Abstract
This study examined the ability of basal nitric oxide activity to suppress intrinsic and vasoconstrictor tone in isolated rings of porcine cerebral artery.
Following stretch of approximately 1 g, NG-nitro-L-arginine methyl ester (L-NAME, 100 μM) produced a rise in tone in endothelium-containing but not endothelium-denuded rings. Thus, intrinsic tone was present and was powerfully suppressed by basal nitric oxide activity.
Nevertheless, when concentration-response curves were constructed to U46619 and 5-hydroxytryptamine (5-HT), no endothelium-dependent depression of vasoconstriction was observed. It therefore appeared that basal nitric oxide activity was able to suppress intrinsic but not vasoconstrictor tone in these vessels.
Stretch-tension curves generated following the application of stretch over the range 0–5.5 g on endothelium-denuded rings showed that tension was stretch-induced. Experiments conducted in the presence of L-NAME (100 μM) revealed that the level of tone present in endothelium-containing rings was substantially higher than in endothelium-denuded rings across the entire range of stretch.
When endothelium-containing and endothelium-denuded rings were set at similar levels of stretch-induced tone, rather than similar levels of stretch, the presence of the endothelium now depressed significantly vasoconstrictor responses to U46619 and 5-HT.
Thus, when endothelium-containing and endothelium-denuded rings of porcine cerebral artery are set at similar points along their respective stretch-tension curves, rather than at similar levels of stretch, basal nitric oxide activity can be seen to inhibit both stretch-induced and vasoconstrictor tone.
Keywords: Cerebral artery, endothelium, nitric oxide, myogenic tone, nitric oxide synthase inhibitor, 5-hydroxytryptamine, U46619
Introduction
Release of nitric oxide from endothelium is known to exert a profound depressant action on the sensitivity of isolated vascular preparations to a wide range of vasoconstrictor agents, including potassium chloride, α-adrenoceptor agonists, 5-hydroxytryptamine (5-HT), prostaglandin F2α, angiotensin II and histamine (see Martin, 1988 for review). In a small number of cases, for example, with norepinephrine or 5-HT in canine and porcine coronary arteries (Cocks & Angus, 1983; Cohen et al., 1983) and with the α2-adrenoceptor agonist, UK 14,304, in canine pulmonary artery and veins (Miller & Vanhoutte, 1985), the depression of vasoconstrictor clearly arises from enhanced release of nitric oxide, stimulated by the vasoconstrictors themselves. In most, however, stimulated release does not occur, demonstrating that a basal release of nitric oxide is responsible for the depression of vasoconstrictor responses (see Martin, 1988 for a review). Moreover, the elevations of blood pressure resulting from administration of inhibitors of nitric oxide synthase (Rees et al., 1990), demonstrate that nitric oxide exerts a tonic vasodepressor action in the vasculature in vivo. Whether such elevations result from blockade of basal release or from agonist- or flow-induced release of nitric oxide, however, remains to be determined.
Although resistance vessels from many vascular sites including the coronary and mesenteric beds exhibit intrinsic (myogenic) tone (Sun et al., 1992; Rajagopalan et al., 1995; Miller et al., 1997), most large systemic vessels do not. Consequently, inhibitors of the actions or synthesis of nitric oxide normally have no effect on the tone of large systemic vessels in the absence of a vasoconstrictor (Martin et al., 1985; Rees et al., 1990). Notable exceptions to this rule, however, are cerebral arteries from a variety of species including rat, cat, rabbit, dog and monkey (Connor & Feniuk, 1989; Alonso et al., 1992; Trezise et al., 1992; Brian & Kennedy, 1993; Toda et al., 1993b; Benyó et al., 1998). In these, direct contractile actions in the absence of vasoconstrictor tone have been observed in endothelium-containing but not endothelium-denuded preparations following treatment with agents that inhibit the actions or synthesis of nitric oxide. Thus, basal nitric oxide activity acts to suppress the intrinsic myogenic tone generated by these cerebral vessels. In addition, as with systemic vessels, basal nitric oxide activity suppresses the vasoconstrictor responses of cerebral arteries to 5-HT, prostaglandin F2α, the thromboxane mimetic U46619, norepinephrine and histamine (Connor & Feniuk, 1989; Trezise et al., 1992; Brian & Kennedy, 1993; Lee et al., 1996; Minato et al., 1995; Yamakawa et al., 1997). In keeping with these findings, infusion of inhibitors of nitric oxide synthase into rats, rabbits, cats and dogs in vivo results in reductions in cerebral blood flow (Prado et al., 1992; Kovach et al., 1992; Seligsohen & Bill, 1993; Toda et al., 1993a). Whether these reductions in blood flow arise from inhibition of synthesis of nitric oxide in endothelial cells, perivascular nitrergic nerves, central neurones or astrocytes, however, remains to be determined (Toda et al., 1993a).
In this study we wished to identify the cellular source of the basal nitric oxide activity in pig isolated cerebral arteries and determine how this influenced the generation of both myogenic and vasoconstrictor tone. A preliminary account of these findings has already been presented (Wallis & Martin, 1999).
