Abstract
The trivalent cation, gadolinium (Gd3+) is commonly used to inhibit stretch-activated channels. In this report, we show that Gd3+ also inhibits ionic current (INaCa), carried by the Na+-Ca2+ exchanger protein. Under selective recording conditions, Gd3+ inhibited both outward and inward INaCa from guinea-pig isolated ventricular myocytes in a dose-dependent manner, with half-maximal inhibition concentrations (IC50) of 30.0±4.0 μM at +60 mV (Hill-coefficient, h=1.04±0.13) and 20.0±2.7 μM at −100 mV (h=1.13±0.16), respectively (P>0.05, n=5–9). Thus, inhibition was not voltage-dependent. The time from Gd3+ application to steady-state effect was slow compared to the divalent blocker Ni2+. The slow time course appeared to reflect gradual Gd3+ accumulation at its binding site on the exchanger, rather than a use-dependent blocking mechanism. This study indicates that for experiments in which Gd3+ is used, its inhibitory effect on INaCa should be taken into account.
Keywords: Na+-Ca2+ exchanger, gadolinium (Gd3+), ventricular myocyte
Introduction
Gadolinium (Gd3+) is a trivalent lanthanide that has been widely used as a stretch-activated channel (SAC) blocker in a wide range of tissue types (Yang & Sachs, 1989). Some reports, however, indicate that the actions of Gd3+ are not restricted to SACs. In ventricular cardiomyocytes, Gd3+ blocks L-type Ca current (ICa,L; Lacampagne et al., 1994) and the rapid delayed rectifier K current (IKr; Pascarel et al., 1998), whilst not inhibiting stretch-induced increase of intracellular Ca2+ (Hongo et al., 1997) or SAC in atrial cells (Zhang et al., 2000). Hongo et al. (1997) suggested that the lack of effect of Gd3+ on stretch-induced increases in [Ca2+]i may result from an additional action: Gd3+ might inhibit Ca2+ extrusion on the sarcolemmal Na+-Ca2+ exchanger, as this plays a vital role in Ca2+ homeostasis (Blaustein & Lederer, 1999).
The exchanger is expressed widely in mammalian tissues e.g. heart, brain, kidney, colon, spleen, lung and pancreas (Blaustein & Lederer, 1999). Thus if Gd3+ can block the exchanger this would be of relevance to studies on a variety of experimental systems. The stoichiometry of the Na+-Ca2+ exchange process (3Na+ ions transported per 1 Ca2+ ion) means that it generates an ionic current (INaCa; Kimura et al., 1986; 1987). The aim of the present study was to utilize direct measurements of INaCa from guinea-pig ventricular myocytes to determine whether or not Gd3+ does inhibit the exchanger.
Methods
Cell isolation
Single ventricular myocytes from hearts of male guinea-pigs (400–600 g) were isolated as previously described (Hobai et al., 1997). The cells were kept at 4°C in high-K+, low Cl− storage medium (Kraft-Brühe, KB medium) containing (in mM): L-glutamate 100, KCl 30, Na-pyruvate 5, taurine 20, creatine 5, succinic acid 5, Na2ATP 2, β-OH butyrate 5, glucose 20, MgCl2 5, EGTA 1, HEPES 10 (pH adjusted to 7.2 with KOH).
Voltage-clamp technique
An aliquot of cell suspension was allowed to settle on the glass bottom of a Perspex chamber on the stage of an inverted microscope (Nikon Diaphot) for several minutes. Then, the bath was continuously superfused at 37°C with Tyrode's solution (see below). Whole-cell patch clamp experiments were performed using an Axopatch 200A amplifier (Axon instruments, U.S.A.). Patch pipettes (Corning 7052 glass, AM Systems) were pulled using a Flaming/Brown P87 puller and fire-polished to final resistance of 1–2 MΩ (Narishige MF 83 microforge, Japan). Protocols were generated and data recorded on-line with p-Clamp 6.0 software via an analogue-to-digital converter (Digidata 1200B, Axon instruments, U.S.A.). Data are expressed as mean±s.e.mean; t-test and ANOVA were used for statistical analysis. P values less than 0.05 were taken as significant.
