Abstract
We investigated the effect of stimulation of H1-receptors with histamine on protein tyrosine phosphorylation levels in guinea-pig left atrium and evaluated the influences of tyrosine kinase inhibitors on the positive inotropic effect mediated by H1-receptors in this tissue.
Histamine induced an increase in tyrosine phosphorylation in four main clusters of proteins with apparent molecular weights of 25, 35, 65 and 150 kDa. Tyrosine phosphorylation of these proteins attained a peak around 2–3 min following histamine stimulation and then declined to or below basal levels. Histamine-induced protein tyrosine phosphorylation was antagonized by the H1-receptor antagonists mepyramine (1 μM) and chlorpheniramine (1 μM), but not by the H2-receptor antagonist cimetidine (10 μM).
The positive inotropic effect of histamine was depressed in a concentration-dependent manner by the tyrosine kinase inhibitors tyrphostin A25 (50 to 100 μM) and genistein (10 to 50 μM) but not by the inactive genistein analogue daidzein (50 μM). The positive inotropic effect of isoprenaline was unchanged by tyrphostin A25 and genistein.
At a concentration of 1 μM histamine produced a dual-component positive inotropic response composed of an initial increasing phase and a second and late developing, greater positive inotropic phase. Treatment with tyrphostin A25 (100 μM) and genistein (50 μM), but not daidzein (50 μM), significantly attenuated the two components of the inotropic response, although genistein suppressed the initial component more markedly than the late component.
We conclude that increased protein tyrosine phosphorylation may play an important role in initiating at least some part of the positive inotropic effect of H1-receptor stimulation in guinea-pig left atrium.
Keywords: Histamine, H1-receptors, positive inotropic effect, tyrosine kinase inhibitors, tyrosine phosphorylation, cardiac muscle
Introduction
Phosphorylation of the tyrosine residues of proteins by tyrosine kinases is widely recognized as a critical step in intracellular signal transduction (Hunter & Cooper, 1985). A family of receptors for growth factors, such as platelet-derived growth factor and epidermal growth factor, have tyrosine kinase activity in their intracellular domain, phosphorylate tyrosine residues of their own receptors, regulatory proteins or structural proteins, and thus activate a cascade of intracellular signalling (Fantl et al., 1993). In addition to receptor tyrosine kinases, cytosolic tyrosine kinases may also play an important role in mediating signal transduction by hetrotrimeric G protein-coupled receptors (Hollenberg, 1994). Indeed, recent studies have suggested that activation of protein tyrosine kinase is involved in smooth muscle contraction induced by angiotensin II and endothelin (Tsuda et al., 1991; Molloy et al., 1993; Ohanian et al., 1997). The presence of protein tyrosine kinases has been also reported in the heart (Maher, 1991; Jin et al., 1994; Liu et al., 1995), and tyrosine kinase inhibitors such as genistein have been shown to modulate several membrane ionic currents in cardiomyocytes (Sorota, 1995; Shuba et al., 1996; Yokoshiki et al., 1996; Wu & Cohen, 1997). Furthermore, an autocrine release of angiotensin II has been proposed to contribute to the stretch-induced increase in tyrosine phosphorylation in cultured neonatal rat ventricular cells (Sadoshima et al., 1993). These studies provide indirect evidence for the possible role of protein tyrosine kinases in the regulation of cardiac cell function.
Histamine produces an increase in cardiac contractility in most mammalian species, including humans, by interacting directly with specific receptors (Hattori, 1999). The positive inotropic effect of histamine is principally due to an H2-receptor-mediated increase in intracellular cyclic AMP (McNeill & Verma, 1974; Reinhardt et al., 1977; Hattori et al., 1991), a mechanism analogous to that well known for β-adrenoceptors. Activation of H1-receptors can also contribute to the positive inotropic effect of histamine in guinea-pig left atria (Reinhardt et al., 1974; Steinberg & Holland, 1975; Verma & McNeill, 1977) and rabbit papillary muscles (Hattori et al., 1988b; 1990). However, less well understood is the mechanism by which activation of H1-receptors produces a positive inotropic effect. The H1-receptor-mediated positive inotropic effect is not associated with an increase in intracellular cyclic AMP (Reinhardt et al., 1977; Verma & McNeill, 1977). Although stimulation of H1-receptors with histamine increases phosphoinositide turnover in cardiac muscles (Sakuma et al., 1988; Hattori et al., 1994), the relationship between H1-receptor-mediated inotropism and phosphoinositide breakdown has been questioned on the basis of our observation that phospholipase C inhibitors can block the histamine-induced phosphoinositide response, but yet leave intact the H1-receptor-mediated inotropic response (Hattori et al., 1989).
Recently, it has been demonstrated that histamine induces tyrosine phosphorylation of four different proteins in swine carotid artery and proposed that tyrosine phosphorylation of these proteins may be involved in the initial phase of smooth muscle contraction in response to histamine (Rembold & Weaver, 1997). This provides evidence suggesting that tyrosine phosphorylation of proteins may be an important step for the signalling pathway following activation of H1-receptors. The present study was designed to determine whether signalling processes for the positive inotropic effect of H1-receptor stimulation involve activation of the tyrosine kinase pathway. For this aim, using a recombinant antiphosphotyrosine antibody, we measured histamine-induced changes in phosphorylation levels of proteins on tyrosine residues in guinea-pig left atrium in which the agonist produces a positive inotropic effect exclusively mediated by H1-receptors (Hattori & Kanno, 1985; Hattori et al., 1989). In addition, we examined the influences of the compounds which inhibit tyrosine kinases on the positive inotropic effect of histamine in this tissue.
