Abstract
We evaluated the role of the inositol 1,4,5-triphosphate (IP3) receptor-mediated Ca2+ release on the positive inotropic effects of α-adrenergic stimulation using a novel, potent, selective membrane-permeable blocker of IP3 receptor, xestospongin C.
Guinea-pig papillary muscle permeabilized with saponin exhibited spontaneous oscillatory contractions in solution buffered with pCa2+ 6.5 by a low concentration of EGTA. The oscillatory activity was increased by adding 100 μM IP3 and abolished by 1 μM ryanodine or 30 μM cyclopiazonic acid. Xestospongin C (3 μM) inhibited the IP3-induced increase in the oscillatory contractions without affecting basal oscillations.
In intact papillary muscle, xestospongin C (3 μM) inhibited the positive inotropic effects of phenylephrine, resulting in a rightward and downward shift of the concentration-response curve for phenylephrine. On the contrary, xestospongin C did not affect the concentration-response curve for phenylephrine obtained in the presence of ryanodine (1 μM).
On the other hand, xestospongin C affected neither basal contractions nor the positive inotropic effects of a high extracellular Ca2+ concentration (3.2 mM) or that of isoprenaline (1 and 10 nM).
These results suggest that the IP3-mediated increase in Ca2+ release is involved in the positive inotropic effects of α-adrenergic stimulation in the guinea-pig cardiac muscle.
Keywords: Xestospongin C; inositol 1,4,5-triphosphate receptor; cardiac muscle; phenylephrine
Introduction
It has been shown that inositol 1,4,5-triphosphate (IP3), increased by the activation of a phosphoinositide signalling cascade, enhances Ca2+ efflux from the sarcoplasmic reticulum (SR) in cardiac muscle (Nosek et al., 1986). Although the increase in Ca2+ release is expected to be involved in receptor-mediated positive inotropy, the role of IP3 on the positive inotropic effects of α-adrenergic stimulation in cardiac muscle is still controversial (for review see Fedida et al., 1993; De Jonge et al., 1995). To determine the role of IP3, it would be useful to have drugs that interfere with the IP3-mediated Ca2+ release. Although heparin and decavanadate have been utilized for this purpose, conclusive evidence has not been obtained because these agents are nonselective (for review see Wilcox et al., 1998). Xestospongin C, isolated from the sponge Xestospongia sp., has recently been shown to be a membrane-permeable blocker of IP3-mediated Ca2+ release (Gafni et al., 1997; Wilcox et al., 1998). This compound is suggested to be a potent and highly sensitive inhibitor of IP3 receptor with IC50 of 350 nM, which is 30 times lower than the IC50 for ryanodine-receptor (Gafni et al., 1997). In the present experiments, we used xestospongin C to examine the role of IP3 receptor on cardiac contraction.
Methods
Measurement of muscle force in papillary muscle
Male guinea-pigs (350–500 g) were anaesthetized with gaseous diethylether and the heart was rapidly removed and washed in ice-cold (4°C) normal physiological salt solution (PSS) (mM) NaCl 145, KCl 5, CaCl2 1.6, MgCl2 1, glucose 10 and HEPES 10. Papillary muscles with a diameter less than 1 mm were excised from the right ventricle. The muscle preparation was perfused continuously (12 ml min−1) with a pre-warmed (30°C), pre-oxygenated physiological salt solution (PSS) solution. Muscle tension was measured isometrically and the resting tension applied to each preparation was adjusted to give 90% of the maximum tension development. During the equilibration period, the muscles were stimulated electrically by 5-ms square pulses at 0.5 Hz with a voltage 1.2 fold greater than the threshold intensity, using an electronic stimulator (SEN-3201, Nihon Kohden, Tokyo, Japan) and a pair of platinum wires. Contractile force was digitized at the sampling rate of 110 points s−1. The amplitudes of isometric contraction elicited by a stimulation frequency of 0.5 Hz in the presence of 1.6 mM Ca2+ were used as the control (100%).
