Abstract
In the absence of selective antagonists, pharmacological characterization of P2Y receptor subtypes has relied heavily upon their distinct agonist profiles. 2-methylthioADP (2-MeSADP) is a selective agonist for the P2Y1 receptor. The agonist action of 2-MeSATP at the P2Y1 receptor has recently been questioned.
The effects of both 2-MeSADP and 2-MeSATP have been studied on rat hepatocytes injected with the bioluminescent Ca2+ indicator, aequorin. Single hepatocytes generate series of repetitive transients in cytosolic free calcium concentration ([Ca2+]i) when stimulated with agonists acting through the phosphoinositide signalling pathway.
The transients induced by 2-MeSADP and 2-MeSATP in the same cell were indistinguishable, indicating that they act at a common receptor. In contrast the transients evoked by ATP and UTP had very different profiles. Treatment of 2-MeSATP with an ATP-regenerating system to remove contaminating 2-MeSADP did not abolish its agonist activity.
Application of the P2Y1 antagonist, adenosine-3′-phosphate-5′-phosphate (A3P5P) inhibited the transients induced by both 2-MeSADP and 2-MeSATP. In contrast the transients induced by ATP and UTP were enhanced by the addition of A3P5P.
These results indicate that both 2-MeSADP and 2-MeSATP are agonists at the rat hepatocyte P2Y1 receptor.
Keywords: Rat hepatocytes, P2Y receptors, intracellular free calcium concentration, calcium transients, ADP, 2-methylthioATP, 2-methylthioADP
Introduction
P2Y receptors are G-protein-coupled receptors responding to extracellular ADP, ATP, UDP and UTP (Burnstock, 1996). Five mammalian P2Y receptor subtypes have been cloned, all of which are coupled to the hydrolysis of phosphatidylinositol 4,5-bisphosphate and hence to inositol 1,4,5-trisphosphate-mediated release of intracellular Ca2+ stores (Boarder & Hourani, 1998). In the absence of selective antagonists, pharmacological characterization of these receptor subtypes has relied heavily upon their agonist profiles. It was thought that 2-MeSATP was a potent agonist at the P2Y1 receptor (Schachter et al., 1996; Burnstock & King, 1996). However, this was challenged by Hechler et al. (1998) who reported that the agonist action of 2-MeSATP on the P2Y1 receptor was due to contamination with 2-MeSADP. Rather than acting as an agonist, they reported that purified 2-MeSATP was an antagonist at the cloned human P2Y1 receptor and the native P2Y1 receptor on rat brain capillary endothelial cells.
Here the effects of both 2-MeSADP and 2-MeSATP have been studied on single rat hepatocytes injected with the Ca2+ indicator, aequorin. In common with many other cell types (Berridge, 1990), single rat hepatocytes generate repetitive transients in cytosolic free calcium concentration ([Ca2+]i) when stimulated with agonists acting through the phosphoinositide signalling pathway (Woods et al., 1986; 1987), including ADP and ATP (Cobbold et al., 1988; Dixon et al., 1990). The duration of the [Ca2+]i transients is dependent on the receptor being activated, so that transients of very different duration can be recorded from the same individual hepatocyte when stimulated with different agonists (Woods et al., 1987). This characteristic of the hepatocyte oscillator can therefore be used as a tool to determine whether 2 agonists are acting at the same receptor or at distinct sites. Thus 2-MeSATP and ADP evoke transients which are indistinguishable, providing evidence for a common site of action, presumably a P2Y1 receptor (Dixon et al., 1995a). Conversely, the response to ATP differs from that induced by ADP, indicating that these nucleotides act at distinct receptors (Dixons et al., 1990; 1993; 1995b; Green et al., 1994). Indeed observations from single cells indicate that ATP does not act as an agonist at the P2Y1 receptor on rat hepatocytes. This conflicts with the findings of Palmer et al. (1998) who reported that ATP was a low efficacy agonist at the P2Y1 receptor.
