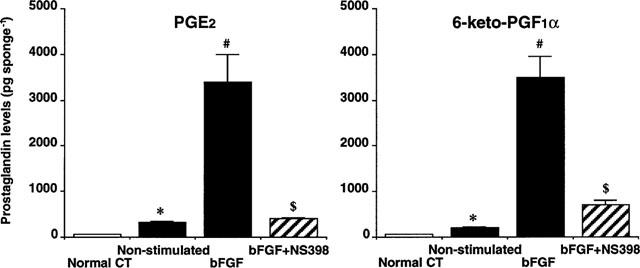
Prostaglandin levels in the granulomas at day-4. Normal CT: cutaneous tissue from normal rats. Non-stimulated: granulation tissue without growth factor stimulation. bFGF: granulation tissues with bFGF stimulation at 1000 ng sponge−1 day−1. bFGF+NS398: granulation tissues with bFGF stimulation at 1000 ng sponge−1 day−1 plus oral administration NS-398 (3 mg kg−1, three times a day). *, compared with Normal CT (P<0.05); #, compared with Non-stimulated (P<0.05); $, compared with bFGF-stimulated (P<0.05). Each column represents the mean±s.e.mean of eight sponges. ANOVA was used.
Figure 6 summarized the inhibitory effects of COX inhibitors on angiogenesis induced in the sponge granulomas. The selective COX-2 inhibitors NS-398, nimesulide, and JTE-522 administered over the experimental period caused significant reductions in angiogenesis stimulated with bFGF, showing equal efficacy to the classical COX inhibitor indomethacin, which can inhibit both COX-1 and COX-2 (Figure 6a,b). The slow angiogenesis observed in sponge granuloma, which was not treated with growth factor over the 14 days of the experiment, was also significantly reduced by prolonged oral administration of NS-398 and nimesulide (Figure 6c).
Figure 6.
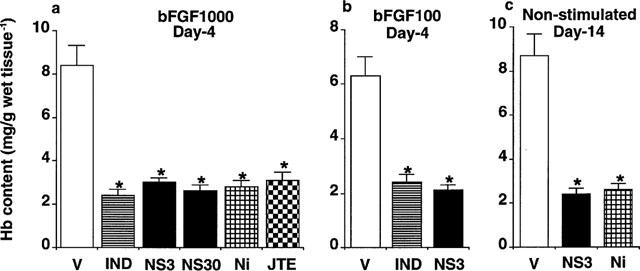
Effect of cyclooxygenase inhibitors on angiogenesis in rat sponge granulomas. Four and 14 days after implantation, the sponges with the surrounding granulation tissue were excised to measure the hemoglobin content as an index of angiogenesis. (a, b, c) Angiogenesis after administration of COX inhibitors. bFGF was topically injected into the sponge at 100 (a) or 1000 (b) ng sponge−1 day−1. COX inhibitors were administered orally. V: Vehicle only administered. IND: Indomethacin, 3 mg kg−1, three times a day. NS3 & NS30: NS-398, 3 & 30 mg kg−1, three times a day. Ni: nimesulide, 3 mg kg−1, three times a day. JTE: JTE-522, 30 mg kg−1, three times a day. Each column represents the mean±s.e.mean of eight sponges (c) and ten sponges (a, b). *P<0.05 (compared with saline-injected sponges or those from vehicle-treated rats, ANOVA).
Further, we investigated the mechanisms of angiogenesis augmentation by PGs generated by COX-2. First, we determined the time course of the expression of vascular endothelial growth factor (VEGF), which is the well-characterized secreted inducer of angiogenesis in vivo, in the granulation tissue. As Figure 7a shows, in the granulation tissue of the sponge implants, without growth factor stimulation, the mRNA level of VEGF detected increased gradually with time, and peaked at day 10. This expression of VEGF mRNA followed that of COX-2 mRNA under the present experimental conditions. The level of VEGF mRNA detected in the sponge granuloma was markedly enhanced by a 4-day treatment with bFGF (Figure 7b). This enhanced expression was strongly inhibited by the concomitant oral administration of NS-398. The topical injections of PGE2 and beraprost sodium markedly increased the expression of VEGF, strongly suggesting that PGs generated by COX-2 may enhance angiogenesis through the induction of VEGF in the granulation tissue.
Figure 7.