The effects of phenylephrine and isoprenaline on contractions were examined in the presence of propranolol (1 μM) and phentolamine (1 μM), respectively, to avoid the interference with the β- and α-adrenoceptor-mediated effects, respectively. Muscles were pretreated with xestospongin C (3 μM) for 30 min, and then phenylephrine was cumulatively applied to obtain a concentration-response curve.
Skinned muscle preparations
Small bundles of muscle tissue (120–150 μm in diameter and 1–2 mm in length) were obtained by teasing tissue from guinea-pig papillary muscle. The bundles were skinned with 50 μg ml−1 saponin for 30 min in relaxing solution (see below). Both ends of each bundle were tied with monofilament silk thread. One end of each bundle was connected to an isometric transducer, and the other end was fixed with a stainless steel pin to the siliconized floor of a chamber under a resting tension of 0.5 mN and equilibrated for 60–90 min. During this period, 10 μM Ca2+ was repeatedly applied until the peak force became reproducible. Experiments were carried out at room temperature (∼22°C).
The relaxing solution contained magnesium (methansulphonate)2 (5.25 mM), potassium methansulphonate (40 mM), ATP (5.3 mM), PIPES (20 mM), phosphocreatine (10 mM), creatine phosphokinase (20 unit ml−1), FCCP (1 μM), E-64 (1 mg ml−1), and EGTA (10 mM). The ionic strength was adjusted to 0.2 M, and the pH was adjusted to 7.1 at 20°C. Free Ca2+ concentration was changed by adding an appropriate amount of Ca2+-(methansulphonate)2. Force oscillations were introduced by exposing the tissue to relaxing solution containing 0.05 mM EGTA in which the free Ca2+ concentration was adjusted to pCa2+ 6.5 by adding Ca2+ (methansulphonate)2. The ionic compositions of these solutions were calculated by a computer program developed by Fabiato (1981) with slight modification. The area of the force oscillations during a 1-min period was used for the quantitative assessment of the effects of IP3 and xestospongin C.
Chemicals
The chemicals used were phenylephrine, phentolamine, isoprenaline, propranolol, cyclopiazonic acid, saponin (Sigma, St. Louis, MO, U.S.A.), EGTA (Wako Pure Chemicals, Tokyo, Japan) and IP3 (Dojindo Laboratories, Kumamoto, Japan). Xestospongin C was isolated and purified from an Okinawan marine sponge, Xestospongia sp. (Kobayashi et al., 1998). Cyclopiazonic acid and xestospongin C were dissolved in dimethylsulphoxide and ethanol, respectively.
Statistics
The numerical data were expressed as mean±s.e.mean. Differences between mean values were evaluated by paired Student's t-test and, where appropriate, analysis of variance (ANOVA) followed by the Bonferroni test. A probability of less than 0.05 was taken as a statistically significant difference.
Results
The effects of xestospongin C on force oscillations in saponin-skinned muscles
In saponin-skinned guinea-pig papillary muscle, force oscillations were observed in pCa2+ 6.5 solution (Figure 1a), and the addition of IP3 (100 pM to 100 μM) augmented the force oscillation in dose-dependent manner (Figure 1a,b), as has been reported by Nosek et al. (1986). Using these force oscillations in saponin-skinned fibre, we examined the pharmacology of xestospongin C on cardiac SR functions.
Figure 1.
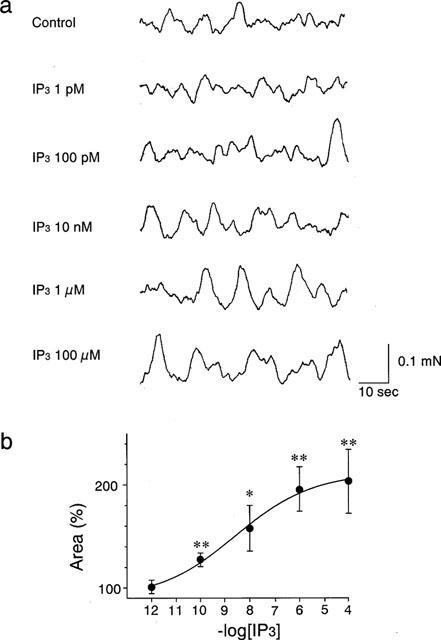
Effects of IP3 (1 pM–100 μM) on the force oscillations in saponin-skinned muscles. Force oscillations were introduced by low-EGTA (0.05 mM) and pCa2+ 6.5 solution. (a) Typical trace of effect of IP3. (b) Summarized data of (a). The area of the force oscillations during 1 min was used for the quantitative assessment of effects of IP3. *P<0.05, **P<0.01 vs the basal force oscillations.