Methods
Contraction experiments
Guinea-pigs of either sex, weighing 250–300 g, were anaesthetized with diethyl ether. The hearts were quickly excised and transferred to a dissection bath filled with oxygenated Krebs-Henseleit solution at room temperature. The composition of the solution was (in mM): NaCl 119, CaCl2 2.5, KCl 4.8, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24.9 and glucose 10.0. The left atrium was carefully dissected from the heart and mounted vertically in a water-jacketed organ bath containing 50 ml of Krebs-Henseleit solution. The solution in the bath was bubbled with 95% O2 and 5% CO2 and its temperature was maintained at 30±1°C. The lower end of the atrium was fixed onto a pair of hook electrodes for stimulation and the other was connected by a silk thread to a force transducer (Nihon Kohden, TB-612T, Tokyo, Japan). Isometric tension developed in the preparation was recorded on a thermal array recorder (Nihon Kohden, RTA-1200) through a preamplifier (Nihon Kohden, RP-5). The resting tension applied to the preparation was adjusted to 2 g. The atrium was electrically driven by rectangular pulses of 1 ms duration and an intensity of 1.5 times threshold voltage at 0.5 Hz in frequency. The pulses were delivered from an electronic stimulator (Sanei-Sokki, 3F46, Tokyo, Japan) through an isolation unit (Sanei-Sokki, 5361). The preparations were allowed to equilibrate for at least 60 min before any experimental procedure was applied.
In a series of experiments carried out to examine the concentration-response curves for the positive inotropic effects of histamine and isoprenaline in the absence or presence of tyrosine kinase inhibitors, only one concentration-response curve was obtained in each preparation. The concentration-response curves were determined in a cumulative manner by increasing the concentration of each agonist in steps of 0.5 log units. The preparation was exposed to each concentration of the agonist until the increase in force of contraction reached a maximum which usually occurred within 10 min. The EC50 value, i.e., the concentration required to produce 50% of the maximal response induced by the agonist, was determined from log-probit plots of the individual response vs concentrations and was expressed as the negative logarithm (pD2 value).
As previously reported (Hattori & Kanno, 1985), in guinea-pig left atrium histamine at concentrations of 1 μM and higher produces a dual-component positive inotropic response (see Results). In order to examine the influences of tyrosine kinase inhibitors on the time course of the inotropic effect of histamine, we compared the time-response curves of 1 μM histamine before and after the addition of each inhibitor. Repeated application of histamine showed no difference in the time course of the inotropic changes induced by histamine (Hattori & Kanno, 1985).
When tyrosine kinase inhibitors were used, they were added to the bath at least 30 min before the administration of histamine or isoprenaline.
Phosphotyrosine immunoblots
Guinea-pig left atrium was prepared and mounted in the organ bath in a manner identical to that used for the contraction experiments. The atrium was removed from the organ bath at a predetermined time after the addition of histamine and was frozen immediately with a clamp that had been cooled with liquid nitrogen. The frozen samples were stored at −80°C until homogenized.
The frozen samples were homogenized in ice-cold lysis buffer (in mM: HEPES 20, NaCl 150, EDTA 2.5, NaF 50, sodium pyrophosphate 10, sodium orthovanadate 1, phenylmethylsulphonyl fluoride 1 and 1% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecylsulphate (SDS), 10% glycerol, 10 μg ml−1 aprotinin) by means of a microhomogenizer (Niti-on, NS310E, Tokyo, Japan). The homogenate was centrifuged at 6000×g for 15 min, and the supernatant was filtered through a single layer of cheese cloth. The protein concentration of the supernatant was determined by the method of Lowry et al. (1951), with bovine serum albumin used as standard. Samples (5 μg) were subjected to a 10% polyacrylamide SDS gel and electroblotted onto polyvinylidene difluoride filter (PVDF) membrane. The PVDF was washed in phosphate-buffered saline (PBS) (in mM: NaCl 137, KCl 2.7, NaH2PO4 8.1) and was blocked for 120 min at a room temperature in 1% bovine serum albumin in PBS-Tween buffer (TPBS) in mM: NaCl 137, KCl 2.7, NaH2PO4 8.1 and 0.05% Tween 20) to reduce non-specific binding. Thereafter, the PVDF was washed twice in TPBS, and incubated for 16 h at 4°C with 2 μg ml−1 mouse polyclonal antiphosphotyrosine antibody (PY20; Transduction Laboratories, Lexington, KY, U.S.A.) in TPBS. The PVDF was washed twice in TPBS then incubated with horseradish peroxidase conjugated anti-mouse antibody (Bio-Rad, Hercules, CA, U.S.A.) diluted at 1 : 6000 in TPBS at room temperature for 60 min. After being washed twice in TPBS, the blots were visualized with the enhanced chemiluminescence detection system (Amersham, Buckinghamshire, UK), exposed to X-ray film for 90 s, and analysed by free software NIH image produced by Wayne Rasband (National Institute of Health, Bethesda, MD, U.S.A.).
Drugs
The following compounds were used: histamine dihydrochloride (Merck, Darmstadt, Germany), (−)-isoprenaline hydrochloride (Sigma, St. Louis, MO, U.S.A.), tyrphostin A25 (Calbiochem, La Jolla, CA, U.S.A.), genistein (Sigma), daidzein (Sigma), mepyramine maleate (Sigma), (+)-chlorpheniramine maleate (Schering, Osaka, Japan) and cimetidine (Sigma). Histamine, isoprenaline and chlorpheniramine were dissolved in distilled water. Tyrosine kinase inhibitors were dissolved in dimethyl sulphoxide. The final concentration of DMSO in the bathing medium did not exceed 1%. Further dilutions to the proper concentrations were made with Krebs-Henseleit solution. Ascorbic acid (0.1 mM) was added to the solution of isoprenaline to retard its auto-oxidation. All materials for SDS-gel electrophoresis were obtained from Bio-Rad or Wako (Osaka, Japan). Other chemicals used in this study were of the highest purity available from Sigma or Wako.
Statistics
The data shown are means±s.e.mean. Two-way analysis of variance (ANOVA) was used to compare concentration-response or time-response curves between groups, Bonferroni's multiple comparison test being used to determine the significance of differences in mean values within each group. Individual points were compared using Student's t-test. P<0.05 was considered significant.
