Abstract
This study has investigated the effects of JTT-501, a peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonist, on the pathogenesis of diabetic complications in the Zucker diabetic fatty (ZDF) rats, a model of type 2 diabetes. Comparison is made with troglitazone, a PPAR-γ agonist.
The ZDF rats exhibited hyperglycaemia and hyperlipidaemia, and developed diabetic complications such as cataract, nephropathy, and neuropathy. Treatment with JTT-501 from the prediabetic stage controlled glycaemia and lipidaemia, and prevented the development of diabetic complications. Troglitazone was less effective in controlling serum cholesterol and neuropathy.
ZDF rats developed diabetic osteopenia with reduced bone turnover, and this was prevented by JTT-501 and troglitazone, possibly mediated by increased bone turnover and bone formation.
Since JTT-501 controlled glycaemia and lipidaemia in ZDF rats and prevented several diabetic complications, it is suggested that treatment with JTT-501, which activates both PPAR-α and PPAR-γ, could provide a valuable therapeutic approach against diabetic complications in type 2 diabetes.
Keywords: Peroxisome proliferator-activated receptor (PPAR)-α and PPAR-γ agonist, JTT-501, troglitazone, diabetic complication, Zucker diabetic fatty (ZDF) rat
Introduction
The chronic detrimental effects of hyperglycaemia in diabetic patients include various microvascular, macrovascular and neuropathic complications (Hanssen, 1991, 1997; Klein, 1995). Tight glycaemic control is of great value in avoiding or delaying the onset and progression of such diabetic complications. Indeed, the Diabetes Control and Complications Trial, the Kumamoto Study, and the U.K. Prospective Diabetes Study have comfirmed that intensive glycaemic control prevents diabetic complication in type 1 and type 2 diabetes (DCCT, 1993; Ohkubo et al., 1995; U.K. Prospective Study Group, 1998a,1998b). There are also significant correlations between raised serum cholesterol and triglyceride concentrations and motor nerve conduction velocity (MNCV) (Hotta et al., 1995). Thus, both glycaemic and lipidaemic control are important in the prevention of diabetic complications.
The thiazolidinediones, peroxisome proliferator-activated receptor (PPAR)-γ agonists, are insulin sensitizing agents which control glycaemia by enhancing insulin sensitivity in peripheral tissues (Saltiel & Olefsky, 1996). A novel insulin sensitizing agent, JTT-501 (4-[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]benzyl]-3,5-isoxazolidinedione) possesses identical properties for enhancing insulin sensitivity in peripheral tissues (Shibata et al., 1998, 1999). This enhancement is mediated by elevating insulin signal transduction (Shibata et al., 1998; Terasaki et al., 1998; Maegawa et al., 1999). Also, JTT-501 possesses a potent lipid lowering action compared with the thiazolidinediones by activating both PPAR-γ and PPAR-α (Shibata et al., 1999). We therefore expected JTT-501 to affect glycaemia and lipidaemia, which might in turn influence diabetic complications.
Accordingly, this study has investigated the effects of glycaemic and lipidaemic control by JTT-501 on diabetic complications such as cataract, nephropathy, neuropathy and on osteopenia. A rodent model of type 2 diabetes, the Zucker diabetic fatty (ZDF) rat was used, and comparison was made with the PPAR-γ agonist troglitazone.
Methods
Materials
JTT-501 was synthesized at Japan Tobacco, Inc. Central Pharmaceutical Research Institute (Osaka, Japan). NOSCAL® tablets (troglitazone 200 mg/tablet) were purchased from Sankyo, Inc. (Tokyo, Japan). Kits for the measurement of metabolites, hormones, ions, and other substances were purchased as follows: glucose, triglyceride, cholesterol, N-acetylglucosaminidase (NAG), creatinine from Boehringer Mannheim (Tokyo, Japan); insulin radioimmunoassay (RIA) from Shionogi (Tokyo, Japan); rat insulin from Linco Research (St. Charles, MO, U.S.A.), haemoglobin A1c (HbA1c) from Seikagaku Kogyo (Tokyo, Japan); protein assay from Bio Rad (Richimond, CA, U.S.A.); rat albumin enzyme-linked immunosorbent assay (ELISA) from Exocell, Inc. (Philadelphia, PA, U.S.A.); calcium from Instrumentation Laboratory (Lexington, MA, U.S.A.); osteocalcin RIA from Biomedical Techonologies, Inc. (Stoughton, MA, U.S.A.); deoxy-pyridinoline (DPD) ELISA from Sumitomo Pharmaceutical (Osaka, Japan), and citrate solution from Fuso Kogyo (3.7% solution, Osaka, Japan).
Animals
These experiments complied with the Guidelines of Animal Experimentation of our laboratories. Male 4–5-week-old ZDF rats (ZDF/Gmi-fa/fa) and lean rats (ZDF/Gmi-lean) were purchased from Charles River Japan, Inc. (Tokyo, Japan). Rats were individually housed in plastic cages and given a standard laboratory chow powdered diet (Clea Japan, Inc., Tokyo, Japan) and water ad libitum in a room controlled for temperature (23±3°C), humidity (55±15%) and lighting (0800 to 2000 h).
Preparations
JTT-501 (0.1 and 0.2%, w w−1) or troglitazone (0.2%, w w−1) were mixed into the powdered diet, and given to rats from 5 weeks of age. Age-matched control rats and lean rats were fed normal powdered diet for this period. Doses of JTT-501 and troglitazone were calculated from body weight and food consumption every week and final doses averaged for 15 weeks. In total, 75 and 147 mg kg−1 day−1 of JTT-501 and 150 mg kg−1 day−1 of troglitazone were administered.
