Abstract
The novel 5-HT7 receptor antagonist, SB-269970-A, potently displaced [3H]-5-CT from human 5-HT7(a) (pKi 8.9±0.1) and 5-HT7 receptors in guinea-pig cortex (pKi 8.3±0.2).
5-CT stimulated adenylyl cyclase activity in 5-HT7(a)/HEK293 membranes (pEC50 7.5±0.1) and SB-269970-A (0.03–1 μM) inhibited the 5-CT concentration-response with no significant alteration in the maximal response. The pA2 (8.5±0.2) for SB-269970-A agreed well with the pKi determined from [3H]-5-CT binding studies.
5-CT-stimulated adenylyl cyclase activity in guinea-pig hippocampal membranes (pEC50 of 8.4±0.2) was inhibited by SB-269970-A (0.3 μM) with a pKB (8.3±0.1) in good agreement with its antagonist potency at the human cloned 5-HT7(a) receptor and its binding affinity at guinea-pig cortical membranes.
5-HT7 receptor mRNA was highly expressed in human hypothalamus, amygdala, thalamus, hippocampus and testis.
SB-269970-A was CNS penetrant (steady-state brain : blood ratio of ca. 0.83 : 1 in rats) but was rapidly cleared from the blood (CLb=ca. 140 ml min−1 kg−1). Following a single dose (3 mg kg−1) SB-269970 was detectable in rat brain at 30 (87 nM) and 60 min (58 nM). In guinea-pigs, brain levels averaged 31 and 51 nM respectively at 30 and 60 min after dosing, although the compound was undetectable in one of the three animals tested.
5-CT (0.3 mg kg−1 i.p.) induced hypothermia in guinea-pigs was blocked by SB-269970-A (ED50 2.96 mg kg−1 i.p.) and the non-selective 5-HT7 receptor antagonist metergoline (0.3–3 mg kg−1 s.c.), suggesting a role for 5-HT7 receptor stimulation in 5-CT induced hypothermia in guinea-pigs.
SB-269970-A (30 mg kg−1) administered at the start of the sleep period, significantly reduced time spent in Paradoxical Sleep (PS) during the first 3 h of EEG recording in conscious rats.
Keywords: Human 5-HT7 receptor, adenylyl cyclase, 5-CT, [3H]-5-CT, SB-269970-A, guinea-pig, rat, hypothermia, sleep, EEG
Introduction
Structural, functional and pharmacological characteristics identify seven major classes of 5-HT receptors. The 5-HT7 receptor has been cloned from mouse, rat, guinea-pig and human and the receptor binding profile is consistent across species (Bard et al., 1993; Plassat et al., 1993; Ruat et al., 1993; Shen et al., 1993; Tsou et al., 1994) and between cloned and native 5-HT7 receptors (To et al., 1995, Boyland et al., 1996). The receptor is encoded by a single gene on chromosome 10 (10q21–q24; Gelernter et al., 1995). 5-HT7 receptors are defined pharmacologically by their high affinity for 5-CT, 5-HT, 5-MeOT and methiothepin, moderate affinity for 8-OH-DPAT and ritanserin and low affinity for pindolol, sumatriptan and buspirone (To et al., 1995). Splice variants have been identified in rat and human but are so far indistinguishable in their pharmacological profile and tissue distribution (Bard et al., 1993; To et al., 1995; Stam et al., 1997; Heidmann et al., 1997). 5-HT7 receptors couple positively to adenylyl cyclase when expressed in cell lines (Hoyer et al., 1994, Thomas et al., 1999), a property shared with 5-HT4 and 5HT6 receptors. A 5-HT receptor which is positively coupled to adenylyl cyclase and shows pharmacological similarities to a 5-HT7 receptor has been reported to be present in guinea-pig hippocampus (Tsou et al., 1994). In addition, the selective 5-HT7 receptor antagonist SB-258719 has been reported to antagonize 5-CT-evoked stimulation of adenylyl cyclase activity in guinea-pig hippocampal membranes. Its potency is consistent with that at the human cloned 5-HT7 receptor (Thomas et al., 1999), suggesting that this 5-CT response is mediated primarily via 5-HT7 receptors.
In brain tissue from various species, 5-HT7 receptor mRNA is discretely localized within thalamus, hypothalamus, limbic and cortical regions (Ruat et al., 1993, Bard et al., 1993, To et al., 1995). Neurochemical lesioning studies indicate that, at least in the hypothalamus, they are located post-synaptically (Clemett et al., 1999). 5-HT7 receptor mRNA is also present in smooth muscle cells from human, porcine and rat blood vessels (Bard et al., 1993, Schoeffter et al., 1996) and has been reported in human gastrointestinal tract (Bard et al., 1993) and rat lumbar dorsal root and sympathetic ganglia (Meuser & Pierce, 1997). Autoradiography, using non-selective radioligands in the presence of appropriate masking ligands, shows a pattern of 5-HT7 binding sites in rat and guinea-pig brain (To et al., 1995, Branchek et al., 1994, Gustafson et al., 1996) which matches, to a large extent, the mRNA distribution.
Receptor distribution studies and pharmacological studies with non-selective compounds have suggested that 5-HT7 receptors may play a role in the control of circadian rhythms (Lovenberg et al., 1993, Tsou et al., 1994, Ying & Rusak, 1998) and smooth muscle relaxation in vascular tissues (Schoeffter et al., 1996, Eglen et al., 1997). These and other observations have led to suggestions that selective 5-HT7 receptor ligands may have potential therapeutic applications in depression (Sleight et al., 1995), migraine (Teron, 1998), schizophrenia (Roth et al., 1994) and pain (Meuser & Pierce, 1997). Although the investigation of receptor function has been hampered by a lack of suitable ligands, compounds have recently been identified which are potent and selective 5-HT7 receptor antagonists (Forbes et al., 1998; Kikuchi et al., 1999). We have recently identified SB-269970-A as a potent and selective 5-HT7 receptor antagonist (Lovell et al., 2000) and report here on its characterization in vitro and in vivo.
Methods
[3H]-5-CT binding to h5-HT7(a)/HEK293 membranes and guinea-pig cerebral cortex membranes
[3H]-5-CT binding to cell membranes prepared from HEK293 stably expressing the h5-HT7(a) receptor (h5-HT7(a)/HEK293) was measured using the method described by Thomas et al. (1998), while [3H]-5-CT binding to guinea-pig cerebral cortex membranes was carried out using a similar method to that described by Thomas et al. (1999), except that WAY-100635 (1 μM) and GR-127935 (10 μM) were included to inhibit [3H]-5-CT binding to 5-HT1A and 5-HT1B/5-HT1D receptors respectively.
Measurement of adenylyl cyclase activity in membrane homogenates from human 5-HT7(a)/HEK293 cells and guinea-pig hippocampus
Adenylyl cyclase activity in h5-HT7(a)/HEK293 cell membranes or guinea-pig hippocampal membranes was determined by measuring the conversion of [α-33P]-ATP to [33P]-cyclic AMP according to the methods described by Thomas et al. (1998; 1999) for the h5-HT7(a)/HEK293 and guinea-pig hippocampal tissues respectively. Following the assay, tubes were stored on ice prior to isolating [33P]-cyclic AMP according to the method of Salomon (1979). Samples were counted using a dual label protocol and the tritium signal was used to correct for per cent column recovery.