Results
Histamine-induced tyrosine phosphorylation
Figure 1 depicts typical patterns of antiphosphotyrosine immunoblots in homogenates of guinea-pig left atrium. There were a number of protein bands on SDS gels that bound antiphosphotyrosine antibody more abundantly when the left atrium was stimulated with 10 μM histamine for 3 min. Quantitation of the relative tyrosine phosphorylation detected four major phosphorylation bands with apparent molecular mass of 25, 35, 65 and 150 kDa, which show reproducible and specific changes following histamine stimulation. When the band derived from unstimulated tissue was assigned a value of 100%, the relative values for these four bands were significantly increased in response to 10 μM histamine for 3 min (see Figures 2–4). Phosphorylation of some other proteins also increased with histamine stimulation. However, changes in intensity in these protein bands were not always consistent. This did not allow accurate and reproducible quantification of the relative tyrosine phosphorylation of the band. It was confirmed that the histamine-induced increases in the phosphorylation levels of these four proteins were nearly completely eliminated when tissues were incubated with 50 μM genistein, a tyrosine kinase inhibitor (Akiyama et al., 1987), for 30 min prior to the addition of histamine (data not shown).
Figure 1.
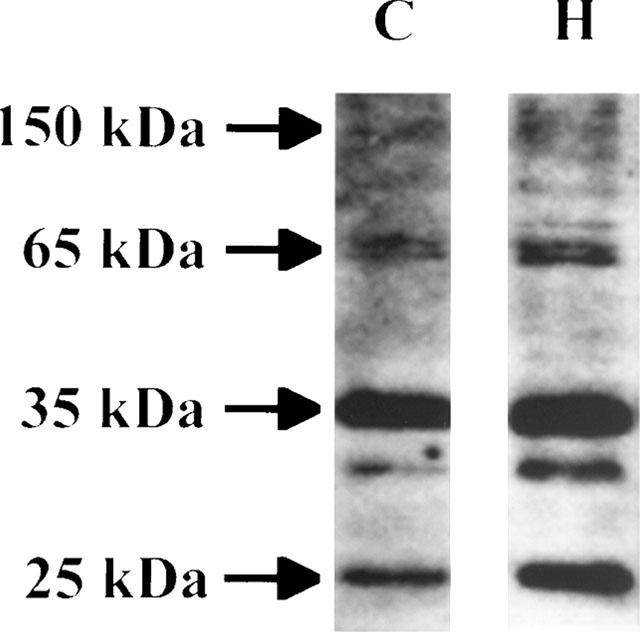
Histamine-induced protein tyrosine phosphorylation in guinea-pig left atrium. Following unexposure (control) or 3 min exposure to 10 μM histamine, the atrium was homogenized, run on SDS-polyacrylamide gel, electrophoretically transferred to PVDF and probed with antiphosphotyrosine antibody as described under ‘Methods'. Lane 1, control (C): lane 2, 10 μM histamine for 3 min (H). The immunoblots shown are representative of four experiments.
Figure 2.
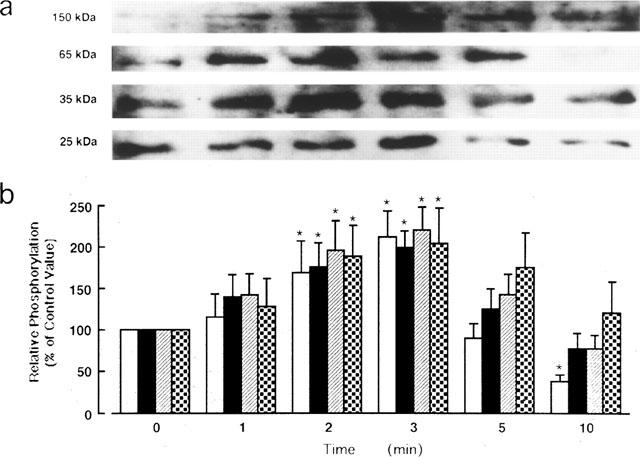
Time course of histamine stimulation of protein tyrosine phosphorylation in guinea-pig left atrium. The tissues were stimulated with 10 μM histamine for various times as indicated. (a) A representative immunoblot showing tyrosine phosphorylation of the 25-, 35-, 65- and 150-kDa protein bands. (b) Quantitation of relative tyrosine phosphorylation levels of the 25-kDa (open bars), 35-kDa (closed bars), 65-kDa (hatched bars) and 150-kDa (dotted bars) protein bands. The values are shown as relative intensity where each band derived from unstimulated tissue is assigned a value of 100%, and are mean±s.e.mean of six experiments. *P<0.05 compared to the corresponding control.
The time course of tyrosine phosphorylation of the four bands when the left atrium was stimulated with 10 μM histamine is shown in Figure 2. The tyrosine phosphorylation level of each protein was elevated above the control value 1 min after stimulation with histamine, although the values were not statistically significant. The phosphorylation levels of all four proteins were significantly increased at 2–3 min. Among the four protein bands, the 25-kDa protein band exhibited the most prominent increase in the tyrosine phosphorylation levels during histamine stimulation. However, the increase in tyrosine phosphorylation did not last long: 5 min later and thereafter the level was gradually declined to or below basal levels.
The maximal effect of histamine on protein tyrosine phosphorylation was obtained at 1 μM, except that a maximum increase in tyrosine phosphorylation of the 150-kDa protein band was produced by 10 μM histamine (Figure 3).
Figure 3.