Measurement of serum parameters
Blood samples were taken from the tail vein in the morning (1000 to 1100 h) each week. Serum was separated by centrifugation, and serum parameters measured. Blood samples were treated in citrate solution for measurement of HbA1c, while in serum glucose, triglyceride, and cholesterol concentrations were determined by Cobas Fara II (Roche, Tokyo, Japan). The serum insulin assay detected a minimum concentation of 0.1 ng ml−1, with intra- and inter-assay variation of less than 5%. Blood HbA1c levels were determined at a wavelength of 415 nm by spectrophotometry. Serum calcium concentrations were determined using a Monarch 2000 (Instrumentation Laboratory), and the serum osteocalcin assay detected a minimum concentation of 1.25 ng ml−1, with intra- and inter-assay variation of less than 5%.
Measurement of urinary parameters
After a 13 week administration period, rats were housed in metabolic cages for 3 days, and given chow and water ad libitum. Urine samples for the last 2 days were used for measurement of urinary parameters and the subsequent values averaged. Urinary albumin concentrations were detectable down to a minimum of 1.56 μg ml−1, with intra- and inter-assay variation of less than 5%. Urinary DPD was detectable to a minimum concentation of 3 nmol l−1, with intra- and inter-assay variation of less than 15%.
Measurement of motor nerve conduction velocity
Measurement of motor nerve conduction velocity (MNCV) was performed using the tail nerve as previously described with minor modifications (Miyoshi & Goto, 1973). Briefly, the animal was lightly anaesthetized by diethyl ether, and the tail was placed on a heated pad maintained surface temperature at 37°C. The tail nerve was stimulated by a stimulator (SEN-7103, intensity=100 V, Nihon-Koden, Tokyo, Japan) through a bipolar electrode (M·T Giken Co., Ltd, Tokyo, Japan) placed at 1 cm from anus. It was used as a bipolar electrode at two points of recording: the first was 4 cm from the stimulus electrode, and the second was 4 cm from the first recording electrode. The muscle action potentials were amplified (AVB-11, Nihon-Koden) and displayed with an amplifier-oscilloscope (VC-10, Nihon-Koden). Groups of 32 potentials were averaged using an averager (DAT-1100, Nihon-Koden) that also provided a chart recorder (RTA-1200, Nihon-Koden). The conduction velocity was calculated from the delta latency between two recording electrodes divided by a distance of 4 cm.
Measurement of bone mineral density
Bone mineral density (BMD) was measured by dual-energy X-ray absorptiometry (DXA) using Dichroma Scan (DCS-600R, Aloka, Tokyo, Japan). The lumbar spine was scanned at the level of vertebrae L4–5. Bone mineral content (BMC) and the scanned area were recorded, and BMD of L4–5 calculated by dividing the sum BMC by the sum area.
Histopathology
Pancreas and kidney were fixed in 10% neutral buffered formalin and the eye fixed in 2.5% glutaraldehyde. Fixed tissues were embedded in paraffin, sectioned, and stained with haematoxylin and eosin for microscopic evaluations. Periodic acid-Schiff stain was also performed on kidney and eye. Pancreas was stained for insulin by the indirect immunoperoxidase method. The sections were placed in absolute methanol containing 0.3% hydrogen peroxide for 30 min at room temperature in inactive endogenous peroxidase activity. The sections were rinsed twice in 0.01 M phosphate buffered saline, pH 7.4, incubated with mouse primary antibody for 30 min. The primary antibody against insulin (Dako Corp., Carpinteria, CA, U.S.A.) diluted to PBS at 1 : 200. After washing in phosphate buffered saline, the sections were covered with peroxidase-conjugated anti-guinea-pig immunoglobulin (1 : 100, Dako Corp.) for 30 min. After washing in PBS, the sections were incubated in Graham–Karnovsky's reaction medium, which contained 3,3′-diaminobenzidine-tetra-hycrochroride and 0.005% hydrogen peroxide in 0.05 M Tris-HCl buffered, pH 7.6, for 3–10 min at room temperature. The sections were counterstained with 2% methyl green.
Statistical analysis
Results are shown as mean±s.e.mean. Significance of difference between two groups was evaluated using Student's t-test. For multiple comparisons, one-way analysis of variance (ANOVA) was used. When ANOVA showed significant differences, post-hoc analysis was performed with Dunnett's test. P<0.05 was considered statistically significant.
Results
Body weight and food intake
Body weight and food intake are shown in Figure 1. Body weight of ZDF rats increased rapidly in young rats, but levelled out at approximately 400 g, the same weight as lean rats (400 g). However, ZDF rats consumed more diet than lean rats during the study period. JTT-501 and troglitazone increased body weight gain in ZDF rats. Growth increased by troglitazone was greater than by JTT-501 during the late period of the study, although food intake between the two groups did not change.
Figure 1.