Solutions
Tyrode's solution contained (in mM): NaCl 140, HEPES 5, glucose 10, KCl 4, CaCl2 2.5, MgCl2 1 (pH adjusted to 7.45 with NaOH). We used similar solutions to Hinde et al. (1999) to measure INaCa. The extracellular solution for recording INaCa was K+-free Tyrode's solution containing 10 μM strophanthidin, 10 μM nifedipine and 1 mM BaCl2 to eliminate K, Ca, background and Na-K pump currents. Solutions were applied using a home-built rapid solution application device. Intracellular solution contained (in mM): Cs-aspartate 113, NaCl 20, MgCl2 0.4, Tris-ATP 5, HEPES 10, glucose 5, BAPTA 10, tetraethylammonium (TEA) 20, CaCl2 1, pH 7.2 (titrated with CsOH). The combination of 10 BAPTA and 1 CaCl2 gave a free pipette Ca concentration of 20 nM (calculated with the Maxchelator program).
Chemicals
Gadolinium chloride was purchased from Sigma and dissolved in the external INaCa solution to the concentrations shown in ‘Results'.
Results
The solutions described above have previously been demonstrated to allow INaCa to be measured as current sensitive to external Ni2+ or 0Na/0Ca solution (Hinde et al., 1999). To determine the effects of Gd3+, we applied descending ramp pulses every 12 s from +80 mV to −120 mV (dV/dt=0.4 V s−1) from a holding potential of −80 mV. Representative net currents before and after Gd3+ are shown in Figure 1A inset. Both outward and inward currents were inhibited by 100 μM Gd3+. The Gd3+-sensitive current showed an outwardly rectifying current-voltage (I–V) relationship with a reversal potential (Erev) close to −60 mV (Figure 1A). The observed Erev was higher than that calculated for INaCa using the free [Ca] and [Na] values for the pipette solution given in the Methods (which was in excess of −150 mV, using equation 6′ of Blaustein & Lederer, 1999). It was, however, consistent with recent data (Convery & Hancox, 1999), which suggest that buffering of subsarcolemmal and bulk [Ca] can differ under selective recording conditions for INaCa. The characteristics of the Gd3+-sensitive current, therefore, resembled those reported previously for INaCa (e.g. Convery & Hancox, 1999; Hinde et al., 1999). To confirm the identity of the Gd3+-sensitive current, extracellular Na+ and Ca2+ were replaced with Li+ and Ba2+ (0Na/0Ca solution to abolish INaCa). With the exchanger inhibited, the residual current was not significantly affected by Gd3+ (Figure 1B). Similar results were obtained when INaCa was completely blocked with the widely used blocker of INaCa Ni2+ (10 mM; data not shown). Collectively, these data showed that the current component inhibited by Gd3+ was INaCa.
Figure 1.

The effect of 100 μM Gd3+ on INaCa. (A) Current-voltage (I-V) relationship of current sensitive to external Gd3+, obtained by subtracting from control the current after 100 μM Gd3+. Inset shows the pulse protocol and representative currents. (B) The effects of Gd3+ under conditions in which INaCa was abolished by 0Na/0Ca solution. Current in the presence of 0Na/0Ca (minus Gd3+) was shown for comparison. Gd3+ did not inhibit residual current. Inset shows representative currents.
The sensitivity of INaCa to Gd3+ was concentration-dependent within the concentration range tested of 2–200 μM. Figure 2A shows the effect of three different [Gd3+] on the I-V relationship for INaCa (n=6–9 cells for these three concentrations). Similar suppression of both outward and inward current amplitudes was observed at particular [Gd3+]. Figure 2B shows the current amplitudes at +60 mV and −100 mV plotted against [Gd3+] between 2–200 μM. Half-maximal inhibition was observed at Gd3+ concentrations (IC50) of 30.0±4.0 μM at +60 mV (Hill-coefficient, h was 1.04±0.13, n=5–9) and 20.0±2.7 μM at −100 mV (h was 1.13±0.16, n=5–9). These values were not significantly different (P>0.05). The similar IC50 values suggest that the inhibition of INaCa by Gd3+ was not voltage-dependent. The Hill-coefficient close to 1 suggests that Gd3+ exerted its inhibitory effect by at least one molecule of Gd3+ binding to one exchanger protein molecule.
Figure 2.
