Abstract
This study compared the effects of flibanserin, a novel 5-HT1A agonist/5-HT2A antagonist; diazepam, a traditional anxiolytic; and imipramine, a traditional antidepressant, on separation-induced ultrasonic vocalizations (USVs), locomotor behaviour, negative geotaxis and body temperature of 7–8-day-old rat pups.
Flibanserin (5, 10, 25 and 50 mg kg−1 s.c.) reduced USVs but had no effects on locomotor behaviour or negative geotaxis. Lower doses of flibanserin (0.5, 1, 2 and 4 mg kg−1 s.c.) had no effect on any behaviour. Diazepam (0.25, 0.5, 1 and 2 mg kg−1 s.c.) not only reduced the USVs but also increased rolling and increased the latency of the negative geotaxic response. Imipramine (10, 15, 20 and 30 mg kg−1 s.c.) reduced USVs, increased total locomotor activity and rolling but had no effect on negative geotaxis. None of the drugs altered body temperature.
Our data showed that flibanserin is as effective in reducing the USVs as diazepam and imipramine but has a lower incidence of motor side effects. This suggests that flibanserin might be effective for the treatment of mood disturbances such as anxiety.
Keywords: Antidepressant, anxiolytic, ultrasonic vocalization, locomotion, negative geotaxis, rat pups
Introduction
Anxiety often occurs with depression and is treated with anxiolytics, antidepressants, or both (Casacalenda & Boulenger, 1998; Stahl 1999). The most common anxiolytics, benzodiazepines (BZs), act via central GABAA/benzodiazepine receptors. Although BZs produce a fast relief of anxiety, they possess unwanted side effects such as sedation, muscle relaxation, synergism with alcohol, and development of tolerance and dependence (Woods et al., 1987; 1995). Buspirone is a clinically available non-benzodiazepine anxiolytic that exerts its action via 5-HT1A receptors (Glennon 1990; Goa & Ward, 1986). Although buspirone lacks the side effects of the benzodiazepines, its clinical use is limited because of its slow onset of action, i.e., treatment for several weeks is necessary to reach the therapeutic effect (Feighner & Boyer, 1989; Rickels 1990). Similarly, traditional antidepressants that block re-uptake of serotonin either specifically (SSRIs) or non-specifically (tricyclic antidepressants) have a slow onset of action and, therefore, their use for fast relief of anxiety is restricted (Schreiber et al., 1998).
The side effects of benzodiazepines and the slow onset of action of clinically available serotonergic therapeutics have stimulated the search for new anxiolytics that are fast acting and free from the unwanted side effects of traditional benzodiazepines (Kunovac & Stahl, 1995; Mosconi et al., 1993). The search for such drugs has focused on serotonin receptor subtypes. Both 5-HT1A agonist and 5-HT2A antagonist drugs possess anxiolytic properties in clinical studies (Gardner 1988; Taylor 1990) although they sometimes fail to produce an anxiolytic effect in animal models of anxiety (Cole & Rodgers, 1994; Griebel et al., 1997a; Wada & Fukuda, 1991). Drugs that act as both 5-HT1A agonists and 5-HT2A antagonists, however, have been shown to produce potent anxiolytic-like effects in animal models of anxiety (Albinsson et al., 1994; Kunovac & Stahl, 1995). Flibanserin (BIMT-17) is a novel drug that acts as a 5-HT1A agonist and a 5-HT2A antagonist in the frontal cortex (Borsini et al., 1998). It has been shown to have antidepressant properties in animal models such as the forced swimming test, learned helplessness paradigm, mild chronic stress test and in the test of ambulation following olfactory bulbectomy (Borsini et al., 1997; Cesana et al., 1995; D'Aquila et al., 1997). Flibanserin has a faster onset of action than the classic antidepressants, as its effect is achieved after a single administration (Borsini et al., 1997; 1998; D'Aquila et al., 1997). Given its unique pharmacological actions on 5-HT1A and 5-HT2A receptors and its fast onset of action, it has been hypothesized that flibanserin might also possess anxiolytic properties. This hypothesis is supported by the findings of Borsini et al. (1999) who found that flibanserin has anxiolytic effects in the light/dark transition box and stress-induced hyperthermia models of anxiety.
The isolation-induced ultrasonic vocalizations (USVs) in infant rodents that are evoked by transient separation from mother and littermates, provide a model for testing the anxiolytic properties of novel psychotropic drugs (Gardner, 1985). Ultrasonic vocalizations are decreased after acute treatment with anxiolytics such as diazepam and chlordiazepoxide (Nastiti et al., 1991) and buspirone (Kramer et al., 1998) as well as with antidepressants such as fluvoxamine, imipramine or clomipramine (Kramer et al., 1998; Olivier et al., 1994; 1998; Winslow & Insel, 1990). Moreover, the side effect profile of putative therapeutic agents can be obtained during tests for USVs by concomitant recording of locomotor behaviours, geotaxic response and neuromuscular reflexes.
The present study examined the effects of flibanserin on the emission of ultrasonic vocalizations, locomotor activity, negative geotaxic response and body temperature in 7–8-day-old rat pups. To test our hypothesis that flibanserin might have anxiolytic properties, its effects were compared to those of a traditional benzodiazepine anxiolytic, diazepam. Since previous studies with flibanserin have shown that it acts as an antidepressant (Borsini et al., 1997; 1998; Cesana et al., 1995), we also compared the effects of flibanserin with those of a traditional antidepressant, imipramine. Because there are individual differences in the frequency of ultrasonic vocalizations emitted by rat pups during baseline tests (Podhorna & Brown, 1999; Winslow et al., 1990), some investigators pre-test pups and then exclude non-vocalizing pups from their experiment (Podhorna & Brown, 1999; Vivian et al., 1997; Winslow et al., 1990). We, however, decided to use all pups in order to determine whether the pups that vocalize at high or low rates in the pre-test have a different reactivity to pharmacological treatments. Thus, we pre-tested pups and divided them into high- and low-vocalizing groups based on the results of this screening test. High-vocalizing (HV) pups were those that emitted 20 or more ultrasonic calls during the 1-min screening period while low-vocalizing (LV) pups emitted 19 or fewer calls.