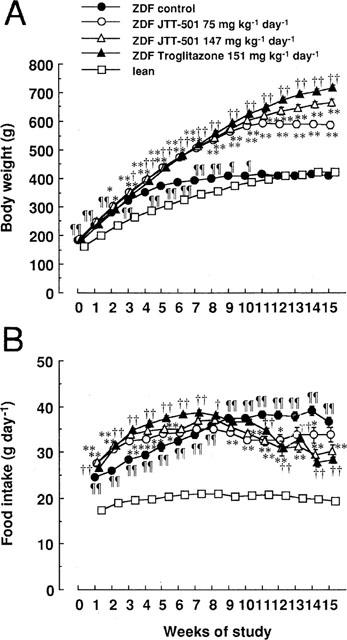
Effects of JTT-501 and troglitazone on body weight and food consumption of ZDF rats. JTT-501 and troglitazone were given to ZDF rats as a food admixture for 15 weeks. Body weight (A) and food consumptions (B) were measured weekly. Each point represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
Serum and blood parameters
ZDF rats were hyperinsulinaemic compared with lean rats (Figure 2A). Insulin levels increased until 3 weeks of study, and thereafter decreased. In ZDF rats, glucose levels were elevated to approximately 33 mM while insulin concentrations were reduced. Accordingly, HbA1c levels were elevated to approximately 15% (Figure 2B,C). ZDF rats were hypertriglyceridaemic and hypercholesterolaemic compared with lean rats (Figure 2D,E). JTT-501 treatment dose-dependently improved these parameters in ZDF rats. Troglitazone treatment was also effective in these parameters; however its cholesterol lowering activity was lower than that of JTT-501, and its efficacy was equivalent to the low dose of JTT-501.
Figure 2.
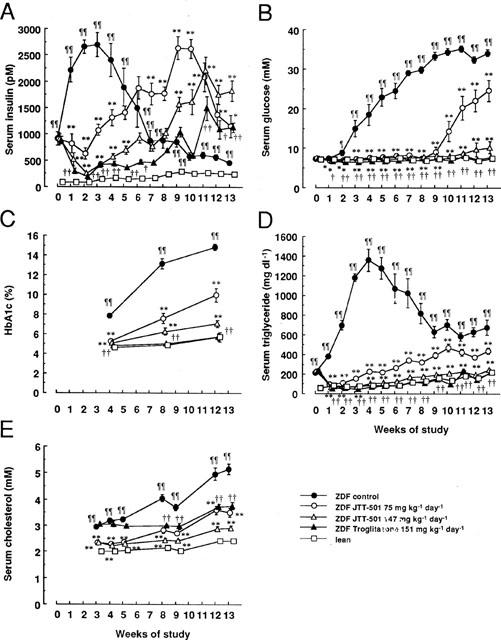
Effects of JTT-501 and troglitazone on blood biochemical parameters in ZDF rats. Blood samples were taken from tail vein weekly. Serum insulin (A), serum glucose (B), blood HbA1c (C), serum triglyceride (D), and serum cholesterol (E) were measured as described in Methods. Each point represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
Pancreatic islet
Pancreatic islets in ZDF rats exhibited multiple irregular projections and contained less insulin compared with those in lean rats (Figure 3). Although multiple irregular projections remained, JTT-501 prevented the reduction of insulin content in islets of ZDF rats.
Figure 3.
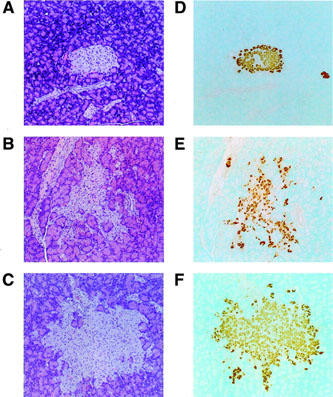
Histopathological analysis of pancreatic islets. Haematoxylin and eosin staining (A–C) and immunohistochemical staining by insulin antibody (D–F) were performed as described in Methods. Original magnification ×320: A, D, lean; B, E, ZDF control; C, F, ZDF treated with JTT-501 (147 mg kg−1 day−1).
Diabetic cataract
In the macroscopic findings, ZDF rats frequently had greater lens opacity (50%) in the pathogenesis of diabetic cataract as compared with lean rats (0%). Lens opacity was not detectable in ZDF rats treated with JTT-501 (0%). From the results of histopathological evaluation, the lens of ZDF rats exhibited degeneration and swelling of fibrae lentis, formation of Morgagnian globules, and stratification of epithelium lentis cells compared with that of lean rats (Figure 4). In ZDF rats treated with JTT-501, these abnormalities were not detected.
Figure 4.
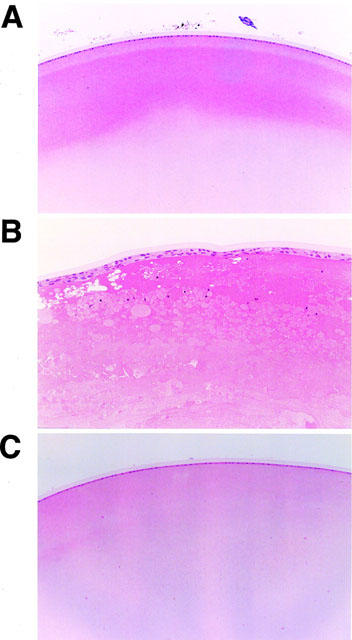
Histopathological analysis of lens by haematoxylin and eosin staining. Original magnification ×160: A, lean; B, ZDF control; C, ZDF treated with JTT-501 (147 mg kg−1 day−1).
Diabetic nephropathy
It was observed that ZDF rats consumed water and excreted urine to a much greater extent than lean rats (Figure 5A,B). ZDF rats also showed hyperproteinuria, hyperalbuminuria, and high activity of NAG compared with lean rats (Figure 5C,E), indicating that ZDF rats exhibited diabetic nephropathy. JTT-501 produced an improvement in these parameters, while troglitazone also showed a similar effect, with an efficacy equivalent to the high dose of JTT-501.