[α-33P]-ATP and [3H]-cyclic AMP were obtained from NEN Du Pont. [3H]-5-CT was obtained from Amersham (U.K.). Stock drug solutions were prepared fresh on the day of assay in de-ionized water or DMSO (the final assay concentration of DMSO not exceeding 0.4%). Drug solutions for adenylyl cyclase assays were prepared in 40 mM Tris buffer (pH 7.4 at 37°C) containing 0.5 mM ascorbic acid.
Data analysis
For receptor binding assays, the concentration of drug inhibiting specific [3H]-5-CT by 50% (IC50) was determined and pKi values (−log of the inhibition constant) were calculated from the IC50 values as described by Cheng & Prusoff (1973), using a KD of 0.4 nM for h5-HT7(a)/HEK293 membranes and 0.7 nM for guinea-pig cerebral cortex membranes. Drug concentration-response curves from adenylyl cyclase assays were fitted to a 4-parameter logistic equation (Grafit, Erithacus Software). Agonist potency was expressed as the pEC50 (−log10 EC50). Apparent pKB values (−log10 of the antagonist equilibrium dissociation constant) for antagonism were determined using the equation: pKB=−log ([antagonist]/(concentration ratio-1)) where concentration ratio=ratio of the agonist EC50s in the presence and absence of antagonist. Data represent the mean±s.e.mean of at least three separate experiments each performed using triplicate (adenylyl cyclase activity) or duplicate (receptor binding) determinations.
TaqMan RT–PCR analysis in human tissues
Human polyA+ mRNA samples extracted from CNS and peripheral tissues were obtained from Clontech (UK). cDNA synthesis was performed in triplicate. For each 20 μl reverse transcription reaction, 200 ng human polyA+ mRNA in 9 μl water was mixed with 1 μl oligodT primer (0.5 μg; Life Technologies) and incubated for 5 min at 65°C. After cooling on ice the solution was mixed with 4 μl 5×first-strand buffer, 2 μl of 0.1 M DTT, 0.5 μl each of dATP, dTTP, dCTP and dGTP (each 10 mM), 1 μl RNAseOUT (40 U; Life Technologies) and 1 μl SuperScript II reverse transcriptase (200 U; Life Technologies). Reactions were performed for 60 min at 42°C and terminated by incubating for 15 min at 70°C. Parallel reactions for each RNA sample were run in the absence of SuperScript II to assess the degree of any contaminating genomic DNA.
TaqMan PCR assays for human 5-HT7 receptor and cyclophilin were performed in triplicate on cDNA samples or genomic DNA standards in 96-well optical plates on an ABI Prism 7700 Sequence Detection system (PE Applied Biosystems). For each 25 μl TaqMan reaction, 1 μl cDNA (or genomic DNA standard) was mixed with 11.25 μl PCR-grade water, 11.25 μl 2×TaqMan Universal PCR Master Mix (PE Applied Biosystems), 0.5 μl sense primer (10 μM), 0.5 μl antisense primer (10 μM) and 0.5 μl TaqMan probe (5 μM). Primer sequences were as follows: 5-HT7 sense primer 5′-AAAACATCTCCATCTTTAAGCGAGAA, 5-HT7 antisense primer 5′-GTAAAGGCCCCGACGATGAT and 5-HT7 probe 5′-AGAAAGCAGCCACCACCCTGGG; cyclophilin sense primer 5′-TGAGACAGCAGATAGAGCCAAGC, cyclophilin antisense primer 5′-TCCCTGCCAATTTGACATCTTC, and cyclophilin probe 5′-CATCACCATTGGCAATGAGCGGTTCC. PCR parameters were 50°C for 2 min, 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were captured using an ABI Prism 7700 sequence detector system and analysed using relative standard curve method (Livak, 1999). Each sample was normalized to cyclophilin to correct for differences in RNA quality and quantity.
In vivo studies
In vivo studies were conducted in compliance with the Home Office Guidance on the operation of the Animals (Scientific Procedures) Act 1986, and were reviewed and approved by the SmithKline Beecham Procedures Review Panel.
Pharmacokinetic studies with SB-269970-A.
Adult male Sprague Dawley rats (Charles River, ca. 250 g body weight) were surgically equipped with a cannula in the jugular vein and femoral vein (Griffiths et al., 1996) and allowed 3 days to recover post-operatively. The CNS penetration of SB-269970-A was investigated following intravenous infusion to steady-state in two rats infused via the femoral vein with SB-269970-A (mono-hydrochloride salt; 0.284 mM, 100 ug free base ml−1 dissolved in normal saline containing 2% (v v−1) DMSO and 10% (w v−1) Encapsin HPB™. The target dose rate was 1.42 umol−kg−h (0.5 mg free base kg−1 h−1) over 12 h.
Blood samples were taken via the jugular vein during the latter part of the infusion to confirm steady-state blood concentrations, and at 12 h the animals were killed by exanguination and the brains removed. Blood samples (50 ul) were diluted with an equal volume of water and brain samples were diluted with two volumes of water and homogenized. All samples were stored at ca. −80°C prior to analysis.
In addition a time-course of the relative CNS penetration of SB-269970-A was conducted. Eighteen adult male Sprague Dawley rats (Charles River, ca. 250 g body weight) and eighteen male guinea-pigs (Dunkin Hartley, ca. 500 g body weight) each received an i.p. injection of SB-269770-A (3 mg kg−1) dissolved in normal saline. At 0.5, 1, 2, 4, 6 and 8 h following drug administration, three animals per time point were killed by exsanguination and the brains removed. Samples of blood and brain were prepared as described previously.
Blood and brain homogenate samples were analysed for SB-269970 using a method based on protein precipitation and LC/MS/MS analysis. To samples of blood and brain homogenate (50 ul), acetonitrile (250 ul) containing an appropriate internal standard was added. Samples were mixed thoroughly (mechanical shaking for 20 min), and then centrifuged (15,500×g for 15 min at room temperature). The resulting supernatant was transferred into a vial containing ammonium acetate buffer (150 ul, 10 mM, pH 4.0). After brief mixing (10 s), samples (40 ul) were assayed for SB-269970 concentrations using LC/MS/MS employing positive-ion electrospray ionisation (Sciex API 300) and a Lightning C18 column (33×3 mm ID; Jones Chromatography). The mobile phase was 32% (v v)−1 acetonitrile/68% (v v)−1 ammonium acetate buffer (10 mM, pH 4.0) at a flow rate of 0.75 ml min−1. The lower limit of quantification was 0.014 uM (5 ng ml−1) and the assay was linear up to 5.600 uM (2000 ng ml−1). A value of 15 ul of blood per g of brain tissue was used to correct the brain concentrations of SB-269970 for any compound present in the residual blood following exanguination (Brown et al., 1986).
Effects of 5-CT on body temperature in guinea-pigs
Adult male Dunkin Harley guinea-pigs (240–350 g, Harlan Olac, U.K.) were housed singly or in pairs at a room temperature of 20–22°C on a 12 h light/dark cycle with lights on between 0700–1900 h. Food (SDS FD1 Diet) and water were available ad libitum. Surgically prepared animals received soft food for 7 days post-operatively.