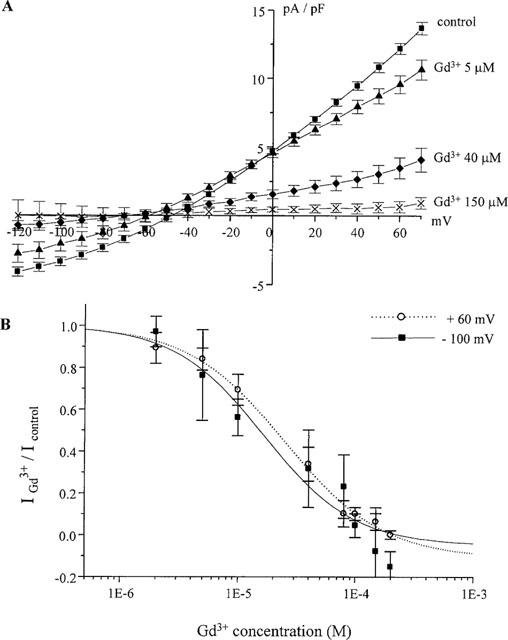
Dose- and voltage-dependence of Gd3+ inhibition of INaCa. (A) Mean I-V relationship of current amplitudes at different Gd3+ concentrations. Gd3+ subsequently suppressed INaCa. (B) Dose-response curves for the effect of Gd3+ on INaCa. Currents were measured at +60 mV and −100 mV. Continuous curves were plotted according to the Michaelis-Menten equation: IGd3+/Icontrol =1/(1+([Gd3+]/IC50)h). h is Hill coefficient.
In contrast to its effects on ICa,L (Lacampagne et al., 1994) or IKr (Hongo et al., 1997), Gd3+ was slow to exert its full effect on INaCa. Application of Gd3+ (100 μM) for up to 2 min only partially suppressed INaCa while the time for its effect to reach the steady-state was over 6 min. The slow time course of inhibition was not attributable to the perfusion system as (a) Ni2+ almost completely suppressed INaCa within 12 s, (b) 100 μM Gd3+ completely blocked ICa,L within only seconds (data not shown). These observations were consistent with previous reports (Lacampagne et al., 1994; Hinde et al., 1999). In addition, reversal of inhibition was rapid on switching to Gd3+-free solution. For 100 μM Gd3+, the current attained 44.6±5.5% (n=6) of the control value within 12 s of washout. This did not increase with repetitive pulsing; thus, under our conditions reversibility was incomplete. The time-course of Gd3+ inhibition of the reverse and forward modes of the exchanger (outward and inward current, respectively) was ascertained by normalizing the mean outward and inward current amplitudes at +60 mV and −100 mV and plotting amplitude relative to control against duration of Gd3+ exposure (Figure 3). The decline of both inward and outward INaCa was fitted best by a bi-exponential function. The initial decline was slightly faster at +60 mV than at −100 mV; but this difference was not significant (P>0.05). The slower phase of decline was similar at the two potentials.
Figure 3.
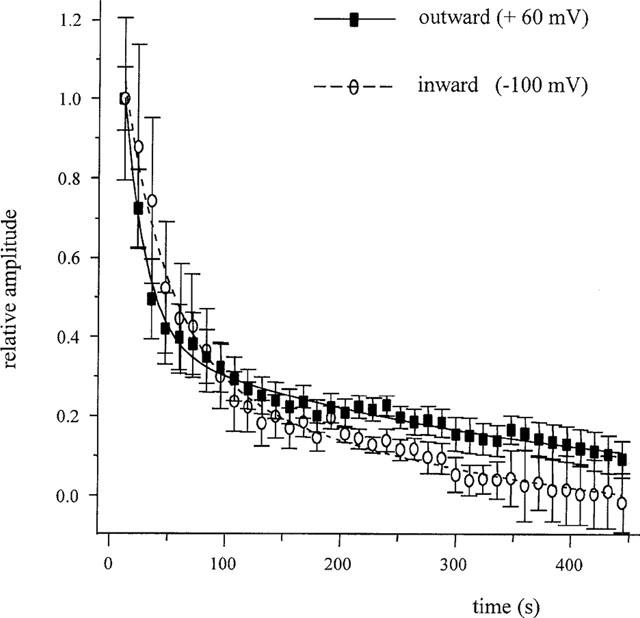
Time-dependence of Gd3+ inhibition of INaCa. Proportion of control INaCa after Gd3+ was applied at +60 mV and −100 mV plotted against time. Both data sets were fitted with a double exponential function: τ1=19.2±0.06 s, τ2=347.4±21.3 s at +60 mV and τ1=38.6±4.3 s, τ2=303.0±36.5 s at −100 mV, n=7.