Figure 5.
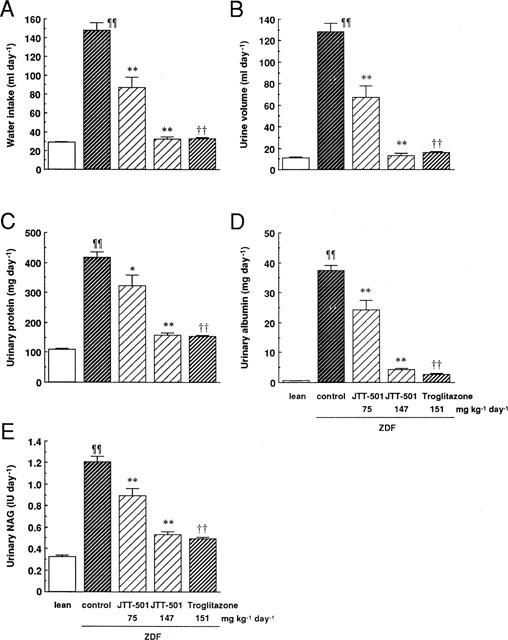
Effects of JTT-501 and troglitazone on water consumption and urinary biochemical parameters in ZDF rats. After administration for 13 weeks, urine samples were taken. Water consumption (A), urine volume (B), urinary protein (C), urinary albumin (D), and urinary NAG (E) were measured as described in Methods. Each column represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
In ZDF rats, increased mesangial matrix and glomerular hyaline droplets were less apparent after treatment with JTT-501 (Figure 6). In addition, ZDF rats exhibited hydropic degeneration of renal tubules; however, no tubular lesions were detected in JTT-501 treated ZDF rats (data not shown).
Figure 6.
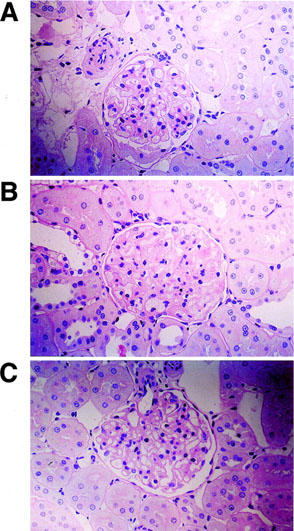
Histopathological analysis of glomerulae by periodic acid-Schiff staining. Original magnification ×320: A, lean; B, ZDF control; C, ZDF treated with JTT-501 (147 mg kg−1 day−1).
Diabetic neuropathy
MNCV was measured to examine nerve disease. The MNCV of ZDF rats was lower than that of lean rats, indicating that ZDF rats exhibited diabetic neuropathy (Figure 7). JTT-501 treatment dose-dependently elevated MNCV, and in particular the high dose of JTT-501 normalized MNCV to the levels of lean rats. Troglitazone did not normalize MNCV despite treatment with effective doses equivalent to JTT-501.
Figure 7.
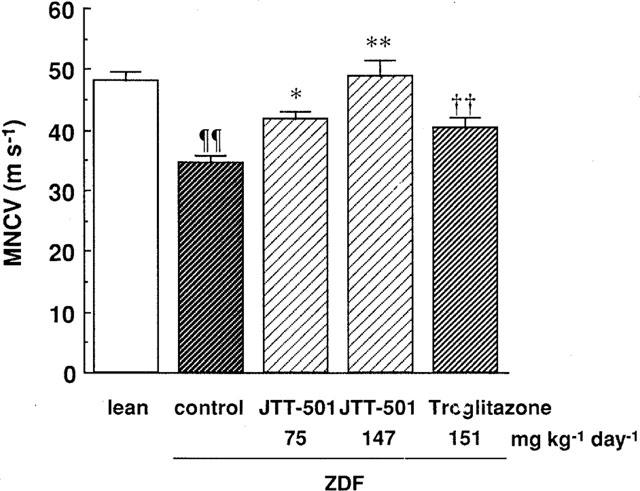
Effect of JTT-501 and troglitazone on MNCV in ZDF rats. After administration for 14 weeks, the measurement MNCV was performed as described in Methods. Each column represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
Diabetic osteopenia
BMD was measured in the 4th and 5th lumbar vertebrae (L4–5) of rats with the DXA analysis to investigate diabetic osteopenia in ZDF rats. BMC area, and BMD levels of ZDF rats were significantly lower than lean rats, indicating that ZDF rats had diabetic osteopenia (Figure 8). Although JTT-501 and troglitazone treatment did not affect the L4–5 area of ZDF rats, such treatment significantly increased BMC, and hence increased BMD compared with ZDF rats.
Figure 8.
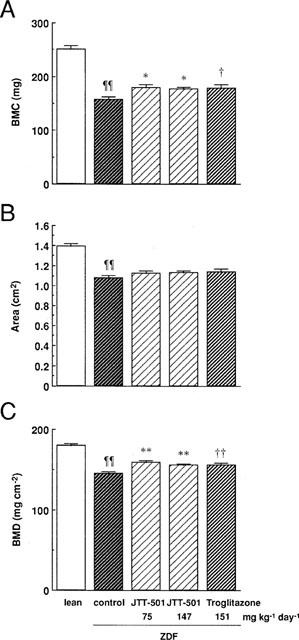
Effects of JTT-501 and troglitazone on BMD in ZDF rats. After administration for 15 weeks, BMC (A) and scanned area (B) were measured as described in Methods. BMD (C) was calculated by BMC dividing by scanned area. Each column represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
ZDF rats had abnormalities of bone metabolism such as hypercalciuria, low levels of serum osteocalcin, and high levels of urinary DPD compared with lean rats in spite of no-change in calcaemia, and these findings were supported by a change in BMD (Figure 9). JTT-501 and troglitazone treatment reduced urinary calcium levels and increased serum osteocalcin levels. JTT-501 treatment also increased urinary DPD levels, while troglitazone treatment had no effect.