Methods
Isolation of porcine cerebral arteries for tension recording
Heads from adult, freshly killed Yorkshire pigs of either sex, weighing 50–75 kg and aged 6–9 months, were obtained from a local slaughterhouse. The entire brain was removed within 30 min and transported to the laboratory in oxygenated Krebs solution at 4°C. Anterior cerebral arteries (approximately 1 mm, outside diameter) were dissected out with the aid of a light microscope, cleared of adhering fat and connective tissue and cut into 2.5 mm wide transverse rings. Some tissues were used that day, while others were stored in oxygenated Krebs solution overnight at 4°C, for use the following day. For some arterial rings, the endothelium was mechanically removed by gentle rubbing of the intimal surface with a piece of stainless steel wire. Rings were mounted under 1 g resting tension (except where otherwise stated in the Results) onto two stainless steel hooks within 10 ml organ baths, and bathed at 37°C in Krebs solution containing (mM): NaCl 118, KCl 4.8, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24, D-glucose 11, and gassed with 95% O2 and 5% CO2. Tension was recorded isometrically with Grass FT03 transducers and displayed on a MacLab (8e Series, AD Instruments). Tissues were allowed to equilibrate for 60–90 min before experiments were carried out, during which time the resting tension was re-adjusted to 1 g, if required.
Assessment of basal nitric oxide activity
The ability of basal nitric oxide activity to suppress tone in porcine cerebral arteries was assessed using three separate protocols. The first involved adding the inhibitor of nitric oxide synthase, L-NAME (100 μM), to endothelium-containing and endothelium-denuded rings in the absence of a vasoconstrictor. The ensuing rise in tone of the endothelium-containing preparations that was not observed in the endothelium-denuded preparations provided an index of the ability of basal nitric oxide activity to suppress intrinsic tone in the vessels. The two other protocols were employed to assess the ability of basal nitric oxide activity to suppress vasoconstrictor tone. One involved constructing full concentration-response curves to two vasoconstrictors, i.e., the thromboxane mimetic U46619 (0.3 nM–10 μM) and 5-hydroxytryptamine (5-HT, 1 nM–10 μM) on endothelium-containing and endothelium-denuded rings and assessing the depression of vasoconstriction produced by the presence of the endothelium. In the other, endothelium-containing and endothelium-denuded rings were pre-constricted with the half maximal effective concentration (EC50) of U46619 (10–30 nM) or 5-HT (10–30 nM). L-NAME (100 μM) was then added and the enhancement of tone in endothelium-containing preparations, which was absent in endothelium-denuded preparations, was taken as an index of basal nitric oxide activity.
Investigation of origin of intrinsic muscle tone
The Results show that in the absence of a vasoconstrictor, the addition of L-NAME (100 μM) to endothelium-containing but not endothelium-denuded vessels resulted in a large rise in tone. This suggested that even in the resting state, intrinsic tone was present in these vessels and that basal nitric oxide activity suppressed this. Experiments were therefore conducted to investigate the possible origins of this tone. The first series investigated the potential role of endothelium-derived constricting factors, such as endothelin and products of cyclooxygenase and lipoxygenase, using appropriate blocking agents. In these experiments, rings were incubated for 30 min with either the mixed ETA/ETB endothelin antagonist SB209670 (1 μM) (Ohlstein et al., 1994), the cyclo-oxygenase inhibitor flurbiprofen (10 μM) (Nozu, 1978), or one of a number of lipoxygenase inhibitors, curcumin, nordihydroguaiaretic acid (NDGA) and MK-886 (3-[1-(chlorobenzyl)-3-t-butylthio-5-isoprpylindol-2-yl]-2,2-dimethylpropanoic acid) (Kim et al., 1992; Ford-Hutchinson et al., 1993; Brouet & Ohishima, 1995), and the effects on the magnitude of the subsequent rise in tone induced by L-NAME (100 μM) assessed. In addition, the potential role of nerve-derived constricting factors was tested by treating rings for 30 min with tetrodotoxin (1 μM) and the effects on the rise in tone induced by L-NAME assessed.
A second series of experiments investigated the possibility that the tone was stretch-induced by constructing stretch-tension response curves. These were constructed by suspending endothelium-containing and endothelium-denuded rings at different levels of stretch between 0 and 5.5 g. Papaverine (100 μM) was then added, and the ensuing relaxation gave a measure of the apparent tone in the vessels (see Figure 1). For endothelium-containing vessels this apparent tone was an underestimate of the total level because of suppression by basal nitric oxide activity. A further series of stretch-tension curves was constructed using L-NAME to assess the total level of tone in these vessels. Again, endothelium-containing and endothelium-denuded rings were suspended at different levels of stretch between 0 and 5.5 g. L-NAME (100 μM) was then added to abolish the suppression of tone by basal nitric oxide activity, and papaverine (100 μM) was subsequently added to abolish all active tone (see Figure 1). The difference (in g) between the level of tone in the presence of L-NAME and after the application of papaverine was taken as a measure of the total tone present in the vessel.
Figure 1.
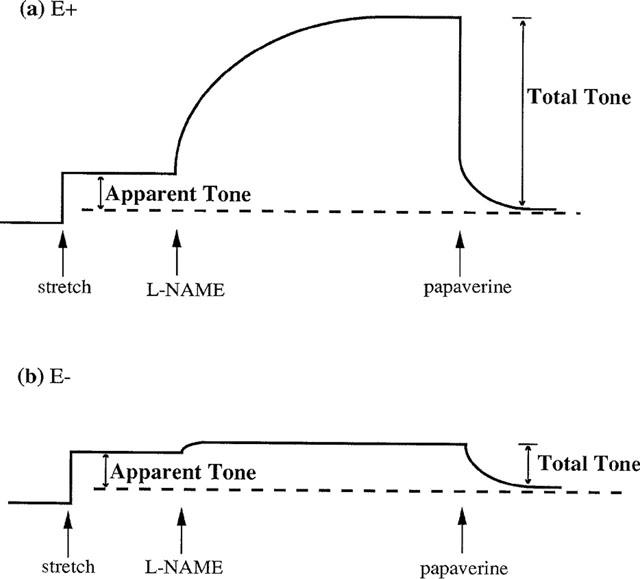
Schematic diagram showing how apparent stretch-induced tone and total stretch-induced tone was calculated in (a) endothelium-containing (E+) and (b) endothelium-denuded (E−) rings of porcine cerebral artery. Apparent tone was taken as the difference between the amount of stretch applied to the vessel and that remaining after the application of papaverine (100 μM). Total tone was taken as the difference between the level of tone in the presence of L-NAME (100 μM) and that remaining after the addition of papaverine (100 μM).