Gd3+-inhibition of calcium current has been reported to depend on the extent of channel activation (Biagi et al., 1990). It seemed possible, therefore, that the slow time-course with which Gd3+ exerted its steady-state effect might have reflected dependence of the inhibition on repeated activation (use-dependence) of the exchanger. The following approach was used to investigate this. First, the net ‘control' INaCa was obtained by measuring the current sensitive to Ni2+ (10 mM) application. In seven cells, current was monitored for 5 min during continuous pulsing. In five cells, the control INaCa was obtained as above, and then Gd3+ (100 μM) applied: cells were exposed to Gd3+ for 5 min, but in the presence of 0Na/0Ca solution to immobilize the exchanger and without application of test pulses. (Figure 4, rested in 0Na/0Ca solution). If the block depended on repeated activation of the exchanger, we reasoned that on readmitting external Na and Ca after 5 min (in the presence of Gd3+), relatively little block would be expected on the first pulse, but would increase with repeated stimulation. In fact, a large reduction of the current amplitude was observed on the first pulse with this method, to an extent that was not significantly different (P>0.05) from that with continuous pulsing. Similar results were also observed with another approach (to measure the Erev for INaCa for each cell and then hold the membrane potential at this value in the presence of Gd3+ (100 μM) for 5 min; the INaCa elicited by the first ramp after this period was then measured). These data suggested that the time course of the effect of Gd3+ could not be accounted for by slow use-dependence of the inhibition.
Figure 4.

Lack of use-dependence of the effect of Gd3+ on INaCa. INaCa amplitudes at +60 mV under three conditions were compared: Control; 100 μM Gd3+ was continuously perfused for 5 min during the time INaCa was repetitively activated by continuous pulsing (n=7); Extracellular Na+ and Ca2+ were replaced by equimolar Li+ and Ba2+ (0Na/0Ca solution, n=5) during 5 min of Gd3+ exposure, then INaCa measured when Na and Ca were readmitted. (****,P<0.00002 with respect to control INaCa; ***P<0.0007 with respect to control INaCa).
Discussion
Whilst there has been no previous study that has shown directly inhibition of the sarcolemmal Na+-Ca2+ exchanger by Gd3+, computer-simulations by Hongo et al. (1997) led them to suggest that the blockade of the exchanger was required to reproduce experimental data showing a stretch-induced increase in guinea-pig ventricular [Ca2+]i. Earlier data had suggested that the trivalent cation La3+ can inhibit the Na+-Ca2+ exchanger (Kimura et al., 1986), but direct information regarding Gd3+ has not until now been available. Our data show that Gd3+ inhibits the cardiac Na+-Ca2+ exchanger with a potency that compares favourably with other divalent or trivalent inorganic inhibitors: e.g. IC50 for Ni2+ of 0.3 mM (Hinde et al., 1999) and inhibition by concentrations >0.25 mM La3+ in smooth muscle cells (Shimizu et al., 1997). The Hill co-efficient values near 1 are consistent with observations on other inorganic inhibitors (Blaustein & Lederer, 1999).
The blocking effect of Gd3+ on the Na+-Ca2+ exchanger was found to be voltage-independent, but was dependent on the duration of exposure to Gd3+, with block increasing to a steady-state value over a period of minutes after initial application. These observations appear to exclude a surface charge mechanism (which would be expected to show voltage dependence) but are most consistent with Gd3+ gradually accumulating at a site that is accessible from the external surface of the cell. On the basis of the experiments shown in Figure 4 it seems reasonable to conclude that the inhibition does not depend on the exchanger being repeatedly activated (in order to facilitate Gd3+ binding), as similar levels of block were observed with exchanger immobilization as with repeated activation. Additionally, preliminary data suggested that Gd3+ might not be able to readily reach its binding site from the cell interior, because Gd3+ in the pipette dialysate (at concentrations up to 5 mM) showed little block of INaCa (data not shown). Intriguingly, the trivalent cation La3+ has been reported to enter cells (Shimizu et al., 1997; Peeters et al., 1989), and therefore might possibly act at the inside of the plasma membrane.
The demonstration that Gd3+ blocks INaCa is important both because the exchanger is present in a range of tissue types, and because Gd3+ is a widely used experimental tool, particularly in studying stretch-related responses. When interpreting its effects in various experimental systems, potentially diverse actions should be considered (Caldwell et al., 1998). To these, it would appear now necessary to add its potent inhibitory effects on INaCa. Further, it is possible that this action of Gd3+ may help increase understanding of the range of roles played by the Na+-Ca2+ exchanger in both physiological and pathological situations.
Acknowledgments
This work was supported by British Heart Foundation Grant PG/98097 awarded to A.J. Levi and J.C. Hancox. J.C. Hancox was supported by a Career Development Fellowship from the Wellcome Trust. We thank Lesley Arberry for help with cell isolation, Mary Convery and Kathryn Yuill for useful discussions and Dr Corné Kros for comments on the manuscript.