Methods
The welfare of the animals as well as the procedure used in this study were under approval of the Dalhousie University Animal Care Committee protocol number 98-054.
Subjects
The offspring of mated pairs of Long-Evans hooded rats originally purchased from Charles River, Canada (St. Constant, Quebec) and bred in the Psychology Department, Dalhousie University were used as subjects. The adults were housed in standard Plexiglas cages (23×45×25 cm) in a vivarium with constant room temperature (22±1°C). They were kept under a reversed 12 : 12 light : dark cycle with lights on from 2100 h to 0900 h 2 weeks prior to mating and throughout the experiment. Pairs of rats were mated for 10 days and then the male was removed. Purina rat chow and water were available ad libitum.
Following birth, each litter was housed with its mother in a Plexiglas cage with wood shavings for bedding and shredded paper for nesting material. Bedding material was not changed from the time of birth until the day after the testing. Litters were culled to 10 pups on day 4 (parturition as day 0) and 28 litters were used for the experiment. All pups were tested once during the dark phase of the light : dark cycle when they were 7–8 days of age. The mean±s.e.mean weight of pups, when tested, was 16.71±0.13 g.
Apparatus
Testing for ultrasonic vocalizations and locomotor behaviour took place in a Plexiglas chamber (26×15×12 cm) with lines at the bottom that divided the floor into eight rectangles (6.5×7.5 cm). To facilitate emission of ultrasonic vocalizations by rat pups, the room temperature was lowered to 20±1°C (Okon 1970; Olivier et al., 1998). Ultrasonic vocalizations were recorded via an ultrasonic microphone connected to an SM2 bat detector (Ultrasound Advice, U.K.). The broadband output of the bat detector was fed into a custom-built four-channel digitizer based on that designed by Harrison & Holman (1978). This digitizer contained four variable band pass filters that were set to 28, 36, 44 and 52 kHz. When input was detected at one of these frequencies, the digitizer produced a pulse for the duration of the signal. The output of the digitizer was connected via a terminal panel and interface card (Strawberry Tree, Sunnyvale, CA, U.S.A.) to a Macintosh 2cx computer on which a custom program written using the Strawberry Tree Workbench Mac software (McGregor, 1996) recorded the occurrence of each ultrasonic vocalization on a minute-by-minute basis.
Procedure
The procedure was similar to that used by Dastur et al. (1999). On the experimental day, pups were transported together with their mother in their home cages to the experimental room and left undisturbed for at least 15 min. Each pup was then weighed, marked with a nontoxic permanent marker and individually placed into the test chamber where its ultrasonic vocalizations were recorded for a 1-min baseline screening period. After all pups from one litter were screened, they were assigned to a treatment, using a split-litter design (one to two pups per litter for each treatment group). Pups were then taken individually from the home cage and their basal temperature (pre-treatment temperature) was measured. This was done by placing a thermocouple, connected to a Physitemp Model B Thermometer, in the left armpit and keeping it there until the temperature on the digital display stabilized. Each pup was then injected with the vehicle or one of the drug doses. After injections, pups were returned to their home cages with their mother and littermates for 30 min.
After the 30 min post-injection interval, pups were removed from their home cage and their temperature was measured a second time (post-treatment temperature). Pups were then individually placed in the centre of the test chamber for 3 min and the number of ultrasonic vocalizations emitted was automatically recorded. Locomotor behaviours were recorded by an observer sitting quietly in the testing room. The behaviours scored were the number of lines crossed (defined as any traversing of a line by a half of the pup's body), rearing (climbing walls of the chamber with front paws on the wall), head raising (raising the head from the horizontal position to an angle of 30°), and rolling (each time a pup rolled onto its back with all four paws up). The total activity score was calculated as the sum of line crossing, rearing and head raising. The frequency of rolling in the 3-min test was used as a measure of ataxia.
At the end of the 3-min test, each pup was removed from the test chamber and tested for the negative geotaxic response. This was done by placing the pup head-down on a wire-mesh covered Plexiglas inclined plane (30° angle). The median latency to turn around in a head-up (±10°) position obtained from five consecutive trials was taken as a measure of the geotaxic response. Pups were tested for a maximum of 60 s and those which failed to show the negative geotaxic response or who fell off of the inclined plane were given this maximum score.
Drugs
Flibanserin (BIMT-17; a gift of Boehringer Ingelheim, Milano, Italy) was dissolved in a solution of 1 N HCl (2.5%), propylene glycol (50%) and distilled water (47.5%). Diazepam (Hoffmann-LaRoche, VALIUM 10 Roche, 5 mg kg−1) was dissolved in a solution of propylene glycol and distilled water (1 : 1). Imipramine (Sigma) was dissolved in saline. All drugs were injected subcutaneously in a volume of 2 ml kg−1 30 min prior to testing. Control groups were treated with the vehicle for the appropriate drug. Each experimental group consisted of 13–15 pups.
The study was done in four experiments. In experiment 1, flibanserin (5, 10, 25 and 50 mg kg−1) was tested at three doses that were previously found to be effective in antidepressant tests (Borsini et al., 1997; D'Aquila et al., 1997), and one high dose (50 mg kg−1) to examine the side effect profile. A dose of 64 mg kg−1 results in serotonergic syndrome (Borsini et al., 1998). Since this wide range of doses produced almost identical effects on behavioural measures, experiment 2 examined effects of lower doses of flibanserin (0.5, 1, 2 and 4 mg kg−1). Diazepam (0.25, 0.5, 1 and 2 mg kg−1) was studied in experiment 3 and imipramine (10, 15, 20 and 30 mg kg−1) in experiment 4.