Drugs
Curcumin, 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α methyl acetate (U46619), 5-hydroxytryptamine hydrochloride (5-HT), NG-nitro-L-arginine methyl ester (L-NAME), nordihydroguaiaretic acid (NDGA), papaverine hydrochloride and tetrodotoxin were obtained from the Sigma Chemical Co., U.K., (Poole, Dorset, U.K.). MK-886 (3-[1-(chlorobenzyl)-3-t-butylthio-5-isoprpylindol-2-yl]-2,2-dimethylpropanoic acid) was obtained from Calbiochem (Nottingham, U.K.). ([(+)-(1S,2R,3S)-3-1-(3,4-methylenedioxyphenyl)-5-(prop-1-yloxy) indene-2-carboxylic acid] (SB209670) and sodium flurbiprofen were kind gifts from SmithKline Beecham Pharmaceuticals, King of Prussia, PA, U.S.A. and Boots Pharmaceuticals, Nottingham, U.K., respectively. All drugs were dissolved in 0.9% saline, with the exception of NDGA (0.1 M), MK-886 (20 mM) and U46619 (10 mM), which were dissolved in absolute ethanol and curcumin (0.1 M) which was dissolved in dimethylsulphoxide. All dilutions were made in saline (0.9%).
Statistical analysis
Results are expressed as mean±s.e.mean of n separate experiments. EC50 and pD2 values were calculated by a computer-based curve-fitting program (GraphPad Prism). Multiple comparisons were made by one-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test. Single comparisons were made using Student's unpaired t-test, as appropriate. Stretch-tension curves were fitted by second-order polynomial analysis and comparisons made using the F-test. A value of P<0.05 was considered significant for all analyses.
Results
Effects of L-NAME and endothelial removal on intrinsic and agonist-induced tone
Following application of approximately 1 g stretch (1.42±0.11 g, n=6) to endothelium-containing rings of porcine cerebral artery, addition of the inhibitor of nitric oxide synthase L-NAME (100 μM) produced a rapid and sustained increase in tone (Figure 2a,b). In contrast, L-NAME (100 μM) had no significant effect on the tone of endothelium-denuded vessels set at a similar level of stretch (1.16±0.15 g, n=6). Furthermore, following induction of approximately 50% of the maximal tone inducible by U46619 (10–30 nM) or 5-HT (10–30 nM), addition of L-NAME (100 μM) resulted in a rapid and sustained increase in tone in endothelium-containing but not endothelium-denuded rings (Figure 3a,b). These data are consistent with a powerful basal release of nitric oxide suppressing the development of tone.
Figure 2.
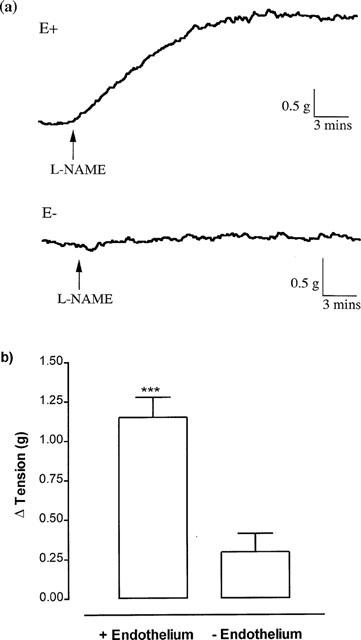
Individual experimental traces (a) and mean data (b) showing the increase in tone obtained when the inhibitor of nitric oxide synthase, L-NAME (100 μM), was added to endothelium-containing (E+) and endothelium-denuded (E−) rings of porcine cerebral artery in the absence of a vasoconstrictor. Each point is the mean±s.e.mean of six observations. ***P<0.001 indicates that L-NAME had a significant effect on tone.
Figure 3.
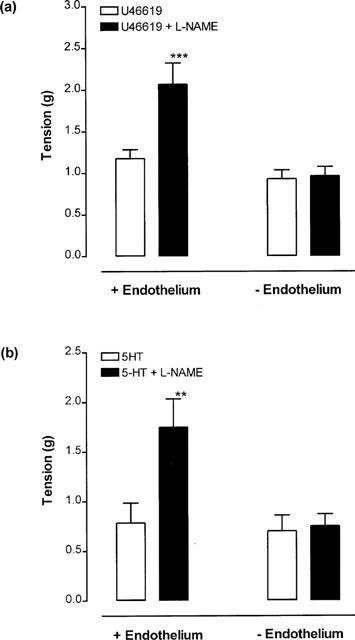
The effects of L-NAME (100 μM) on the tone of endothelium-containing and endothelium-denuded rings of porcine cerebral artery, pre-constricted with the half-maximal effective concentration (EC50) of (a) U46619 (10–30 nM) or (b) 5-HT (10–30 nM). Each value is the mean±s.e.mean of 6–10 observations. **P<0.01 and ***P<0.001 respectively indicate where L-NAME produced a significant augmentation of 5-HT- and U46619-induced tone.