Guinea-pigs were anaesthetized with 4% isoflurane and maintained under surgical anaesthesia with 2–3% isoflurane and nitrous oxide. Animals were positioned in a Kopf stereotaxic frame with the incisor bar lowered 0.5 cm below zero. An incision was made over the skull exposing the bregma to which 2% mepivicaine hydrochloride (Intra Epicaine™, National Veterinary Supplies) was applied to provide local anaesthesia. A 22-gauge stainless steel/perspex guide cannula (Semat) was implanted unilaterally into the lateral ventricle according to the following coordinates: AP +0.98 cm from the intraaural line, L±0.23 cm from the midline and DV −0.4 cm from the skull surface. The guide cannula was held in place by anchor screws, secured using Densply Poly F Plus™ zinc carboxylate covered by a thin layer of methyl methacrylate acrylic cement. Buprenorphine (0.3 mg kg−1s.c.) (Vetergesic™, Reckitt & Coleman) was administered to provide post-operative analgesia. A dummy cannula was inserted into the guide to maintain patency prior to drug administration, which commenced at least 7 days after surgery.
Core body temperature was electronically measured (COMARK thermometer, model 9001) in lightly restrained guinea-pigs using a rectal probe (COMARK, model BS4937K) immediately prior to and at intervals after drug or vehicle administration, until peak treatment effects were observed. Following lubrication with water, the temperature probe was inserted approximately 5 cm into the rectum and held in position for typically 10–15 s until a stable temperature reading was obtained. Mean change in body temperature was calculated for each treatment group (n=4 for intracerebroventricular and n=6–12 for systemic treatments) at every time-point measured. In agonist/antagonist interaction studies data for each group were expressed as maximal change (mean±s.e.mean) in body temperature over the whole testing period.
The effect of 5-CT on core body temperature in guinea-pigs was determined following both central (2–10 μg i.c.v.) and systemic (0.03–3 mg kg−1 i.p.) administration. Subsequently, the effects of the 5-HT7 receptor antagonist SB-269970-A (1–30 mg kg−1 i.p.), the mixed 5-HT antagonist metergoline (0.3–3 mg kg−1 s.c.), the 5-HT1A/1B receptor antagonist pindolol (2–8 mg kg−1 s.c.), and the 5-HT1B/1D receptor antagonist GR 125743 (0.1–3 mg kg−1 i.p.) on 5-CT-induced hypothermia (0.3 mg kg−1 i.p.) in guinea-pigs were investigated. Drug treatments were evaluated between 1000 and 1700 h. Changes in body temperature were compared using one-way ANOVA followed by Dunnett's t-test, with P<0.05 considered significant.
Sleep studies
Male Hooded Lister rats (Charles River, 200–250 g) were housed in groups of four on reverse 12 h light–dark cycle (0600 : 1800 h dark) for at least 21 days prior to surgery. Access to food and water was allowed ad libitum. Rats were anaesthetized with a mixture of Domitor® (medetomidine HCl, 0.4 mg kg−1 sc; Pfizer) and Sublimaze® (fentanyl, 0.45 mg kg−1 i.p.; Janssen-Cilag) and prepared for chronic EEG and EMG recordings as previously described (Hagan et al., 1999). After recovery animals were housed in pairs in a temperature controlled environment (20±1°C) with access to food and water ad libitum. At least 7 days elapsed before further testing.
Sleep recording
During sleep studies, rats were housed singly and acclimatized to recording chambers with non-restraining leads for 24 h with food and water available ad libitum. On test days, the rats were dosed on a randomized crossover basis with vehicle or SB-269970-A (30 mg kg−1 i.p.) at 1800 or 0900 h, with at least 4 days between treatments. EEG/EMG signals were recorded for the remainder of the 12 h period, and the following 12 h. Ten second epochs of EEG and EMG signals were captured continuously, using an amplifier/filter system (PA400, Biodata) which was linked to a PC via an A/D convertor card (LPM 16, National Instruments).
Sleep stage analysis
Automated analysis of the EEG and EMG (Sleep Stage Analysis v3.03, SmithKline Beecham) was used to define four sleep stages; arousal, slow wave sleep (SWS) 1 & 2 and paradoxical sleep (PS) as described previously (Hagan et al., 1999).
Analysis of sleep data
Mean±s.e.mean percentage time spent in each sleep stage per hour of recording was calculated and used to determine area under the curve (AUC) for the light–dark phases. The number and duration (seconds) of bouts of PS was quantified. Latency (minutes) to the first 10 s epoch of PS was also quantified. Statistical significance (P<0.05) between SB-269970-A and the corresponding vehicle control was assessed by ANOVA followed by Contrast Analysis (Crowder & Hand, 1996).
Drugs
SB-258719 ((R)-3,N-dimethyl-N-[1-methyl-3-(4-methylpiperidin-1-yl)propyl]benzene-sulphonamide), SB-269970-A ((R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol)), N-[4-methoxy-3-(4-methyl-1-piperazinyl) phenyl]-3-methyl-4-(4-pyridinyl)benzamide (GR 125743), GR-125743 and WAY-100635 were synthesized at SmithKline Beecham (Harlow, U.K.). 5-hydroxytryptamine HCl (5-HT), 5-carboxamidotryptamine (5-CT) and methiothepin mesylate were obtained from Sigma-Aldrich (Poole, U.K). Metergoline and (±)pindolol were purchased from Research Biochemicals International, Natick, U.S.A. and Sigma, Poole, U.K., respectively.
For in vivo studies, drugs were dissolved in appropriate solvents (shown in parentheses); 5-CT (0.9% saline); SB-269970-A (distilled water); pindolol and metergoline (1% glacial acetic acid, neutralized with sodium hydroxide) and GR 125743 (1% tartaric acid, neutralized with sodium hydroxide). A 1 ml kg−1 dose volume was used for all systemic treatments. For intracerebroventricular administration drugs were infused into the right or left lateral ventricle at a rate of 2.5 ul min−1 for 2 min through a 28 gauge stainless steel injection cannula which remained in position for 1 min after infusion to ensure adequate drug diffusion. All doses are expressed as free base.
Results
[3H]-5-CT binding to h5-HT7(a)/HEK293 membranes and guinea-pig cerebral cortex membranes
[3H]-5-CT binding to h5-HT7(a)/HEK293 membranes displayed a single saturable binding component with a KD of 0.42±0.04 nM and Bmax of 6190±940 fmoles mg−1 protein (data not shown). [3H]-5-CT (0.5 nM) binding to h5-HT7(a)/HEK293 membranes was potently inhibited by SB-269970 with a pKi of 8.9±0.1 (Table 1). [3H]-5-CT binding to guinea-pig cerebral cortex membranes, measured in the presence of WAY-100635 (1 μM) and GR-127935 (10 μM), also revealed a single saturable binding component with a KD of 0.67±0.12 nM and Bmax of 143±19 fmoles mg−1 protein (data not shown). A number of standard 5-HT receptor agonists and antagonists, including the selective 5-HT7 receptor antagonist, SB-258719, inhibited the binding with a rank order of potency corresponding to that reported for the human cloned 5-HT7 receptor (Thomas et al., 1998) (Table 1). SB-269970-A was a potent inhibitor of [3H]-5-CT binding to guinea-pig cerebral cortex membranes (pKi 8.3±0.2), consistent with its high affinity for the human cloned 5-HT7 receptor (Table 1). For all compounds tested, Hill slope values for inhibition of [3H]-5-CT binding to guinea-pig cerebral cortex membranes were close to unity. Absolute pKi values for antagonists to inhibit [3H]-5-CT binding to guinea-pig cerebral cortex membranes were approximately 0.5 log units lower than the corresponding values for the human cloned 5-HT7 receptor.
