Abstract
Nitric oxide (NO) modulates the levels of various neurotransmitters in the CNS. Here we determined whether the specific nitric oxide synthase (NOS) inhibitor 7-nitroindazole (7-NI), the non-selective inhibitor of guanylate cyclase (GC) and NOS, methylene blue (MB), the NO-precursor L-arginine (L-Arg), and the selective soluble GC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) affect extracellular levels of serotonin (5-HT), dopamine (DA), 5-hydroxyindoleacetic acid (5-HIAA), and homovanillic acid (HVA) in the rat ventral hippocampus by using microdialysis in freely moving animals.
Local perfusion of 7-NI (1 mM) and MB (1 mM) significantly increased extracellular level of 5-HT, whereas DA was increased by 7-NI only. Systemic administration of 7-NI (50 mg kg−1) and MB (30 mg kg−1) increased the extracellular levels of 5-HT and DA. Extracellular levels of 5-HIAA was not influenced by local or systemic MB or 7-NI. In contrast, extracellular level of HVA was decreased by systemic MB and retrodialyzed MB, but was not influenced by 7-NI.
Retrodialysis of L-Arg (2 mM) decreased the levels of 5-HT, DA, 5-HIAA and HVA in the hippocampus. Systemic administration of L-Arg (250 mg kg−1) decreased the level of 5-HT, but failed to influence DA, 5-HIAA and HVA.
Local perfusion of ODQ (400 μM) did not affect 5-HT overflow in the hippocampus.
We conclude that NOS inhibitors increased extracellular levels of 5-HT and DA in the rat ventral hippocampus after local or systemic administration, whereas the NO precursor L-Arg had the opposite effect. Thus, endogenous NO may exert a negative control over the levels of 5-HT and DA in the hippocampus. However, this effect is probably not mediated by cyclic GMP.
Keywords: Serotonin, dopamine, nitric oxide, 7-nitroindazole, methylene blue, ODQ, microdialysis, hippocampus
Introduction
Nitric oxide (NO) is an unconventional transmitter molecule in the nervous system, which is synthesized from L-arginine (L-Arg) by nitric oxide synthase (NOS) (Garthwaite et al., 1988). NO has been suggested to have multiple possible targets, among which the soluble guanylate cyclase (GC) is the most extensively characterized (Yun et al., 1997).
Several in vitro studies have demonstrated that NO modulates the extracellular levels of various neurotransmitters in the central nervous system, e.g. serotonin (5-HT), dopamine (DA), γ-aminobutyric acid (GABA), and glutamate (Kaehler et al., 1999; Strasser et al., 1994; Lorrain & Hull, 1993; Segovia et al., 1994). In addition, NO can inactivate the rate limiting enzyme in the synthesis of 5-HT, tryptophan hydroxylase (Kuhn & Arthur, 1996) and has been suggested to stimulate synaptic vesicle release from hippocampal synaptosomes (Meffert et al., 1994). Furthermore, NO inhibits uptake of [3H]-DA by striatal synaptosomes (Lonart & Johnson, 1994) and transforms 5-HT into an inactive form (Fossier et al., 1999; Pogun et al., 1994). It is currently obscure which of the many possible targets of NO are important in vivo.
To date, there are no in vivo studies describing the role of NO in regulating hippocampal levels of 5-HT and DA. However, local perfusion of N-methyl-D-aspartate (NMDA) into hippocampus, which is believed to increase the production of NO (Luo et al., 1993), significantly decreased the extracellular levels of 5-HT and DA (Whitton et al., 1994a,1994b; Tao & Auerbach, 1996). These findings indirectly indicate that NO may interact with the serotonergic and dopaminergic neurotransmission by suppressing the overflow of 5-HT and DA in the hippocampus.
Further characterization of NO's actions in the hippocampus could have several neurobiological implications, in light of the alleged role of NOergic and serotonergic mechanisms in anxiety and depression (Wiley et al., 1995; Volke et al., 1997; Faria et al., 1997; Harkin et al., 1999; Mongeau et al., 1997).
The aim of present study was, using microdialysis in freely moving animals, to elucidate the possible role of NO in the regulation of extracellular levels of 5-HT and DA in ventral hippocampus after local and systemic application of drugs that affects the NO level.
Methods
Animals
All animal procedures were accepted by the Danish National Committee for Ethics in Animal Experimentation (j.no. 1998-561-44). Male Sprague-Dawley rats (MB Breeding Centre, Denmark) weighing 280–400 g were used. They were housed individually at 20±2°C in 12 h light/dark cycle (light on at 0700). Tap water and chow pellets were available ad libitum. The animals were kept for at least 2 weeks in the animal colony before entering experiments.