Here the effect of 2-MeSADP is compared with that of 2-MeSATP in the same hepatocyte. The possibility that the agonist action of 2-MeSATP is due to contaminating 2-MeSADP is also investigated. In addition the effect of the selective P2Y1 antagonist, adenosine-3′-phosphate-5′-phosphate (A3P5P; Boyer et al., 1996) on [Ca2+]i transients induced by 2-MeSADP and 2-MeSATP is reported. The results provide evidence that both 2-MeSADP and 2-MeSATP are agonists at the P2Y1 receptor on rat hepatocytes.
Methods
Single hepatocytes were isolated from fed, male Wistar-strain rats (150–250 g) by collagenase perfusion as described previously (Dixon et al., 1995a). Briefly, the hepatic portal vein was cannulated and an initial Ca2+-free perfusion was followed by perfusion with collagenase (0.04% w v−1) and Ca2+ (3.8 mM) for 15 min. The perfusion rate was 30 ml min−1 throughout. The cells were harvested and incubated at 37°C at low density (∼103 cells ml−1) in 2% type IX agarose in William's medium E (WME). Single hepatocytes were prepared for microinjection with the bioluminescent Ca2+ indicator, aequorin as described previously (Cobbold & Lee, 1991). The injected cell was transferred to a perfusable cup held at 37°C, positioned under a cooled, low-noise photomultiplier, and continuously superfused with WME, to which agonists were added. Photon counts were sampled every 50 ms by computer. At the end of an experiment, the total aequorin content of each cell was determined by discharging the aequorin by lysing the cell. The data were normalized retrospectively by computer, by calculating the photon counts per second divided by the total counts remaining. The computed fractional rate of aequorin consumption could then be plotted as [Ca2+]i using in vitro calibration data and exponential smoothing with time constants: for resting [Ca2+]i, 12 s; for transients, 1 s. The duration of transients was determined at their base by computer using custom-written software.
Materials
Aequorin was provided by Professor O. Shimomura (Marine Biological Laboratory, Woods Hole, MA, U.S.A.). Collagenase was obtained from Boehringer (Lewes, U.K.) and WME from GIBCO (Paisley, U.K.). Agarose and nucleotides were purchased from Sigma-Aldrich (Poole, U.K.). A stock solution of 2-MeSATP (4 mM) was treated with an ATP-regenerating system (20 units ml−1 creatine phosphokinase (CPK) and 20 mM phosphocreatine (PC); Sigma-Aldrich) for a minimum of 2 h, to remove contaminating 2-MeSADP (Hechler et al., 1998). Samples of the treated 2-MeSATP stock solution were analysed by HPLC to confirm its composition.
Results
It has previously been demonstrated that 2-MeSATP induces [Ca2+]i transients in hepatocytes which are indistinguishable from those induced by ADP and which contrast with those induced by ATP (Dixon et al., 1995a). In the present study 2-MeSADP is also shown to induce [Ca2+]i transients in aequorin-injected hepatocytes. As illustrated in Figure 1, these transients resemble those induced by 2-MeSATP (and therefore ADP) in the same cell, but contrast with those induced by ATP and UTP. The duration of transients recorded from the same cell was (mean±s.e.mean) 8.2±0.4 s (n=10) for 2-MeSATP and 8.1±0.2 s (n=23) for 2-MeSADP, where n is the number of individual transients. Comparison by Student's t-test revealed no significant difference (P>0.05). There was a considerable degree of cell-to-cell variability in the threshold concentration for transient generation in response to both 2-MeSADP and 2-MeSATP, which ranged from 0.1–10 μM. Notably, however, within the same cell the thresholds for the 2 agonists were similar. As shown previously for ADP (Cobbold et al., 1988) and 2-MeSATP (Dixon et al., 1995a), the response to a maximal dose of 2-MeSADP was composed of high frequency transients of short duration, which were initially superimposed on an elevated baseline (data not shown). This maximal response is achieved at similar concentrations of either 2-MeSATP or 2-MeSADP, which for a given cell is ∼10–20 fold above threshold. This type of response is in contrast to the effect of high concentrations of ATP when transients are replaced by a sustained rise in [Ca2+]i (Cobbold et al., 1988; Dixon et al., 1995a).