Table 1.
Comparison of the affinity of ligands for the human 5-HT7(a) receptor and guinea-pig cerebral cortex 5-HT7 receptor determined by competition with [3H]-5-CT
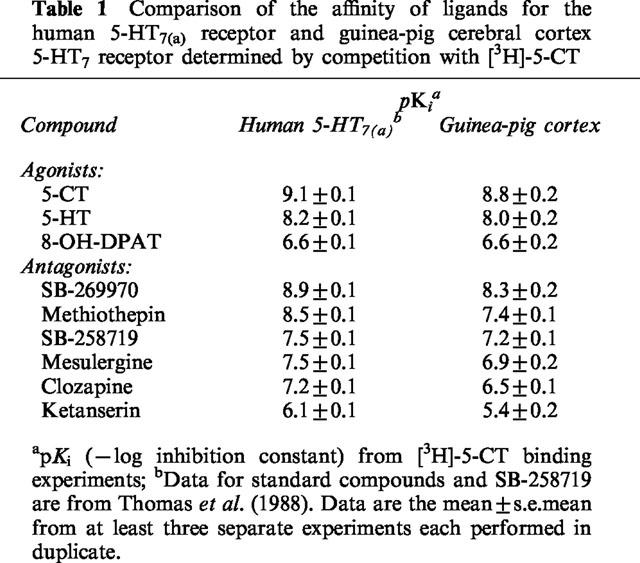
Adenylyl cyclase activity in membrane homogenates from human 5-HT7(a)/HEK293 cells
It has previously been reported that the functional profile for the h5-HT7(a) receptor, as determined by measurement of adenylyl cyclase activity in washed 5-HT7(a)/HEK293 membranes, is consistent with a 5-HT7 receptor-mediated functional response (Thomas et al., 1998). 5-CT stimulated adenylyl cyclase activity in 5-HT7(a)/HEK293 membranes by 500±80% (mean±s.e.mean) and with a pEC50 of 7.5±0.1 (Figure 1). SB-269970-A (0.03, 0.1, 0.3 and 1 μM) produced a concentration-related rightward-shift of the 5-CT concentration-response curve with no significant alteration in the maximal response to 5-CT. Schild analysis of the antagonism by SB-269970-A gave a pA2 of 8.5±0.2 and slope (0.8±0.1, 95% confidence interval 0.70–0.97) which was marginally significantly different from 1 (P<0.05), (Figure 1, inset). The pA2 determined for SB-269970-A was in good agreement with the corresponding pKi determined from [3H]-5-CT binding studies (Table 1). In addition to antagonizing the 5-CT response, SB-269970-A also produced a small inhibition of basal adenylyl cyclase activity in the absence of added 5-CT (Figure 1).
Figure 1.
Stimulation of adenylyl cyclase activity in human 5-HT7/HEK293 membranes by 5-CT alone and in the presence of SB-269970-A (0.03, 0.1, 0.3 and 1 μM). Data points represent the mean±s.e.mean of at least three separate experiments each performed using duplicate determinations. Results are expressed as % of the maximal 5-CT response. Inset: Schild analysis of the same data.
Adenylyl cyclase activity in membrane homogenates from guinea-pig hippocampus
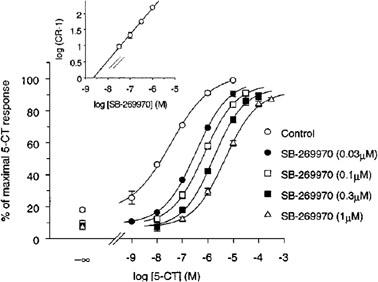
5-CT produced a 21% stimulation of adenylyl cyclase activity in guinea-pig hippocampus with a pEC50 of 8.4±0.2 (Figure 2). SB-269970-A (0.3 μM) produced a parallel rightward-shift of the 5-CT concentration-response curve (Figure 2). The effect of SB-269970-A was surmountable at high concentrations of 5-CT, consistent with competitive antagonism. The calculated pKB for SB-269970-A of 8.3±0.1 was in good agreement with both its antagonist potency at the human cloned 5-HT7 receptor and its potency to inhibit [3H]-5-CT binding to 5-HT7 receptors in guinea-pig cortical membranes (Table 1). In addition to antagonizing the effect of 5-CT, SB-269970-A produced a small inhibition of basal adenylyl cyclase activity (Figure 2).
Figure 2.
Stimulation of adenylyl cyclase activity in guinea-pig hippocampal membranes by 5-CT alone and in the presence of SB-269970-A (0.3 μM). Data points represent the mean±s.e. mean values from three separate experiments each performed using triplicate determinations. Results are expressed as % of the maximal 5-CT response.
Taqman analysis of the distribution of 5-HT7 mRNA in human tissues
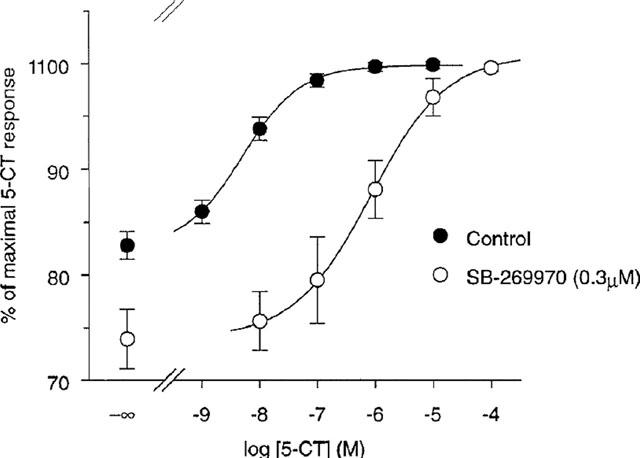
Preliminary results showed that cyclophilin was a suitable housekeeping gene for normalization since the variation of expression between 30 CNS and peripheral tissues was minimal (data not shown). 5-HT7 receptor mRNA was expressed in a variety of tissues but the expression levels were highest in hypothalamus, amygdala, thalamus, hippocampus and testis. Lower expression was observed in most other CNS tissues and some but not all peripheral tissues (Figure 3).
Figure 3.
Taqman analysis of the distribution of 5-HT7 mRNA in human tissues. TaqMan PCR assays for human 5-HT7 receptor and cyclophilin were performed in triplicate on cDNA samples or genomic DNA standards in 96-well optical plates on an ABI Prism 7700 Sequence Detection system.
Pharmacokinetic studies with SB-269970-A
The CNS penetration of SB-269970-A was determined at steady-state in the rat (n=2) following i.v. infusion of the SB-269970-A (0.5 mg free base kg−1 h−1) over 12 h. Analysis of blood samples obtained during the latter part of the infusion confirmed steady-state conditions with mean brain and blood concentrations of 0.136 and 0.163 uM respectively. SB-269970 was therefore CNS penetrant with a steady-state brain:blood ratio of ca. 0.83 : 1. The compound was rapidly cleared in the rat with a CLb of ca. 140 ml min−1 kg−1.