Microdialysis and analytical technique
Under the fentanyl-fluanisone (0.0945 and 0.3 mg kg−1, respectively)+midazolam (0.25 mg kg−1) anaesthesia a guide cannula (CMA/12) was implanted just above the ventral hippocampus at AP −4; L −4.5; DV −5 relative to bregma (Paxinos & Watson, 1982) and was secured using three screws and dental acrylic. Immediately after the operation, the animal was injected with buprenorphin at 0.03 mg kg−1 s.c. After 3–5 days, the rat was sedated with fentanyl-fluanisone+midazolam, and a concentric style (I-shaped) microdialysis probe (CMA/12 – 4 mm tip length) was inserted through the guide cannula and was secured with plastic glue. The animals were attached to a dual channel swivel (Instech technology, Plymouth, PA, U.S.A.), allowing unrestricted movement in the test cage.
The probes were perfused in situ overnight (14–16 h) with artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 145, KCl 3, CaCl2 1.2, MgCl2 1. In order to study release of 5-HT, 1 μM citalopram was added to the ACSF. During the experiment, ACSF was pumped at a rate of 1.5 μl min−1 and samples were collected continuously for intervals of 20 min. Analysis of the samples was carried out within 1–12 h after collection. Before and after the experimental microdialysis session, recoveries of all probes were assessed by examining the amount of neurotransmitters from a known standard solution passing through the probe-membrane to the dialysate. All microdialysis disposables were obtained from CMA/Microdialysis AB (Stockholm, Sweden).
High pressure liquid chromotography (HPLC) with electrochemical detection was used for sample analysis (ESA Coulochem II with 5014B microdialysis coulometric analytic cell; ESA Inc. MA, U.S.A.). The mobile phase was composed of 50 mM NaH2PO4, 740 μM 1-octanesulphonic acid, 108 μM Na-EDTA, 80 ml l−1 acetonitrile and 100 μl l−1 triethylamine with pH adjusted to 3.5 using H3PO4.
Histology
At the end of the experiment the rat was anaesthetized with chloral hydrate (2.5 mM kg−1 i.p.) and cresyl-violete was perfused (1.0 μl min−1) through a dialysis probe for about 10 min to stain the surrounding tissue. Using this procedure only tissue intimately in contact with the probe was stained. After removal, the brain was fixed in a 4% formalin solution for at least 2 days. Subsequently, the brain was sliced on a microtome and the probe location was inspected under a microscope. Animals with misplaced probes were excluded from the study.
Drugs
Drugs were administered directly via the probe, injected intraperitoneally (i.p.) or subcutaneously (s.c.). Doses and routes of systemic administration were chosen according to behavioural studies showing the drugs to have the intended effect (Volke et al., 1997; 1998; Eroglu & Caglayan, 1997). All chemicals were of reagent grade or higher. L-Arg, 7-NI, triethylamine, 1-octanesulphonic acid, Tween-80 (Polyoxyethylenesorbitan monooleate), and NaH2PO4 were obtained from Sigma (St. Louis, MO, U.S.A.). 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) was purchased from Calbiochem-Novabiochem (San Diego, CA, U.S.A.). Chloralhydrate, acetonitrile, dimethyl sulphoxide (DMSO) and Na-EDTA were obtained from Merck (Darmstadt, Germany). MB stock solution (10 mg methylthionine chloride per ml sterile water) was purchased from the local hospital pharmacy. Citalopram-HBr was kindly donated by H. Lundbeck A/S, Valby, Copenhagen.
ODQ was dissolved in DMSO and was made up to final volume by addition of ACSF. The final concentration of DMSO in the perfusion media was 0.3%. 7-NI was dissolved in a few drops of Tween-80, and was made up to final volume by addition of isotonic saline (injection studies) or directly dissolved in ACSF by a slight (50–60°C) heating procedure (perfusion studies). All other chemicals used for animal experimentation were dissolved in the ACSF or isotonic saline.
Statistics
Microdialysis results are expressed as mean percentage change from the average of four sequential baseline measurements±standard error of the mean (s.e.mean). Statistical analysis (SPSS version 9.0) on dialysate 5-HT and DA-extracellular levels was carried out by comparing drug-treated and control animals for significant differences using analysis of variance with repeated measures over time. The data were corrected for multiple comparisons using the Bonferroni procedure (Grove & Andreasen, 1982). Differences were considered statistically significant when P was less than 0.05. The numbers of animals in each group are given in figure legends.