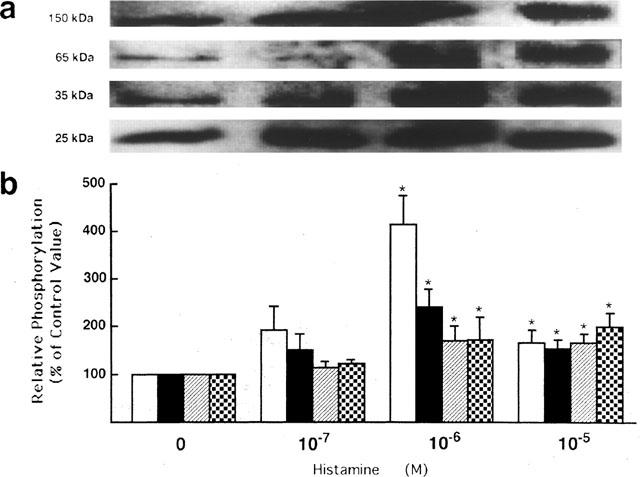
Concentration relationship of histamine stimulation of protein tyrosine phosphorylation in guinea-pig left atrium. The tissues were stimulated for 3 min with different concentrations of histamine as indicated. (a) A representative immunoblot showing tyrosine phosphorylation of the 25-, 35-, 65- and 150-kDa protein bands. (b) Quantitation of relative tyrosine phosphorylation levels of the 25-kDa (open bars), 35-kDa (closed bars), 65-kDa (hatched bars) and 150-kDa (dotted bars) protein bands. The values are shown as relative intensity where each band derived from unstimulated tissue is assigned a value of 100%, and are mean±s.e.mean of six experiments. *P<0.05 compared to the corresponding control.
As shown in Figure 4, histamine-stimulated protein tyrosine phosphorylation was greatly inhibited by the H1-receptor antagonists mepyramine (1 μM) and chlorpheniramine (1 μM). On the other hand, the H2-receptor antagonist cimetidine (10 μM) did not modify the stimulatory effect of histamine on protein tyrosine phosphorylation.
Figure 4.
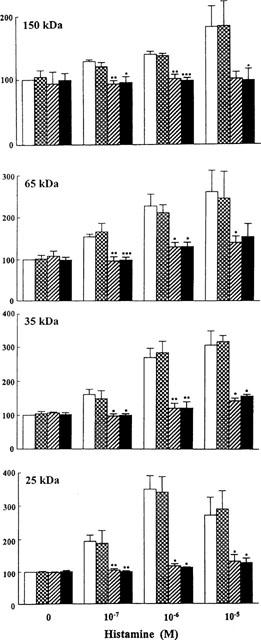
Concentration-dependent effects of histamine on tyrosine phosphorylation of the 25-, 35-, 65- and 150-kDa protein bands in the absence (open bars) and presence of 10 μM cimetidine (dotted bars), 1 μM mepyramine (hatched bars) and 1 μM chlorpheniramine (closed bars) in guinea-pig left atrium. The tissues were stimulated for 3 min with different concentrations of histamine. The antagonists were given at least 30 min before the addition of histamine. The values are shown as relative intensity where each band derived from unstimulated tissue in the absence of any antagonist is assigned a value of 100% and are mean±s.e.mean of at least three experiments. *P<0.05, **P<0.01 and ***P<0.001 compared to the corresponding value without any antagonist.
Influences of tyrosine kinase inhibitors on the positive inotropic effect of histamine
As shown in Figure 5a, histamine produced a concentration-dependent increase in force of contraction in guinea-pig left atrium electrically driven at 0.5 Hz. Treatment with 100 μM tyrphostin A25, a tyrosine kinase inhibitor (Gazit et al., 1989), resulted in a downward shift of the concentration-response curve for the positive inotropic effect of histamine. The sensitivity to histamine was unchanged by treatment with the tyrosine kinase inhibitor. Thus, the pD2 value for histamine was 6.58±0.10 (n=6) in the absence and 6.56±0.29 (n=6) in the presence of 100 μM tyrphostin A25. On the other hand, the maximum increase in force of contraction induced by histamine was markedly decreased from 162±13% (n=6) to 99±33% (n=4) and 62±19% (n=6) by treatment with 50 and 100 μM tyrphostin A25, respectively. However, the positive inotropic effect of isoprenaline was not modified by 100 μM tyrphostin A25 (Figure 5b).
Figure 5.
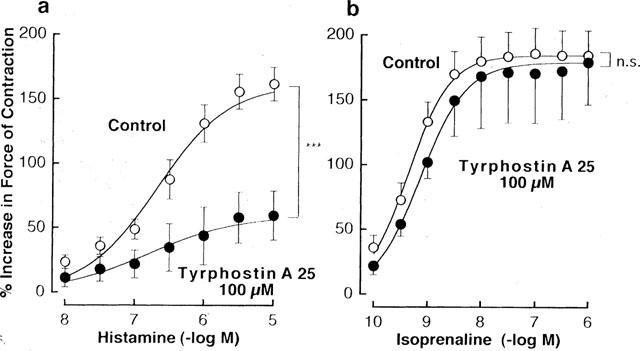
Influences of 100 μM tyrphostin A25 on the concentration-response curves for the positive inotropic effects of histamine (a) and isoprenaline (b) in guinea-pig left atrium electrically driven at 0.5 Hz. Tyrphostin A25 and its vehicle were applied to the tissues at least 30 min before the addition of the agonists. Points represent the net increases in force of contraction expressed as a percentage of the basal value recorded before any treatment and are shown as mean±s.e.mean of 4–6 experiments. Basal force of contraction was 527±36 mg (n=22). ***P value indicates that the curves obtained in the presence of tyrphostin A25 and its vehicle are significantly different from each other (<0.001); n.s.=not significant.
Another tyrosine kinase inhibitor, genistein (Akiyama et al., 1987), also caused a marked downward shift of the response to histamine (Figure 6a). The maximum response to histamine was reduced to 124±14% (n=4), 108±9% (n=5) and 90±15% (n=6) in the presence of 10, 25 and 50 μM genistein, respectively. In similar to tyrphostin A25, genistein had no apparent effect on the positive inotropic response to isoprenaline (data not shown). Treatment with 50 μM daidzein, a genistein analogue with little effect on tyrosine kinases (Peterson & Barnes, 1993), had no significant effect on the positive inotropic response to histamine (Figure 6b).
Figure 6.