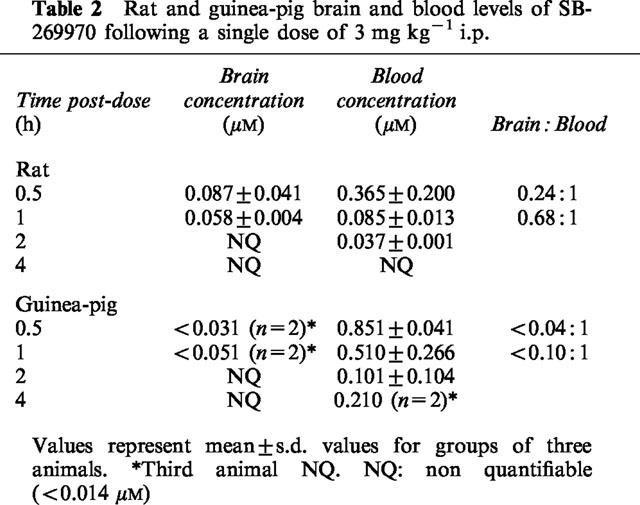
Measurement of blood and brain levels of SB-269970, following a single i.p. dose of SB-269970-A (3 mg free base kg−1) in rats showed that SB-269970 was rapidly distributed into the brain (Table 2). The compound was rapidly eliminated with blood concentrations decreasing 4 fold after the first 0.5 h and no compound was measurable in the brain after 1 h. The pharmacokinetic profile following a single i.p. dose in guinea-pigs also showed rapid distribution into the brain, although brain concentrations were lower than in rats and in one of the three animals studies the compound was below the lower limit of detection (Table 2). The compound was rapidly eliminated, with blood concentrations decreasing 8 fold in the 2 h following dosing. The brain to blood concentration ratios were lower in guinea-pig than in rat (Table 2).
Table 2.
Rat and guinea-pig brain and blood levels of SB-269970 following a single dose of 3 mg kg−1 i.p.
5-CT-induced hypothermia in guinea-pigs
Vehicle-treated animals maintained a stable body temperature, which fluctuated by no more than 0.5°C over the 2 h recording period. Systemic (0.03–3 mg kg−1 i.p., Figure 4) and intracerebroventricular (2–10 μg i.c.v., Figure 5) administration of 5-CT-induced a pronounced, dose-dependent reduction in body temperature. The minimum statistically significant dose was 0.03 mg kg−1 i.p. and 10 μg i.c.v. At a dose of 0.3 mg kg−1 i.p., 5-CT produced a submaximal response which, in this (Figure 4) and subsequent studies (Table 3), was found to lower body temperature by 1.2–2.3°C, at 90 min post-dose.
Figure 4.
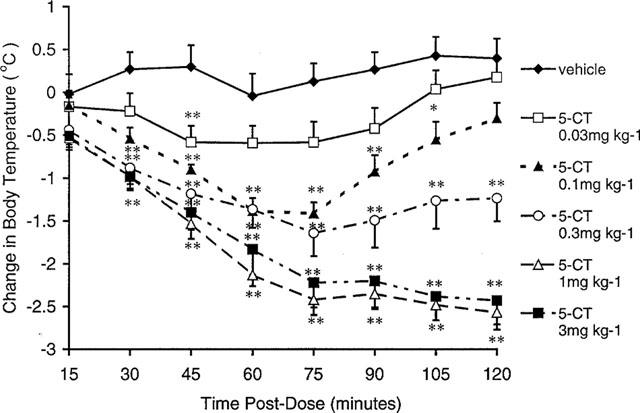
Effect of 5-CT on body temperature in the guinea-pig following i.p. administration. Data represent mean±s.e.mean values for groups of six guinea-pigs. *P<0.05 and **P<0.01 compared to vehicle-treated control animals.
Figure 5.
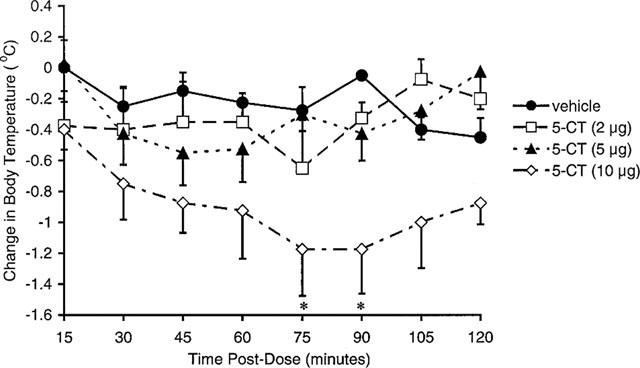
Effect of 5-CT on body temperature in the guinea-pig following i.c.v. administration. Data represent mean±s.e.mean values in groups of four guinea-pigs. *P<0.05 compared to vehicle-treated control animals.
Table 3.
Effect of metergoline, pindolol and GR 125743 on 5-CT-induced hypothermia in guinea-pigs
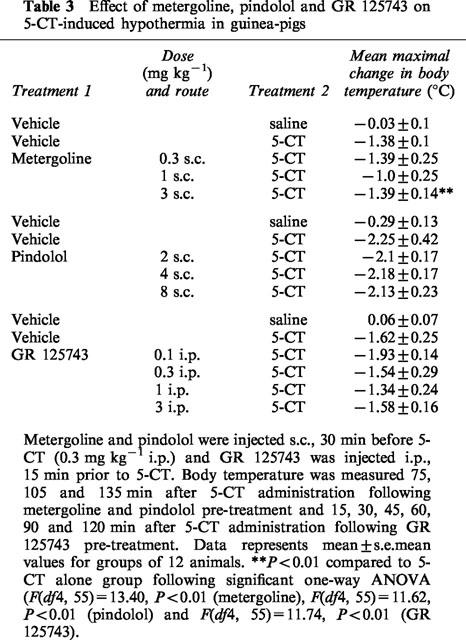
The non-selective 5-HT1A/1B receptor antagonist, pindolol (2–8 mg kg−1 s.c.) and the 5-HT1B/1D receptor antagonist GR 125743 (0.1–3 mg kg−1 i.p.) had no effect on 5-CT-induced hypothermia (Table 3). In marked contrast, the mixed 5-HT receptor antagonist metergoline (0.3–3 mg kg−1 s.c., Table 3) and the selective 5-HT7 receptor antagonist SB-269970-A (1–30 mg kg−1 i.p., Figure 6) both dose-dependently and almost completely inhibited the 5-CT-induced hypothermic response (0.3 mg kg−1 i.p.) with ED50 values of 1.8±0.1 and 2.96±0.2 mg kg−1, respectively. Neither compound had any effect on body temperature when tested alone (data not shown). In addition, at 3 mg kg−1 i.p., SB-269970-A demonstrated peak activity within 1 h post-dose (81% inhibition of the 5-CT response) and exhibited activity for ⩾4 h in this model (Figure 7).
Figure 6.