Figure 1.
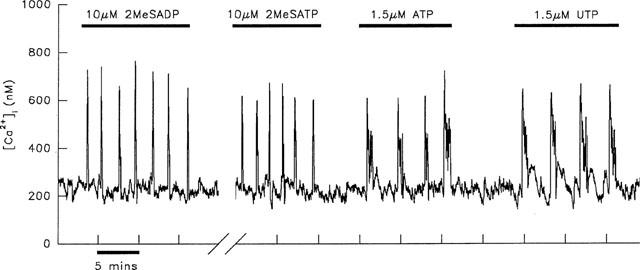
Similarities in the [Ca2+]i response of a single rat hepatocyte to 2-MeSADP and 2-MeSATP, and the contrasting effects of ATP and UTP. Transients were recorded from a single aequorin-injected hepatocyte in response to stimulation with agonists at the concentrations indicated. The short duration transients induced by 10 μM 2-MeSADP were found to be indistinguishable from those induced by 10 μM 2-MeSATP. In contrast stimulation with either 1.5 μM ATP or 1.5 μM UTP induced transients of much longer duration in the same hepatocyte. The break in the x-axis represents a period of 15 min when the cell was superfused with WME alone. This result is typical of 20 experiments.
Hechler et al. (1998) recently reported that the apparent agonist activity of 2-MeSATP was due to contamination with 2-MeSADP; treatment of 2-MeSATP with an ATP-regenerating system rendered it inactive. As depicted in Figure 1, stimulation of hepatocytes with the same dose of both 2-MeSADP and 2-MeSATP led to transients at similar frequencies. Over 20 experiments, there was no significant difference in the frequency of transients induced by the same concentration of these two agonists. This argues against the effect of 2-MeSATP being due to contaminating 2-MeSADP, as the frequency of transients is dose-dependent. Therefore, the proportion of contaminating 2-MeSADP in the stock 2-MeSATP would need to be approaching 100% for transients to be elicited at similar frequencies in response to the same concentration of each of these agonists. In fact HPLC analysis of the purchased 2-MeSATP revealed an approximate 10% contamination with 2-MeSADP (Figure 2b); stimulation of the cell depicted in Figure 1 with 1 μM 2-MeSADP failed to induce a [Ca2+]i response. Nevertheless to eliminate the possibility that the response to 2-MeSATP was due to the presence of 2-MeSADP, single hepatocytes were stimulated with 2-MeSATP which had been pre-treated with 20 units ml−1 CPK and 20 mM PC. In contrast to the results of Hechler et al. (1998) this treatment did not abolish the agonist activity of 2-MeSATP in any cell tested (n=9). This is illustrated in Figure 2a which depicts the transients induced by untreated 2-MeSATP and subsequently with the same concentration of agonist from a stock solution treated with the ATP-regenerating system for 3 h. HPLC analysis confirmed that this treatment effectively removed the 2-MeSADP contamination in the stock of 2-MeSATP (Figure 2c). There was no difference in the duration of transients induced by untreated 2-MeSATP (8.6±0.8 s; n=8) or treated 2-MeSATP (8.7±0.4 s; n=9).
Figure 2.