Figure 9.
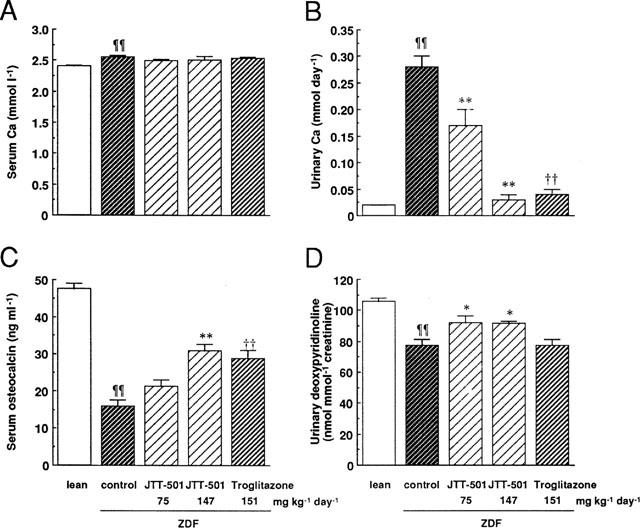
Effects of JTT-501 and troglitazone on serum or urinary calcium, serum osteocalcin, and urinary DPD in ZDF rats. Using serum and urine samples at 13–15 weeks administration, serum calcium (A), urinary calcium (B), serum osteocalcin (C), and urinary DPD (D) were measured as described in Methods. Each column represents the mean±s.e.mean (n=8). ¶P<0.05, ¶¶P<0.01 vs lean (Student's t-test). *P<0.05, **P<0.01 vs ZDF control (ANOVA followed by Dunnett's test). †P<0.05, ††P<0.01 vs ZDF control (Student's t-test).
Discussion
The present study investigated the effectiveness of glycaemic and lipidaemic control by insulin sensitizing agents against diabetic complications. Using the ZDF rat as a model of type 2 diabetes, the effect of JTT-501 (PPAR-γ and PPAR-α agonist) was compared with troglitazone (PPAR-γ agonist). ZDF rats exhibited diabetes and diabetic complications, which were in agreement with previous reports (Clark et al., 1983; Peterson et al., 1990a,1990b; Unger, 1991; Slieker et al., 1992; Danis & Yang, 1993; Ohneda et al., 1995; Sturis et al., 1995; Tokuyama et al., 1995). JTT-501 prevented the development of diabetic cataract, neuropathy, nephropathy, and osteopenia by controlling the glycaemia and lipidaemia, while troglitazone was not effective against cholesterol or neuropathy.
ZDF rats exhibited hyperinsulinaemia for 4 weeks of study (at 8–9 weeks old) after treatment initiation and thereafter decreased insulin levels, which is in agreement with previous reports (Slieker et al., 1992; Sturis et al., 1995; Tokuyama et al., 1995). Serum glucose and lipid levels were elevated by the decreased insulin levels in ZDF rats. Reduced insulin concentration in ZDF rats appears to be caused by defective function of pancreatic β-cells, associated with decreased insulin content. By the end of the study, JTT-501 increased insulin content in the islets of ZDF rats. Since JTT-501 enhances insulin sensitivity in peripheral tissues (Shibata et al., 1998, 1999; Terasaki et al., 1998; Maegawa et al., 1999), it is considered that JTT-501 suppresses the ‘overworking' of β-cells through improving insulin sensitivity, followed by prevention of hyperglycaemia and hyperlipidaemia.
In this study, JTT-501 prevented not only hyperglycaemia but also hyperlipidaemia in ZDF rats. The Diabetes Control and Complications Trial, the Kumamoto Study, and the U.K. Prospective Diabetes Study indicated that intensive glycaemic control prevents the development of diabetic complications (DCCT, 1993; Ohkubo, 1995; UK Prospective Study Group, 1998a,1998b). On the other hand, Hotta et al. (1995) reported that reduction of lipidaemia improves diabetic complications, and in particular, neuropathy. Such evidence suggests that controlling not only glycaemia but also lipidaemia is important to avoid the development of diabetic complications. Indeed, JTT-501 potently prevented the elevation of serum cholesterol levels and improved MNCV compared with troglitazone. Our findings suggest the lipidaemic control is a principal factor for the development of diabetic neuropathy.
Recently, it has been reported that PPAR-α regulates lipid metabolism (Keller et al., 1993; Desvergne et al., 1998). In fact, JTT-501 activates not only PPAR-γ but also PPAR-α, in contrast to troglitazone (Shibata et al., 1999). This evidence leads us to conclude that the cholesterol lowering effect of JTT-501 is based on activating PPAR-α. Our results indicate that the agonist against both PPAR-α and PPAR-γ, JTT-501, is more comprehensively effective in preventing the development of diabetic complications compared with the agonist for PPAR-γ alone, the thiazolidinediones.