Data analysis
Data sets that met the criterion for homogeneity of variance were analysed using one-way ANOVA, and non-homogenous data were transformed using the square root transformation before analysis. Data that still did not meet the criteria for parametric statistics were analysed using a non-parametric Kruskal-Wallis (H) test. Post-hoc comparisons between vehicle- and drug-treated groups were carried out using parametric or non-parametric versions of Bonferroni's test (Glantz, 1992). Changes in body temperature for each drug dose were analysed using a paired t-test.
For each experiment, data from all subjects were first analysed and then the data were separated into two groups based on the number of vocalizations of each pup in the 1-min screening test. Pups that emitted 20 or more ultrasonic calls during the screening period were classified as ‘high-vocalizing' (HV) while pups emitting 19 or fewer calls were classified as ‘low-vocalizing' (LV). Data from each of these two sub-groups of pups was analysed using the statistical methods described above.
Results
Screening responding
From a total of 273 pups tested in the 1-min-screening period, 159 were classified as high-vocalizing while the other 114 pups were classified as low-vocalizing. The mean±s.e.mean number of ultrasonic vocalizations emitted during the screening period by all pups was 43.50±2.60, by high-vocalizing pups was 68.20±3.18 and by low-vocalizing pups was 8.03±0.55.
Vehicle treated rat pups emitted a mean of 56.45 ultrasounds during the 1-min screening period and a mean of 169.8 ultrasounds during the 3-min test period. There was no significant difference between the ultrasonic vocalization from the screening period (multiplied by 3) and the ultrasonic vocalization from the test period in vehicle treated rat pups (P=0.51; Wilcoxon test).
Experiment 1
Flibanserin
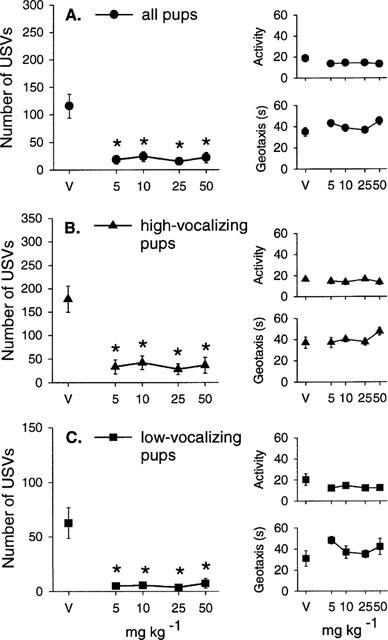
At the doses used in this experiment (5, 10, 25 and 50 mg kg−1), flibanserin had no effect on body temperature (Table 1A). Flibanserin suppressed the number of ultrasonic vocalizations (H(4)=20.56, P<0.001; Figure 1A) without affecting total activity (F(4, 62)=1.23, NS) or latency to show the geotaxic response (F(4, 62)=1.71, NS; Figure 1A). There was no effect of flibanserin on the frequency of rolling (H(4)=0.32, NS; Table 2A).
Table 1.
Pre- and post-treatment body temperature (mean °C±s.e.mean) of rat pups following vehicle (V) or drug treatment
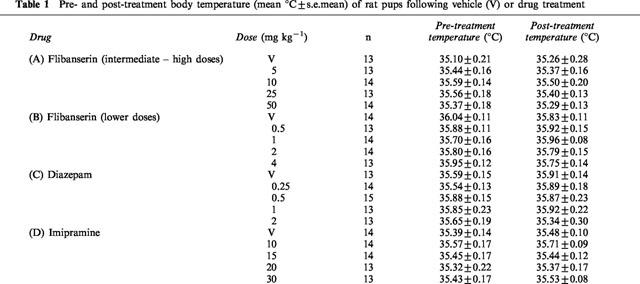
Figure 1.
Mean±s.e.mean number of ultrasonic vocalizations (USVs), total activity score (Activity) and the latency to show the geotaxic response in seconds (Geotaxis) in (A) all pups, (B) high-vocalizing pups and (C) low-vocalizing pups 30 min following flibanserin injections (5, 10, 25 and 50 mg kg−1). The number of animals (n) in each group is shown in Table 2A. *P<0.05 in comparison with vehicle-treated group (V).
Table 2.
Mean±s.e.mean frequency of rolling following drug treatment in rat pups
The effects of flibanserin on the behavioural measures were the same in both high-vocalizing (HV) and low-vocalizing (LV) pups. Thus, flibanserin at doses of 5, 10, 25 and 50 mg kg−1 decreased ultrasonic vocalizations in both HV (F(4, 27)=12.376, P<0.0001; Figure 1B) and LV rat pups (H(4)=11.53, P<0.01; Figure 1C) but had no effect on total locomotor activity (HV: F(4, 27)=0.41, NS; LV: F(4, 29)=1.08, NS) or on the latency to show the negative geotaxic response (HV: F(4, 27)=1.53, NS; LV: F(4, 29)=1.39, NS) in either group of pups (Figure 1B,C). Flibanserin had no significant effect on the frequency of rolling (HV: H(4)=0.97, NS; LV: H(4)=2.36, NS; Table 2A).