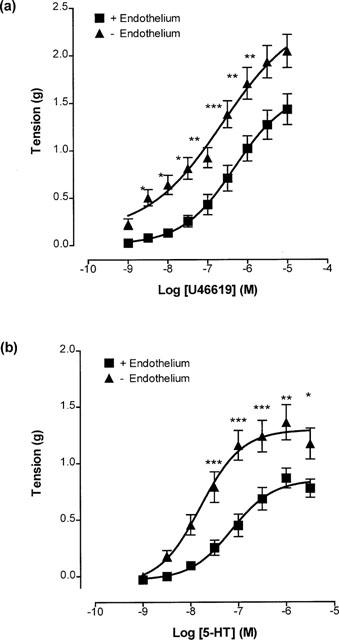
Surprisingly, when rings of cerebral artery were placed under an initial 1 g of stretch, full concentration-response curves to U46619 (0.3 nM–10 μM) constructed on endothelium-containing and endothelium-denuded rings were not significantly different (maximum contractions 2.25±0.30 g and 1.73±0.30 g; pD2 values 7.21±0.15 and 7.42±0.21, respectively; Figure 4a). Similarly, 5-HT (1 nM–10 μM)-induced contractions in endothelium-containing and endothelium-denuded rings were not significantly different (maximum contractions 1.48±0.24 g and 1.30±0.26 g; pD2 values 7.30±0.15 and 7.72±0.17, respectively; Figure 4b).
Figure 4.
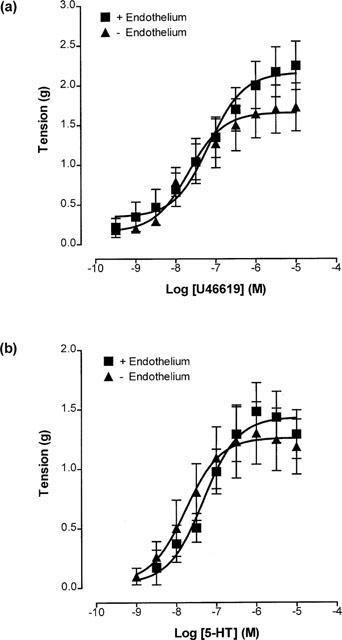
Concentration-response curves showing contractions to (a) U46619 (0.3 nM–10 μM) and (b) 5-HT (1 nM–10 μM) on endothelium-containing and endothelium-denuded rings of porcine cerebral artery, placed under 1 g of stretch. Each point is the mean±s.e.mean of 6–8 observations.
Origin of the intrinsic muscle tone in cerebral artery rings
The above data suggested that the intrinsic muscle tone was more effectively suppressed than vasoconstrictor tone by basal nitric oxide activity. The possibility that this intrinsic tone was stretch-induced was investigated by generating stretch-tension curves on endothelium-containing and endothelium-denuded rings. These were constructed (see Figure 1) by mounting rings at different levels of applied stretch (0–5.5 g) and estimating the apparent level of muscle tone present from the relaxation produced by the addition of papaverine (100 μM). Bestline fit polyomial analysis (Figure 5) shows that stretch did indeed induce muscle tone and that the levels generated were similar in endothelium-containing and endothelium-denuded rings except at high levels of stretch (>4 g), where the latter appeared to plateau. The levels observed on endothelium-containing rings were, however, an underestimate of the total level of muscle tone present because of suppression by basal nitric oxide activity. The total levels of muscle tone were therefore determined by treating rings with L-NAME (100 μM) to remove basal nitric oxide and measuring the subsequent fall in tone produced by papaverine (100 μM). Following this procedure the total levels of muscle tone present (Figure 5) were substantially higher in endothelium-containing than in endothelium-denuded rings across the entire range of stretch.
Figure 5.
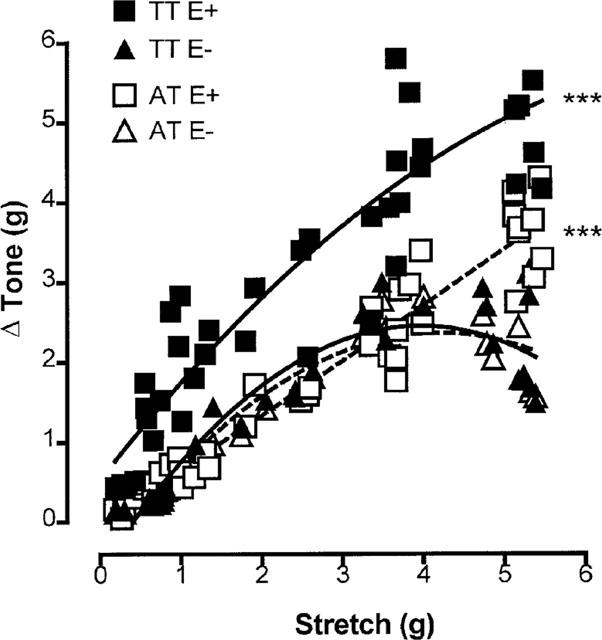
Stretch-tension curves generated on endothelium-containing (E+) and endothelium-denuded (E−) rings of porcine cerebral artery, showing the apparent level of stretch-induced tone (AT) revealed by the addition of papaverine (100 μM) and the total level of tone (TT) revealed upon removal of the depressant action of nitric oxide by L-NAME followed by the addition of papaverine (100 μM). Each data point was generated using a single arterial ring. Solid lines indicate TT and dotted lines AT. Lines were fitted using second-order polynomial analysis. ***(P<0.001, F-test) indicates that TT E+ was significantly different from TT E− across the entire range of stretch and that AT E+ was only different from AT E− at levels of stretch above 4 g.