Figure 1.
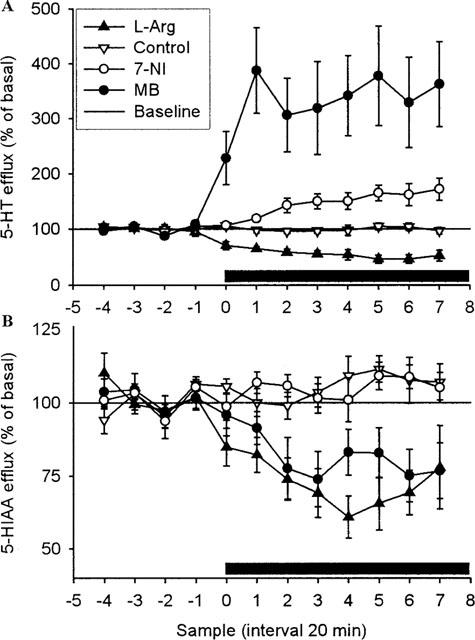
Effect of local perfusion of L-Arg (2 mM), 7-NI (1 mM), MB (1 mM) or artificial CSF on 5-HT (A) (n=8, n=12, n=7 and n=13, respectively) and 5-HIAA (B) (n=6, n=7, n=7 and n=10, respectively) overflow in ventral hippocampus of rats. Drugs were infused into ventral hippocampus as indicated by bar. Results are expressed as per cent of basal efflux±s.e.mean.
Results
Hippocampal 5-HT release is increased by local perfusion of 7-NI or MB, and decreased by L-Arg (Figure 1)
In the first series of experiments, we studied the effects of 7-NI (1 mM), MB (1 mM), and L-Arg (2 mM) on extracellular levels of 5-HT and 5-HIAA in the ventral hippocampus. Retrodialysis of MB caused an immediate and marked elevation of released 5-HT to a plateau ca. 350% of base level (P<0.001). Also 7-NI produced a significant increase in released 5-HT (P<0.05), albeit the time-course was slower and the maximal level reached was about 200% of baseline. No changes were observed in the extracellular 5-HIAA level after administration of 7-NI and MB.
In contrast, administration of L-Arg decreased 5-HT to 50% of baseline (P<0.001); the reduction occurred immediately and quickly reached a plateau, which was maintained throughout the experiment. The extracellular level of the metabolite 5-HIAA was significantly decreased by L-Arg; in a way similar to 5-HT (P<0.001).
Figure 2.
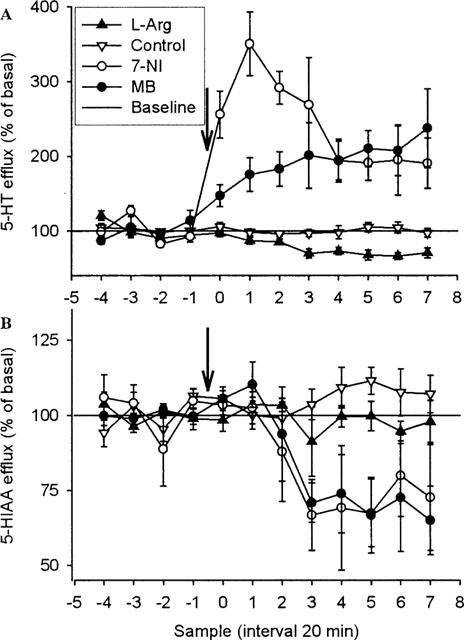
Effect of systemic injection of 7-NI (50 mg kg−1, i.p.), MB (30 mg kg−1,s.c.), L-Arg (250 mg kg−1, s.c.) or vehicle on 5-HT (A) (n=5, n=6, n=7 and n=13, respectively) and 5-HIAA (B) (n=4, n=3, n=9 and n=13, respectively) overflow in ventral hippocampus of rats. Drugs were injected as indicated by the arrow. Results are expressed as per cent of basal efflux±s.e.mean.
Hippocampal 5-HT release is increased by systemic administration of 7-NI or MB, and decreased by L-Arg (Figure 2)
7-NI (50 mg kg−1, i.p.) and MB (30 mg kg−1, s.c.) given systemically caused a significant increase in 5-HT release (P<0.001 and P<0.01, respectively). The increase in 5-HT release occurred immediately after injection of 7-NI and peaked within 1 h at 350% of baseline, whereas MB caused a slower but continuous increase in 5-HT release. Both 7-NI and MB failed to affect the extracellular level of 5-HIAA. In contrast, injection of L-Arg (250 mg kg−1, s.c.) significantly decreased the extracellular level of 5-HT (P<0.01), but failed to influence 5-HIAA.