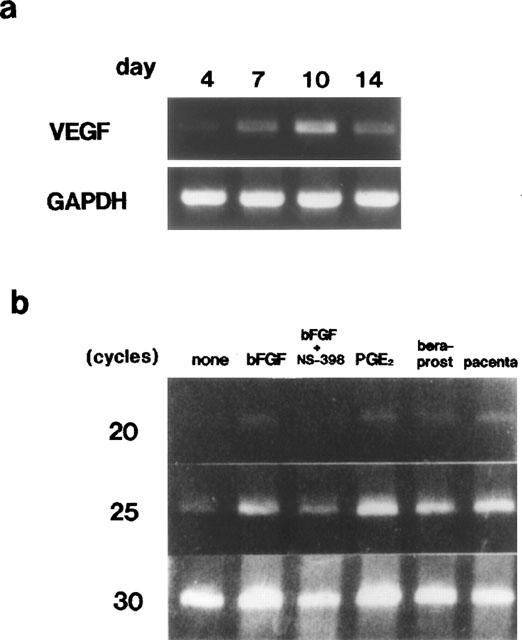
Expression of VEGF in granulomas. (a) Expression of VEGF at four time points in granulomas without growth factor stimulation, assessed by RT–PCR. (b) VEGF expression after topical injections of bFGF (1000 ng sponge−1 day−1), bFGF plus oral administration NS-398 (3 mg kg−1, three times a day), PGE2 (50 nmole sponge−1 day−1) and beraprost sodium (50 nmole sponge−1 day−1) for 4 days. The placenta isolated female pregnant Sprague-Dawley strain rats was used as a positive control for VEGF RT–PCR.
The contribution of VEGF to the enhancement of angiogenesis was further examined by means of topical injections of anti-sense oligonucleotide against VEGF (Figure 8a,b). Topical daily injections of VEGF anti-sense oligonucleotide into the sponge resulted in a marked reduction in the angiogenesis under bFGF stimulation (Figure 8a). By contrast, injections of randomized control oligonucleotide did not affect the process of angiogenesis at all (Figure 8a). The haemoglobin concentrations in the granulation tissue receiving the anti-sense oligonucleotide were significantly lower than in that receiving randomized control oligonucleotide (Figure 8b).
Figure 8.
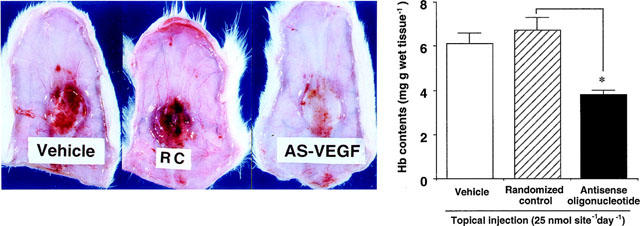
Effects of topical injections of anti-sense oligonucleotide against VEGF on angiogenesis. bFGF (1000 ng sponge−1 day−1) was topically administered for 4 days. Anti-sense oligonucleotide against VEGF (25 nmole sponge−1 day−1), randomized control oligonucleotide (25 nmole sponge−1 day−1), or vehicle solution was simultaneously administered into the sponge for 4 days. Values from anti-sense oligonucleotide-treated sponges were compared to those from randomized control oligonucleotide-treated sponges. Each column represents the mean±s.e.mean of ten sponges. *: P<0.05, analysed with ANOVA.
To ascertain what type of cells expressed VEGF, we conducted an immunohistochemical study using rat VEGF antibody (Figure 9). Positive staining was observed in the fibroblast-like cells around the neovasculized blood vessels in the sponge granulation tissue receiving randomized control oligonucleotide (Figure 9a). During the treatment with anti-sense oligonucleotide, the neovascularization was markedly suppressed, and the population of positive-stained cells was also markedly reduced (Figure 9b).
Figure 9.
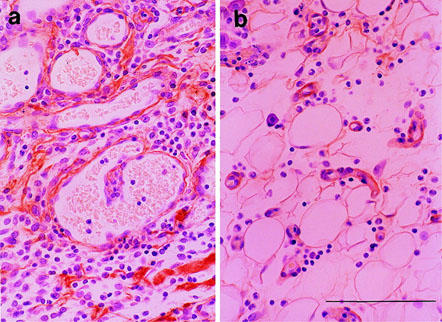
Immunohistochemical localization of VEGF in the granulomas at day 4. (a) Treated with bFGF and randomized control oligonucleotide. (b) Treated with bFGF and anti-sense oligonucleotide. Note the presence of VEGF immunoreactivity (brown) in the fibroblast-like cells around the newly formed microvessels. Bar=50 μm.