Abbreviations
- Erev
reversal potential
- Gd3+
gadolinium
- ICa,L
L-type calcium current
- IKr
rapid delayed rectifier current
- INaCa
Na+-Ca2+ exchanger current
- SAC
stretch-activated channel
References
- BIAGI B.A., ENYEART J.J. Gadolinium blocks low- and high-threshold calcium currents in pituitary cells. Am. J. Physiol. 1990;259:C515–C520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- BLAUSTEIN M.P., LEDERER W.J. Sodium/calcium exchange: its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- CALDWELL R.A., CLEMO H.F., BAUMGARTEN C.M. Using gadolinium to identify stretch-activated channels: technical considerations. Am. J. Physiol. 1998;275:C619–C621. doi: 10.1152/ajpcell.1998.275.2.C619. [DOI] [PubMed] [Google Scholar]
- CONVERY M.K., HANCOX J.C. Comparison of Na+-Ca2+ exchange current elicited from isolated rabbit ventricular myocytes by voltage ramp and step protocols. Pflügers. Arch. 1999;437:944–954. doi: 10.1007/s004240050866. [DOI] [PubMed] [Google Scholar]
- HINDE A.K., PERCHENET L., HOBAI I.A., LEVI A.J., HANCOX J.C. Inhibition of Na/Ca exchange by external Ni in guinea-pig ventricular myocytes at 37°C, dialysed internally with cAMP-free and cAMP-containing solutions. Cell Calcium. 1999;25:321–331. doi: 10.1054/ceca.1999.0035. [DOI] [PubMed] [Google Scholar]
- HOBAI I.A., HOWARTH F.C., PABBATHI V.K., DALTON G.R., HANCOX J.C., ZHU J.Q., HOWLETT S.E., FERRIER G.R., LEVI A.J. “Voltage-activated Ca release” in rabbit, rat and guinea-pig cardiac myocytes, and modulation by internal cAMP. Pflügers. Arch. 1997;435:164–173. doi: 10.1007/s004240050496. [DOI] [PubMed] [Google Scholar]
- HONGO K., PASCAREL C., CAZORLA O., GANNIER F.L.E., GUENNEC J.Y., WHITE E. Gadolinium blocks the delayed rectifier potassium current in isolated guinea-pig ventricular myocytes. Exp. Physiol. 1997;82:647–656. doi: 10.1113/expphysiol.1997.sp004053. [DOI] [PubMed] [Google Scholar]
- KIMURA J., MIYAMAE S., NOMA A. Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J. Physiol. (Lond) 1987;384:199–222. doi: 10.1113/jphysiol.1987.sp016450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIMURA J., NOMA A., IRISAWA H. Na-Ca exchange current in mammalian heart cells. Nature. 1986;319:596–597. doi: 10.1038/319596a0. [DOI] [PubMed] [Google Scholar]
- LACAMPAGNE A., GANNIER F., ARGIBAY J., GARNIER D., LE GUENNEC J.Y. The stretch-activated ion channel blocker gadolinium also blocks L-type calcium channels in isolated ventricular myocytes of the guinea-pig. Biochim. Biophys. Acta. 1994;1191:205–208. doi: 10.1016/0005-2736(94)90250-x. [DOI] [PubMed] [Google Scholar]
- PASCAREL C., HONGO K., CAZORLA O., WHITE E., LE GUENNEC J.Y. Different effects of gadolinium on IKR, IKS and IK1 in guinea-pig isolated ventricular myocytes. Br. J. Pharmacol. 1998;124:356–360. doi: 10.1038/sj.bjp.0701835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEETERS G.A., KOHMOTO O., BARRY W.H. Detection of La3+ influx in ventricular cells by indo- 1 fluorescence. Am. J. Physiol. 1989;256:C351–C357. doi: 10.1152/ajpcell.1989.256.2.C351. [DOI] [PubMed] [Google Scholar]
- SHIMIZU H., BORIN M.L., BLAUSTEIN M.P. Use of La3+ to distinguish activity of the plasmalemmal Ca2+ pump from Na+/Ca2+ exchange in arterial myocytes. Cell Calcium. 1997;21:31–41. doi: 10.1016/s0143-4160(97)90094-4. [DOI] [PubMed] [Google Scholar]
- YANG X.C., SACHS F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1989;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- ZHANG Y.H., YOUM J.B., SUNG H.K., LEE S.H., RYU S.Y., HO W.K., EARM Y.E. Stretch-activated and background non-selective channels in rat atrial myocyte. J. Physiol. (Lond.) 2000;523:607–619. doi: 10.1111/j.1469-7793.2000.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