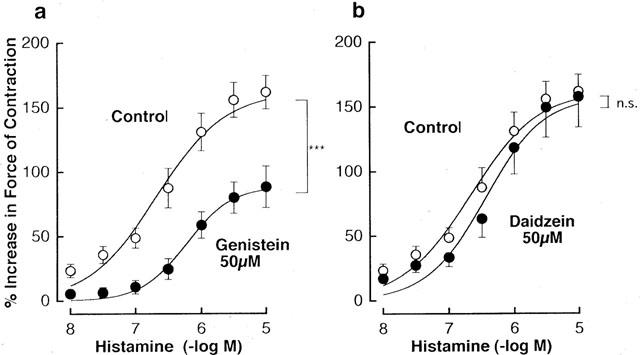
Influences of 50 μM genistein (a) and 50 μM daidzein (b) on the concentration-response curves for the positive inotropic effect of histamine in guinea-pig left atrium electrically driven at 0.5 Hz. Genistein, daidzein and their vehicles were applied to the tissues at least 30 min before the addition of histamine. Points represent the net increases in force of contraction expressed as a percentage of the basal value recorded before any treatment and are shown as mean±s.e.mean of six experiments. Basal force of contraction was 603±69 mg (n=18). ***P value indicates that the curves obtained in the presence of the drugs and their vehicles are significantly different from each other (<0.001); n.s.=not significant.
Genistein caused a moderate increase in the basal force of contraction in a concentration-dependent manner. Thus, when exposed to 10, 25 and 50 μM genistein, the basal force of contraction was increased by 14±3% (n=6), 28±5% (n=6) and 47±7% (n=10), respectively. The addition of 100 μM tyrphostin A25 and 50 μM daidzein also increased the basal force of contraction by 17±8% (n=10) and 18±8% (n=6), respectively. However, these contractile effects of tyrphostin A25 and daidzein appeared to be due to dimethyl sulphoxide used as a solvent. The vehicle increased the basal force of contraction by 22±7% (n=12).
As demonstrated in our previous reports (Hattori & Kanno, 1985; Hattori et al., 1988a), histamine at concentrations of 1 μM and higher exhibits a dual-component positive inotropic response that is entirely mediated by H1-receptors. As illustrated in Figure 7, the initial increase in force of contraction appeared immediately after the addition, reaching a maximum level within 1 min. A second increase in force of contraction appeared later on and was observed to emerge from the initial increase. The late component developed more slowly than the initial one, reaching a maximum level at 8–10 min. In the presence of tyrphostin A25, the magnitudes of the two components were reduced to the same extent (Figure 7a). Thus, the peak amplitudes of the initial and late components of the inotropic response to 1 μM histamine in the presence of 100 μM tyrphostin A25 were 57±7 and 56±8% (n=10) of the corresponding control response, respectively. Treatment with 50 μM genistein suppressed the initial component of the histamine inotropic response more markedly than the late component. The peak amplitude of each component was decreased to 36±6 and 71±6% (n=8) of controls. As a result, genistein apparently fused the two components of the positive inotropic effect produced by 1 μM histamine into one (Figure 7b). Treatment with 50 μM daidzain did not modify the overall pattern and extent of the dual-component positive inotropic response to 1 μM histamine (Figure 7c).
Figure 7.
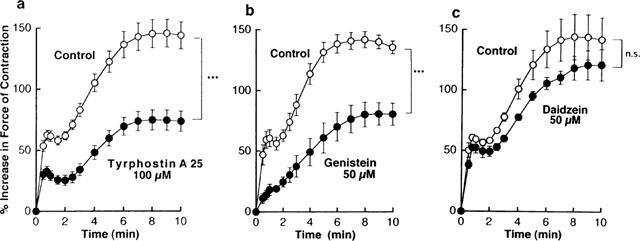
Influences of 100 μM tyrphostin A25 (a), 50 μM genistein (b) and 50 μM daidzein (c) on the time course of changes in force of contraction after application of 1 μM histamine in guinea-pig left atrium electrically driven at 0.5 Hz. These drugs and their vehicles were applied to the tissues at least 30 min before the addition of the agonists. Ordinates: increases in force of contraction expressed as a percentage of the basal value recorded before any treatment. Abscissae: time after the addition of histamine. Basal force of contraction was 518±32 mg (n=23). Points are mean±s.e.mean of 5–10 experiments. ***P value indicates that the curves obtained in the presence of the drugs and their vehicles are significantly different from each other (<0.001); n.s.=not significant.
Discussion
In the present study, tyrosine phosphorylation was estimated by measuring relative levels of the binding of antiphosphotyrosine antibodies to proteins that were extracted from guinea-pig left atrium prior to and following histamine stimulation and were separated by SDS gel electrophoresis. We found four bands with approximate molecular weights of 25, 35, 65 and 150 kDa in which tyrosine phosphorylation increased in response to histamine. In swine carotid artery, histamine is capable of increasing tyrosine phosphorylation of four proteins of molecular weights of 75, 85, 110 and 120 kDa (Rembold & Weaver, 1997). Thus, histamine can phosphorylate a number of proteins on tyrosine residues in both cardiac and vascular smooth muscles, but the phosphorylated proteins appears to be somewhat different between the two tissues. Histamine-induced increases in phosphorylation of cardiac proteins on tyrosine residues were blocked by the H1-receptor antagonists mepyramine and chlorpheniramine. At the concentration employed in this study (1 μM), these antagonists selectively antagonize the H1-receptor-mediated cardiac responses without affecting the H2-receptor-mediated ones (Hattori et al., 1988b; 1991; 1994). On the other hand, the H2-receptor antagonist cimetidine (10 μM) did not affect the stimulatory effect of histamine on protein tyrosine phosphorylation. These results imply mediation through H1-receptors. To our knowledge, this study is the first to demonstrate that activation of H1-receptors increases tyrosine phosphorylation of myocardial proteins.