Figure 3.
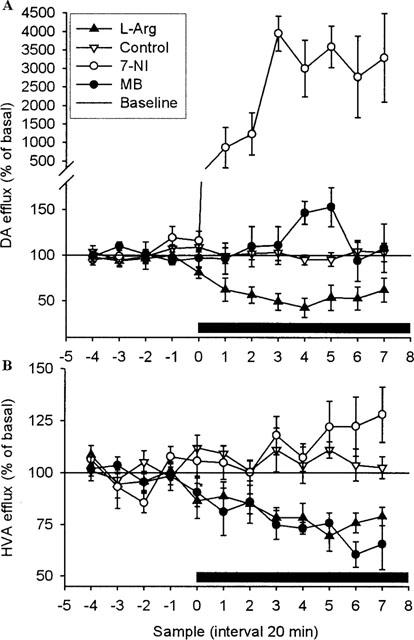
Effect of local perfusion of L-Arg (2 mM), 7-NI (1 mM), MB (1 mM) or artificial CSF on DA (A) (n=6, n=7, n=7 and n=10, respectively) and HVA (B) (n=10, n=7, n=7 and n=10, respectively) overflow in ventral hippocampus of rats. Drugs were infused into ventral hippocampus as indicated by bar. Results are expressed as per cent of basal efflux±s.e.mean.
Hippocampal DA is increased by local perfusion of 7-NI or MB, and decreased by L-Arg (Figure 3)
Retrodialysis of the ventral hippocampus with 7-NI (1 mM) caused an immediate and marked elevation of the level of DA to about 4000% of baseline (P<0.001). MB (1 mM) failed, however, to affect the extracellular DA. No changes were observed in extracellular HVA levels after administration of 7-NI, but a decrease was seen in the group retrodialyzed with MB (P<0.05). L-Arg decreased the extracellular DA levels to 50% of baseline (P<0.05). The reduction occurred immediately and it slowly progressed to a through after 3 h. In addition, the extracellular level of HVA was reduced by L-Arg (P<0.01).
Figure 4.
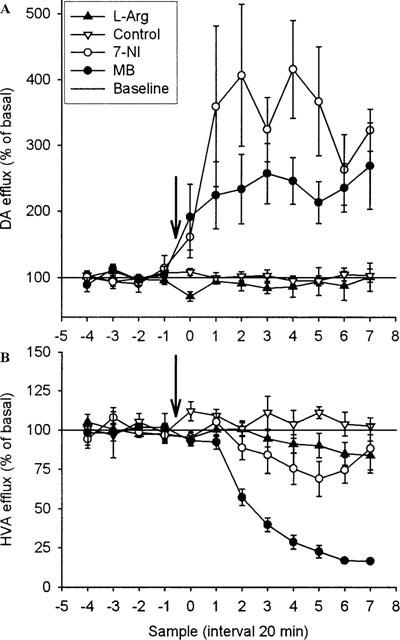
Effect of systemic injection of 7-NI (50 mg kg−1, i.p.), MB (30 mg kg−1, s.c.), L-Arg (250 mg kg−1, s.c.) or vehicle on DA (A) (n=5, n=5, n=4 and n=10, respectively) and HVA (B) (n=6, n=4, n=5 and n=9, respectively) overflow in ventral hippocampus of rats. Drugs were injected as indicated by the arrow. Results are expressed as per cent of basal efflux±s.e.mean.
Hippocampal DA is increased by systemic administration of 7-NI or MB (Figure 4)
Systemic administration of 7-NI (50 mg kg−1, i.p.) and MB (30 mg kg−1, s.c.) caused a significant increase in the extracellular level of DA (P<0.01 and P<0.05, respectively). The increase occurred immediately after injection of either drug and rapidly reached a plateau at 250% (MB) and 400% (7-NI) of baseline. MB caused a marked decrease in extracellular levels of HVA, which reached a plateau at 20% of basal (P<0.001). The extracellular level of HVA after was not influenced following the 7-NI injection. Systemic administration of L-Arg failed to influence DA and HVA.
Figure 5.