The possibility that endothelium-derived vasoconstrictors were responsible for the greater levels of actual muscle tone in endothelium-containing than in endothelium-denuded rings was investigated using the mixed ETA/ETB endothelin receptor antagonist SB209670, the cyclo-oxygenase inhibitor flurbiprofen or the lipoxygenase inhibitors curcumin, NDGA and MK-866. Moreover, the potential involvement of nerve-derived constrictors was investigated using tetrodotoxin. When artery rings were mounted at an initial stretch of 1 g and pre-treated for 30 min with either SB209670 (1 μM), flurbiprofen (10 μM), tetrodotoxin (1 μM) or curcumin (30 μM), the endothelium-dependent rise in tone induced by L-NAME was unaffected (Figure 6). In contrast, MK-886 (30 μM) and NDGA (30 μM) abolished the rise in tone induced by L-NAME. Unlike the other agents, these last two also had effects by themselves on endothelium-containing rings; MK-886 produced a powerful fall in tone (0.43±0.7 g, n=9, P<0.001) and depressed subsequent contraction to 5-HT and U46619 (data not shown), and NDGA produced an increase in tone (0.28±0.14, n=8, P<0.05).
Figure 6.
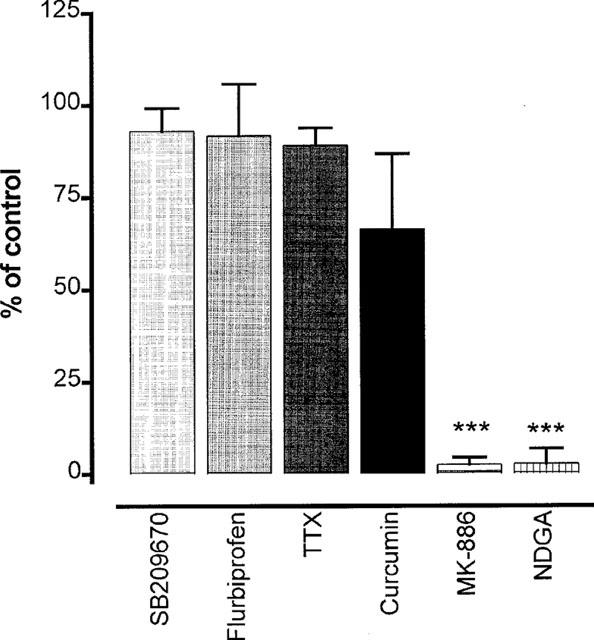
Graph showing how the increase in tone resulting from addition of L-NAME (100 μM) to endothelium-containing rings of porcine cerebral artery was affected following a 30 min pre-treatment with the mixed ETA/ETB endothelin receptor blocker SB209670 (1 μM), the cyclo-oxygenase inhibitor flurbiprofen (10 μM), the neurotoxin tetrodotoxin (1 μM), or the lipoxygenase inhibitors curcumin (30 μM), MK-886 (30 μM) and NDGA (30 μM). Each value is the mean±s.e.mean. 7–10 observations. ***P<0.001 indicates a significant reduction.
U46619- and 5-HT-induced contraction on endothelium-containing and endothelium-denuded rings set at similar levels of muscle tone
Experiments were conducted to determine if basal nitric oxide activity depressed vasoconstrictor responses to U46619 and 5-HT when endothelium-containing and endothelium-denuded rings were mounted at different levels of stretch but at similar levels of stretch-induced tone. Using data derived from Figure 5, endothelium-denuded rings were placed under approximately 1 g (1.39±0.04 g and 1.28±0.03 g for experiments with U46619 and 5-HT, respectively) of stretch, and the corresponding stretch giving the same level of total tone (∼0.7 g) for endothelium-containing rings was 0.3 g (0.36±0.02 g and 0.33±0.02 g, respectively). When concentration-response curves were constructed under these conditions, endothelium-containing rings were significantly less sensitive than endothelium-denuded rings to both U46619 (1 nM–10 μM; maximum contractions 1.430±0.162 g and 2.043±0.174 g; pD2 values 6.45±0.12 and 6.717±0.07, respectively, P<0.01) and 5-HT (1 nM–3 μM; maximum contractions: 0.87±0.09 g and 1.36±0.16 g; pD2 values 7.20±0.13 and 7.88±0.10, respectively, P<0.01) (Figure 7a,b).
Figure 7.
Concentration-response curves showing contractions to (a) U46619 (1 nM–10 μM) and (b) 5-HT (1 nM–3 μM) on endothelium-containing and endothelium-denuded rings of porcine cerebral artery set at the same level of stretch-induced tone. In these experiments, endothelium-denuded rings were stretched to a resting tension of 1 g. The stretch applied to endothelium-containing rings was 0.3 g since the data displayed on Figure 5 indicated that this generates the same level of total tone as an endothelium-denuded vessel stretched to 1 g. Each point is the mean±s.e.mean of 7–12 observations. *P<0.05, **P<0.01, and ***P<0.001 respectively indicate that contractions were significantly greater in endothelium-denuded rings.
Discussion
We found that in the absence of a vasoconstrictor, but in the presence of an applied stretch of approximately 1 g, addition of the nitric oxide synthase inhibitor, L-NAME, to endothelium-containing rings of porcine cerebral artery resulted in an immediate and sustained increase in tone of around 1 g. Such rises in resting tone in response to agents which either inhibit the actions or synthesis of nitric oxide have previously been found in cerebral arteries from other species, including, rat, cat, rabbit, dog and monkey (Connor & Feniuk, 1989; Alonso et al., 1992; Trezise et al., 1992; Brian & Kennedy, 1993; Toda et al., 1993b; Benyó et al., 1998). Together, these reports suggest two things, i.e. that these vessels exhibit intrinsic tone and that the powerful tonic influence of basal nitric oxide continuously suppresses the development of this tone. There are two potential sources of nitric oxide in these cerebral arteries, i.e. the vascular endothelium and the perivascular nitrergic nerves, and both structures are known to release nitric oxide under basal conditions (Martin, 1988; Gillespie et al., 1989; Li & Rand, 1989). Our finding that endothelial removal abolished the ability of L-NAME to elevate tone in porcine cerebral artery is, however, in keeping with previous reports on cerebral arteries from other species (Connor & Feniuk, 1989; Alonso et al., 1992; Trezise et al., 1992; Brian & Kennedy, 1993; Toda et al., 1993b), indicating that the major, if not exclusive, source of this basal nitric oxide is the endothelium.