The present study showed that the tyrosine kinase inhibitors tyrphostin A25 and genistein significantly reduced the positive inotropic effect of histamine in guinea-pig left atrium which is exclusively mediated through H1-receptors. With regard to the specificity of the inhibitors used in this study, several lines of evidence indicate that while both tyrphostin A25 and genistein in the concentrations used in the current study can inhibit the activity of protein tyrosine kinases (Akiyama et al., 1987; Gazit et al., 1989; Sauro & Thomas, 1993; Di Salvo et al., 1993), they have no significant effect on other enzymes, including myosin light chain kinase (Di Salvo et al., 1993), protein kinase A (Akiyama et al., 1987; Gazit et al., 1989; Di Salvo et al., 1993) or protein kinase C (Akiyama et al., 1987; Gazit et al., 1989). Indeed, the failure to inhibit the positive inotropic effect of isoprenaline supports that the inhibitors did not produce their effects nonspecifically. The inhibitors, in the concentrations used in this study, had no apparent depressive effect on the basal force of contraction, suggesting that they do not directly interfere with the excitation-contraction coupling process. Furthermore, daidzein, an inactive analogue of genistein (Peterson & Barnes, 1993), scarcely modified the positive inotropic response to histamine. Therefore, we interpret the present results to indicate that activation of protein tyrosine kinases may be a key step in signaling processes for the positive inotropic effect of H1-receptor stimulation.
Tyrosine phosphorylation of four protein bands started to elevate 1 min after the addition of histamine, peaked at 2∼3 min, and then tended to decrease to or below basal levels. On the other hand, the positive inotropic effect induced by histamine revealed a biphasic time course. The initial component of the positive inotropic effect peaked within 1 min and declined gradually, whereas the late component appeared about 2 min after agonist stimulation and required 8 to 10 min to reach a maximal level that was well sustained. Thus, histamine-induced changes in tyrosine phosphorylation did not apparently show a temporal correlation with the changes in force of contraction. Especially, even when histamine-induced increases in tyrosine phosphorylation of four proteins returned toward the basal value, force of contraction was still increased as the late component. However, an important prerequisite for a causal relation between the increase in protein tyrosine phosphorylation and the positive inotropic effect mediated by H1-receptors might be that the former can precede the latter. When the atrial muscle was stimulated with histamine, the increase in tyrosine phosphorylation levels preceded the appearance of the late component of the positive inotropic response. Therefore, we assume that even though dephosphorylation of the phosphoproteins occurred during the development of force of contraction, a transitory but preceding increase in protein tyrosine phosphorylation may be important as a key step of signalling cascade to initiate the late component of the H1-receptor-mediated positive inotropism. The mechanism underlying the late components of the positive inotropic effect of H1-receptor activation is associated with an increased Ca2+ influx via L-type Ca2+ channels resulting from action potential prolongation (Hattori et al., 1988a; Yoshimoto et al., 1998). In this regard, it is interesting to note that one mechanism by which tyrosine phosphorylation may regulate smooth muscle contraction is through effects on ion channels and Ca2+ influx (Wijetunge & Hughes, 1995; Smirnov & Aaronson, 1995). However, functional experiments using tyrphostin A25 and genistein revealed that the initial component of the histamine inotropic response was depressed to the same extent as the late component by these tyrosine kinase inhibitors. Although genistein was stronger than tyrphostin A25 in inhibiting the initial component, this difference might include quantitative (partial inhibition of tyrosine kinases) and/or qualitative aspects (inhibition of different kinases). Nevertheless, the functional antagonism would suggest that protein tyrosine phosphorylation may be also involved as the signalling cascade in initial components of the histamine-induced positive inotropic response. Thus, a small change in tyrosine phosphorylation immediately after histamine application, although not being detectable by our immunoblotting, may be responsible for the generation of the initial component of the H1-receptor-mediated inotropism. The mechanism of the initial component is clearly different from that of the late component in that the initial component is resistant to dihydropyridine Ca2+ channel antagonists (Hattori & Kanno, 1985; Hattori et al., 1988a). Even if protein tyrosine phosphorylation is involved in the establishment of both components of the H1-receptor-mediated positive inotropic effect, it remains unclear how this signalling pathway links to the two different intracellular mechanisms.
A number of recent reports suggest that angiotensin II and phenylephrine, which stimulate heterotrimeric G protein-linked receptors, may directly induce protein tyrosine kinase activation in cardiac myocytes (Thorburn & Thorburn, 1994; Sadoshima & Izumo, 1996). Thus, the pathway leading to activation of protein tyrosine kinases may be mediated through activated G proteins, but it is certainly possible that some intermediates, as the upstream effectors, may be involved in this pathway. Furthermore, it cannot be ruled out that the primary signal induced by the receptor agonists may be inhibition of tyrosine phosphatases. In any event, activation of tyrosine kinases is known to phosphorylate phospholipase C-γ, resulting in its activation, consequent phosphoinositide hydrolysis, Ca2+ mobilization and protein kinase C activation (Auger et al., 1989; Homma et al., 1993). One of the proteins that were phosphorylated on tyrosine residues when stimulated with histamine in this study represents a molecular mass of 150 kDa that is roughly consistent with the size for phospholipase C-γ (Blayney et al., 1996), although the identity of our phosphorylated protein is currently unclear. However, it seems unlikely that the positive inotropic effect of H1-receptor stimulation is related to tyrosine phosphorylation of phospholipase C-γ, because neither activators nor inhibitors of protein kinase C alters substantially the positive inotropic effect of histamine in guinea-pig left atrium (Hattori et al., 1989). Another ‘downstream' event from protein tyrosine phosphorylation would be the important mechanism by which activation of H1-receptors increases cardiac contractility.
In conclusion, we demonstrated that stimulation of H1-receptors with histamine increased protein tyrosine phosphorylation in guinea-pig left atrium. The increase in tyrosine phosphorylation was transient, but appeared to precede the late component of the positive inotropic effect of H1-receptor stimulation with histamine. Furthermore, the tyrosine kinase inhibitors specifically attenuated the positive inotropic effect of histamine. Although further study must focus on identifying the phosphorylated proteins and their function, the present data are consistent with a role for the protein tyrosine phosphorylation signalling cascade in at least some part of the H1-receptor-mediated positive inotropism.