Discussion
COX is a key enzyme of PG formation, and two isozymes, COX-1 and COX-2, have been identified (Herschman, 1994). COX-2 was reported to be induced in the cultured cells in response to various stimuli, including administration of LPS, cytokines (Hla & Neilson, 1992), promoter phorbol ester (Kujubu et al., 1993), and growth factor (O'Banion et al., 1992). COX-2 is called as an inflammatory COX, since its expression was suppressed by an anti-inflammatory steroid, dexamethasone. In a chronic inflammatory model, air pouch granuloma, the induction of COX-2 was reported, and the roles of COX-2 in PG formation were discussed (Masferrer et al., 1994). Although angiogenesis was observed during the development of the granuloma formation in a chronic inflammatory model, the roles of COX-2 in angiogenesis have not been fully investigated, and still require clarification.
The assay for angiogenesis used in the present study was designed to quantify angiogenesis. Angiogenesis in the granuloma tissues can be estimated by the amount of dye injected intravenously (Andre et al., 1987). Alternatively, the haemoglobin content of the sponge granuloma tissues is also a good marker for angiogenesis in this model (Majima et al., 1997). Temporal and spatial neovascularization in the sponge granuloma tissues was also confirmed by the histology and immunohistochemical study using antibody raised for factor VIII, which is a marker of endothelial cells (data not shown). With this quantitative approach, we estimated the time course of the angiogenesis after implantation. The neovascularization with topical administrations of bFGF and EGF was enhanced in a dose-dependent manner, suggesting that the method used in the present experiment was reliable to quantify.
We first detected COX-2 mRNA in the sponge granuloma tissues by RT–PCR analysis (Figure 3a). In the repairing phase of mouse gastric ulcer, the increase in COX-2 gene expression was detected in the gastric ulcer lesions induced by acetic acid (Mizuno et al., 1997). We previously reported that expression of COX-2 was also confirmed in the endometrium, in which the thickness of tissues was increased during estrus (Shouda et al., 1995). However, there is no observation of angiogenesis in these tissues. The growth factors, such as bFGF, EGF, and transforming growth factor alpha (TGFα) were reported to increase the expressions of COX-2 in cultured cells (Angerman-Stewart et al., 1995; DuBois et al., 1995; Hamasaki & Eling 1995; Kawaguchi et al., 1995). Even in this sponge granuloma, the induction of COX-2 was enhanced by bFGF (Figure 3b). The intense positive staining obtained in immunohistochemical study for immunoreactive COX-2 in the vasculatures of granulation tissues under bFGF treatment also confirmed the increased induction of COX-2 (Figure 4). It was previously reported that the positive staining of COX was identified using a non-selective antibody for isozymes on the endothelium of vessels in the synovial tissues of the joints of rheumatoid arthritis patients (Sano et al., 1992). A more recent report using a specific COX-2 antibody revealed that the positive stain in the patient synovial tissues was also located on the endothelium (Crofford et al., 1994). These previous reports were consistent with the findings of our present study on the localization of the induced COX, COX-2. Compared with COX-2 mRNA, the expression of mRNA of COX-1 was fairly kept constant (Figure 3a), suggesting that COX-1 was constitutively expressed in this model.
Next, the contribution of these COX moieties to endogenous PG generation was investigated. The levels of PGs were artificially elevated with ease by mechanical stimulation (Suzuki et al., 1990). We therefore froze the granulation tissue, including the sponges, with liquid nitrogen, and then these frozen tissues were pulverized in a liquid nitrogen cooled steel cylinder. Lipids, including PGs, were extracted from the pulverized tissues with ethanol, and after purification with a silica mini-column and high performance liquid chromatography, PG levels were determined by ELISA. Normal rat cutaneous tissue had quite low levels of PGE2 and 6-keto-PGF1α, a metabolite of PGI2. The implantation of a sponge increased these PGs, and topical injections of bFGF, which can enhance the expression of COX-2, increased these levels markedly (Figure 5). Effect of NS-398 on levels of PGE2 and 6-keto-PGF1α indicated that both PGs were generated by COX-2 in the granulation tissues (Figure 5).