Despite exhibiting powerful basal nitric oxide activity, we did not observe the expected endothelium-dependent depression of vasoconstrictor tone when full concentration-response curves were generated to U46619 and 5-HT on rings of porcine cerebral artery; responses were, in fact, not significantly different on endothelium-containing and endothelium-denuded rings. Others have, however, reported that the endothelium in cerebral arteries does indeed suppress vasoconstrictor responses to these agents (Connor & Feniuk, 1989; Trezise et al., 1992; Brian & Kennedy, 1993). We did find, however, that when porcine cerebral arteries were contracted by approximately the EC50 concentrations of U46619 or 5-HT, the subsequent addition of L-NAME led to an immediate rise in tone in endothelium-containing but not endothelium-denuded rings. The magnitude of these rises were, however, similar to those that would have been expected if L-NAME had simply been added to endothelium-containing rings in the absence of a vasoconstrictor. Thus, it was likely that these rises in tone resulted from loss of the suppression by basal nitric oxide of intrinsic rather than vasoconstrictor tone. In other words, it seemed that under our experimental conditions, basal nitric oxide activity was able to suppress intrinsic but not vasoconstrictor tone.
These findings led us to investigate the nature of the intrinsic tone exhibited by rings of porcine cerebral artery. Myogenic tone is known to be generated in vascular smooth muscle in response to an increase in transmural pressure or stretch (D'Angelo & Meininger, 1994). We therefore constructed stretch-tension curves on rings of porcine cerebral artery. This was done by applying stretch over the range 0–5.5 g to both endothelium-containing and endothelium-denuded rings and determining the level of tone present by adding a maximally effective concentration of papaverine. We found that the tone did indeed develop almost linearly in proportion to the applied stretch, i.e. it was stretch-induced. Moreover, the magnitude of the tone induced was identical in endothelium-containing and endothelium-denuded rings, except at very high levels of stretch (>4 g), where the magnitude of the latter began to decline.
It must be remembered, however, that the presence of the endothelium powerfully depresses the stretch-induced tone in these vessels, and so the levels assessed by the above analysis gave only apparent and not total levels of tone in the endothelium-containing vessels. In order to determine the total levels of stretch-induced tone present, a new set of stretch-tension curves was generated in which following application of the initial stretch (0–5.5 g), L-NAME was added to inhibit the synthesis of nitric oxide. As before, L-NAME elevated the tone in endothelium-containing but not endothelium-denuded rings, and the total level of stretch-induced tone present was assessed following the addition of papaverine. Comparison of the curves now showed that across the full range of applied stretch, the total levels of stretch-induced tone present in endothelium-containing rings was substantially higher than in endothelium-denuded rings.
Our experiments on endothelium-denuded vessels showed clearly that the presence of the endothelium is not an absolute requirement for the generation of stretch-induced tone. Nevertheless, some workers have suggested that endothelium-derived vasoconstrictors may contribute to the development of myogenic tone. For example, Benyó et al. (1998) provided evidence that endothelium-derived thromboxane A2 contributes to myogenic tone in the rat cerebral artery, but inhibitors of cyclooxygenase have no effect in canine (Connor & Feniuk, 1989) or rabbit (Trezise et al., 1992) cerebral arteries. Moreover, NDGA abolished stretch-induced tone in porcine cerebral artery, suggesting the involvement of a lipoxygenase product (Kim et al., 1992). Endothelin has also been considered as a potential mediator of myogenic tone in human pial arteries (Thorin et al., 1998). Our experiments showed clearly, however, that the cyclooxygenase inhibitor flurbiprofen (Nozu, 1978), and the mixed ETA/ETB receptor antagonist SB209670 (Ohlstein et al., 1994), had no effect on the L-NAME-induced rise in tone in endothelium-containing rings of porcine cerebral artery. Thus, neither a cyclooxygenase product nor endothelin appears to contribute to the generation of myogenic tone in porcine cerebral artery. Moreover, the results with tetrodotoxin ruled out the involvement of a nerve-derived vasoconstrictor. The results with lipoxygenase inhibitors were, however, equivocal; curcumin had no effect, but both NDGA and MK-886 abolished the endothelium-dependent rise in tone induced by L-NAME. The effects of NDGA and MK-886 may, however, have resulted from non-selective actions rather than blockade of lipoxygenase, since the former produced a rise in tone consistent with its ability to inhibit the activity of nitric oxide (Furchgott, 1984) and the latter depressed both stretch-induced and vasoconstrictor tone. Thus, it is not possible to conclude for certain that a lipoxygenase product contributes to stretch-induced tone in the porcine cerebral artery.
