Abstract
Interleukin-12 (IL-12) plays a central role in the immune system by driving the immune response towards T helper 1 (Th1) type responses characterized by high IFN-γ and low IL-4 production. In this study we investigated whether retinoid-mediated inhibition of interleukin-12 production in mouse macrophages could regulate cytokine profile of antigen (Ag)-primed CD4+ Th cells.
Pretreatment with retinoids (9-cis-RA, all-trans-RA, TTNPB) significantly inhibited IL-12 production by mouse macrophages stimulated with lipopolysaccharide (LPS) or heated-killed Listeria monocytogenes (HKL). Retinoid-pretreated macrophages reduced their ability to induce IFN-γ and increased the ability to induce IL-4 in Ag-primed CD4+ T cells.
Addition of recombinant IL-12 to cultures of retinoid-pretreated macrophages and CD4+ T cells restored IFN-γ production in CD4+ T cells.
The in vivo administration of 9-cis-RA resulted in the inhibition of IL-12 production by macrophages stimulated in vitro with either LPS or HKL, leading to the inhibition of Th1 cytokine profile (decreased IFN-γ and increased IL-4 production) in CD4+ T cells.
These findings may explain some known effects of retinoids including the inhibition of encephalitogenicity, and point to a possible therapeutic use of retinoids in the Th1-mediated immune diseases such as autoimmune diseases.
Keywords: Retinoids, interleukin-12, macrophage, T helper cell, cytokine profile
Introduction
The existence of Th1/Th2 subsets in Th lymphocytes that differs in their cytokine secretion patterns and effector functions provides a framework for understanding normal and pathological immune responses (Mosmann, 1991). The Th1 subset of CD4+ T cells secretes cytokines usually associated with inflammation such as interferon-γ (IFN-γ) and tumour necrosis factor-β (TNF-β), and induces cell-mediated immune responses. The Th2 subset produces cytokines such as IL-4 and IL-5 that help B cells to proliferate and differentiate and is associated with humoral immune responses (Seder & Paul, 1994; Constant & Bottomly, 1997). Recent studies indicate that the ratio of these two Th cell types, Th1 and Th2, is closely correlated with the outcome of many diseases (Romagnani, 1996; D'Elios & Del Prete, 1998). Th1 responses predominate in organ-specific autoimmune disorders, acute allograft rejection, and in some chronic inflammatory disorders of unknown aetiology (Hayashi et al., 1995; Lafaille, 1998). In contrast, Th2 responses predominate in Omenn's syndrome, transplantation tolerance, chronic graft versus host disease, systemic sclerosis, and allergic diseases (Grewe et al., 1998).
The nature of Th1 or Th2 polarizing signals is not yet fully understood. However, the cytokines that are present in the environment of the CD4+ T cell at the time it encounters the antigen significantly regulate the differentiation of Th cells into either Th1 or Th2 subsets (O'Garra, 1998). IL-12 promotes Th1 differentiation, while IL-4 plays a key role in the differentiation of the precursor CD4+ T cells toward a Th2 phenotype. Recent evidence points to a critical role for IL-12 in the pathogenesis of rodent models of Th1-mediated autoimmune diseases such as type-1 diabetes, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease, and acute graft-versus-host disease. IL-12 has also been implicated in the pathogenesis of sarcoidosis, a multisystem disorder characterized by non-caseating granulomatous inflammation and dominant type-1 cytokine expression (Gately et al., 1998; Trinchieri, 1998). Thus, pharmacological control of IL-12 production may be a key strategy in modulating specific immune-mediated diseases dominated by type-1 cytokine responses.
Retinoids are a group of naturally occurring and synthetic compounds with vitamin A-like biological activity. Retinoids regulate the growth and differentiation of a wide variety of cell types (Rogers, 1997). Numerous clinical studies have demonstrated beneficial effects of retinoids not only in dermatology but also in oncology, diabetes, autoimmune diseases, and diseases associated with the human papilloma virus (Chandraratna, 1998; Evans & Kaye, 1999). Retinoids exert most of their effects by binding to specific receptors and modulating gene expression (Minucci & Ozato, 1996).