The roles of COX-2 expressed in the granulation tissues on angiogenesis were estimated by using several selective COX-2 inhibitors (Figure 6). A previous report that the angiogenesis in sponge implantation enhanced by EGF or bFGF was suppressed by a non-selective COX inhibitor, naproxen supported this possibility, since not only COX-1 but also COX-2 are inhibited by naproxen (Allison & Kowalski, 1993). In the present experiment, the non-selective inhibitor, indomethacin also markedly suppressed the stimulated angiogenesis, as determined from the haemoglobin content of the sponges (Figure 6a,b). Furthermore, the stimulated angiogenesis was markedly reduced with the selective COX-2 inhibitors. Since a lower dose of NS-398, which had the same effect as a high dose, was equipotent with indomethacin (Figure 6a,b), it is plausible that COX-2 but not COX-1 has a role in neovascularization in this model.
Further, we also investigated the mechanisms of angiogenesis augmentation by PGs generated by COX-2. It is well known that several growth factors can enhance neovascularization in vivo. Vascular endothelial growth factor (VEGF), which is also known as vascular permeability factor, is a 38-to-46-kDa heparin-binding homodimeric glycoprotein belonging to the PDGF growth factor family (Ferrara et al., 1992; Senger et al., 1990). It is one of the major proteins that are specific endothelial mitogens in vitro (Connolly et al., 1989). VEGF is the well-characterized secreted inducer of angiogenesis in vivo (Banai et al., 1994). Since prostaglandin E2 is reported to increase the expression of VEGF in osteoblasts and synovial fibroblasts in vitro (Ben-Av et al., 1995; Harada et al., 1994), VEGF may be involved in the present angiogenesis model, in which PGE2 and PGI2 were generated. The increase in VEGF expression in the granulation tissue without growth factor stimulation followed that of COX-2 (Figures 3a and 7a). The level of VEGF mRNA detected in the sponge granuloma was markedly enhanced by bFGF (Figure 7b), and was inhibited by administration of COX-2 inhibitors. These suggested that PGs generated by COX-2 may enhance angiogenesis through the induction of VEGF in the granulation tissue. The actual contribution of VEGF to the enhancement of angiogenesis was confirmed by topical injections of anti-sense oligonucleotide against VEGF. This sponge model is useful because it has two particular advantages: easy quantitative estimation of angiogenesis from the haemoglobin concentration; and easy topical injection of biologically active substances in order to test their effects on angiogenesis. The latter advantage is a result of a high concentration of active substance being maintained in the sponge matrix because this is enclosed by the granulation tissue during angiogenesis. Topical daily injections of VEGF anti-sense oligonucleotide into the sponge resulted in a marked reduction in the angiogenesis (Figure 8a). By contrast, injections of randomized control oligonucleotide did not affect the process of angiogenesis at all (Figure 8a). The contribution of VEGF in this model was also confirmed by topical injections of anti-VEGF antibody (data not shown). An immunohistochemical study using rat VEGF antibody revealed that VEGF positive cells were the fibroblast-like cells around the neovasculized blood vessels (Figure 9). The results on the localization of COX-2 and VEGF suggest that paracrine communication of the PGs formed by COX-2 mainly on the endothelial cells takes place to the fibroblast-like cells present around the neovasculized vessels, and that VEGF is induced in the fibroblast-like cells in response to PGs, augmenting the neovascularization.
We conclude that, in this in vivo angiogenesis model with chronic and proliferative characteristics of inflammation, COX-2 was induced, and that PGs generated by induced COX-2 participated in angiogenesis through the induction of vascular endothelial growth factor. Our results clearly indicate that a selective COX-2 inhibitor may be useful for controlling angiogenesis-related human pathological states, such as granulation tissue formation, which may induce the bone absorption causing the destruction or dysfunction of the joints, and is a characteristic of rheumatoid arthritis.
Acknowledgments
The authors thank Ms Michiko Ogino, Ms Keiko Nakamigawa, Mr Osamu Katsumata and Mr Masaki Soma for their skillful technical assistance. We also express thanks to Mr C.W.P. Reynolds for correcting the English of this manuscript and to Japan Tobacco Inc. (Osaka, Japan) for supplying JTE-522. This work was supported by the grant from Academic Frontier Project of The Ministry of Education, Science, Sports and Culture.