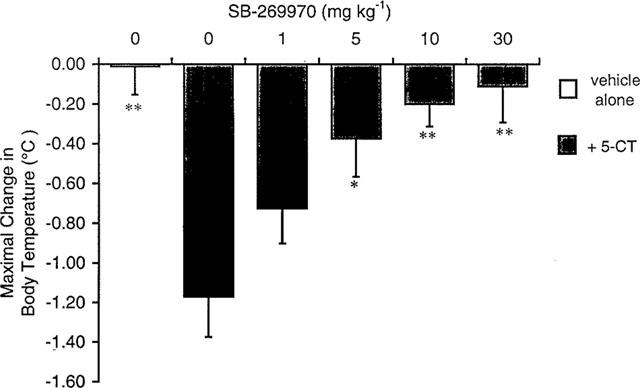
Effect of SB-269970-A on 5-CT-induced hypothermia in guinea-pigs. SB-269970-A (1, 5, 10, 30 mg kg−1) was injected i.p. 60 min before 5-CT (0.3 mg kg−1 i.p.). Body temperature was measured 55, 85 and 115 min after 5-CT administration. Data are mean±s.e.mean values for groups of 7–8 animals. *P<0.05, **P<0.01 compared to 5-CT alone group following significant one-way ANOVA (F(df5, 41)=6.39, P=0.00018).
Figure 7.
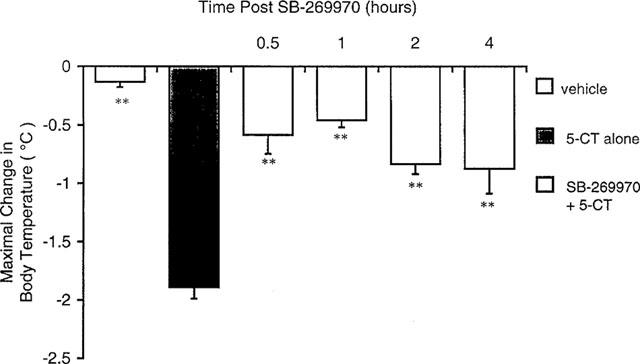
Time course of the effects of SB-269970-A on 5-CT-induced hypothermia in guinea-pigs. SB-269970-A (3 mg kg−1) was administered 0.5, 1, 2 or 4 h prior to 5-CT (0.3 mg kg−1 i.p.). Body temperature was then measured 60, 90 and 120 min after 5-CT administration. Data are mean±s.e.mean for groups of 11–12 animals. **P<0.01 compared to 5-CT alone group following significant 1-way ANOVA (F(df5, 65)=16.65, P<0.01).
Sleep studies
When administered at 0900 h, 3 h into the arousal period (lights off), SB-269970-A (30 mg kg−1 i.p.) had no significant effects on the distribution of the four sleep stages throughout the remainder of the recording period or during the ensuing 12 h (data not shown). In contrast, the same dose of SB-269970-A given at the start of the sleep period (1800 h) produced a marginal increase in the level of arousal (Figure 8), and a significant (P<0.05, F 1,176=4.49) reduction in the overall time spent in PS during the first 3 h of recording (Figure 8, Table 4). These changes in PS were characterized by a trend towards decreased numbers of PS occurrences with an inconsistent effect on occurrence length. PS latency, although variable, was increased by 88% (Table 4). There were no statistically significant effects on the distribution of SWS 1 or 2 and no effects on the sleep–wake cycle in the following awake period (data not shown).
Figure 8.
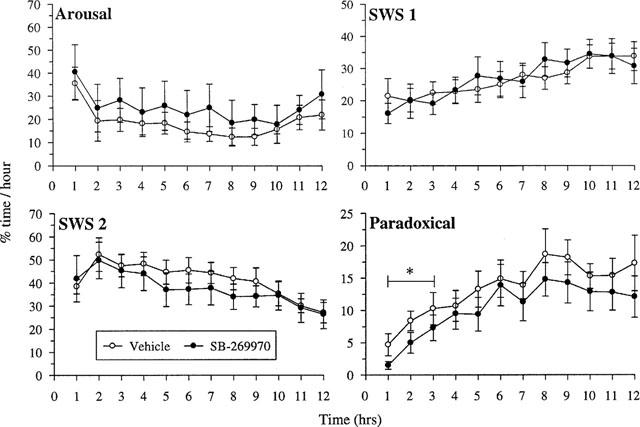
Effect of SB-269970-A on sleep stage distribution during the normal sleep phase. Vehicle or SB-269970-A (30 mg kg−1) were dosed intraperitoneally at the beginning of the sleep phase (1800 h). Data are percentage time spent in each sleep phase for each of the remaining 9 h during the awake period.
Table 4.
Effect of SB-269970-A on paradoxical (PS) sleep during the normal sleep phase

Discussion
SB-269970-A has been shown to be a potent and selective ligand for the human cloned 5-HT7(a) receptor (pKi of 8.9±0.1; Table 1) and for the 5-HT7 receptor in the guinea-pig cortex (pKi 8.3±0.2; Table 1). In radioligand binding assays, the compound was at least 100-fold selective against a wide range of receptors and enzymes except for the human 5-ht5A receptor for which selectivity was 50-fold (Lovell et al., 2000). The 5-HT7(a) receptor isoform is the dominant form in human and rat tissue (Heidemann et al., 1997) and the rank order or potency at the human 5-HT7(a) and guinea-pig receptor agree well, although absolute pKi values for antagonists to inhibit [3H]-5-CT binding to guinea-pig cerebral cortex membranes were approximately 0.5 log units lower than the corresponding values for the human cloned 5-HT7 receptor. The reason for this difference is unclear but may be related to the higher receptor expression level in the recombinant system.
SB-269970-A caused a concentration-dependent rightward-shift of the dose-response curve to 5-CT in the human 5-HT7(a) receptor adenylyl cyclase assay, indicating competitive, surmountable antagonism of the response. The calculated pA2 (8.5±0.2) is in good agreement with the affinity of the compound in radioligand binding studies at the same receptor. In addition to antagonizing the 5-CT response, SB-269970-A also produced a small inhibition of basal adenylyl cyclase activity in the absence of added 5-CT. The inhibition of basal adenylyl cyclase activity by SB-269970-A in this system is consistent with inverse agonism to reduce constitutive receptor-G-protein coupling, as has previously been reported for SB-258719 and a number of non-selective 5-HT receptor antagonists (Thomas et al., 1998). In the guinea-pig native tissue assay the potency of SB-269970-A to inhibit 5-CT-induced stimulation (pKB 8.3±0.1) of adenylyl cyclase was comparable to its potency as an antagonist at the human 5-HT7(a) receptor. The inhibition of basal adenylyl cyclase activity evident in the guinea-pig hippocampal assay may be due to antagonism of tonic 5-HT7 receptor stimulation by endogenous 5-HT, which may not be completely removed during the membrane preparation procedure (see Thomas et al., 1999 for discussion).
Pharmacokinetic studies demonstrated that SB-269970-A has good CNS penetration, with a maximum achievable brain:blood ratio of 0.83 : 1 determined under steady-state conditions. However, the compound was rapidly cleared in the rat (CLb=ca 140 ml min−1 kg−1). CNS penetration was confirmed following i.p. administration in rat and guinea-pig studies in which brain levels of SB-269970 were found to decline rapidly after a single i.p. injection. Blood levels in rat were maximal at the 30 min post dose time point (365 nM), falling to 10% of peak (37 nM) by 2 h. In the rat brain the compound reached concentrations of approximately 60–80 nM in the first hour (Table 2). In the guinea-pig brain levels were lower and the brain:blood ratio was also lower. The concentration of the compound in guinea-pig brain was below the level of detection in one out of three animals tested. Nevertheless, despite rapid elimination after i.p. injection, concentrations of SB-269970-A were achieved in blood and brain which would be predicted to attain substantial occupancy of 5-HT7 receptors.