Experiment 2
Lower doses of flibanserin
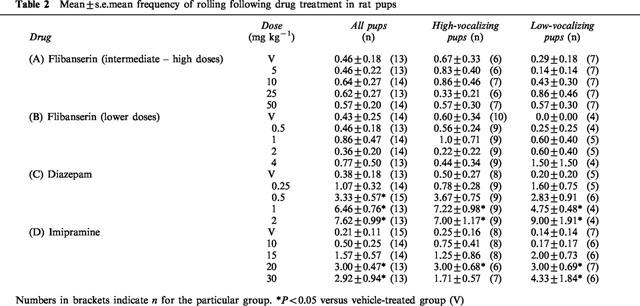
Over all pups, the lower doses of flibanserin (0.5, 1, 2 and 4 mg kg−1) had no significant effect on body temperature (Table 1B), ultrasonic vocalizations (√transformation: F(4, 63)=2.27, P=0.07; Figure 2A), total activity (F(4, 63)=0.49, NS; Figure 2A), the latency to show the geotaxic response (F(4, 63)=0.88, NS; Figure 2A) or the frequency of rolling (H(4)=0.53, NS; Table 2B).
Figure 2.
Mean±s.e.mean number of ultrasonic vocalizations (USVs), total activity score (Activity) and the latency to show the geotaxic response in seconds (Geotaxis) in (A) all pups, (B) high-vocalizing pups and (C) low-vocalizing pups 30 min following flibanserin injections (0.5, 1, 2 and 4 mg kg−1). The number of animals (n) in each group is shown in Table 2B. †P<0.05 in comparison with lowest dose of flibanserin (0.5 mg kg−1).
In HV pups, one-way ANOVA revealed a significant effect of flibanserin on the number of ultrasonic vocalizations (F(4, 41)=4.44, P<0.01; Figure 2B). However, post-hoc comparisons (Bonferroni) showed that the significant difference was found between the 1, 2 and 4 mg kg−1 versus 0.5 mg kg−1 but not versus the vehicle treated group (Figure 1B). There was no effect of flibanserin on ultrasonic vocalization in LV rat pups. The doses of flibanserin used in this experiment had no effect on total activity levels or the latency to show the geotaxic response in HV or LV pups (Figure 2B,C), nor did flibanserin affect the frequency of rolling (Table 2B).
Experiment 3
Diazepam
There was no effect of diazepam (0.25, 0.5, 1 and 2 mg kg−1) on body temperature in rat pups (Table 1C). Diazepam produced a dose-dependent decrease in the number of ultrasonic vocalizations (H(4)=33.92, P<0.0001; Figure 3A) but had no significant effect on total activity (F(4, 63)=0.38, NS; Figure 3A). Diazepam did, however, increase the latency to show the geotaxic response and this effect was evident at the lowest dose (0.25 mg kg−1) in all pups (H(4)=33.9, P<0.0001, Figure 3A). When individual locomotor behaviours were examined, diazepam had no significant effect on the frequency of line crossing (H(4)=6.14, NS) or rearing (H(4)=0.83, NS) but decreased the frequency of head raising (H(4)=17.78, P<0.0001). The three highest doses of diazepam also significantly increased the frequency of rolling (H(4)=45.61, P<0.0001; Table 2C).
Figure 3.
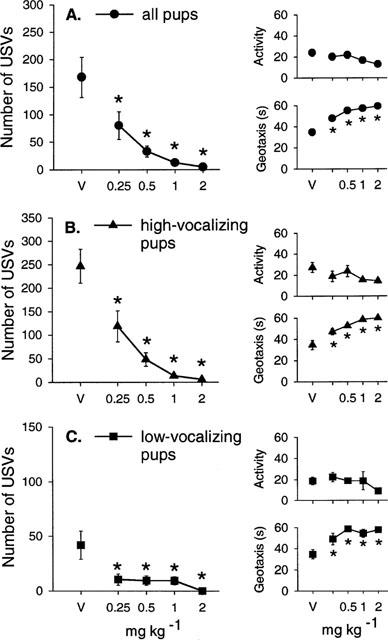
Mean±s.e.mean number of ultrasonic vocalizations (USVs), total activity score (Activity) and the latency to show the geotaxic response in seconds (Geotaxis) in (A) all pups, (B) high-vocalizing pups and (C) low-vocalizing pups 30 min following diazepam injections (0.25, 0.5, 1 and 2 mg kg−1). The number of animals (n) in each group is shown in Table 2C. *P<0.05 in comparison with vehicle-treated group (V).
In high-vocalizing (HV) rat pups, diazepam produced a dose-dependent decrease of USVs (H(4)=32.43, P<0.0001; Figure 3B), prolonged latency to show the geotaxic response (H(4)=24.9, P<0.0001, Figure 3B), and an increased frequency of rolling (H(4)=29.25, P<0.0001, Table 2C), with no change in total activity (F(4, 39)=1.72, NS; Figure 3B). In LV pups, diazepam also reduced the number of USVs emitted (F(4, 19)=5.06, P<0.01; Figure 3C) and increased the latency of the geotaxic response (F(4, 19)=7.38, P<0.001; Figure 3C) without altering total activity of pups (F(4, 19)=1.11, NS; Figure 3C). The frequency of rolling was also increased by the two highest doses of diazepam in LV pups (F(4, 19)=11.48, P<0.0001; Table 2C).