In this study we have demonstrated that pretreatment with retinoids inhibited IL-12 production in macrophages stimulated with LPS or HKL. Importantly, inhibition of IL-12 production in retinoid-pretreated macrophages resulted in the inhibition of Th1 cytokine profile (decreased IFN-γ and increased IL-4 production) in Ag-primed CD4+ T cells.
Methods
Preparation of splenic macrophages stimulated with either lipopolysaccharide (LPS) or heat-killed Listeria monocytogenes (HKL)
Splenic macrophages were isolated from DBA/2 mice and stimulated, as previously described (Na et al., 1999). In brief, spleen cells were cultured at 106 cells ml−1 for approximately 3 h in Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum at 37°C in a 5% CO2 humidified air atmosphere. The non-adherent cells were removed by washing with warm DMEM until visual inspection revealed a lack of lymphocytes (>98% of the cell population). The adherent cells were removed from plates by incubating for 15 min with ice-cold phosphate-buffered saline solution and rinsing repeatedly. The isolated adherent cell population was stimulated with 5 μg ml−1 LPS in the absence or presence of retinoids at 10−10, 10−9, 10−8, 10−7, 10−6 M at 1×105 cells per well in 96-well culture plates for 48 h. For some experiments, the cells were stimulated with HKL at 2×106 bacteria per well.
Purification and induction of cytokine synthesis in antigen-primed CD4+ T cells
Draining axillary, popliteal, and inguinal lymph nodes were removed from mice 9 days after priming with 100 μg keyhole limpet haemocyanin (KLH) in complete Freunds adjuvant (CFA) in the footpads. Lymph node cells were depleted of B cells by adherence to goat anti-mouse Ig-coated dishes for 1 h at 4°C. Nonadherent cells were depleted of CD8+ T cells and other antigen-presenting cells by treating the cells with a mixture of anti-CD8 and anti-class II MAbs on ice for 30 min, followed by addition of low toxicity rabbit complement (Pel Freeze, Rogers, AR, U.S.A.) and incubation at 37°C for 45 min. More than 95% of the cells were CD4+ T cells, as demonstrated by cytofluorometric analysis using anti-CD4 MAb (PharMingen). Purified CD4+ T cells were incubated in 96-well plates at 4×105 cells per well with macrophages (1×105 cells per well) and KLH (10 μg ml−1). Culture supernatants were harvested after 2 days (for IL-12 p70) or after 4 days (for IFN-γ and IL-4), and assayed by an enzyme-linked immunosorbent assay (ELISA).
Cytokine assays
The quantities of IFN-γ, IL-4, IL-10, and IL-12 p70 in culture supernatants were determined by a sandwich ELISA using MAbs specific for each cytokine as previously described (Kim et al., 1997). The MAbs for coating the plates and the biotinylated second MAbs were as follows: for IFN-γ, rat anti-mouse IFN-γ (HB170) and biotinylated rat anti-mouse IFN-γ (XMG1.2); for IL-4, rat anti-mouse IL-4 (BVD4-1D11) and biotinylated anti-mouse IL-4 (BVD6); for IL-10, rat anti-mouse IL-10 (JES-2A5) and biotinylated anti-mouse IL-10 (SXC-1); for IL-12 p70, anti-mouse IL-12 (p35/p70) (Red-T/G297-289) and rat anti-mouse IL-12 p40 (C17.8). Standard curves were generated using recombinant cytokines. Murine rIL-12 was provided by Dr S. Wolf (Genetics Institute, Cambridge, MA, U.S.A.), and rIFN-γ, rIL-4 and rIL-10 were purchased from PharMingen. The lower limit of detection was 125 pg ml−1 for IFN-γ, 3 pg ml−1 for IL-4, 0.2 ng ml−1 for IL-10, and 50 pg ml−1 for IL-12 p70.
Statistical analysis
The Student t-test was used for statistical comparisons between the two groups. In multiple groups, data were analysed with one-way ANOVA followed by the Bonferroni multiple comparison method. Data are represented as mean±s.e.mean, and differences were considered statistically significant at P<0.05.