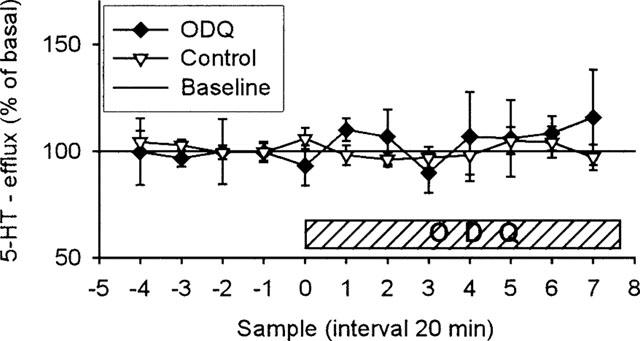
Effect of local perfusion of ODQ (400 μM, n=5) or artificial CSF (n=13) on 5-HT overflow in ventral hippocampus of rats. ODQ was infused into ventral hippocampus as indicated by bar. Results are expressed as per cent of basal efflux±s.e.mean.
Local perfusion of 1H-[1,2,4]oxadiazolo [4,3-a]quinoxalin-1-one (ODQ) fails to affect hippocampal 5-HT release (Figure 5)
In order to clarify whether the possible effect of NO on the extracellular levels of 5-HT and DA is mediated by cyclic GMP, we examined the effect of addition of the selective NO-sensitive-sGC inhibitor ODQ to the perfusion media (containing 0.3% DMSO). In agreement with previous studies (Fedele et al., 1996), DMSO alone at this concentration had no effect on 5-HT release (results not shown), so DMSO was not included in the perfusion media for the controls. Addition of ODQ to the perfusion media (400 μM) did not influence the release of 5-HT compared with controls. However, due to the co-elusion of ODQ with DA in our chromatographic system, we were not able to detect changes in DA levels.
Discussion
The main finding of the present study is that both local and systemic administration of NOS inhibitors, 7-NI or MB, increased the efflux of 5-HT and DA from the ventral hippocampus in freely moving rats. These results agree with previous studies in which the NMDA receptor antagonists, MK-801 and D-AP5, increased extracellular levels of 5-HT and DA in the ventral hippocampus in vivo (Whitton et al., 1992a,1992b; 1994a). Some studies have also shown similar effects of NOS inhibitors in other areas of the brain (Silva et al., 1995; Desvignes et al., 1999; Kaehler et al., 1999). Thus, it appears that under basal conditions, endogenous NO plays an important role in negatively controlling extracellular levels of 5-HT and DA in the hippocampus. Retrodialysis of the ventral hippocampus with 7-NI induced a very large magnitude of increase in extracellular DA compared with the increase in 5-HT. However, as the perfusion media contained citalopram, the basal level of 5-HT was already elevated. Thus, it cannot be excluded that the true elevation of 5-HT produced by 7-NI was partially masked by citalopram.
In accordance with the effects of NOS inhibitors, perfusion with the endogenous NO-precursor, L-Arg, decreased the extracellular levels of 5-HT, DA, 5-HIAA and HVA. This probably represents the true effect of facilitated NO synthesis as L-Arg (1 mM) has been shown to increase NOS activity in the hippocampus (Vallebuona & Raiteri, 1994). Similar effects on the levels of 5-HT and DA have been obtained with local perfusion of NMDA into the ventral hippocampus (Whitton et al., 1994a,1994b; Tao & Auerbach, 1996).
However, contradictory evidence also exists concerning the role of NO on the levels of the monoamines. For example, the NO donor hydroxylamine has been shown to increase DA release from striatal slices (Lonart et al., 1993) which, however, may not depend on NO, since low L-Arg concentrations, had no effect on DA release itself (Silva et al., 1995). Furthermore, L-Arg has been shown to induce DA release from the striatum and increase the extracellular levels of 5-HT and DA in the medial preoptic area in vivo (Strasser et al., 1994; Lorrain & Hull, 1993). We believe that NO serves different roles in regulating transmitter levels in distinct brain areas and, at least in the ventral hippocampus, increased NO synthesis may result in suppression of 5-HT and DA overflow.
Two distinct isoforms of NOS, neuronal (nNOS) and endothelial NOS (eNOS), with different anatomical and intracellular localization, have been described in neurons (Dinerman et al., 1994). It is likely that eNOS and nNOS serve different roles in the regulation of neurotransmitter release (Kano et al., 1998). 7-NI has often been referred to as being a selective inhibitor of neuronal NOS in vivo. This assumption may be incorrect, however, since it is based solely on the fact that 7-NI in some studies does not increase blood pressure of animals (Moore et al., 1993). Moreover, direct evidence is missing as to whether 7-NI affects eNOS activity in neurons. By using knockout mice, Kano et al. (1998) have indirectly shown that 7-NI seems to inhibit both nNOS and eNOS in the cerebral cortex when administered locally. Thus, the contribution of different isoforms of NOS to the regulation of the levels of 5-HT and DA in the hippocampus remains to be established.