Acknowledgments
Experimental work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan. The authors are very grateful to Mr Kinya Kudo for technical assistance.
Abbreviations
- PBS
phosphate-buffered saline
- PVDF
polyvinylidene difluoride filter
- SDS
sodium dodecylsulphate
- TPBS
PBS-Tween buffer
References
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M. , FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- AUGER K.R., SERUNIAN L.A., SOLTOFF S.R., LIBBY P. , CANTLEY L.C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. doi: 10.1016/0092-8674(89)90182-7. [DOI] [PubMed] [Google Scholar]
- BLAYNEY L.M., GAPPER P.W. , NEWBY A.C. Phospholipase C isoforms in vascular smooth muscle and their regulation by G-proteins. Br. J. Pharmacol. 1996;118:1003–1011. doi: 10.1111/j.1476-5381.1996.tb15499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI SALVO J., STEUSLOFF A., SEMENCHUK L., SATOH K., KOLQUIST K. , PFITZER G. Tyrosine kinase inhibitors suppress agonist-induced contraction in smooth muscle. Biochem. Biophys. Res. Commun. 1993;190:968–974. doi: 10.1006/bbrc.1993.1144. [DOI] [PubMed] [Google Scholar]
- FANTL W.J., JOHNSON D.E. , WILLIAMS L.T. Signalling by receptor tyrosine kinases. Annu. Rev. Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- GAZIT A., YAISH P., GILON C. , LEVITZKI A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- HATTORI Y. Cardiac histamine receptors: Their pharmacological consequences and signal transduction pathways. Methods Find. Exp. Clin. Pharmacol. 1999;21:123–131. doi: 10.1358/mf.1999.21.2.529239. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., ENDOU M., SHIROTA M. , KANNO M. Dissociation of phosphoinositide hydrolysis and positive inotropic effect of histamine mediated by H1-receptors in guinea-pig left atria. Naunyn-Schmiedeberg' Arch. Pharmacol. 1989;340:196–203. doi: 10.1007/BF00168969. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., GANDO S., ENDOU M. , KANNO M. Characterization of histamine receptors modulating inotropic and biochemical activities in rabbit left atria. Eur. J. Pharmacol. 1991;196:29–36. doi: 10.1016/0014-2999(91)90405-f. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., GANDO S., NAGASHIMA M. , KANNO M. Histamine receptors mediating a positive inotropic effect in guinea pig and rabbit ventricular myocardium: Distribution of the receptors and their possible intracellular coupling processes. Jpn. J. Pharmacol. 1994;65:327–336. doi: 10.1254/jjp.65.327. [DOI] [PubMed] [Google Scholar]
- HATTORI Y. , KANNO M. Effect of Ni2+ on the multiphasic inotropic responses to histamine mediated by H1-receptors in left atria of guinea pigs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;329:188–194. doi: 10.1007/BF00501211. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., NAKAYA H., ENDOU M. , KANNO M. Inotropic, electrophysiological and biochemical responses to histamine in rabbit papillary muscles: Evidence for coexistence of H1- and H2-receptors. J. Pharmacol. Exp. Ther. 1990;253:250–256. [PubMed] [Google Scholar]
- HATTORI Y., NAKAYA H., TOHSE N. , KANNO M. Effects of Ca2+ channel antagonists and ryanodine on H1-receptor mediated electromechanical response to histamine in guinea-pig left atria. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988a;337:323–330. doi: 10.1007/BF00168846. [DOI] [PubMed] [Google Scholar]
- HATTORI Y., SAKUMA I. , KANNO M. Differential effects of histamine mediated by histamine H1- and H2-receptors on contractility, spontaneous rate and cyclic nucleotides in the rabbit heart. Eur. J. Pharmacol. 1988b;153:221–229. doi: 10.1016/0014-2999(88)90609-7. [DOI] [PubMed] [Google Scholar]
- HOLLENBERG M.D. Tyrosine kinase pathways and the regulation of smooth muscle contractility. Trends Pharmacol. Sci. 1994;15:108–114. doi: 10.1016/0165-6147(94)90046-9. [DOI] [PubMed] [Google Scholar]
- HOMMA Y., SAKAMOTO H., TSUNODA M., AOKI M., TAKENAWA T. , OOYAMA T. Evidence for involvement of phospholipase C-gamma 2 in signal transduction of platelet-derived growth factor in vascular smooth muscle cells. Biochem. J. 1993;290:649–653. doi: 10.1042/bj2900649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER T. , COOPER J.A. Protein-tyrosine kinases. Annu. Rev. Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- JIN Y., PASUMARTHI K.B., BOCK M.E., LYTRAS A., KARDAMI E. , CATTINI P.A. Cloning and expression of fibroblast growth factor receptor-1 isoforms in the mouse heart: evidence for isoform switching during heart development. J. Mol. Cell. Cardiol. 1994;26:1449–1459. doi: 10.1006/jmcc.1994.1164. [DOI] [PubMed] [Google Scholar]
- LIU L., PASUMARTHI K.B., PADUA R.R., MASSAELI H., FANDRICH R.R., PIERCE G.N., CATTINI P.A. , KARDAMI E. Adult cardiomyocytes express functional high-affinity for basic fibroblast growth factor. Am. J. Physiol. 1995;268:H1927–H1938. doi: 10.1152/ajpheart.1995.268.5.H1927. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L. , RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAHER P.A. Tissue-dependent regulation of protein tyrosine kinase activity during embryonic development. J. Cell. Biol. 1991;112:955–963. doi: 10.1083/jcb.112.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNEILL J.H. , VERMA S.C. Blockade by burimamide of the effects of histamine analogs on cardiac contractility, phospholyase activation and cyclic adenosine monophosphate. J. Pharmacol. Exp. Ther. 1974;188:180–188. [PubMed] [Google Scholar]