Materials
Retinoids (9-cis-RA, all-trans-RA, TTNPB) and LPS (from E. coli 0111:B4) were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). KLH was obtained from the Calbiochem. (San Diego, CA, U.S.A.) Anti-mIL-4 (BVD4 and BVD6) and anti-mIFN-γ MAbs (R46A2 and XMG1.2) were purified from ascitic fluids by ammonium sulfate precipitation followed by diethylaminoethyl-Sephacel chromatography (Sigma). Anti-mouse IL-10 (JES-2A5 and SXC-1) and anti-mouse IL-12 (p35/p70) (Red-T/G297-289) were obtained from PharMingen (San Diego, CA, U.S.A.), and rat anti-mouse IL-12 p40 (C17.8) was kindly donated by Dr G. Trinchieri (Wistar Institute, Philadelphia, PA, U.S.A.). MAb-secreting hybridomas were obtained from the ATCC (American Type Culture Collection, Rockville, MD, U.S.A.). The cells were maintained at 37°C in a humidified 5% CO2 in RPMI 1640 or Dulbecco's modified Eagle's medium (DMEM) containing 10% foetal bovine serum and antibiotics (Gibco BRL, Grand Island, NY, U.S.A.). Six- to eight-week-old female DBA/2 mice were obtained from the SLC Japan (Tokyo, Japan), and maintained in pathogen-limited conditions. The mice were maintained and treated according to National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Results
Pretreatment with retinoids inhibited IL-12 production from mouse macrophages stimulated with either LPS or HKL
To determine whether retinoids could affect the production of T cell cytokine indirectly via an effect on IL-12 production by antigen-presenting cells (APCs) such as macrophages, we first investigated the effect of retinoids on IL-12 production in mouse macrophages. Macrophages were pretreated with varying amounts of retinoids for 4 h. After washing out the retinoids in the cultures, the cells were stimulated with either LPS or HKL for 48 h. As shown in Figure 1, LPS or HKL readily induced IL-12 p70 production, as expected. Pretreatment of macrophages with retinoids significantly inhibited this induced IL-12 production in a dose-dependent manner (P<0.05 at 10−8, 10−7 and 10−6 M retinoids, relative to an untreated group). 9-cis-RA and all-trans-RA were significantly more effective than (E)-4-[2-(5,6,7,8-tetrahydro-5,5,8,8,-tetramethyl-2-naphthylenyl)-1-propenyl] benzoic acid (TTNPB) (P<0.01 at 10−7 and 10−6 M). IL-12 p40 production was inhibited by retinoids to the same extent as IL-12 p70 (data not shown). In contrast, treatment with retinoids did not significantly inhibit IL-10 production from mouse macrophages (the levels of IL-10 in the absence or presence of 10−6 M 9-cis-RA, all-trans-RA, or TTNPB were 870 ng/ml±65, 760±78, 752±82, 826±58, respectively), suggesting that the inhibition of IL-12 production by retinoids was not the result of a general dampening of cellular activation.
Figure 1.
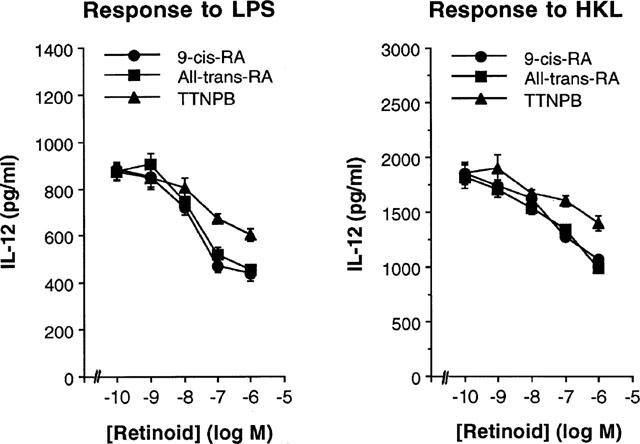
Pretreatment with retinoids inhibits IL-12 production in mouse macrophages. Primary macrophages were treated with varying concentrations of retinoids or left untreated. After 4 h, the cells were washed and stimulated with either LPS (5 μg ml−1) or HKL (2×106 cells well−1) for 48 h. Culture supernatants were harvested and IL-12 levels were evaluated by ELISA. The results are presented as mean±s.e.mean (n=3).