Stimulation of 5-HT1B receptors in guinea-pigs has previously been shown to cause hypothermia (Hagan et al., 1997; Skingle et al, 1994). Although 5-CT, has 5-HT1B and 5-HT1D receptor agonist properties, its hypothermic effects were not blocked by 5-HT1B/D receptor antagonists (Skingle et al, 1994) suggesting an alternative mechanism of action. Our data confirm that 5-CT has a hypothermic effect in guinea-pigs. Although the CNS penetration of 5-CT is believed to be poor, there is some evidence to suggest that 5-CT is brain penetrant following systemic administration in guinea-pigs (Lawrence & Marsden, 1992). Our observation that, when injected directly into the lateral ventricles, 5-CT significantly reduced core body temperature by 1.2°C after a dose of 10 ug i.c.v./guinea-pig, suggests that the hypothermic response to 5-CT has a CNS component.
The inability of the 5-HT1A/1B receptor antagonist pindolol and the 5-HT1B/1D receptor antagonist GR 125743 to antagonize the hypothermic effect of 5-CT confirms that 5-HT1A, 5-HT1B or 5-HT1D receptor stimulation is unlikely to be involved in mediating this effect. In contrast, the selective 5-HT7 receptor antagonist, SB-269970-A, dose-dependently inhibited the 5-CT response, indicating that the hypothermic effect of 5-CT may be mediated by stimulation of 5-HT7 receptors. In support of this hypothesis metergoline, which has high 5-HT7 receptor affinity (Bard et al., 1993; To et al., 1995) but lacks selectivity, also antagonized the hypothermic effects of 5-CT. Although the potential for the metabolites of SB-269970-A to contribute to the pharmacological profile of the compound has not been excluded, the findings with metergoline, which is structurally diverse from SB-269970-A, support the hypothesis that it is 5-HT7 receptor blockade which accounts for the actions of SB-269970-A. Furthermore, the effect of SB-269970-A was evident after 30 min and 1 h, which corresponds well with peak plasma and brain concentrations. Further studies are required to determine whether the blockade of 5-CT induced hypothermia is mediated by central or peripheral 5-HT7 receptors. Thus, despite its rapid metabolism the time course of the effects of SB-269970-A in the hypothermia model suggests that the compound has a duration of action which renders it suitable for studying the functions of 5-HT7 receptors in vivo.
5-HT7 receptors have been implicated in mediating 5-HT induced phase shifts of neuronal activity in the suprachiasmatic nucleus of rat hypothalamus (Lovenberg et al., 1993, Tsou et al., 1994, Ying & Rusak, 1997). Furthermore, the distribution of the receptor in thalamic nuclei suggests that it may play a role in the control of sleep. Our data from both the Taqman analysis of the distribution of the mRNA for the 5-HT7 receptor and the sleep studies reported here support this hypothesis. When injected prior to the normal sleep phase the compound significantly reduced paradoxical sleep, without effects on other sleep stages. These data further support the hypothesis that 5-HT7 receptors may play important roles in sleep control, although further studies using selective 5-HT7 receptor antagonists with improved pharmacokinetic profiles are required to confirm this.
There is evidence that depression is characterized by a shortening of rapid eye movement (REM) sleep latency (Le Bon et al., 1997), and an increased REM density. Conversely, many antidepressant drugs have been demonstrated to increase REM latency and reduce REM density in patients with depression (Staner et al., 1999). In rats a single administration of fluoxetine, for example, has been shown to induce a dose-related suppression of REM sleep that persisted for 4–5 h (Pastel & Fernstrom, 1987). Interestingly, fluoxetine did not modify the length of each REM sleep period, but significantly reduced the number of REM periods, and increased REM latency. Whilst SB-269970-A did not induce a significant increase in PS latency, it did reduce PS density, without diminishing PS occurrence length. Thus, these observations with SB-269970-A, although preliminary, support the hypothesis that 5-HT7 receptor antagonists have potential utility for the treatment of depression and/or circadian rhythm disorders.
In summary, SB-269970-A has been identified as a potent, selective 5-HT7 receptor antagonist in vitro. The compound is cleared rapidly in vivo but has good CNS penetration. Preliminary data suggest that 5-CT induced hypothermia in guinea-pig is mediated by 5-HT7 receptor stimulation. Paradoxical sleep in rats is reduced when SB-269970-A is administered prior to the normal sleep phase, suggesting that 5-HT7 receptors play a role in the control of the sleep cycle. Further studies of selective 5-HT7 receptor antagonists with improved pharmacokinetic profiles are required to confirm this hypothesis.
Abbreviations
- AC
adenylyl cyclase
- EEG
electroencephalogram
- HEK293
human embryonic kidney 293
- PS
paradoxical sleep
- REM sleep
rapid eye movement sleep
- SB-258719
(((R)-3,N-dimethyl-N-[1-methyl-3-(4-methylpiperidin-1-yl)propyl]benzene-sulphonamide)), 5MeOT (5-methoxy-tryptamine)
- SB-269970
(((R)-3-(2-(2-(4-methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulphonyl)-phenol)), N-[4-methoxy-3-(4-methyl-1-piperazinyl)phenyl]-3-methyl-4-(4-pyridinyl)benzamide)
- SWS
slow wave sleep
- 5-CT
5-carboxytryptamine
- 8-OH-DPAT
8-hydroxy-2-[N-n-dipropyl-N-(3′-iodo-2′-propenyl)amino]tetralin)
References
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BOYLAND P.S., EASTWOOD S., ELLIS C., BERGSMA D., JONES B.J., GLOGER I.S., UPTON N., MIDDLEMISS D.N. High specific activity [3H]-5-CT binding: correlation of guinea-pig cortex with human cloned 5-HT7 receptors. Br. J. Pharmacol. 1996;117:132P. [Google Scholar]
- BRANCHEK T.A., GUSTAFSON E.L., DURKIN M.M., BARD J.A., WEINSHANK R.L. Autoradiographic localisation of 5-HT7 and its mRNA in rat CNS by radioligand binding and in situ hybridization histochemistry. Br. J. Pharmacol. 1994;112 Proc. Suppl:100P. [Google Scholar]
- BROWN E.A., GRIFFITHS R., HARVEY C.A., OWEN D.A.A. Pharmacological studies with SK&F 93944 (temelastine), a novel histamine H1-receptor antagonist with negligible ability to penetrate the central nervous system. Br. J. Pharmacol. 1986;87:569–578. doi: 10.1111/j.1476-5381.1986.tb10199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHENG Y., PRUSOFF W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CLEMETT D.A., KENDALL D.A., COCKETT M.I., MARSDEN C.A., FONE K.C.F. Pindolol-insensitive [3H]-5-hydroxytryptamine binding in the rat hypopthalamus; identity with 5-hydroxytryptamine7 receptors. Br. J. Pharmacol. 1999;127:236–242. doi: 10.1038/sj.bjp.0702503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWDER M.J., HAND D.J. Analysis of repeated measures. London: Chapman and Hall; 1996. [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. TiPS. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- FORBES I.T., DABBS S., DUCKWORTH D.M., JENNINGS A.J., KING F.D., LOVELL P.J., COLLIN L., BROWN A.M., HAGAN J.J., MIDDLEMISS D.N., RILEY G.J., THOMAS D.R., UPTON N. (R)-3,N-Dimethyl-N-[1-methyl-3-(4-methylpiperidin-1-yl)propyl]benzene sulfonamide: The First Selective 5-HT7 Receptor Antagonist. J. Med. Chem. 1998;41:655–657. doi: 10.1021/jm970519e. [DOI] [PubMed] [Google Scholar]