The basal spontaneous force oscillations were completely inhibited by 1 μM ryanodine (Figure 2a) or by 30 μM cyclopiazonic acid, a SR Ca2+ -ATPase inhibitor (data not shown), suggesting that the force oscillations were induced by the rhythmic Ca2+ release from the SR. The force oscillations stimulated by 100 μM IP3 were also abolished by 30 μM cyclopiazonic acid. On the other hand, the basal force oscillations were not changed by 3 μM xestospongin C. However, in the presence of xestospongin C, IP3 failed to enhance the force oscillations (Figure 2c). These results are summarized in Figure 2d.
Figure 2.
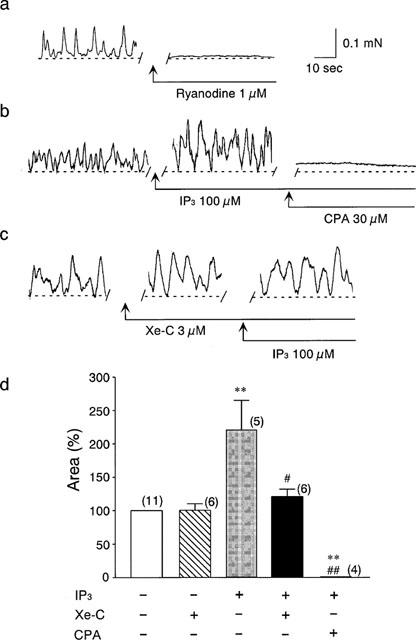
Effects of xestospongin C (Xe-C, 3 μM) on the force oscillations in saponin-skinned muscles. (a) Typical trace of effects of 1 μM ryanodine on the force oscillations. (b) Typical trace of 100 μM IP3-induced potentiation of oscillations and the effects of addition of cyclopiazonic acid (CPA, 30 μM). (c) Typical trace of the effect of 100 μM IP3 in the presence of 3 μM xestospongin C. (d) Summarized data of (b) and (c). The numbers of the experiments is shown near each column. **P<0.01 vs the basal force oscillations. #P<0.05, ##P<0.01 vs the results obtained in the presence of IP3.
The effects of xestospongin C in intact muscle
The application of 3 μM xestospongin C had no effect on the peak force of contraction in normal PSS (Figure 3a). An increase in extracellular Ca2+ concentration ([Ca2+]o) from 1.6 to 3.2 mM increased the peak force. Xestospongin C (3 μM) did not affect the positive inotropic effect of the elevation in [Ca2+]o (Figure 3a,b).
Figure 3.
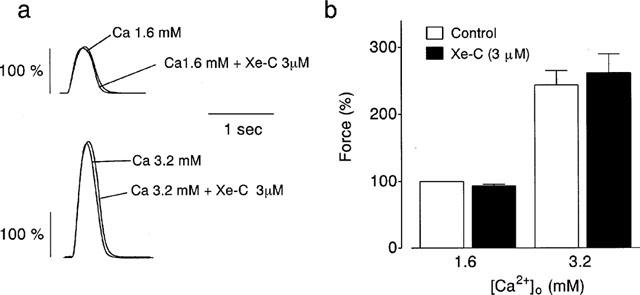
Effects of 3 μM xestospongin C (Xe-C) on the force of contractions in 1.6 and 3.2 mM [Ca2+]o. (a) Typical tracings of the force. (b) Summarized data (n=4). Each column represents the mean±s.e.mean. Values obtained in normal PSS at 0.5 Hz were taken as 100%.