Abbreviations
- bFGF
basic fibroblast growth factor
- COX
cyclo-oxygenase
- PG
prostaglandin
- VEGF
vascular endothelial growth factor
References
- ALLISON A.C., KOWALSKI W.J.Vascular Endothelium Receptors and Transduction Mechanisms 1993New York: Plenum Press; 99–110.ed. Catravas, J.D., Gillis, C.N. & Ryan, U.S. pp [Google Scholar]
- ANDRE S.P., FAN T.-P.D., LEWIS G.P. Quantitative method for measuring adjuvant-induced granuloma angiogenesis in insulin-treated diabetic mice. Br. J. Exp. Pathol. 1987;68:755–766. [Google Scholar]
- ANGERMAN-STEWART J.A., ELING T.E., GLASGOW W.C. Prostaglandin H synthase-2 is induced in Syrian hamster embryo cells in response to basic fibroblast growth factor. Arch. Biochem. Biophys. 1995;318:378–386. doi: 10.1006/abbi.1995.1243. [DOI] [PubMed] [Google Scholar]
- BANAI S., JAKLITSCH M.T., SHOU M., LAZAROUS D.F., SCHEINOWITZ M., BIRO S., EPSTEIN S.E., UNGER E.F. Angiogenetic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth in dog. Circulation. 1994;89:2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- BEN-AV P., CROFFORD L.J., WILDER R.L., HLA T. Induction of vascular endothelial growth factor expression in synovial fibroblasts by prostaglandin E and interleukin-1: a potent mechanism for inflammatory angiogenesis. FEBS Lett. 1995;372:83–87. doi: 10.1016/0014-5793(95)00956-a. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., SCOTT D.L. Angiogenesis and rheumatoid arthritis: pathogenic and therapeutic implications. Ann. Rheum. Dis. 1992;51:919–925. doi: 10.1136/ard.51.7.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONNOLLY D.T., HEUVELMAN D.M., NELSON R., OLANDER J.V., EPPLEY B.L., DELFINO J.J., SIEGEL N.R., LEIMGRUBER R.M., FEDER J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J. Clin. Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROFFORD L.J., WIELDER R.N., RISTIMAKI A.P., SANO H., REMMERS E.F., EPPS H.R., HLA T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. J. Clin. Invest. 1994;93:1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS R.N., TSUJII M., BISHOP P., AWAD J.A., MAKITA K., LANAHAN A. Cloning and characterization of a growth factor-inducible cyclooxygenase gene from rat intestinal epithelial cells. Am. J. Physiol. 1995;266:G822–G827. doi: 10.1152/ajpgi.1994.266.5.G822. [DOI] [PubMed] [Google Scholar]
- FERRARA N., HOUCK K., JAKEMAN L., LEUNG D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endcrinol. Rev. 1992;13:18–70. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- FOLKMAN J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- FORM D.M., AUERBACH R. PGE2 and angiogenesis. Prog. Soc. Exp. Biol. Med. 1983;172:214–218. doi: 10.3181/00379727-172-41548. [DOI] [PubMed] [Google Scholar]
- HAMASAKI Y., ELING T.E. EGF and TPA stimulate de novo synthasis of PGHS-1 and PGSH-2 through different signal transduction pathways. Prostaglandins Leukot. Essent. Fatty Acids. 1995;53:225–229. doi: 10.1016/0952-3278(95)90121-3. [DOI] [PubMed] [Google Scholar]
- HARADA S., NAGY J.A., SULLIVAN K.A., THOMAS K.A., ENDO N., RODAN G.A., RODAN S.B. Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblast. J. Clin. Invest. 1994;93:2490–2496. doi: 10.1172/JCI117258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTNER A., GOPPELT-STRUEBE M., HILGERS K.F. Coordinate expression of cyclooxygenase-2 and renin in the rat kidney in renovascular hypertension. Hypertension. 1998;31:201–205. doi: 10.1161/01.hyp.31.1.201. [DOI] [PubMed] [Google Scholar]