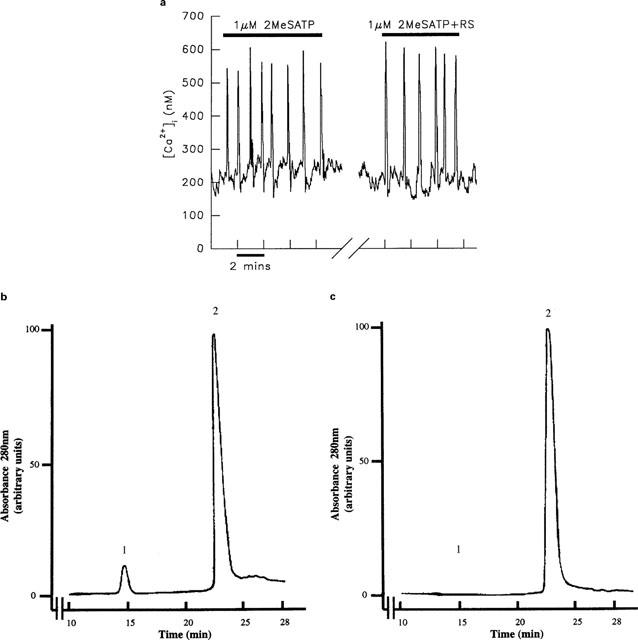
(a) Purification of 2-MeSATP to remove contaminating 2-MeSADP does not alter its ability to evoke [Ca2+]i transients in rat hepatocytes. The 2-MeSATP stock was treated with an ATP-regenerating (RS) for 3 h and applied to an aequorin-injected hepatocyte. Stimulation with the same concentration of treated or untreated nucleotide evoked transients which were indistinguishable. The break in the x axis represents a period of 10 min when the cell was superfused with WME alone. This trace is representative of nine experiments. (b) HPLC profile of unpurified 2-MeSATP showing 2-MeSADP contamination. Peaks: (1) 2-MeSADP; (2) 2-MeSATP. (c) HPLC profile of 2-MeSATP after incubation with 20 units ml−1 CPK and 20 mM PC for 3 h. 2-MeSADP contamination has been effectively removed (1). This sample was taken from the stock used to stimulate the cell depicted in (a).
The effect of the selective P2Y1 receptor antagonist, A3P5P was studied in cells responding to 2-MeSADP and 2-MeSATP. Application of 10 μM A3P5P had similar inhibitory effects on the transients induced by these 2 agonists, leading to either a decrease in frequency of transients (Figure 3), or their abolition. Increasing the antagonist concentration to 50 μM led to a further reduction in frequency or blocking of transients (Figure 3). In contrast to these inhibitory effects, transients induced by both ATP and UTP were enhanced by addition of 10 μM A3P5P. This is illustrated in Figure 4, which depicts the lengthening of UTP-induced transients in response to this treatment. Application of 50 μM A3P5P alone was without effect on [Ca2+]i, and transients induced by the α1-adrenergic agonist, phenylephrine were unaffected by the application of 10 μM A3P5P (data not shown).
Figure 3.
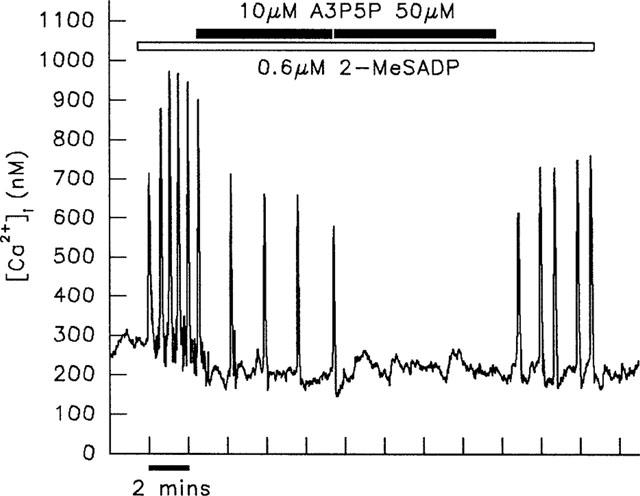
Inhibitory effects of A3P5P on [Ca2+]i transients induced by 2-MeSADP. Application of 10 μM A3P5P to a single cell responding to 2-MeSADP resulted in a decrease in frequency of transients. Increasing the concentration of A3P5P to 50 μM led to abolition of transients. This result is typical of five experiments. Transients induced by 2-MeSATP were affected similarly by addition of A3P5P (n=5).
Figure 4.