Pretreatment of macrophages with retinoids inhibited IFN-γ production and enhanced IL-4 production by antigen-primed CD4+ T cells
Since IL-12 has been known to potently enhance IFN-γ and inhibit IL-4 in CD4+ T cells, we asked if the cytokine profiles of CD4+ T cells responding to Ag presented by retinoid-treated macrophages would be altered. Direct effects of retinoids on CD4+ T cells were eliminated by pretreatment of macrophages in vitro with retinoids for 4 h and washing them to remove retinoids, before culture with syngeneic CD4+ T cells purified from lymph nodes of KLH-primed mice and the Ag KLH. Figure 2A shows that IL-12 production in cultures of retinoid-treated macrophages was significantly decreased in comparison with untreated macrophages. In the absence of retinoids treatment, stimulation with KLH resulted in the development of T cells producing high levels of IFN-γ. However, pretreatment of macrophages with retinoids for 4 h greatly inhibited their capacity to induce IFN-γ production by CD4+ T cells (Figure 2B) and significantly increased IL-4 production (Figure 2C). No cytokine production by CD4+ T cells was detected in the absence of macrophages, demonstrating that the retinoid-treated macrophages regulated the cytokine production by CD4+ T cells. Thus, pretreatment of macrophages with retinoids enhances their capacity to inhibit Th1 and to enhance Th2 cytokine synthesis.
Figure 2.
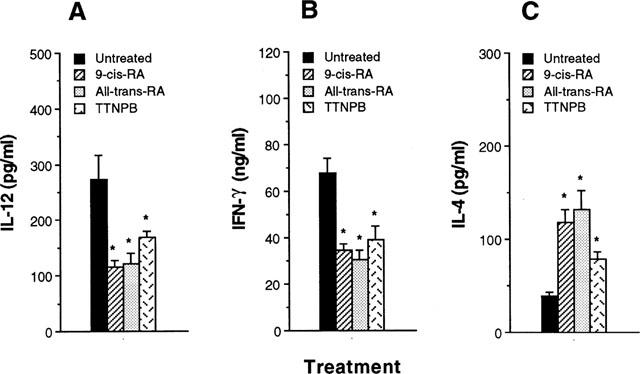
Macrophages pretreated with retinoids inhibit IFN-γ and enhance IL-4 production by Ag-primed CD4+ T cells. Macrophages (1×105 cells well−1) were pretreated with media or 10−7 M retinoids. After 4 h, the cells were washed and incubated with KLH-primed CD4+ T cells (5×105 cells well−1) and KLH (10 μg ml−1). Supernatants were harvested after 2 days for IL-12 (A) or after 4 days for IFN-γ (B) and IL-4 (C), and assayed by cytokine-specific ELISA. The results are presented as mean±s.e.mean (n=3). *P<0.01, relative to an untreated group.
Addition of recombinant IL-12 (rIL-12) to cultures of retinoid-pretreated macrophages and CD4+ T cells restored the levels of IFN-γ production in CD4+ T cells
To determine whether the reduced ability of retinoid-pretreatment macrophages to induce IFN-γ synthesis in CD4+ T cells was a result of their diminished production of IL-12, we reconstituted cultures of retinoid-pretreated macrophages and CD4+ T cells with rIL-12 (10 pg ml−1). Figure 3 shows that addition of rIL-12 to cultures of retinoid-pretreatment macrophage and CD4+ T cells significantly increased IFN-γ and reduced IL-4 production to levels seen in untreated control cultures. These results suggest that reduction of IL-12 synthesis by retinoid-treated macrophages was a major effect that affected the ability of macrophages to regulate cytokine synthesis in CD4+ T cells.
Figure 3.