Experiment 4
Imipramine
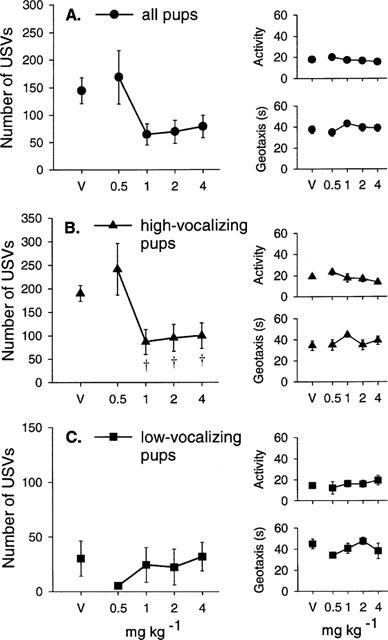
Imipramine had no effect on body temperature at any dose used (Table 1D). Imipramine decreased the number of USVs emitted (H(4)=20.4, P<0.001; Figure 4A) but increased the frequency of locomotor activity (F(4, 63)=4.63, P<0.01; Figure 4A) without affecting the latency to show the geotaxic response (F(4, 63)=0.68, NS; Figure 4A). In particular, imipramine increased the frequency of line crossing (F(4, 63)=4.41, P<0.01). At the two highest doses, imipramine also increased the frequency of rolling (H(4)=21.46, P<0.0001, Table 2D).
Figure 4.
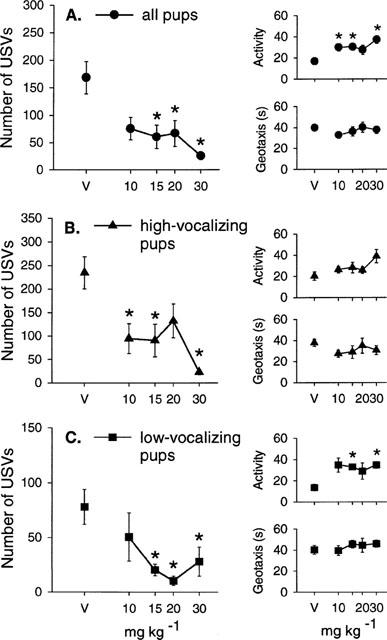
Mean±s.e.mean number of ultrasonic vocalizations (USVs), total activity score (Activity) and the latency to show the geotaxic response in seconds (Geotaxis) in (A) all pups, (B) high-vocalizing pups and (C) low-vocalizing pups 30 min following imipramine injections (10, 15, 20 and 30 mg kg−1). The number of animals (n) in each group is shown in Table 2D. *P<0.05 in comparison with vehicle-treated group (V).
In high-vocalizing (HV) pups, imipramine reduced the number of USVs (F(4, 32)=6.44, P<0.001; Figure 4B) without affecting the latency to show the geotaxic response (F(4, 32)=0.82, NS; Figure 4B). Although imipramine increased activity levels in HV pups, this effect did not reach significance (F(4, 32)=2.29, NS; Figure 4B). Imipramine (20 mg kg−1) increased the frequency of rolling (F(4, 32)=3.05, P<0.05; Table 2D) in HV pups. In low-vocalizing (LV) pups, imipramine decreased the number of USVs (F(4, 27)=5.26, P<0.01; Figure 4C), and increased total activity (H(4)=10.6, P<0.05; Figure 4C) with no significant effect on the latency to show the geotaxic response (F(4, 26)=0.32, NS; Figure 4C). The frequency of rolling was also increased in LV pups at the two highest doses of imipramine (H(4)=16.1, P<0.01; Table 2D).
Discussion
Psychotropic drugs that alleviate symptoms of anxiety and depression in humans suppress the emission of separation-induced ultrasonic vocalizations in rodent pups (Gardner 1985; Kramer et al., 1998; Olivier et al., 1998; Winslow & Insel, 1991). Concomitant recording of locomotor behaviour, the latency to show the geotaxic response and changes in body temperature provides information on the side effects of psychotropic drugs tested in this model. Our results show that intermediate to high doses of the novel 5-HT1A agonist/5-HT2A antagonist, flibanserin, reduced ultrasonic vocalizations in rat pups and that this effect was not due to changes in body temperature, and did not result in concomitant changes in activity or ataxia. The effect of flibanserin on the USVs was comparable to that of the clinically used anxiolytic, diazepam, and the antidepressant, imipramine. While diazepam and imipramine both produced motor impairment, flibanserin showed no apparent sedative, muscle relaxant or ataxic effects at any dose.
Flibanserin reduced ultrasonic vocalizations in an ‘all-or-none' way, presumably due to its action as a mixed 5-HT1A agonist/5-HT2A antagonist (Borsini et al., 1998). Both 5-HT1A agonists, such as buspirone, gepirone and ipsapirone, and 5-HT2 antagonists, such as ritanserin, show anxiolytic effects in clinical studies (Gardner, 1988; Taylor, 1990) but produce inconsistent effects in animal models of anxiety. For example, the 5-HT1A agonist buspirone showed anxiolytic-like effects in the T-maze (Graeff et al., 1998) and in the operant conflict paradigm (Kleven & Koek, 1996) but not in the elevated plus-maze (Cole & Rodgers, 1994; Wada & Fukuda, 1991). The 5-HT2 antagonists show either a weak anxiolytic effect in the elevated plus maze (Griebel et al., 1997b), are ineffective in the light/dark transition box and the Vogel punished drinking paradigm (Griebel et al., 1997a) or even anxiogenic in the infant rodent ultrasonic vocalization model of anxiety (Olivier et al., 1998). The mixed 5-HT1A agonist/5-HT2A antagonist FG5983 produced a potent anxiolytic-like effect in the separation-induced ultrasonic vocalization model of anxiety (Albinsson et al., 1994) which is in agreement with our results. Although we cannot rule out the possibility that the anxiolytic effect of mixed 5-HT1A agonists/5-HT2A antagonists is due to the predominant effect on 5-HT1A receptors, we hypothesize that the interaction between the two receptors plays a role in this phenomenon. With regard to our hypothesis, it is interesting that a low active dose of the 5-HT2 antagonist, ritanserin, enhanced the anticonflict effects of the 5-HT1A agonists, 8-OH-DPAT and buspirone in the operant conflict paradigm (Kleven & Koek, 1996). Moreover, it has been proposed that simultaneous targeting both 5-HT1A and 5-HT2A receptors produces fast and robust anxiolytic effect (Kunovac & Stahl, 1995).