- HERSCHMAN H.R. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metastasis Rev. 1994;13:241–256. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- HLA T., NEILSON K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. USA. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAGUCHI H., PILBEAM C.C., ABREU G., FLETCHER B.S., HERSCHMAN R.H., RAISZ R.L., HURLEY M.M. Transcriptional induction of prostaglandin G/H synthase-2 by basic fibroblast growth factor. J. Clin. Invest. 1995;96:923–930. doi: 10.1172/JCI118140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUJUBU D.A., REDDY S.T., FLETCHER B.S., HERSCHMAN H.R. Expression of the protein product of the prostaglandin synthase-2/TIS10 gene in mitogen stimulated Swiss 3T3 cells. J. Biol. Chem. 1993;268:5425–5430. [PubMed] [Google Scholar]
- MAJIMA M., ISONO M., IKEDA Y., HAYASHI I., HATANAKA K., HARADA Y., KATSUMATA O., YAMASHINA S., KATORI M., YAMAMOTO S. Significant roles of inducible cyclooxygenase (COX)-2 in angiogenesis in rat sponge implants. Jpn. J. Pharmacol. 1997;75:105–114. doi: 10.1254/jjp.75.105. [DOI] [PubMed] [Google Scholar]
- MASFERRER J.L., ZWEIFEL B.S., MANNING P.T., HAUSER S.D., LEAHY K.M., SMITH W.G., ISAKSON P.C., SEIBERT K. Selective inhibition of inducible cyclooxygenase 2 in vivo is anti-inflammatory and nonulcerogenic. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHAELSON I.C. The mode of development the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol., U.K. 1948;68:137–180. [Google Scholar]
- MIZUNO H., SAKAMOTO C., MATSUDA K., WADA K., UCHIDA T., NOGUCHI H., AKAMATSU T., KASUGA M. Induction of cyclooxygenase-2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997;112:387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- NARKO K., RISTIMAKI A., MACPHEE M., SMITH E., HAUDENSCHILD C.C., HLA T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-overexpression. J. Biol. Chem. 1997;272:21455–21460. doi: 10.1074/jbc.272.34.21455. [DOI] [PubMed] [Google Scholar]
- O'BANION M.K., WINN V.D., YOUNG D.A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory COX. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEACOCK D.J., BANQUERIGO M.L., BRAHN E. Angiogenesis inhibition suppresses collagen arthritis. J. Exp. Med. 1992;175:1135–1138. doi: 10.1084/jem.175.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO H., HLA T., MAIER J.A.M., CROFFORD L.J., CASE J.P., MACIAG T., WILDER R.L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J. Clin. Invest. 1992;89:97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., PERKINS W., LEE L., ISAKSON P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. U.S.A. 1994;91:12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENGER D.R., CONNOLY D.T., WATER L., FEDER J., DVORAK H.F. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer. Res. 1990;50:1774–1778. [PubMed] [Google Scholar]
- SHOUDA T., HATANAKA K., SAITO M., MAJIMA M., OGINO M., HARADA Y., NISHIJIMA M., KATORI M., YAMAMOTO S. Induction of cyclooxygenase Type-2 (COX-2) in rat endometrium at the peak of serum estradiol during the estrous cycle. Jpn. J. Pharmacol. 1995;69:289–291. doi: 10.1254/jjp.69.289. [DOI] [PubMed] [Google Scholar]
- SUZUKI Y., UENO A., KAWAMURA M., NISHIYAMA K., KATORI M., OKABE H. Prostaglandin levels in the rat resting gastric wall and enhancement of prostaglandin E2 generation after administration of mild hyperosmotic saline solution into gastric lumen. Eicosanoids. 1990;3:23–27. [PubMed] [Google Scholar]
- TUJII M., KAWANO S., TSUJI S., SAWAOKA H., HORI H., DUBOIS R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- WALLANCE J.L. Selective COX-2 inhibitors: is the water becoming muddy. Tips. 1999;20:4–6. doi: 10.1016/s0165-6147(98)01283-8. [DOI] [PubMed] [Google Scholar]
- WISE G.N. Retinal neovascularization. Trans. Am. Ophthalmol. Soc. 1956;54:729–826. [PMC free article] [PubMed] [Google Scholar]
- ZICHE M., JONES J., GULLINO P.M. Role of prostaglandin E1 and copper in angiogenesis. J. Natl. Cancer. Inst. 1982;69:475–482. [PubMed] [Google Scholar]