Whatever the origins of stretch-induced tone, the stretch-tension curves generated on porcine cerebral artery show that when suspended at our standard resting stretch of 1 g, endothelium-containing and endothelium-denuded rings exhibit markedly different levels of tone (roughly 1.8 and 0.7 g, respectively). Thus, when we failed to observe the expected endothelium-dependent depression of vasoconstriction to U46619 and 5-HT, the endothelium-containing and endothelium-denuded vessels had been set at different points along their respective stretch-tension curves. Consequently, in order to make a more appropriate comparison of the effects of the vasoconstrictors on endothelium-containing and endothelium-denuded rings, a new set of experiments was conducted in which the two groups of vessels were suspended at different levels of stretch but at similar levels of stretch-induced tone. Furthermore, a relatively low level of stretch was chosen since basal nitric oxide activity would then be working against a low level of stretch-induced tone. Specifically, endothelium-denuded vessels were suspended at the standard stretch of 1 g, generating roughly 0.7 g of tone. Endothelium-containing vessels were stretched to roughly 0.3 g, since the data in Figure 5 indicate that this produces the same level of total stretch-induced tone (0.7 g) as denuded vessels stretched to 1 g. When concentration response curves were constructed to U46619 and 5-HT under these conditions, endothelium-containing rings were observed to be significantly less sensitive than endothelium-denuded rings. Thus, when set at the same level of stretch-induced tone, rather than at a similar degree of stretch, endothelium-dependent depression of vasoconstrictor responses was clearly seen. We should point out that our experiments were conducted under isometric conditions and at high oxygen tension (95% O2). Consequently, further experiments will be required to determine if the conditions found here for optimal demonstration of suppression of both stretch-induced and vasoconstrictor tone by basal nitric oxide apply also to vessels in situ. Moreover, shear stress (flow)-induced tone may also be present in vivo and it would be of interest to determine if this too was modulated by basal nitric oxide activity.
In conclusion, the porcine cerebral artery generates tone in response to stretch and this is powerfully suppressed in endothelium-containing vessels by high basal nitric oxide activity. Endothelium-containing and endothelium-denuded rings suspended at similar levels of stretch therefore exhibit strikingly different levels of tone, and this can mask the ability of basal nitric oxide to suppress vasoconstrictor tone. If, however, endothelium-containing and endothelium-denuded rings are suspended at the same level of stretch-induced tone, the endothelium-dependent depression of vasoconstrictor tone mediated by basal nitric oxide activity is readily observed. These findings have important implications for the study of blood vessels in which stretch-induced tone and basal nitric activity are both powerful.
Acknowledgments
We are grateful to the Wellcome Trust and the British Heart Foundation for support.
Abbreviations
- cyclic GMP
cyclic guanosine 3′,5′-monophosphate
- 5-HT
5-hydroxytryptamine
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- L-NOARG
NG-nitro-L-arginine
References
- ALONSO M.J., SALAICES M., SANCHEZ-FERRER C.F., MARIN J. Predominant role of nitric oxide in the relaxation induced by acetylcholine in cat cerebral arteries. J. Pharmacol. Exp. Ther. 1992;261:12–20. [PubMed] [Google Scholar]
- BENYÓ Z., GORLACH C., WAHL M. Involvement of thromboxane A2 in the mediation of the contractile effect induced by inhibition of nitric oxide synthesis in isolated rat middle cerebral arteries. J. Cereb. Blood Flow Metab. 1998;18:616–618. doi: 10.1097/00004647-199806000-00003. [DOI] [PubMed] [Google Scholar]
- BRIAN J.E., KENNEDY R.H. Modulation of cerebral arterial tone by endothelium-derived relaxing factor. Am. J. Physiol. 1993;264:H1245–H1250. doi: 10.1152/ajpheart.1993.264.4.H1245. [DOI] [PubMed] [Google Scholar]
- BROUET I., OHSHIMA H. Curcumin, an anti-tumour promoter and anti-inflammatory agent, inhibits induction of nitric oxide synthase in activated macrophages. Biochem. Biophys. Res. Commun. 1995;206:533–540. doi: 10.1006/bbrc.1995.1076. [DOI] [PubMed] [Google Scholar]
- COCKS T.M., ANGUS J.A. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., SHEPHERD J.T., VANHOUTTE P.M. 5-Hydroxytryptamine can mediate endothelium-dependent relaxation of coronary arteries. Am. J. Physiol. 1983;245:H1077–H1080. doi: 10.1152/ajpheart.1983.245.6.H1077. [DOI] [PubMed] [Google Scholar]
- CONNOR H.E., FENIUK W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5-HT agonists in canine basilar artery. Br. J. Pharmacol. 1989;96:170–178. doi: 10.1111/j.1476-5381.1989.tb11797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ANGELO G., MEININGER G.A. Transduction mechanisms involved in the regulation of myogenic activity. Hypertension. 1994;23:1096–1105. doi: 10.1161/01.hyp.23.6.1096. [DOI] [PubMed] [Google Scholar]