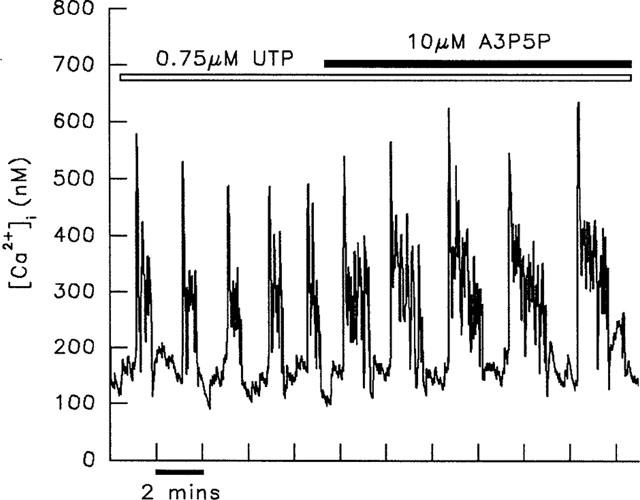
Enhancement of UTP-induced [Ca2+]i transients by the addition of A3P5P. Application of 10 μM A3P5P to a cell responding to UTP, led to an increase in duration of individual [Ca2+]i transients. This result is typical of three experiments. ATP-induced transients were affected similarly by the addition of A3P5P (n=3).
Discussion
Previous studies on single rat hepatocytes have established that the duration of [Ca2+]i transients can be used to indicate the receptor activated by an agonist; activation of a given receptor results in transients of consistent duration both in an individual cell, and also from one cell to another (Woods et al., 1987). This characteristic of the hepatocyte [Ca2+]i oscillator has been exploited here to investigate the action of these two agonists. The transients induced by 2-MeSATP and 2-MeSADP were indistinguishable, indicating action at a single receptor. Likewise, similarities have previously been demonstrated in the hepatocyte response to ADP and 2-MeSATP (Dixon et al., 1995a). In contrast, ATP and UTP produce a different pattern of response as illustrated here and previously (Dixon et al., 1990; 2000). In combination these results suggest that a single receptor is responsible for mediating the effects of 2-MeSADP, 2-MeSATP and ADP.
Unlike 2-MeSATP which is known to be an agonist at the P2Y11 receptor (Communi et al., 1997), 2-MeSADP is selective for the P2Y1 receptor. HPLC analysis showed that 2-MeSADP was essentially free of contaminating 2-MeSATP (data not shown). Combined with the observed inhibition of the response to both 2-MeSADP and 2-MeSATP by the selective P2Y1 antagonist A3P5P, this provides strong evidence that their effects are mediated by a P2Y1 receptor.
Purification of the 2-MeSATP stock to remove contaminating 2-MeSADP did not abolish its ability to evoke a Ca2+i response. Furthermore, in the experiments reported here, the combination of continuous flow and the single cell being held in isolation from any others, makes it extremely unlikely that inter-conversion of nucleotides by extracellular enzymes will occur. Such inter-conversions can complicate the interpretation of results from population studies (Harden et al., 1997). Here the cell is constantly superfused with medium which thereby provides a continuous supply of fresh agonist and simultaneously removes any breakdown products. It therefore seems likely that 2-MeSATP acts directly as an agonist at the P2Y1 receptor on the rat hepatocyte.
This conclusion is apparently inconsistent with the findings of Hechler et al. (1998). They reported that 2-MeSATP, purified of contaminating 2-MeSADP, was an antagonist not an agonist at the cloned human P2Y1 receptor transfected into Jurkat cells, and at the endogenous P2Y1 receptor of rat brain capillary endothelial cells. In contrast, Palmer et al. (1998) reported that 2-MeSATP was an agonist at the cloned human P2Y1 receptor transfected into 1321N1 cells, although less potent than 2-MeSADP. They explained this difference on the basis of receptor reserve, arguing that the higher expression of P2Y1 receptors by 1321N1 cells compared with the Jurkat cells used by Hechler et al. (1998), led to the detection of agonists with lower efficacy than 2-MeSADP or ADP. When the level of expression was reduced by the down-regulation of P2Y1 receptor by treatment with ADPβS, the agonist activity of ATP at the P2Y1 receptor was abolished.