The finding that diazepam dose-dependently decreased the number of USVs in rat pups confirmed previous findings from other studies using rat pups (Gardner, 1985; Klint & Andersson, 1994; Vivian et al., 1997), guinea-pig pups (Kramer et al., 1998) and mouse pups (Nastiti et al., 1991). Diazepam also increased the latency to show the negative geotaxic response and increased the frequency of rolling, demonstrating its well-documented muscle relaxant and ataxic properties (Gardner, 1985; Nastiti et al., 1991). We did not observe any sedation caused by diazepam at the doses used in our experiment, as spontaneous locomotion (line crossing) was not significantly decreased by up to 2 mg kg−1 diazepam. Our results are in agreement with those of Vivian et al. (1997) who found that up to 3 mg kg−1, diazepam had no effect on line crossing in rat pups. None of the doses of diazepam tested in our study altered armpit temperature of rat pups although some authors have reported decreased rectal temperature in rodent pups after diazepam treatment (Nastiti et al., 1991; Vivian et al., 1997). The different methods of measuring body temperature may explain this discrepancy. We used armpit temperature to eliminate the stress caused by measuring rectal temperature (van der Heyden et al., 1997).
Imipramine (10–30 mg kg−1), the non-selective serotonin and noradrenaline re-uptake inhibitor, reduced the number of USVs in 7–8-day-old rat pups. Olivier et al. (1998) found that imipramine reduced USVs in 9–11-day-old rat pups at low doses (1 and 3 mg kg−1) but not at a dose of 10 mg kg−1, which was the highest dose they used. Imipramine also decreased ultrasonic calls in guinea-pig pups (Kramer et al., 1998) and produced a trend toward decreased USVs in mouse pups (Benton & Nastiti, 1988). In contrast to other studies using adult rodents (de Angelis, 1996; Merlo Pich & Samanin, 1989; Mogensen et al., 1994), we found increased activity (line crossing) in rat pups after treatment with imipramine. At higher doses (20 and 30 mg kg−1), imipramine produced motor impairment as it increased the frequency of rolling, although not as much as diazepam. In agreement with our results, acute and chronic imipramine treatment has been shown to produce motor impairment as it reduced rearing behaviour in the open field (de Angelis 1996; Mogensen et al., 1994) and in the plus maze tests (Cole & Rodgers, 1995).
The variability between and within litters in the number of USVs emitted by rodent pups is a well-known phenomenon (Olivier et al., 1998; Vivian et al., 1997; Winslow et al., 1990). Some researchers overcome this problem by screening for USVs prior to experimental manipulations, and eliminate low-vocalizing pups from their studies (Podhorna & Brown, 1999; Vivian et al., 1997; Winslow et al., 1990). In our experiment, we compared the effects of drugs on behavioural measures in low- and high-vocalizing rat pups and found virtually no difference in the effects of drugs on the USVs in LV and HV rat pups. Dose-response curves for the number of USVs in LV and HV pups did not differ, although the mean number of USVs emitted was higher in HV pups than in LV pups. In general, the effects of drugs on locomotor behaviours and negative geotaxis in LV and HV rat pups did not differ, except for imipramine which increased the frequency of line crossing in LV pups but not in HV pups.
The effect of the 5-HT1A agonist/5-HT2A antagonist, flibanserin, in the rat pup ultrasonic vocalization model of anxiety supports the hypothesis that, in addition to its putative antidepressant effects (Borsini et al., 1997; Cesana et al., 1995; D'Aquila et al., 1997), flibanserin might also have anxiolytic effects. The anxiolytic effects of flibanserin are not restricted to the ultrasonic vocalization model as flibanserin also produced anxiolytic effects in the light/dark transition and stress-induced hyperthermia models of anxiety (Borsini et al., 1999). Our results confirm the findings of other investigators (Borsini et al., 1997; 1998; 1999; Cesana et al., 1995) that flibanserin is effective within 30 min and has no severe locomotor side effects at active doses. Since FG5983, another mixed 5-HT1A agonist/5-HT2A antagonist, has also shown potent anxiolytic-like effects in rats (Albinsson et al., 1994), it appears that drugs with mixed 5-HT1A agonistic/5-HT2A antagonistic actions might be effective for the treatment of anxiety disorders.
Acknowledgments
This research was supported by an NSERC of Canada research grant to Richard E. Brown. We would like to thank Dr M. Turconi (Boehringer Ingelheim, Italy) for the gift of flibanserin and Lesley Newhook for helping to test rat pups.