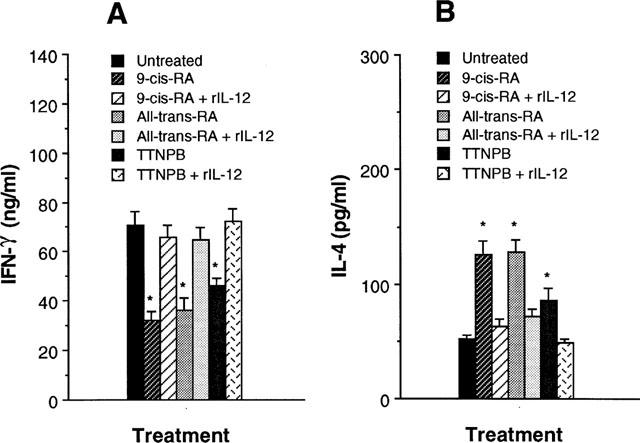
Addition of recombinant IL-12 restores the decreased IFN-γ production of T cells in cultures of retinoid-pretreated macrophages and Ag-primed CD4+ T cells. KLH-primed CD4+ T cells were cultured with 10−7 M retinoid-pretreated macrophages in the presence of rIL-12 (10 pg ml−1) and KLH (10 μg ml−1). Supernatants were harvested 4 days later and assayed for their IFN-γ (A) and IL-4 (B) content by ELISA. The results are presented as mean±s.e.mean (n=3). *P<0.01, relative to each retinoid-treated group in the presence of rIL-12.
Macrophages from mice treated in vivo with 9-cis-RA also inhibited Th1 cytokine profile by Ag-primed CD4+ T cells
To demonstrate that retinoids had a consequential effect on macrophages in an in vivo system, mice were injected intraperitoneally (i.p.) with 100 μg 9-cis-RA per mouse. After 24 h, splenic macrophages were purified from the 9-cis-RA-treated mice, or from saline-injected control mice. Figure 4 shows that macrophages from 9-cis-RA-treated mice induced significantly lower amounts of IL-12 in response to either LPS or HKL than macrophages from control mice (P<0.001).
Figure 4.
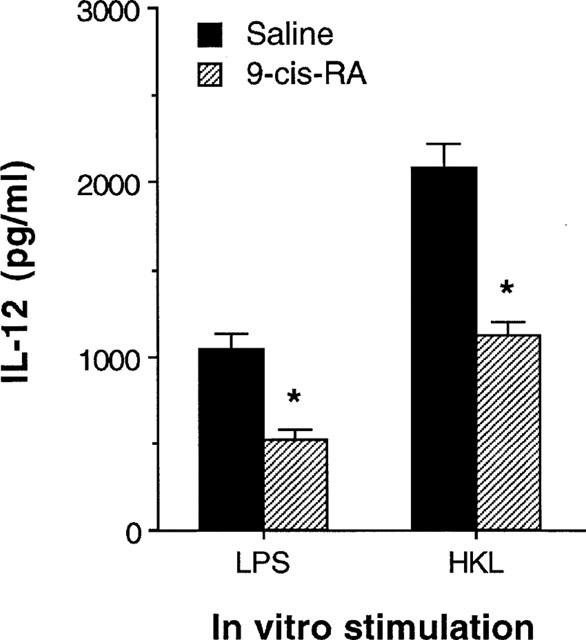
Macrophages exposed to 9-cis-RA in vivo decreased levels of IL-12 production. Mice were treated in vivo with 9-cis-RA (100 μg per mouse, i.p.). After 24 h, macrophages were purified and stimulated with either LPS (5 μg ml−1) or HKL (2×106 bacteria well−1). Culture supernatants were harvested 48 h later and IL-12 content was assayed by ELISA. The results are presented as mean±s.e.mean (n=5). *P<0.001, relative to a saline-injected group.
To determine whether cytokine production by Ag-primed CD4+ T cells would differ in the presence of Ag presented by macrophages from mice treated in vivo with 9-cis-RA, macrophages were purified from DBA/2 mice 24 h following i.p. injection with 9-cis-RA (100 μg per mouse), and cultured with CD4+ T cells from KLH-primed mice, and KLH. Figure 5 demonstrates that production of IL-12 in cultures containing Ag-primed CD4+ T cells and macrophages from 9-cis-RA-treated mice was greatly reduced compared with those from saline-injected control mice. Moreover, macrophages from mice injected with 9-cis-RA significantly induced lower amounts of IFN-γ and greater amounts of IL-4 than macrophages from control mice. Macrophages from mice treated in vivo with either all-trans-RA or TTNPB also inhibited Th1 cytokine profile by Ag-primed CD4+ T cells (data not shown). These results show that in vivo treatment with retinoids regulates the ability of macrophages to control IFN-γ and IL-4 production.
Figure 5.