The GC inhibitor ODQ did not affect the 5-HT release. The dose of ODQ used has been shown to decrease the hippocampal cyclic GMP level by 50% (Fedele et al., 1996). Thus, it seems likely that cyclic GMP does not have a major role in mediating the effects of NO on hippocampal transmitter levels under basal conditions. We cannot, however, exclude the possible role of cyclic GMP in case of increased concentration of NO. Further studies are warranted to clarify this matter.
Interestingly, not only local, but also systemic administration of 7-NI and MB increased the efflux of 5-HT and DA. There are several studies reporting potent anxiolytic and antidepressant-like effects of NOS inhibitors in various behavioural models (Wiley et al., 1995; Volke et al., 1997; Faria et al., 1997; Harkin et al., 1999). Furthermore, MB has been used as an experimental antidepressant in patients suffering from bipolar disorder (Narsapur & Naylor, 1983; Naylor et al., 1987). The present study raises the question whether the neurochemical basis of this action may be the change in 5-HT turnover similar to what is seen after established antidepressant therapy (Kreiss & Lucki, 1995). Proper answer to this question does, however, require further work. As conventional anxiolytic drugs are expected to suppress levels of 5-HT in the hippocampus (Nishikawa & Scatton, 1986) we believe that the mechanism underlying the anxiolytic-like activity of NOS inhibitors is not related to the change in the 5-HT efflux.
We conclude that NOS inhibitors increase the extracellular levels of 5-HT and DA in the rat ventral hippocampus after local or systemic administration whereas NO precursor L-Arg had the opposite effect. Thus, endogenous NO seems to exert a negative control over the levels of 5-HT and DA in the hippocampus. However, this effect is probably not mediated by the cyclic GMP.
Acknowledgments
This study was supported in part by Danish Medical Association Research Fund (086.51), Hørslev Fonden (1999), Eli Lillys Psychiatric Research Foundation (97.17.11), The Novo Nordisk Foundation (1999), Psykiatrisk Forskningsfond (1999), Konsul Johannes Fogh-Nielsen og hustru fru Ella Fogh-Nielsens Legat (1999-721-014), Aarhus University Research Foundation (F-1999-SUN-1-4) and Estonian Science Foundation (2980). We thank Donald F. Smith for constructive comments and Bodil Schmidt for skilful technical assistance.
Abbreviations
- DA
dopamine
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
serotonin
- HVA
homovanillic acid
- L-Arg
L-arginine
- MB
methylene blue
- 7-NI
7-nitroindazole
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- s.e.mean
standard error of the mean
References
- DESVIGNES C., BERT L., VINET L., DENOROY L., RENAUD B., LAMBAS-SENAS L. Evidence that the neuronal nitric oxide synthase inhibitor 7-nitroindazole inhibits monoamine oxidase in the rat: in vivo effects on extracellular striatal dopamine and 3,4-dihydroxyphenylacetic acid. Neurosci. Lett. 1999;264:5–8. doi: 10.1016/s0304-3940(99)00139-1. [DOI] [PubMed] [Google Scholar]
- DINERMAN J.L., DAWSON T.M., SCHELL M.J., SNOWMAN A., SNYDER S.H. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EROGLU L., CAGLAYAN B. Anxiolytic and antidepressant properties of methylene blue in animal models. Pharmacol. Res. 1997;36:381–385. doi: 10.1006/phrs.1997.0245. [DOI] [PubMed] [Google Scholar]
- FARIA M.S., MUSCARA M.N., MORENO J.H., TEIXEIRA S.A., DIAS H.B., DE OLIVEIRA B., GRAEFF F.G., DE NUCCI G. Acute inhibition of nitric oxide synthesis induces anxiolysis in the plus maze test. Eur. J. Pharmacol. 1997;323:37–43. doi: 10.1016/s0014-2999(97)00027-7. [DOI] [PubMed] [Google Scholar]