Abbreviations
- HV
high-vocalizing pups
- LV
low-vocalizing pups
- USV
ultrasonic vocalizations
References
- ALBINSSON A., BJORK A., SVARTENGREN J., KLINT T. , ANDERSSON G. Preclinical pharmacology of FG5893: a potential anxiolytic drug with high affinity for both 5-HT1A and 5-HT2A receptors. Eur. J. Pharmacol. 1994;261:285–294. doi: 10.1016/0014-2999(94)90119-8. [DOI] [PubMed] [Google Scholar]
- BENTON D. , NASTITI K. The influence of psychotropic drugs on the ultrasonic calling of mouse pups. Psychopharmacol. 1988;95:99–102. doi: 10.1007/BF00212775. [DOI] [PubMed] [Google Scholar]
- BORSINI F., BRAMBILLA A., CECI A., CESANA R., GIRALDO E., LADINSKY H., MONFERINI E. , TURCONI M. Flibanserin. Drugs Fut. 1998;23:9–16. [Google Scholar]
- BORSINI F., BRAMBILLA A., GRIPPA N. , PITSIKAS N. Behavioural effects of flibanserin (BIMT 17) Pharmacol. Biochem. Behav. 1999;64:137–146. doi: 10.1016/s0091-3057(99)00072-6. [DOI] [PubMed] [Google Scholar]
- BORSINI F., CESANA R., KELLY J., LEONARD B.E., MCNAMARA M., RICHARDS J. , SEIDEN L. BIMT 17: a putative antidepressant with a fast onset of action. Psychopharmacol. 1997;134:378–386. doi: 10.1007/s002130050474. [DOI] [PubMed] [Google Scholar]
- CASACALENDA N. , BOULENGER J.P. Pharmacologic treatments effective in both generalized anxiety disorder and major depressive disorder: clinical and theoretical implications. Can. J. Psychiatry. 1998;43:722–730. doi: 10.1177/070674379804300707. [DOI] [PubMed] [Google Scholar]
- CESANA R., CIPRANDI C. , BORSINI F. The effect of BIMT 17, a new potential antidepressant, in the forced swimming test in mice. Behav. Pharmac. 1995;6:688–694. [PubMed] [Google Scholar]
- COLE J.C. , RODGERS R.J. Ethological evaluation of the effects of acute and chronic buspirone treatment in the murine elevated plus-maze test: comparison with haloperidol. Psychopharmacol. 1994;114:288–296. doi: 10.1007/BF02244851. [DOI] [PubMed] [Google Scholar]
- COLE J.C. , RODGERS R.J. Ethological comparison of the effects of diazepam and acute/chronic imipramine on the behaviour of mice in the elevated plus-maze. Pharmacol. Biochem. Behav. 1995;52:473–478. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- D'AQUILA P., MONLEON S., BORSINI F., BRAIN P.F. , WILLNER P. Anti-anhedonic actions of the novel serotonergic agent flibanserin, a potential rapidly-acting antidepressant. Eur. J. Pharmacol. 1997;340:121–132. doi: 10.1016/s0014-2999(97)01412-x. [DOI] [PubMed] [Google Scholar]
- DASTUR F.N., MCGREGOR I.S. , BROWN R.E. Dopaminergic modulation of rat pup ultrasonic vocalizations. Eur. J. Pharmacol. 1999;382:53–67. doi: 10.1016/s0014-2999(99)00590-7. [DOI] [PubMed] [Google Scholar]
- DE ANGELIS L. Experimental anxiety and antidepressant drugs: the effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn Schmiedebergs Arch. Pharmacol. 1996;354:379–383. doi: 10.1007/BF00171072. [DOI] [PubMed] [Google Scholar]
- FEIGHNER J.P. , BOYER W.F. Serotonin-1A anxiolytics: an overview. Psychopathol. 1989;22 Suppl. 1:21–26. doi: 10.1159/000284623. [DOI] [PubMed] [Google Scholar]
- GARDNER C.R. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J. Pharmacol. Meth. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- GARDNER C.R. Potential use of drugs modulating 5HT activity in the treatment of anxiety. Gen. Pharmacol. 1988;19:347–356. doi: 10.1016/0306-3623(88)90027-4. [DOI] [PubMed] [Google Scholar]
- GLANTZ S.A. Primer of Biostatistics. New York: McGraw Hill; 1992. [Google Scholar]
- GLENNON R.A. Serotonin receptors: Clinical implications. Neurosci. Biobehav. Rev. 1990;14:35–47. doi: 10.1016/s0149-7634(05)80158-7. [DOI] [PubMed] [Google Scholar]
- GOA K.L. , WARD A. Buspirone: A preliminary review of its pharmacological properties and therapeutic efficacy as an anxiolytic. Drugs. 1986;32:114–129. doi: 10.2165/00003495-198632020-00002. [DOI] [PubMed] [Google Scholar]
- GRAEFF F.G., NETTO C.F. , ZANGROSSI H., Jr The elevated T-maze as an experimental model of anxiety. Neurosci. Biobehav. Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- GRIEBEL G., PERRAULT G. , SANGER D.J. A comparative study of the effects of selective and non-selective 5-HT2 receptor subtype antagonists in rat and mouse models of anxiety. Neuropharmacol. 1997a;36:793–802. doi: 10.1016/s0028-3908(97)00034-8. [DOI] [PubMed] [Google Scholar]
- GRIEBEL G., RODGERS R.J., PERRAULT G. , SANGER D.J. Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol. Biochem. Behav. 1997b;57:817–827. doi: 10.1016/s0091-3057(96)00402-9. [DOI] [PubMed] [Google Scholar]
- HARRISON G.E. , HOLMAN S.D. A device to enable the automatic recording of rodent ultrasonic vocalizations. Behav. Res. Meth. Instr. 1978;10:689–692. [Google Scholar]
- KLEVEN M.S. , KOEK W. Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT2A/2C antagonist anxiolytics. I. Antipunishment effects in the pigeon. J. Pharmacol. Exp. Ther. 1996;276:388–397. [PubMed] [Google Scholar]