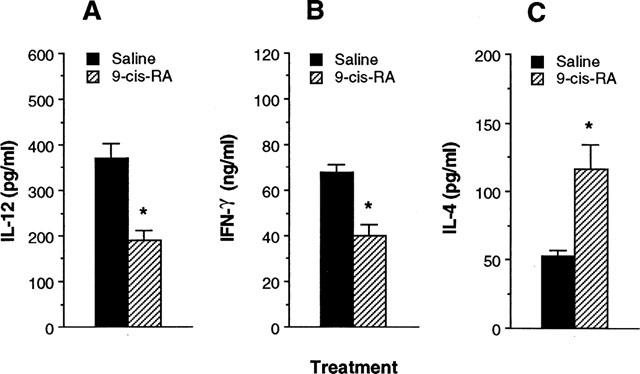
Macrophages purified from mice treated in vivo with 9-cis-RA regulate cytokine production in Ag-primed CD4+ T cells. DBA/2 mice were treated in vivo with either 9-cis-RA (100 μg per mouse, i.p.) or saline. After 24 h, macrophages were purified and incubated with KLH-primed CD4+ T cells and KLH (10 μg ml−1). After 4 days, culture supernatants were harvested and assayed for IL-12 (A), IFN-γ (B) and IL-4 (C) content by ELISA. The results are presented as mean±s.e.mean (n=5). *P<0.01, relative to a saline-injected group.
Discussion
Inhibiting the action of IL-12 has been shown to prevent development and block progression of disease in experimental models of autoimmunity (Caspi, 1998). These findings have raised great interest in identifying inhibitors of IL-12 production for the treatment of Th1-mediated diseases such as type-1 diabetes, multiple sclerosis, rheumatoid arthritis, inflammatory bowel disease and acute graft-versus-host disease. In this study we demonstrated that pretreatment of macrophages with retinoids significantly inhibited IL-12 production from macrophages, resulting in a reduced ability to induce IFN-γ and an increased ability to induce IL-4 in CD4+ T cells. These results suggest that retinoid-mediated inhibition of IL-12 production led to the inhibition of Th1 and enhancement of Th2 cytokine synthesis in CD4+ T cells. Since the cytokine profile of Th cells plays an important role to determine the outcome of many diseases, retinoids may have therapeutic potential to treat Th1-mediated diseases. Conversely, retinoids may trigger or enhance Th2-mediated diseases because of their ability to increase IL-4 production.
Although retinoids may affect cytokine production in CD4+ T cells in several ways, we believe that inhibition of IL-12 production in macrophages is a major mechanism by which retinoids affect cytokine production in CD4+ T cells, particularly since IL-12 is extremely potent in enhancing IFN-γ and inhibiting IL-4 in CD4+ T cells (Marshall et al., 1995; Gerosa et al., 1996; Umetsu et al., 1996). In our cultures, the effect of retinoids on cytokine production in CD4+ T cells was indirect since the CD4+ T cell in these cultures were never directly exposed to the retinoids. Similar studies showed that β2-adrenergic compounds including salbutamol inhibited IL-12 production from human monocytes or dendritic cells by increasing intracellular cyclic AMP levels, leading to the inhibition of Th1 development while promoting Th2 cell differentiation (Panina-Bordignon et al., 1997). Corticosteroids have been known to enhance the capacity of macrophages to induce IL-4 synthesis in CD4+ T cells by inhibiting IL-12 production (DeKruyff et al., 1998). However, retinoids may also directly affect the cytokine production of CD4+ T cells. Racke et al. (1995) reported that the presence of all-trans-RA during in vitro activation of antigen-specific lymph node cells increased IL-4 levels in culture supernatants, while mRNAs for IL-2, TNF-α and IFN-γ were decreased. They suggested that T cell activation in the presence of all-trans-RA resulted in the development of T cells with Th2 phenotype, which might be responsible for the decrease in the encephalitogenicity of antigen-specific T cells.