- FORD-HUTCHINSON A.W., TAGARI P., CHING S.V., ANDERSON C.A., COLEMAN J.B., PETER C.P. Chronic leukotriene inhibition in the rat fails to modify the toxicological effects of a cyclo-oxygenase inhibitor. Can. J. Physiol. Pharmacol. 1993;71:806–810. doi: 10.1139/y93-120. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F. The role of endothelium in the responses of vascular smooth muscle to drugs. Ann. Rev. Pharmacol. Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- GILLESPIE J.S., LIU X., MARTIN W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus to NANC nerve stimulation. Br. J. Pharmacol. 1989;98:1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM P., SHIMOKAWA H., VANHOUTTE P.M. Dietary ω-3 fatty acids and endothelium-dependent responses in porcine cerebral arteries. Stroke. 1992;23:407–413. doi: 10.1161/01.str.23.3.407. [DOI] [PubMed] [Google Scholar]
- KOVACH A.G.B., SZABO C., BENYO Z., CSAKI C., GREENBERG J.H., REIVICH M. Effects of NG-nitro-L-arginine and L-arginine on regional cerebral blood flow in the cat. J. Physiol. 1992;449:183–196. doi: 10.1113/jphysiol.1992.sp019081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE T.J.F., SARWINSKI S.J., ISHINE T., LAI C.C., CHEN F.-Y. Inhibition of cerebral neurogenic vasodilatation by L-glutamine and nitric oxide synthase inhibitors and its reversal by L-citrulline. J. Pharmacol. Exp. Ther. 1996;276:353–358. [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Evidence for a role of nitric oxide in the neurotransmitter system mediating relaxation of the rat anococcygeus muscle. Clin. Exp. Pharmacol. Physiol. 1989;16:933–938. doi: 10.1111/j.1440-1681.1989.tb02404.x. [DOI] [PubMed] [Google Scholar]
- MARTIN W.Basal release of endothelium-derived relaxing factor Relaxing and Contracting Factors 1988Clifton, New Jersey: Humana Press; 159–178.ed. Vanhoutte, P.M. pp [Google Scholar]
- MARTIN W., VILLANI G.M., JOTHIANANDAN D., FURCHGOTT R.F. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J. Pharmacol. Exp. Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- MILLER F.J., DELLSPERGER K.C., GUTTERMAN D.D. Myogenic constriction of human coronary arterioles. Am. J. Physiol. 1997;273:H257–H264. doi: 10.1152/ajpheart.1997.273.1.H257. [DOI] [PubMed] [Google Scholar]
- MILLER V.M., VANHOUTTE P.M. Endothelial α2-adrenoceptors in canine pulmonary and systemic blood vessels. Eur. J. Pharmacol. 1985;118:123–129. doi: 10.1016/0014-2999(85)90670-3. [DOI] [PubMed] [Google Scholar]
- MINATO H., HASHIZUME M., MASUDA Y., HOSOKI K. Modulation of extraluminally induced vasoconstrictions by endothelium-derived nitric oxide in the canine basilar artery. Arzneim.-Forsch. 1995;45:675–678. [PubMed] [Google Scholar]
- NOZU K. Flurbiprofen: highly potent inhibitor of prostaglandin synthesis. Biochem. Biophys. Acta. 1978;529:493–496. [PubMed] [Google Scholar]
- OHLSTEIN E.H., NAMBI P., DOUGLAS S.A., EDWARDS R.M., GELLAI M., LAGO A., LEBER J.D., COUSINS R.D., GAO A., FRAZEE J.S., PEISHOFF C.E., BEAN J.W., EGGLESTON D.S., ELSHOURBAGY N.A., KUMAR C., LEE J.A., YUE T.-L., LOUDEN C., BROOKS D.P., WEINSTOCK J., FEUERSTEIN G., POSTE G., RUFFOLO R.R., GLEASON J.G., ELLIOTT J.D. SB 209670, a rationally designed potent nonpeptide endothelin receptor antagonist. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8052–8056. doi: 10.1073/pnas.91.17.8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRADO R., WATSON B.D., KULUZ J., DIETRICH W.D. Endothelium-derived nitric oxide synthase inhibition: effects on cerebral blood flow, pial artery diameter, and vascular morphology in rats. Stroke. 1992;23:1118–1124. doi: 10.1161/01.str.23.8.1118. [DOI] [PubMed] [Google Scholar]
- RAJAGOPALAN S., DUBE S., CANTY J.M. Regulation of coronary diameter by myogenic mechanisms in arterial microvessels greater than 100 μm in diameter. Am. J. Physiol. 1995;268:H788–H793. doi: 10.1152/ajpheart.1995.268.2.H788. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELIGSOHEN E.E., BILL A. Effects of NG-nitro-L-arginine methyl ester on the cardiovascular system of the anesthetised rabbit and on the cardiovascular response to thyrotropin-releasing hormone. Br. J. Pharmacol. 1993;109:1219–1225. doi: 10.1111/j.1476-5381.1993.tb13752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN D., MESSINA E.J., KALEY G., KOLLER A. Characteristics and origin of myogenic response in isolated mesenteric arterioles. Am. J. Physiol. 1992;263:H1486–H1491. doi: 10.1152/ajpheart.1992.263.5.H1486. [DOI] [PubMed] [Google Scholar]
- THORIN E., NGUYEN T.-D., BOUTHILLIER A. Control of vascular tone by endogenous endothelin-1 in human pial arteries. Stroke. 1998;29:175–188. doi: 10.1161/01.str.29.1.175. [DOI] [PubMed] [Google Scholar]
- TODA N., AYAJIKI K., OKAMURA T. Neural mechanism underlying basilar artery constriction by intracisternal L-NNA in anesthetised dogs. Am. J. Physiol. 1993a;265:H103–H107. doi: 10.1152/ajpheart.1993.265.1.H103. [DOI] [PubMed] [Google Scholar]
- TODA N., AYAJIKI K., OKAMURA T. Endothelial modulation of contractions caused by oxyhemoglobin and NG-nitro-L-arginine in isolated dog and monkey cerebral arteries. Stroke. 1993b;24:1584–1588. doi: 10.1161/01.str.24.10.1584. [DOI] [PubMed] [Google Scholar]
- TREZISE D.J., DREW G.M., WESTON A.H. Analysis of the depressant effect of the endothelium on contractions of rabbit isolated basilar artery to 5-hydroxytryptamine. Br. J. Pharmacol. 1992;106:587–592. doi: 10.1111/j.1476-5381.1992.tb14380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLIS S.J., MARTIN W. Suppression of myogenic and vasoconstrictor tone in porcine cerebral artery by basal nitric oxide activity. Br. J. Pharmacol. 1999;128:62P. doi: 10.1038/sj.bjp.0703351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAKAWA N., OHASHI M., WAGA S., ITOH T. Role of endothelium in regulation of smooth muscle membrane potential and tone in the rabbit middle cerebral artery. Br. J. Pharmacol. 1997;121:1315–1322. doi: 10.1038/sj.bjp.0701285. [DOI] [PMC free article] [PubMed] [Google Scholar]