- MOLLOY C.J., TAYLOR D.S. , WEBER H. Angiotensin II stimulation of rapid protein tyrosine phosphorylation and protein kinase activation in rat aortic smooth muscle cells. J. Biol. Chem. 1993;268:7338–7345. [PubMed] [Google Scholar]
- OHANIAN J., OHANIAN V., SHAW L., BRUCE C. , HEAGERTY A.M. Involvement of tyrosine phosphorylation in endothelin-1-induced calcium-sensitization in rat small mesenteric arteries. Br. J. Pharmacol. 1997;120:653–661. doi: 10.1038/sj.bjp.0700950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON G. , BARNES S. Genistein and biochanin A inhibit the growth of human prostate cancer cell but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate. 1993;22:335–345. doi: 10.1002/pros.2990220408. [DOI] [PubMed] [Google Scholar]
- REINHARDT D., SCHMIDT U., BRODDE O.E. , SCHÜMANN H.J. H1-and H2-receptor mediated responses to histamine on contractility and cyclic AMP of atrial and papillary muscles from guinea-pig hearts. Agents Actions. 1977;7:1–12. doi: 10.1007/BF01964874. [DOI] [PubMed] [Google Scholar]
- REINHARDT D., WAGNER J. , SCHÜMANN H.J. Differentiation of H1-and H2-receptors mediating positive chrono- and inotropic responses to histamine on atrial preparations of the guinea-pig. Agents Actions. 1974;4:217–221. doi: 10.1007/BF01965222. [DOI] [PubMed] [Google Scholar]
- REMBOLD C.M. , WEAVER B.A. Tyrosine phosphorylation and regulation of swine carotid artery contraction. J. Vasc. Res. 1997;34:1–10. doi: 10.1159/000159196. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J. , IZUMO S. The hetrotrimeric Gq protein-coupled angiotensin II receptor activates p21ras via the tyrosine kinase-Shc-Grb2-Sos pathway in cardiac myocytes. EMBO J. 1996;15:775–787. [PMC free article] [PubMed] [Google Scholar]
- SADOSHIMA J., XU Y., SLAYTER H.S. , IZUMO S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- SAKUMA I., GROSS S.S. , LEVI R. Positive inotropic effect of histamine on guinea pig left atrium: H1-receptor-induced stimulation of phosphoinositide turnover. J. Pharmacol. Exp. Ther. 1988;247:466–472. [PubMed] [Google Scholar]
- SAURO M.D. , THOMAS B. Tyrphostin attenuates platelet-derived growth factor-induced contraction in aortic smooth muscle through inhibition of protein tyrosine kinase(s) J. Pharmacol. Exp. Ther. 1993;267:1119–1125. [PubMed] [Google Scholar]
- SHUBA L.M., ASAI T., PELZER S. , MCDONALD T.F. Activation of cardiac chloride conductance by the tyrosine kinase inhibitor, genistein. Br. J. Pharmacol. 1996;119:335–345. doi: 10.1111/j.1476-5381.1996.tb15991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRNOV S.V. , AARONSON P.I. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ. Res. 1995;76:310–316. doi: 10.1161/01.res.76.2.310. [DOI] [PubMed] [Google Scholar]
- SOROTA S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflügers Arch. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- STEINBERG M.I. , HOLLAND D.R. Separate receptors mediating the positive inotropic and chronotropic effect of histamine in guinea-pig atria. Eur. J. Pharmacol. 1975;34:95–104. doi: 10.1016/0014-2999(75)90229-0. [DOI] [PubMed] [Google Scholar]
- THORBURN J. , THORBURN A. The tyrosine kinase inhibitor, genistein, prevents alpha-adrenergic-induced cardiac muscle cell hypertrophy by inhibiting activation of the Ras-MAP kinase signaling pathway. Biochem. Biophys. Res. Commun. 1994;202:1586–1591. doi: 10.1006/bbrc.1994.2113. [DOI] [PubMed] [Google Scholar]
- TSUDA T., KAWAHARA Y., SHII K., KOIDE M., ISHIDA Y. , YOKOYAMA M. Vasoconstrictor-induced protein-tyrosine phosphorylation in cultured vascular smooth muscle cells. FEBS Lett. 1991;285:44–48. doi: 10.1016/0014-5793(91)80721-e. [DOI] [PubMed] [Google Scholar]
- VERMA S.C. , MCNEILL J.H. Cardiac histamine receptors: Differences between left and right atria and right ventricle. J. Pharmacol. Exp. Ther. 1977;200:352–362. [PubMed] [Google Scholar]
- WIJETUNGE S. , HUGHES A.D. pp60c-src increases voltage-operated calcium channel currents in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1995;217:1039–1044. doi: 10.1006/bbrc.1995.2874. [DOI] [PubMed] [Google Scholar]
- WU J.-Y. , COHEN I.S. Tyrosine kinase inhibition reduces if in rabbit sinoatrial node myocytes. Pflügers Arch. 1997;434:509–514. doi: 10.1007/s004240050430. [DOI] [PubMed] [Google Scholar]
- YOKOSHIKI H., SUMII K. , SPERELAKIS N. Inhibition of L-type calcium current in rat ventricular cells by the tyrosine kinase inhibitor, genistein and its inactive analog, daidzein. J. Mol. Cell. Cardiol. 1996;28:807–814. doi: 10.1006/jmcc.1996.0075. [DOI] [PubMed] [Google Scholar]
- YOSHIMOTO K., HATTORI Y., HOUZEN H., KANNO M. , YASUDA K. Histamine H1-receptor-mediated increase in the Ca2+ transient without a change in the Ca2+ current in electrically stimulated guinea-pig atrial myocytes. Br. J. Pharmacol. 1998;124:1744–1750. doi: 10.1038/sj.bjp.0702008. [DOI] [PMC free article] [PubMed] [Google Scholar]