In the reconstitution experiment of retinoid-treated macrophages with rIL-12, a small amount of rIL-12 (10 pg ml−1) could effectively reverse cytokine production by the activated CD4+ T cells if present at the initiation of cultures. Delayed addition of rIL-12 to the cultures was significantly less effective because the corresponding T cells became more activated and the capacity to produce IL-4 or IFN-γ became more established in vitro (DeKruyff et al., 1995). Addition of 5 pg ml−1 rIL-12 to cultures of dexamethasone-treated macrophages was also known to restore the induction of IFN-γ and inhibit the induction of IL-4 synthesis by T cells (DeKruyff et al., 1998). In addition, rIL-12 might enhance IL-4 production in CD4+ T cells by increasing IL-10 production in the cultures of the retinoid-treated macrophages and T cells. IL-12 has been reported to increase IL-10 production by T cells in systems using established T cell lines (Jeannin et al., 1996). However, IL-10 production increased by IL-12 appears to occur mainly when very high concentrations of IL-12 (10 ng/ml, 1000 fold higher than the amount used in our study) are used (Windhagen et al., 1996). Therefore, reduction of IL-12 production by retinoids was a major immunoregulatory mechanism that led to the inhibition of Th1 cytokine profile in CD4+ T cells.
In conclusion, we have shown that pretreatment of macrophages with retinoids inhibited IL-12 production in a dose-dependent manner, leading to the inhibition of Th1 cytokine profile in CD4+ T cells. These results suggest that retinoid-mediated inhibition of IL-12 production in macrophages may explain some known biological effects of retinoids, and retinoids may be useful in treatment of Th1-mediated immunological disorders.
Acknowledgments
We would like to thank Drs X. Ma, G. Trinchieri, S. Wolf for providing valuable reagents. This work was supported by a grant from the KOSEF (HRC 1998G0201 to T.S. Kim).
Abbreviations
- Ag
antigen
- APC
antigen-presenting cell
- ELISA
enzyme-linked immunosorbent assay
- HKL
heat-killed Listeria monocytogenes
- IFN-γ
interferon-gamma
- IL
interleukin
- KLH
keyhole limpet haemocyanin
- LPS
lipopolysaccharide
- MAb
monoclonal antibody
- RA
retinoic acid
- Th
T helper
- TTNPB
(E)-4-[2-(5,6,7,8-tetrahydro-5,5,8,8,-tetramethyl-2-naphthylenyl)-1-propenyl] benzoic acid
References
- CASPI R.R. IL-12 in autoimmunity. Clin. Immunol. Immunopathol. 1998;88:4–13. doi: 10.1006/clin.1998.4540. [DOI] [PubMed] [Google Scholar]
- CHANDRARATNA R.A. Current research and future developments in retinoids: oral and topical agents. Cutis. 1998;61:40–45. [PubMed] [Google Scholar]
- CONSTANT S.L., BOTTOMLY K. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 1997;15:297–322. doi: 10.1146/annurev.immunol.15.1.297. [DOI] [PubMed] [Google Scholar]
- DEKRUYFF R.H., FANG F., UMETSU D.T. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J. Immunol. 1998;160:2231–2237. [PubMed] [Google Scholar]
- DEKRUYFF R.E., FANG F., WOLF S.E., UMETSU D.T. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J. Immunol. 1995;154:2578–2587. [PubMed] [Google Scholar]
- D'ELIOS M., DEL PRETE G. Th1/Th2 balance in human disease. Transplant Proc. 1998;30:2373–2379. doi: 10.1016/s0041-1345(98)00659-9. [DOI] [PubMed] [Google Scholar]
- EVANS T.R., KAYE S.B. Retinoids: present role and future potential. Br. J. Cancer. 1999;80:1–8. doi: 10.1038/sj.bjc.6690312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GATELY M.K., RENZETTI L.M., MAGRAM J., STERN A.S., ADORINI L., GUBLER U., PRESKY D.H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu. Rev. Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- GEROSA F., PAGANIN C., PERITT D., PAIOLA F., SCUPOLI M.T., ASTE-AMEZAGA M., FRANK I., TRINCHIERI G. Interleukin-12 primes human CD4+ and CD8+ T cell clones for high production of both interferon-gamma and interleukin-10. J. Exp. Med. 1996;183:2559–2569. doi: 10.1084/jem.183.6.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREWE M., BRUIJNZEEL-KOOMEN C.A., SCHOPF E., THEPEN T., LANGEVELD-WILDSCHUT A.G., RUZICKA T., KRUTMANN J. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol. Today. 1998;19:359–361. doi: 10.1016/s0167-5699(98)01285-7. [DOI] [PubMed] [Google Scholar]