Diabetic osteopenia is an established fact in type 1 diabetes (Wiske et al., 1982; Hui et al., 1985; Piepkorn et al., 1997), but is more controversial in type 2 diabetes, in which case BMD may be increased (Johnston et al., 1985; van Daele et al., 1995; Barrett-Connor & Kritz-Silverstein, 1996), decreased (Leivin et al., 1976; Ishida et al., 1985; Okuno et al., 1991; Wakasugi et al., 1993), or unchanged (Weinstock et al., 1989). It has been reported that the change of BMD in type 2 diabetes is mainly dependent on circulating insulin levels (Barrett-Connor & Kritz-Silverstein, 1996; Reid et al., 1993; Cornish et al., 1996; Fukunaga et al., 1997). Such evidence suggests that the development of diabetic osteopenia is reflected by a difference in the diabetic condition in type 2 diabetes, as hyperinsulinaemia or impaired insulin secretion. In this study, although BMD levels were decreased in ZDF rats, it is considered that such a phemomenon is due to impaired insulin secretion. JTT-501 treatment prevented a decrease in BMD associated with improved insulin secretion.
In ZDF rats, serum osteocalcin levels, which relate to bone formation, and urinary DPD levels, which relate to bone resorption, were lower than those in lean rats. Furthermore, ZDF rats excreated urinary calcium at high levels compared to lean rats. These results suggest that ZDF rats exhibit diabetic osteopenia with low bone turnover. JTT-501 elevated osteocalcin and DPD and reduced urinary calcium in ZDF rats. This indicates that JTT-501 shifts the bone metabolism of ZDF rats from low turnover to high turnover. It has been reported that the insulin receptor is expressed in osteoblasts, which are associated with bone formation, and insulin up-regulates bone formation (Ituarte et al., 1989). In addition, insulin treatment elevates bone formation in vivo (Cornish et al., 1996). Thomas et al. (1998) reported that the insulin receptor is present in osteoclasts, which are associated with bone resorption, and that insulin enhances bone resorption. In fact, JTT-501 elevates insulin signal transduction (Shibata et al., 1998; Terasaki et al., 1998; Maegawa et al., 1999). This evidence supports the hypothesis that JTT-501 influences bone formation, which is mediated by enhanced insulin signalling through the insulin receptor. Troglitazone influenced bone formation alone in this study although troglitazone, like JTT-501, enhances insulin signalling (Saltiel & Olefsky, 1996). We do not have any evidence with regard to the different effects. However, we speculate that the high turnover bone metabolism by JTT-501, as well as that in lean rats, may be a more advantageous effect to that of troglitazone.
The present study is the first to report that diabetic complications and diabetic osteopenia in a type 2 diabetic model (ZDF rat) are prevented by use of insulin sensitizing agents. Furthermore, JTT-501 was effective in avoiding the progression of diabetic complications, and in particular, was more effective against neuropathy than troglitazone. The PPAR-α and PPAR-γ agonist, JTT-501, may therefore be more effective against diabetic complications than PPAR-γ agonists such as the thiazolidinedione, troglitazone.
In conclusion, glycaemic and lipidaemic control by JTT-501 treatment from the prediabetic stage prevented diabetic cataract, neuropathy, nephropathy, and osteopenia in ZDF rats. JTT-501 also effectively reduced cholesterol and improved diabetic neuropathy. We suggest that treatment with JTT-501, which activates both PPAR-α and PPAR-γ, could be a valuable theraputic approach in patients with diabetic complications in type 2 diabetes.
Acknowledgments
The authors thank Atsuhiro Uemura, Takashi Nakagawa, and Katsuhiro Miyajima for excellent technical assistance.
Abbreviations
- BMC
bone mineral content
- BMD
bone mineral density
- DPD
deoxy-pyridinoline
- DXA
dual-energy X-ray absorptiometry
- ELISA
enzyme-linked immunosorbent assay
- HbA1c
haemoglobin A1c
- MNCV
motor nerve conduction velocity
- NAG
N-acetylglucosaminidase
- RIA
radioimmunoassay
References
- BARRETT-CONNOR E., KRITZ-SILVERSTEIN D. Dose hyperinsulinemia preserve bone. Diabetes Care. 1996;19:1388–1392. doi: 10.2337/diacare.19.12.1388. [DOI] [PubMed] [Google Scholar]
- CLARK J.B., PALMER C.J., SHAW W.N. The diabetic Zucker fatty rat (41611) Proc. Soc. Exp. Biol. Med. 1983;173:68–75. doi: 10.3181/00379727-173-41611. [DOI] [PubMed] [Google Scholar]
- CORNISH J., CALLON K.E., REID I.R. Insulin increases histomorphometric indices of bone formation in vivo. Calcif. Tissue Int. 1996;59:492–495. doi: 10.1007/BF00369216. [DOI] [PubMed] [Google Scholar]