- GELERNTER J., RAO P.A., PAULS D.L., HAMBLIN M.W., SIBLEY D.R., KIDD K.K. Assignment of the 5-HT7 receptor gene (HTR7) to chromosome 10q and exclusion of genetic linkage with Tourette syndrome. Genomics. 1995;26:207–209. doi: 10.1016/0888-7543(95)80202-w. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS R.G., LEWIS A., JEFFREY P.Models of drug absorption in situ and in conscious animals Models for Assessing Drug Absorption and Metabolism 1996New York: Plenum Press; eds. Borchardt, RT et al [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.A., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacology. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., LESLIE R.A., PATEL S., EVANS M.L., WATTAM T.A., HOLMES S., BENHAM C.D., TAYLOR S.G., ROUTLEDGE C., HEMMATI P., MUNTON R.P., ASHMEADE T.E., SHAH A.S., HATCHER J.P., HATCHER P.D., JONES D.N.C., SMITH M.I., PIPER D.C., HUNTER A.J., PORTER R.A., UPTON N. Orexin-A Activates Locus Coeruleus Cell Firing and Increases Arousal in the Rat. Proc. Natl. Acad. Sci. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., SLADE P.D., HATCHER J., GASTER L., JEFFREY P. 5-HT1B (formerly 5-HT1Dβ) receptors mediate the hypothermia induced by the 5-HT1B/D agonist SKF 99101H in the Guinea-Pig. Eur. J. Pharmacol. 1997;331:169–174. doi: 10.1016/s0014-2999(97)01055-8. [DOI] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., METCALF M.A., KOHEN R., HAMBLIN M.W. Four 5-Hydroxytryptamine7 (5HT7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron-exon organisation. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA F.R., HUMPHREY P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–243. [PubMed] [Google Scholar]
- KIKUCHI C., NAGASO H., HIRANU A.T., KOYAMA M. Tetrahydrobenzindoles: Selective antagonists of the 5-HT7 receptor. J. Med. Chem. 1999;42:533–535. doi: 10.1021/jm980519u. [DOI] [PubMed] [Google Scholar]
- LAWRENCE A.J., MARSDEN C.A. Terminal autoreceptor control of 5-hydroxytryptamine release as measured by in vivo microdialysis in the conscious guinea-pig. J. Neurochem. 1992;58:142–146. doi: 10.1111/j.1471-4159.1992.tb09289.x. [DOI] [PubMed] [Google Scholar]
- LE BON O., STANER L., MURPHY J.R., HOFFMAN G., PULL C.H., PELC I. Critical analysis of the theories advanced to explain short REM latencies and other sleep anomalies in several psychiatric conditions. J. Psychiat. Res. 1997;31:433–450. doi: 10.1016/s0022-3956(97)00017-4. [DOI] [PubMed] [Google Scholar]
- LIVAK K.J. Allelic discrimination using fluorogenic probes and the 5′-nuclease assay. Gen Anal. Biomol. Engin. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- LOVELL P.J., BROMIDGE S.M., DABBS S., DUCKWORTH D.M., FORBES I.T., JENNINGS A.J., KING F.D., MIDDLEMISS D.N., RAHMAN S.K., SAUNDERS D.V., COLLIN L.L., HAGAN J.J., RILEY G.J., THOMAS D.R. A Novel, Potent and Selective 5-HT7 Antagonist: (R)-3-(2-(2-(4-Methyl-piperidin-1-yl)ethyl)-pyrrolidine-1-sulfonyl)-phenol (SB-269970) J. Med. Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythyms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- MEUSER T., PIERCE P.A. 5-Hydroxytryptamine-7 (5-HT7) receptor distribution in dorsal root ganglion. Anaesthesiology. 1997;87:A816. [Google Scholar]
- PASTEL R.H., FERNSTROM J.D. Short-term effects of fluoxetine and trifluoromethylphenylpiperazine on electroencephalographic sleep in the rat. Brain Res. 1987;436:92–102. doi: 10.1016/0006-8993(87)91560-5. [DOI] [PubMed] [Google Scholar]
- PLASSAT J., AMLAIKY N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- ROTH B.L., CRAIGO S.C., CHOUDHARY M.S., ULUER A., MONSMA F.J. , JR, SHEN Y., MELTZER H.Y., SIBLEY D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharm. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.-M., SCWARTZ J.-C. Molecular cloning, characterisation, and localisation of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALOMON Y. Adenylate cyclase assay. Adv. Cyclic Nuc. Res. 1979;10:35–55. [PubMed] [Google Scholar]
- SCHOEFFTER P., ULLMER C., BOBIRNAC I., GABBIANI G., LUBBERT H. Functional, endogenously expressed 5-hydroxytryptamine 5-ht7 receptors in human vascular smooth muscle cells. Br. J. Pharmacol. 1996;117:993–994. doi: 10.1111/j.1476-5381.1996.tb16687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SKINGLE M., HIGGINS G.A., FENIUK W. Stimulation of central 5-HT1D receptors causes hypothermia in the guinea-pig. J. Psychopharm. 1994;8:14–21. doi: 10.1177/026988119400800103. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A.J., CAROLO C., PETIT N., ZWINGELSTEIN C., BOURSON A. Identification of 5-HT7 receptor binding sites in rat hypothalamus. Mol. Pharmacol. 1995;47:99–103. [PubMed] [Google Scholar]
- STAM N.J., ROESINK C., DIJCKS F., GARRITSEN A., VAN HERPEN A., OLIJVE W. Human serotonin 5-HT7 receptor: cloning and pharmacological characterisation of two receptor variants. FEBS Letts. 1997;413:489–494. doi: 10.1016/s0014-5793(97)00964-2. [DOI] [PubMed] [Google Scholar]
- STANER L., LUTHRINGER R., MARCHER J.-P. Effects of antidepressant drugs on sleep EEG in patients with major depression. CNS Drugs. 1999;11:49–60. [Google Scholar]
- TERON J.A. Involvement of the 5-HT7 receptor in craniovascular vasodilatation: Potential in migraine. Proc. West. Pharmacol. Soc. 1998;41:247–251. [PubMed] [Google Scholar]
- THOMAS D.R., GITTINS S.A., COLLIN L.L., MIDDLEMISS D.N., RILEY G., HAGAN J., GLOGER I., ELLIS C.E., FORBES I.T., BROWN A.M. Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br. J. Pharmacol. 1998;124:1300–1306. doi: 10.1038/sj.bjp.0701946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS D.R., MIDDLEMISS D.N., TAYLOR S.G., NELSON P., BROWN A.M. 5-CT stimulation of adenylyl cyclase activity in guinea pig hippocampus: evidence for involvement of 5-HT7 and 5-HT1A receptors. (1999) Br. J. Pharmacol. 1999;128:158–164. doi: 10.1038/sj.bjp.0702759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterisation and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU A.P., KOSAKA A., BACH C., ZUPPAN P., YEE C., TOM L., ALVAREZ R., RAMSEY S., BONHAUS D.W., STEFANICH E., JAKEMAN L., EGLEN R.M., CHAN H.W. Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenyly cyclase. J. Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- YING S.W., RUSAK B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmatic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]