- HAYASHI M., MARTINEZ O.M., GARCIA-KENNEDY R., SO S., ESQUIVEL C.O., KRAMS S.M. Expression of cytokines and immune mediators during chronic liver allograft rejection. Transplantation. 1995;60:1533–1538. doi: 10.1097/00007890-199560120-00027. [DOI] [PubMed] [Google Scholar]
- JEANNIN P., DELNESTE Y., SEVESO M., LIFE P., BONNEFOY J.Y. IL-12 synergizes with IL-2 and other stimuli in inducing IL-10 production by human T cells. J. Immunol. 1996;156:3159–3165. [PubMed] [Google Scholar]
- KIM T.S., DEKRUYFF R.H., RUPPER R., MAECKER H.T., LEVY S., UMETSU D.T. An ovalbumin-IL-12 fusion protein is more effective than ovalbumin plus free recombinant IL-12 in inducing a T helper cell type 1-dominated immune response and inhibiting antigen-specific IgE production. J. Immunol. 1997;158:4137–4144. [PubMed] [Google Scholar]
- LAFAILLE J.J. The role of helper T cell subsets in autoimmune diseases. Cytokine Growth Factor Rev. 1998;9:139–151. doi: 10.1016/s1359-6101(98)00009-4. [DOI] [PubMed] [Google Scholar]
- MARSHALL J.D., SECRIST H., DEKRUYFF R.H., WOLF S.F., UMESTU D.T. IL-12 inhibits the production of IL-4 and IL-10 in allergen-specific human CD4+ T lymphocytes. J. Immunol. 1995;155:111–117. [PubMed] [Google Scholar]
- MINUCCI S., OZATO K. Retinoid receptors in transcriptional regulation. Curr. Opin. Genet. Dev. 1996;6:567–574. doi: 10.1016/s0959-437x(96)80085-2. [DOI] [PubMed] [Google Scholar]
- MOSMANN T.R. Cytokine secretion phenotypes of TH cells: how many subsets, how much regulation. Res. Immunol. 1991;142:9–13. doi: 10.1016/0923-2494(91)90003-2. [DOI] [PubMed] [Google Scholar]
- NA S.-Y., KANG B.Y., CHUNG S.W., HAN S.-J., MA X., TRINCHIERI G., IM S.-Y., LEE J.W., KIM T.S. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J. Biol. Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- O'GARRA A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- PANINA-BORDIGNON P., MAZZEO D., LUCIA P.D., D'AMBROSIO D., LANG R., FABBRI L., SELF C., SINIGAGLIA F. β2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Invest. 1997;100:1513–1519. doi: 10.1172/JCI119674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RACKE M.K., BURNETT D., PAK S.H., ALBERT P.S., CANNELLA B., RAINE C.S., MCFARLIN D.E., SCOTT D.E. Retinoid treatment of experimental allergic encephalomyelitis. IL-4 production correlates with improved disease course. J. Immunol. 1995;154:450–458. [PubMed] [Google Scholar]
- ROGERS M.B. Life and death decisions influenced by retinoids. Curr. Top. Dev. Biol. 1997;35:1–46. doi: 10.1016/s0070-2153(08)60255-0. [DOI] [PubMed] [Google Scholar]
- ROMAGNANI S. Th1 and Th2 in human diseases. Clin Immunol. Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- SEDER R.A., PAUL W.E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu. Rev. Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- TRINCHIERI G. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 1998;16:365–396. doi: 10.3109/08830189809043002. [DOI] [PubMed] [Google Scholar]
- UMETSU D.T., GIENI R., DEKRUYFF R.H. Effects of IL-12 in memory CD4+ T lymphocyte responses. Ann. NY Acad. Sci. 1996;795:88–99. doi: 10.1111/j.1749-6632.1996.tb52658.x. [DOI] [PubMed] [Google Scholar]
- WINDHAGEN A., ANDERSON D.E., CATTIZOSA A., WILLIAMS R.E., HAFLER D.A. IL-12 induces human T cells secreting IL-10 with IFN-γ. J. Immunol. 1996;157:1127–1131. [PubMed] [Google Scholar]