- FEDELE E., JIN Y., VARNIER G., RAITERI M. In vivo microdialysis study of a specific inhibitor of soluble guanylyl cyclase on the glutamate receptor/nitric oxide/cyclic GMP pathway. Br. J. Pharmacol. 1996;119:590–594. doi: 10.1111/j.1476-5381.1996.tb15713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOSSIER P., BLANCHARD B., DUCROCQ C., LEPRINCE C., TAUC L., BAUX G. Nitric oxide transforms serotonin into an inactive form and this affects neuromodulation. Neuroscience. 1999;93:597–603. doi: 10.1016/s0306-4522(99)00165-7. [DOI] [PubMed] [Google Scholar]
- GARTHWAITE J., CHARLES S.L., CHESS-WILLIAMS R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- GROVE W.M., ANDREASEN N.C. Simultaneous tests of many hypotheses in exploratory research. J. Nerv. Ment. Dis. 1982;170:3–8. doi: 10.1097/00005053-198201000-00002. [DOI] [PubMed] [Google Scholar]
- HARKIN A.J., BRUCE K.H., CRAFT B., PAUL I.A. Nitric oxide synthase inhibitors have antidepressant-like properties in mice. 1. Acute treatments are active in the forced swim test. Eur. J. Pharmacol. 1999;372:207–213. doi: 10.1016/s0014-2999(99)00191-0. [DOI] [PubMed] [Google Scholar]
- KAEHLER S.T., SINGEWALD N., SINNER C., PHILIPPU A. Nitric oxide modulates the release of serotonin in the rat hypothalamus. Brain Res. 1999;835:346–349. doi: 10.1016/s0006-8993(99)01599-1. [DOI] [PubMed] [Google Scholar]
- KANO T., SHIMIZU-SASAMATA M., HUANG P.L., MOSKOWITZ M.A., LO E.H. Effects of nitric oxide synthase gene knockout on neurotransmitter release in vivo. Neuroscience. 1998;86:695–699. doi: 10.1016/s0306-4522(98)00179-1. [DOI] [PubMed] [Google Scholar]
- KREISS D.S., LUCKI I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J. Pharmacol. Exp. Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- KUHN D.M., ARTHUR R.E.J. Inactivation of brain tryptophan hydroxylase by nitric oxide. J. Neurochem. 1996;67:1072–1077. doi: 10.1046/j.1471-4159.1996.67031072.x. [DOI] [PubMed] [Google Scholar]
- LONART G., CASSELS K.L., JOHNSON K.M. Nitric oxide induces calcium-dependent [3H]dopamine release from striatal slices. J. Neurosci. Res. 1993;35:192–198. doi: 10.1002/jnr.490350210. [DOI] [PubMed] [Google Scholar]
- LONART G., JOHNSON K.M. Inhibitory effects of nitric oxide on the uptake of [3H]dopamine and [3H]glutamate by striatal synaptosomes. J. Neurochem. 1994;63:2108–2117. doi: 10.1046/j.1471-4159.1994.63062108.x. [DOI] [PubMed] [Google Scholar]
- LORRAIN D.S., HULL E.M. Nitric oxide increases dopamine and serotonin release in the medial preoptic area. Neuroreport. 1993;5:87–89. doi: 10.1097/00001756-199310000-00024. [DOI] [PubMed] [Google Scholar]
- LUO D., KNEZEVICH S., VINCENT S.R. N-methyl-D-aspartate-induced nitric oxide release: an in vivo microdialysis study. Neuroscience. 1993;57:897–900. doi: 10.1016/0306-4522(93)90035-e. [DOI] [PubMed] [Google Scholar]
- MEFFERT M.K., PREMACK B.A., SCHULMAN H. Nitric oxide stimulates Ca(2+)-independent synaptic vesicle release. Neuron. 1994;12:1235–1244. doi: 10.1016/0896-6273(94)90440-5. [DOI] [PubMed] [Google Scholar]
- MONGEAU R., BLIER P., DE MONTIGNY C. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res. Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., BABBEDGE R.C., WALLACE P., GAFFEN Z.A., HART S.L. 7-Nitro indazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br. J. Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARSAPUR S.L., NAYLOR G.J. Methylene blue. A possible treatment for manic depressive psychosis. J. Affect. Disord. 1983;5:155–161. doi: 10.1016/0165-0327(83)90008-3. [DOI] [PubMed] [Google Scholar]
- NAYLOR G.J., SMITH A.H., CONNELLY P. A controlled trial of methylene blue in severe depressive illness. Biol. Psychiatry. 1987;22:657–659. doi: 10.1016/0006-3223(87)90194-6. [DOI] [PubMed] [Google Scholar]