A true comparison of the potency of 2-MeSATP and 2-MeSADP in rat hepatocytes is not possible from the studies presented here. Transients in [Ca2+]i in hepatocytes are frequency-modulated; as the degree of receptor activation increases so does the frequency of transients (Woods et al., 1986). However, considerable cell-to-cell variability exists in the frequency of transients induced by a given dose of agonist as seen in Figures 1 and 2a. Thus in the cell depicted in Figure 1, the frequency of transients induced by 10 μM 2-MeSATP is lower than the frequency of transients induced by only 1 μM 2-MeSATP in the cell shown in Figure 2a. For this reason the pooling of data from a number of individual cells to generate concentration-response curves would not be informative. Receptor desensitization presents problems for the construction of accurate concentration-response curves based on data from a single cell. However, three observations argue against there being a major difference in the potency or efficacy of 2-MeSADP and 2-MeSATP: (1) the threshold for the two agonists within the same cell is similar; (2) transients are induced at similar frequencies within an individual cell, by the same sub-maximal dose of 2-MeSADP and 2-MeSATP; and (3) the magnitude of the maximal response, and the concentration at which it is achieved, is similar for both agonists. The agonist action of 2-MeSATP reported here is therefore difficult to reconcile with this nucleotide being a low efficacy agonist (compared with 2-MeSADP), stimulating a system with a high receptor reserve, as concluded by Palmer et al. (1998). It is conceivable that the difference reflects a species difference as Palmer et al. (1998) studied the human homologue of the P2Y1 receptor expressed in 1321N1 cells.
Palmer et al. (1998) determined that ATP and 2-MeSATP were both low efficacy agonists at the human P2Y1 receptor. In contrast to this, observations from single rat hepatocytes indicate that ATP does not act at the P2Y1 receptor (Dixon et al., 1990; 1993; 1995b; Green et al., 1994). So, although 2-MeSADP and ADP both target the P2Y1 receptor, ATP does not behave like its 2-methylthio derivative. This is supported by the observation that the P2Y1 receptor antagonist, A3P5P did not have any inhibitory effect on the ATP-induced response. Indeed, both ATP- and UTP- induced transients were enhanced by the application of A3P5P. This P2Y1 antagonist is a partial agonist at the turkey P2Y1 receptor, although not at the human P2Y1, P2Y2 or P2Y4 receptors (Boyer et al., 1996). It could be argued that if A3P5P acts as a partial agonist at the rat P2Y receptor mediating the effects of ATP and UTP, its addition would effectively increase the agonist concentration acting at this receptor. However, the effect seen is not that simply of raising agonist concentration, which leads to an increase in the frequency of transients, without any change in their duration. Furthermore, at the concentrations employed in this study, A3P5P alone had no effect on [Ca2+]i. Transients mediated by the α1-adrenergic receptor, were unaffected by the addition of A3P5P indicating that this antagonist did not have a non-specific action on the phosphoinositide signalling pathway.
The results reported here are particularly important since they were conducted on primary cells natively expressing P2Y receptors, rather than a cell line or transfected cells. In addition the [Ca2+]i transients studied are generated in response to physiological doses of agonists. The finding that both diphosphate and triphosphate 2-thioether derivatives act on the rat P2Y1 receptor may have important implications for the development of drugs targeting this receptor.
Acknowledgments
I am grateful to The Wellcome Trust for funding. I would also like to thank Dr John Roberts for HPLC analysis and Alex Laude for help with the preparation of figures.