- DANIS R.P., YANG Y. Microvascular retinopathy in the Zucker diabetic fatty rat. Invest. Ophthalmol. Vis. Sci. 1993;34:2367–2371. [PubMed] [Google Scholar]
- DESVERGNE B., IJPENBERG A., DEVCHAND P.R., WAHLI W. The peroxisome proliferator-activated receptors at the cross-road of diet and hormonal signalling. J. Steroid. Biochem. Molec. Biol. 1998;65:65–74. doi: 10.1016/s0960-0760(97)00182-9. [DOI] [PubMed] [Google Scholar]
- FUKUNAGA Y., MINAMIKAWA J., INOUE D., KOSHIYAMA H. Dose insulin use increase bone mineral density in patients with non-insulin-dependent diabetes mellitus. Arch. Intern. Med. 1997;157:2668–2669. doi: 10.1001/archinte.157.22.2668a. [DOI] [PubMed] [Google Scholar]
- HANSSEN K.F.The determinants of microvascular complications in diabetes: an overview Textbook of Diabetes 1991Oxford: Blackwell Scientific Publications; 519–525.ed Pickup, J.C. and Williams, G pp [Google Scholar]
- HANSSEN K.F. Blood glucose control and microvascular and macrovascular complications in diabetes. Diabetes. 1997;46 Suppl. 2:S101–S103. doi: 10.2337/diab.46.2.s101. [DOI] [PubMed] [Google Scholar]
- HOTTA N., NAKAMURA J., KAKUTA H., FUKASAWA H., KOH N., SAKAKIBARA F., MORI K., SAKAMOTO N. Niceritrol prevents the decrease in red blood cell 2,3-diphosphoglycerate and neuropathy in streptozotocin-induced diabetic rats. J. Diab. Comp. 1995;9:133–139. doi: 10.1016/1056-8727(95)00049-8. [DOI] [PubMed] [Google Scholar]
- HUI S.L., EPSTEIN S., JOHNSTON C.C. A prospective study of bone mass in patients with Type 1 diabetes. J. Clin. Endocrinol. Metab. 1985;60:74–80. doi: 10.1210/jcem-60-1-74. [DOI] [PubMed] [Google Scholar]
- ISHIDA H., SEINO Y., MATSUKURA S., IKEDA M., YAWATA M., YAMASHITA G., ISHIZUKA S., IMURA H. Diabetic osteopenia and circulating levels of vitamin D metabolites in type 2 (noninsulin-dependent) diabetes. Metabolism. 1985;34:797–801. doi: 10.1016/0026-0495(85)90101-5. [DOI] [PubMed] [Google Scholar]
- ITUARTE E.A., HALSTEAD L.R., IIDA-KLEIN A., ITUARTE H.G., HAHN T.J. Glucose transport system in UMR-106-01 osteoblastic osteosarcoma cells: regulation by insulin. Calcif. Tissue Int. 1989;45:27–33. doi: 10.1007/BF02556657. [DOI] [PubMed] [Google Scholar]
- JOHNSTON C.C., HUI S.L., LONGCOPE C. Bone mass and sex steroid concentrations in postmenopausal caucasian diabetes. Metabolism. 1985;34:544–550. doi: 10.1016/0026-0495(85)90192-1. [DOI] [PubMed] [Google Scholar]
- KELLER H., DREYER C., MEDIN J., MAHFOUDI A., OZATO K., WAHLI W. Fatty acids and retinoids control lipid metabolism through activation of peroxisome proliferator-activated receptor-retinoid X receptor heterodimers. Proc. Natl.Acad. Sci. U.S.A. 1993;90:2160–2164. doi: 10.1073/pnas.90.6.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18:258–268. doi: 10.2337/diacare.18.2.258. [DOI] [PubMed] [Google Scholar]
- LEIVIN M.E., BOISSEAU V.C., AVIOLI L.V. Effects of diabetes mellitus on bone mass in juvenile and adult-onset diabetes. N. Engl. J. Med. 1976;294:241–245. doi: 10.1056/NEJM197601292940502. [DOI] [PubMed] [Google Scholar]
- MAEGAWA H., OBATA T., SHIBATA T., FUJITA T., UGI S., MORINO K., NISHIO Y., KOJIMA H., HIDAKA H., HANEDA M., YASUDA H., KIKKAWA R., KASHIWAGI A. A new antidiabetic agent (JTT-501) rapidly stimulates glucose disposal rates by enhancing insulin signal transduction in skeleral muscle. Diabetologia. 1999;42:151–159. doi: 10.1007/s001250051133. [DOI] [PubMed] [Google Scholar]
- MIYOSHI T., GOTO I. Serial in vivo determinations of nerve conduction velocity in rat tails: physiological and pathological changes. Electroencephalogr. Clin. Neurophysiol. 1973;35:125–131. doi: 10.1016/0013-4694(73)90168-5. [DOI] [PubMed] [Google Scholar]
- OHKUBO Y., KISHIKAWA H., ARAKI E., MIYATA T., ISAMI S., MOTOYOSHI S., KOJIMA Y., FURUYOSHI N., SHICHIRI M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res. Clin. Prac. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- OHNEDA M., INMAN L.R., UNGER R.H. Caloric restriction in obese pre-diabetic rats prevents beta-cell depletion, loss of beta-cell GLUT 2 and glucose incompetence. Diabetologia. 1995;38:173–179. doi: 10.1007/BF00400091. [DOI] [PubMed] [Google Scholar]
- OKUNO Y., NISHIZAWA Y., SEKITA K., HAGIWARA S., MIKI T., MORII H. Total and regional bone mineral content in patients with non-insulin dependent diabetes mellitus. J. Nutr. Sci. Vitaminol. 1991;37:S43–S49. doi: 10.3177/jnsv.37.supplement_s43. [DOI] [PubMed] [Google Scholar]