- NISHIKAWA T., SCATTON B. Neuroanatomical site of the inhibitory influence of anxiolytic drugs on central serotonergic transmission. Brain Res. 1986;371:123–132. doi: 10.1016/0006-8993(86)90817-6. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. Academic: Sydney; 1982. [DOI] [PubMed] [Google Scholar]
- POGUN S., BAUMANN M.H., KUHAR M.J. Nitric oxide inhibits [3H]dopamine uptake. Brain Res. 1994;641:83–91. doi: 10.1016/0006-8993(94)91818-x. [DOI] [PubMed] [Google Scholar]
- SEGOVIA G., PORRAS A., MORA F. Effects of a nitric oxide donor on glutamate and GABA release in striatum and hippocampus of the conscious rat. Neuroreport. 1994;5:1937–1940. doi: 10.1097/00001756-199410000-00024. [DOI] [PubMed] [Google Scholar]
- SILVA M.T., ROSE S., HINDMARSH J.G., AISLAITNER G., GORROD J.W., MOORE P.K., JENNER P., MARSDEN C.D. Increased striatal dopamine efflux in vivo following inhibition of cerebral nitric oxide synthase by the novel monosodium salt of 7-nitro indazole. Br. J. Pharmacol. 1995;114:257–258. doi: 10.1111/j.1476-5381.1995.tb13219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSER A., MCCARRON R.M., ISHII H., STANIMIROVIC D., SPATZ M. L-arginine induces dopamine release from the striatum in vivo. Neuroreport. 1994;5:2298–2300. doi: 10.1097/00001756-199411000-00023. [DOI] [PubMed] [Google Scholar]
- TAO R., AUERBACH S.B. Differential effect of NMDA on extracellular serotonin in rat midbrain raphe and forebrain sites. J. Neurochem. 1996;66:1067–1075. doi: 10.1046/j.1471-4159.1996.66031067.x. [DOI] [PubMed] [Google Scholar]
- VALLEBUONA F., RAITERI M. Extracellular cGMP in the hippocampus of freely moving rats as an index of nitric oxide (NO) synthase activity. J. Neurosci. 1994;14:134–139. doi: 10.1523/JNEUROSCI.14-01-00134.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLKE V., SOOSAAR A., KOKS S., BOURIN M., MANNISTO P.T., VASAR E. 7-Nitroindazole, a nitric oxide synthase inhibitor, has anxiolytic-like properties in exploratory models of anxiety. Psychopharmacol (Berl.) 1997;131:399–405. doi: 10.1007/s002130050309. [DOI] [PubMed] [Google Scholar]
- VOLKE V., SOOSAAR A., KOKS S., VASAR E., MANNISTO P.T. L-arginine abolishes the anxiolytic-like effect of diazepam in the elevated plus-maze test in rats. Eur. J. Pharmacol. 1998;351:287–290. doi: 10.1016/s0014-2999(98)00364-1. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., BIGGS C.S., PEARCE B.R., FOWLER L.J. MK-801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J. Neurochem. 1992a;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., BIGGS C.S., PEARCE B.R., FOWLER L.J. Regional effects of MK-801 on dopamine and its metabolites studied by in vivo microdialysis. Neurosci. Lett. 1992b;142:5–8. doi: 10.1016/0304-3940(92)90607-9. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., MAIONE S., BIGGS S.C., FOWLER L.J. N-methyl-d-aspartate receptors modulate extracellular dopamine concentration and metabolism in rat hippocampus and striatum in vivo. Brain Res. 1994a;635:312–316. doi: 10.1016/0006-8993(94)91453-2. [DOI] [PubMed] [Google Scholar]
- WHITTON P.S., RICHARDS D.A., BIGGS C.S., FOWLER L.J. N-methyl-D-aspartate receptors modulate extracellular 5-hydroxytryptamine concentration in rat hippocampus and striatum in vivo. Neurosci. Lett. 1994b;169:215–218. doi: 10.1016/0304-3940(94)90395-6. [DOI] [PubMed] [Google Scholar]
- WILEY J.L., CRISTELLO A.F., BALSTER R.L. Effects of site-selective NMDA receptor antagonists in an elevated plus-maze model of anxiety in mice. Eur. J. Pharmacol. 1995;294:101–107. doi: 10.1016/0014-2999(95)00506-4. [DOI] [PubMed] [Google Scholar]
- YUN H.Y., DAWSON V.L., DAWSON T.M. Nitric oxide in health and disease of the nervous system. Mol. Psychiatry. 1997;2:300–310. doi: 10.1038/sj.mp.4000272. [DOI] [PubMed] [Google Scholar]