- KLINT T. , ANDERSSON G. Ultrasound vocalization is not related to corticosterone response in isolated rat pups. Pharmacol. Biochem. Behav. 1994;47:947–950. doi: 10.1016/0091-3057(94)90301-8. [DOI] [PubMed] [Google Scholar]
- KRAMER M.S., CUTLER N., FEIGHNER J., SHRIVASTAVA R., CARMAN J., SRAMEK J.J., REINES S.A., LIU G., SNAVELY D., WYATT KNOWLES E., HALE J.J., MILLS S.G., MACCOSS M., SWAIN C.J., HARRISON T., HILL R.G., HEFTI F., SCOLNICK E.M., CASCIERI M.A., CHICCHI G.G., SADOWSKI S., WILLIAMS A.R., HEWSON L., SMITH D. , RUPNIAK N.M. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- KUNOVAC J.L. , STAHL S.M. Future directions in anxiolytic pharmacotherapy. Psychiatr. Clin. North Am. 1995;18:895–909. [PubMed] [Google Scholar]
- MCGREGOR I.S. Using Strawberry Tree WorkbenchMac and Workbench PC software for data acquisition and control in the animal learning laboratory. Behav. Res. Meth. Instr. Comp. 1996;28:38. [Google Scholar]
- MERLO PICH E. , SAMANIN R. A two-compartment exploratory model to study anxiolytic/anxiogenic effects of drugs in the rat. Pharmacol. Res. 1989;21:595–602. doi: 10.1016/1043-6618(89)90201-6. [DOI] [PubMed] [Google Scholar]
- MOGENSEN J., PEDERSEN T.K. , HOLM S. Effects of chronic imipramine on exploration, locomotion, and food/water intake in rats. Pharmacol. Biochem. Behav. 1994;47:427–435. doi: 10.1016/0091-3057(94)90139-2. [DOI] [PubMed] [Google Scholar]
- MOSCONI M., CHIAMULERA C. , RECCHIA G. New anxiolytics in development. Int. J. Clin. Pharm. Res. 1993;13:331–344. [PubMed] [Google Scholar]
- NASTITI K., BENTON D. , BRAIN P.F. The effects of compounds acting at the benzodiazepine receptor complex on the ultrasonic calling of mouse pups. Behav. Pharmac. 1991;2:121–128. [PubMed] [Google Scholar]
- OKON E.E. The effect of environmental temperature on the production of ultrasounds by isolated non-handled albino mouse pups. J. Zool. 1970;162:71–83. [Google Scholar]
- OLIVIER B., MOLEWIJK E., VAN DER HEYDEN J., VAN OORSCHOT R., RONKEN E., MOS J. , MICZEK K.A. Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci. Biobehav. Rev. 1998;23:215–227. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- OLIVIER B., MOLEWIJK E., VAN OORSCHOT R., VAN DER POEL G., ZETHOF T., VAN DER HEYDEN J. , MOS J. New animal models of anxiety. Eur. Neuropsychopharmacol. 1994;4:93–102. doi: 10.1016/0924-977x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- PODHORNA J. , BROWN R.E. Inhibition of nitric oxide synthase reduces ultrasonic vocalizations of rat pups. Eur. J. Pharmacol. 1999;382:143–150. doi: 10.1016/s0014-2999(99)00595-6. [DOI] [PubMed] [Google Scholar]
- RICKELS K. Buspirone in clinical practice. J. Clin. Psychiatry. 1990;51 Suppl.:51–54. [PubMed] [Google Scholar]
- SCHREIBER R., MELON C. , DE VRY J. The role of 5-HT receptor subtypes in the anxiolytic effects of selective serotonin reuptake inhibitors in the rat ultrasonic vocalization test. Psychopharmacol. 1998;135:383–391. doi: 10.1007/s002130050526. [DOI] [PubMed] [Google Scholar]
- STAHL S.M. Antidepressants: the blue-chip psychotropic for the modern treatment of anxiety disorders. J. Clin. Psychiatry. 1999;60:356–358. doi: 10.4088/jcp.v60n0601. [DOI] [PubMed] [Google Scholar]
- TAYLOR D.P. Serotonin agents in anxiety. Ann. N. Y. Acad. Sci. 1990;600:545–557. doi: 10.1111/j.1749-6632.1990.tb16909.x. [DOI] [PubMed] [Google Scholar]
- VAN DER HEYDEN J.A., ZETHOF T.J. , OLIVIER B. Stress-induced hyperthermia in singly housed mice. Physiol. Behav. 1997;62:463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- VIVIAN J.A., BARROS H.M., MANITIU A. , MICZEK K.A. Ultrasonic vocalizations in rat pups: modulation at the gamma-aminobutyric acidA receptor complex and the neurosteroid recognition site. J. Pharmac. Exp. Ther. 1997;282:318–325. [PubMed] [Google Scholar]
- WADA T. , FUKUDA N. Effects of DN-2327, a new anxiolytic, diazepam and buspirone on exploratory activity of the rat in an elevated plus-maze. Psychopharmacol. 1991;104:444–450. doi: 10.1007/BF02245647. [DOI] [PubMed] [Google Scholar]
- WINSLOW J.T. , INSEL T.R. Serotonergic and catecholaminergic reuptake inhibitors have opposite effects on the ultrasonic isolation calls of rat pups. Neuropsychopharmacol. 1990;3:51–59. [PubMed] [Google Scholar]
- WINSLOW J.T. , INSEL T.R. The infant rat separation paradigm: a novel test for novel anxiolytics. TiPS. 1991;12:402–404. doi: 10.1016/0165-6147(91)90616-z. [DOI] [PubMed] [Google Scholar]
- WINSLOW J.T., INSEL T.R., TRULLAS R. , SKOLNICK P. Rat pup isolation calls are reduced by functional antagonists of the NMDA receptor complex. Eur. J. Pharmacol. 1990;190:11–21. doi: 10.1016/0014-2999(90)94107-9. [DOI] [PubMed] [Google Scholar]
- WOODS J.H., KATZ J.L. , WINGER G. Abuse liability of benzodiazepines. Pharmacol. Rev. 1987;39:251–413. [PubMed] [Google Scholar]
- WOODS J.H., KATZ J.L. , WINGER G.Abuse and therapeutic use of benzodiazepines and benzodiazepine-like drugs Psychopharmacology: The Fourth Generation of Progress 1995New York: Raven Press; 1777–1791.ed. Bloom, F.E. & Kupfer, D.J. pp [Google Scholar]