- PETERSON R.G., NEEL M.A., LITTLE L.A., KINCAID J.C., EICHBERG J.Neuropathic complications in the Zucker diabetic fatty rat (ZDF/Drt-fa) Frontiers in diabetic research. Lesson from animal diabetes III 1990aLondon: Smith-Gordon; 456–458.ed. Shafrir, E. pp [Google Scholar]
- PETERSON R.G., SHAW W.N., NEEL M.A., LITTLE L.A., EICHBERG J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR News. 1990b;32:16–19. doi: 10.1093/ilar.32.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIEPKORN B., KANN P., FORST T., ANDREAS J., PFÜTZNER A., BEYER J. Bone mineral density and bone metabolism in diabetes mellitus. Horm. Metab. Res. 1997;29:584–591. doi: 10.1055/s-2007-979106. [DOI] [PubMed] [Google Scholar]
- REID I.R., EVANS M.C., COOPER G.J.S., AMES R.W., STAPLETON J. Circulating insulin levels are related to bone density in normal postmenopausal women. Am. J. Physiol. 1993;265:E655–E659. doi: 10.1152/ajpendo.1993.265.4.E655. [DOI] [PubMed] [Google Scholar]
- SALTIEL A.R., OLEFSKY J.M. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- SHIBATA T., MATSUI K., NAGAO K., SHINKAI H., YONEMORI F., WAKITANI K. Pharmacological profiles of a novel oral antidiabetic agent, JTT-501, an isoxazolidinedione derivative. Eur. J. Pharmacol. 1999;364:221–219. doi: 10.1016/s0014-2999(98)00832-2. [DOI] [PubMed] [Google Scholar]
- SHIBATA T., MATSUI K., YONEMORI F., WAKITANI K. JTT-501, a novel antidiabetic agent, improves insulin resistance in genetic and non-genetic insulin-resistant models. Br. J. Pharmacol. 1998;125:1744–1750. doi: 10.1038/sj.bjp.0702253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLIEKER L.J., SUNDELL K.L., HEATH W.F., OSBORNE E., BUE J., MANETTA J., SPORTSMAN J.R. Glucose transporter levels in tissues of spontaneously diabetic Zucker fa/fa rat (ZDF/drt) and viable yellow mouse (Avy/a) Diabetes. 1992;41:187–193. doi: 10.2337/diab.41.2.187. [DOI] [PubMed] [Google Scholar]
- STURIS J., PUGH W.L., TANG J., POLONSKY K.S. Prevention of diabetes does not completely prevent insulin secretory defects in the ZDF rat. Am. J. Physiol. 1995;269:E786–E792. doi: 10.1152/ajpendo.1995.269.4.E786. [DOI] [PubMed] [Google Scholar]
- TERASAKI J., ANAI M., FUNAKI M., SHIBATA T., INUKAI K., OGIHARA T., ISHIHARA H., KATAGIRI H., ONISHI Y., SAKODA H., FUKUSHIMA Y., YAZAKI Y., KIKUCHI M., OKA Y., ASANO T. Role of JTT-501, a new insulin sensitiser, in restoring impaired GLUT4 translocation in adipocytes of rats fed a high fat diet. Diabetologia. 1998;41:400–409. doi: 10.1007/s001250050922. [DOI] [PubMed] [Google Scholar]
- THE DIABETES CONTROL AND COMPLICATIONS TRIAL RESEARCH GROUP The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- THOMAS D.M., UDAGAWA N., HARDS D.K., QUINN J.M.W., MOSELEY J.M., FINDLAY D.M., BEST J.D. Insulin receptor expression in primary and cultured osteoclast-like cells. Bone. 1998;23:181–186. doi: 10.1016/s8756-3282(98)00095-7. [DOI] [PubMed] [Google Scholar]
- TOKUYAMA Y., STURIS J., DEPAOLI A.M., TAKEDA J., STOFFEL M., TANG J., SUN X., POLONSKY K.S., BELL G.I. Evolution of β-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–1457. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- UK PROSPECTIVE DIABETES STUDY GROUP Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998a;352:837–853. [PubMed] [Google Scholar]
- UK PROSPECTIVE DIABETES STUDY GROUP Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998b;352:854–865. [PubMed] [Google Scholar]
- UNGER R.H. Role of impaired glucose transport by β cells in the pathogenesis of diabetes. J. NIH Res. 1991;3:77–80. [Google Scholar]
- VAN DAELE P.L.A., STOLK R.P., BURGER H., ALGRA D., GROBBEE D.E. , HOFMAN A., BIRKENHÄGER J.C., POLS H.A.P. Bone density in non-insulin-dependent diabetes mellitus.: The Rotterdam Study. Ann. Intern. Med. 1995;122:409–414. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- WAKASUGI M., WAKAO R., TAWATA M., GAN N., KOIZUMI K., ONAYA T. Bone mineral density measured by dual energy xray absorptiometry in patients with non-insulin-dependent diabetes mellitus. Bone. 1993;14:29–33. doi: 10.1016/8756-3282(93)90252-6. [DOI] [PubMed] [Google Scholar]
- WEINSTOCK R.S., GOLAND R.S., SHANE E., CLEMENS T.L., LINDSAY R., BILEZIKIAN J.P. Bone mineral density in women with type II diabetes mellitus. J. Bone Miner. Res. 1989;4:97–101. doi: 10.1002/jbmr.5650040114. [DOI] [PubMed] [Google Scholar]
- WISKE P.S., WENTWORTH S.M., NORTON J.A., EPSTEIN S., JOHNSTON C.C. Evaluation of bone mass and growth in young diabetes. Metabolism. 1982;31:848–854. doi: 10.1016/0026-0495(82)90086-5. [DOI] [PubMed] [Google Scholar]