Positive inotropic effects were also elicited by 1 nM isoprenaline in the presence of 1 μM phentolamine (Figure 4a). Xestospongin C (3 μM) did not change the isoprenaline-induced inotropism (Figure 4a). The effects of xestospongin C on 1 and 10 nM isoprenaline-induced inotropic action are summarized in Figure 4b.
Figure 4.
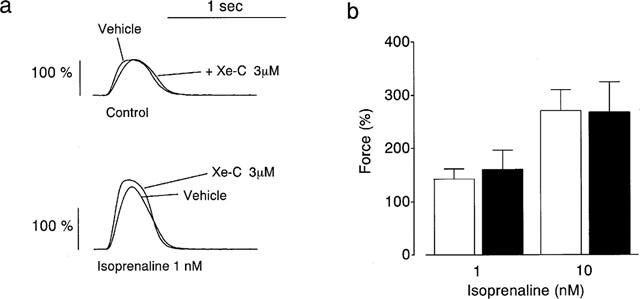
Effects of 3 μM xestospongin C (Xe-C) on the positive inotropic effects of isoprenaline (Iso). (a) Typical tracings of the force. (b) Summarized data (n=5–10). Each column represents the mean±s.e.mean. Values obtained in normal PSS at 0.5 Hz were taken as 100%.
As shown in Figure 5a, phenylephrine (1 and 10 μM), in the presence of 1 μM propranolol, increased the twitch contractions in a concentration-dependent manner. Xestospongin C partially but significantly inhibited the positive inotropic effect of phenylephrine. Figure 5b shows the effects of various concentrations of phenylephrine on twitch contractions in the absence or presence of 3 μM xestospongin C. The results indicate that the concentration-response curve for phenyle-phrine was shifted to the lower right by xestospongin C.
Figure 5.
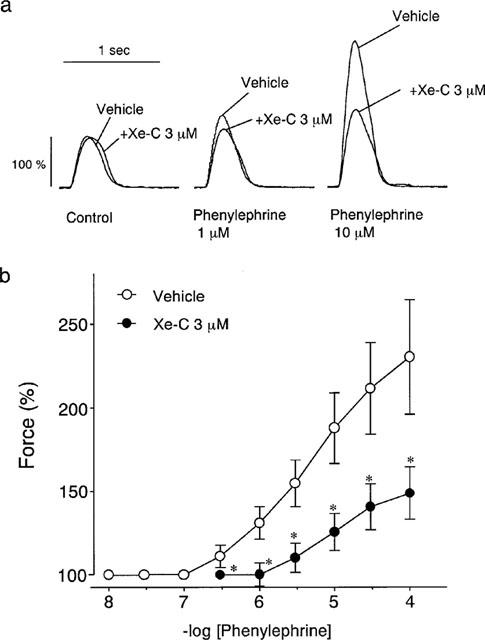
Effects of 3 μM xestospongin C (Xe-C) on the positive inotropic effects of phenylephrine. (a) Typical tracings of the xestospongin C on the contractions in the presence of 1 and 100 μM phenylephrine. (b) Effects of xestospongin C on the concentration-response curve of phenylephrine. Open and closed symbols represent the results in the absence and presence of xestospongin C, respectively. Each value represents the mean±s.e.mean. *P<0.05 vs the vehicle. Vehicle, n=8. Xe-C, n=10.
Ryanodine (1 μM) decreased the twitch contraction in normal PSS, and xestospongin C (3 μM) alone did not change the contractions obtained in the presence of ryanodine (Figure 6). In the presence of ryanodine, the positive inotropy of phenylephrine was inhibited, resulting in the downward shift of the dose-response curve for phenylephrine. As shown in Figure 6, xestospongin C had no effects on the dose-response curve for phenylephrine obtained in the presence of ryanodine.
Figure 6.