Abbreviations
- A3P5P
adenosine-3′-phosphate-5′-phosphate
- [Ca2+]i
intracellular free Ca2+ concentration
- CPK
creatine phosphokinase
- 2-MeSADP
2-methylthio-ADP
- 2-MeSATP
2-methylthio-ATP
- PC
phosphocreatine
- WME
William's medium E
References
- BERRIDGE M.J. Calcium oscillations. J. Biol. Chem. 1990;265:9583–9586. [PubMed] [Google Scholar]
- BOARDER M.R., HOURANI S.M.O. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol. Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., ROMERO-AVILA T., SCHACHTER J.B., HARDEN T.K. Identification of competitive antagonists of the P2Y receptor. Mol. Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- BURNSTOCK G.P2 purinoceptors: historical perspective and classification P2 purinoceptors: localization, function and transduction mechanisms 1996198Chichester: John Wiley & Sons Ltd; 1–29.Ciba Foundation Symposium [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KING B.F. The numbering of cloned P2 receptors. Drug Dev. Res. 1996;38:67–71. [Google Scholar]
- COBBOLD P.H., LEE J.A.C.Aequorin measurements of cytoplasmic free calcium Cellular Calcium: A Practical Approach 1991Oxford: I.R.L. Press; 55–81.ed. McCormack, J.G. & Cobbold, P.H. pp [Google Scholar]
- COBBOLD P., WOODS N., WAINWRIGHT J., CUTHBERTSON K.S.R. Single cell measurements in research on calcium-mobilising purinoceptors. J. Receptor Res. 1998;8:481–491. doi: 10.3109/10799898809049006. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., GOVAERTS C., PARMENTIER M., BOEYNAEMS J.-M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J. Biol. Chem. 1997;272:31969–31973. doi: 10.1074/jbc.272.51.31969. [DOI] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Adenosine 5′-[αβ-methylene]triphosphate potentiates the oscillatory cytosolic Ca2+ responses of hepatocytes to ATP but not ADP. Biochem. J. 1993;293:757–760. doi: 10.1042/bj2930757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Actions of ADP, but not ATP, on cytosolic free Ca2+ in single rat hepatocytes mimicked by 2-methylthioATP. Br. J. Pharmacol. 1995a;116:1979–1984. doi: 10.1111/j.1476-5381.1995.tb16401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., COBBOLD P.H., GREEN A.K. Oscillations in cytosolic free Ca2+ induced by ADP and ATP in single rat hepatocytes display differential sensitivity to application of phorbol ester. Biochem. J. 1995b;309:145–149. doi: 10.1042/bj3090145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Evidence for two calcium-mobilizing purinoceptors on rat hepatocytes. Biochem. J. 1990;269:499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON C.J., WOODS N.M., WEBB T.E., GREEN A.K. Evidence that rat hepatocytes co-express functional P2Y1 and P2Y2 receptors. Br. J. Pharmacol. 2000;129:764–770. doi: 10.1038/sj.bjp.0703103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN A.K., COBBOLD P.H., DIXON C.J. Elevated intracellular cyclic AMP exerts different modulatory effects on cytosolic free Ca2+ oscillations induced by ADP and ATP in single hepatocytes. Biochem. J. 1994;302:949–955. doi: 10.1042/bj3020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDEN T.K., LAZAROWSKI E.R., BOUCHER R.C. Release, metabolism and interconversion of adenine and uridine nucleotides: implications for G-protein-coupled P2 receptor agonist selectivity. Trends Pharmacol. Sci. 1997;18:43–46. [PubMed] [Google Scholar]
- HECHLER B., VIGNE P., LEON C., BREITTMAYER J.-P., GACHET C., FRELIN C. ATP derivatives are antagonists of the P2Y1 receptor: similarities to the platelet ADP receptor. Mol. Pharmacol. 1998;53:727–733. [PubMed] [Google Scholar]
- PALMER R.K., BOYER J.L., SCHACHTER J.B., NICHOLAS R.A., HARDEN T.K. Agonist action of adenosine triphosphates at the human P2Y1 receptor. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- SCHACHTER J.B., LI Q., BOYER J.L., NICHOLAS R.A., HARDEN T.K. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1-purinoceptor. Br. J. Pharmacol. 1996;118:167–173. doi: 10.1111/j.1476-5381.1996.tb15381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986;319:600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]
- WOODS N.M., CUTHBERTSON K.S.R., COBBOLD P.H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987;8:79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]