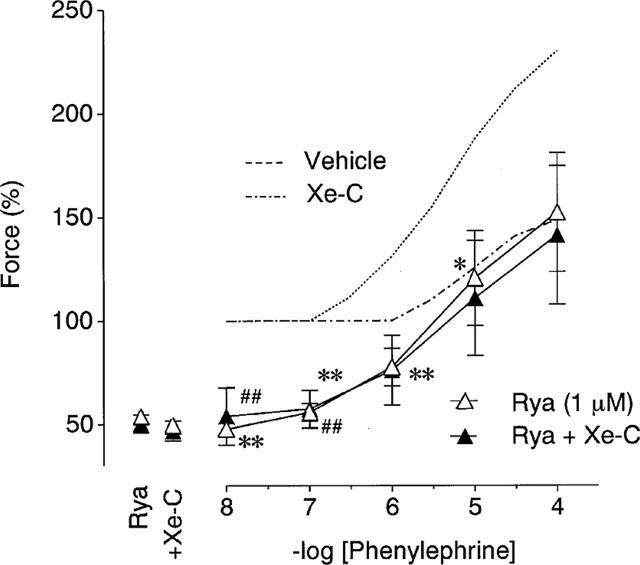
Effects of 3 μM xestospongin C (Xe-C) on the positive inotropic effects of phenylephrine in the presence of ryanodine. Open and closed symbols represent the results in the absence and presence of xestospongin C, respectively. Control responses in the absence of ryanodine (dashed lines) are adopted from Figure 5b. Each value represents the mean±s.e.mean (n=7). *P<0.05, **P<0.01 vs the vehicle data in Figure 5b. ##P<0.01 vs the data obtained in the presence of xestospongin C in Figure 5b.
Discussion
In saponin-skinned papillary muscles, spontaneous force oscillations were observed in a low-EGTA/pCa2+ 6.5 solution and these oscillations were completely abolished by ryanodine or cyclopiazonic acid (Figure 2). These results suggest that the force oscillations are mediated by the spontaneous Ca2+ release from the SR through the ryanodine-receptor, as has been reported by Nosek et al. (1986) and Vites & Pappano (1990). In guinea-pig papillary muscle, IP3 (100 pM–100 μM) augmented the force oscillation in a dose-dependent manner (Figure 1) and 3 μM xestospongin C inhibited the IP3-induced potentiation in force oscillations without affecting the basal force oscillations (Figure 2). These results suggest that in guinea-pig papillary muscle skinned with saponin retains the IP3-mediated pathway for Ca2+-release from the SR. The lack of the effect of xestospongin C on the steady state oscillations suggests the high selectivity of this inhibitor for IP3-receptor over the ryanodine-receptor (Gafni et al., 1997).
In the intact papillary muscle, 3 μM xestospongin C inhibited the phenylephrine-induced positive inotropic action. However, xestospongin C had no effects on the basal twitch contractions or the positive inotropic effects elicited by the increase in [Ca2+]o. Furthermore, the positive inotropic action of isoprenaline, which is mediated by increasing the activities of voltage-dependent Ca2+ channels and SR Ca2+-ATPase, was also unaffected by xestospongin C (Figure 4). These results suggest that IP3-mediated Ca2+ release is involved only in an α-adrenoceptor mediated positive inotropy.
Ryanodine inhibited not only the basal contraction, but also the positive inotropy of phenylephrine, suggesting that Ca2+ induced Ca2+ release (CICR) contributes to the positive inotropy of phenylephrine. Xestospongin C, however, did not inhibit the positive inotropic effect of phenylephrine in the presence of ryanodine (Figure 6). These results suggest that IP3-mediated Ca2+ release and Ca2+-induced Ca2+ release are derived from the same intracellular Ca2+ source in cardiac muscle. In addition, these results support the notion that xestospongin C (up to 3 μM) has no effect on cellular functions in the absence of SR function. In this study, we examined the effects of 10 μM xestospongin C and found that this concentration of xestospongin C decreased the contraction in normal PSS to approximately 70% (our unpublished observation), suggesting that higher concentration of xestospongin C might interfere with other components of the contractile responses.
The present data also showed that 3 μM xestospongin C, which almost completely inhibits IP3 induced Ca2+ release in vitro, only partially inhibited the inotropic action of α-adrenergic stimulation. This result suggests that other mechanisms, such as the activation of protein kinase C, contribute to the α-adrenoceptor mediated positive inotropy, since it has been shown that not only [Ca2+]i (Woo & Lee, 1999) but also a myofilament Ca2+ sensitivity (Gambassi et al., 1992; De Jonge et al., 1995) is increased through the activation of protein kinase C. Alternatively, the membrane permeability is not high enough to access SR IP3 receptors.
In conclusion, our results provide initial evidence of the role of the IP3 receptor on the positive inotropy elicited by α-adrenergic stimulation.
Abbreviations
- IP3
inositol 1,4,5-triphosphate
- SR
sarcoplasmic reticulum
- PSS
physiological salt solution
- CICR
Ca2+-induced Ca2+-release
References
- DEJONGE H, W., VAN HEUGTEN H.A.A., LAMERS J.M.J. Signal transduction by the phosphatidylinositol cycle in myocardium. J. Mol. Cell. Cardiol. 1995;27:93–106. doi: 10.1016/s0022-2828(08)80010-7. [DOI] [PubMed] [Google Scholar]
- FABIATO A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J. Gen. Physiol. 1981;78:457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDIDA D., BRAUN A.P., GILES W.R. α1-adrenoceptors in myocardium: Functional aspects and transmembrane signaling mechanisms. Physiol. Rev. 1993;73:469–487. doi: 10.1152/physrev.1993.73.2.469. [DOI] [PubMed] [Google Scholar]
- GAFNI J., MUNSCH J.A., LAM T.H., CATLIN M.C., COSTA L.G., MOLINSKI T.F., PESSAH L.N. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-triphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- GAMBASSI G., SPURGEON H.A., LAKATTA E.G., BLANK P.S., CAPOGROSSI M.C. Different effects of α- and β-adrenergic stimulation on cytosolic pH and myofilament responsiveness to Ca2+ in cardiac myocytes. Circ. Res. 1992;71:870–882. doi: 10.1161/01.res.71.4.870. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI M., MIYAMOTO Y., AOKI S., MURAKAMI N., IN K., ISHIDA T. Isomerization of dimeric 2,9-disubstituted 1-oxa-quinolizidine alkaloids and structural revision of aragusponjines B and E, isolated from a marine sponge of Xesospongia sp. Heterocycles. 1998;47:195–203. [Google Scholar]
- NOSEK T.M., WILLIAMS M.F., ZIEGLER S.T., GODT R.E. Inositol triphosphate enhances calcium release in skinned cardiac and skeletal muscle. Am. J. Physiol. 1986;250:C807–C811. doi: 10.1152/ajpcell.1986.250.5.C807. [DOI] [PubMed] [Google Scholar]
- VITES A.M., PAPPANO A. Inositol 1,4,5-triphosphate releases intracellular Ca2+ in permeabilized chicken atria. Am. J. Physiol. 1990;258:H1745–H1752. doi: 10.1152/ajpheart.1990.258.6.H1745. [DOI] [PubMed] [Google Scholar]
- WILCOX R.A., PRIMROSE W.U., NAHORSKI S.R., CHALLISS R.A.J. New developments in the molecular pharmacology of the myo-inositol 1,4,5-triphosphate receptor. Trends Pharmacol. Sci. 1998;19:467–475. doi: 10.1016/s0165-6147(98)01260-7. [DOI] [PubMed] [Google Scholar]
- WOO S.H., LEE C.O. Role of PKC in the effects of α1-adrenergic stimulation on Ca2+ transients, contraction and Ca2+ current in guinea-pig ventricular myocytes. Pflügers Arch. 1999;437:335–344. doi: 10.1007/s004240050787. [DOI] [PubMed] [Google Scholar]


