Abstract
The smooth muscle relaxant responses to the mixed β3-, putative β4-adrenoceptor agonist, (−)-CGP 12177 in rat colon are partially resistant to blockade by the β3-adrenoceptor antagonist SR59230A suggesting involvement of β3- and putative β4-adrenoceptors. We now investigated the function of the putative β4-adrenoceptor and other β-adrenoceptor subtypes in the colon, oesophagus and ureter of wildtype (WT) and β3-adrenoceptor knockout (β3KO) mice.
(−)-Noradrenaline and (−)-adrenaline relaxed KCl (30 mM)-precontracted colon mostly through β1-and β3-adrenoceptors to a similar extent and to a minor extent through β2-adrenoceptors. In colon from β3KO mice, (−)-noradrenaline was as potent as in WT mice but the effects were mediated entirely through β1-adrenoceptors. (−)-CGP 12177 relaxed colon from β3KO mice with 2 fold greater potency than in WT mice. The maintenance of potency for (−)-noradrenaline and increase for (−)-CGP 12177 indicate compensatory increases in β1- and putative β4-adrenoceptor function in β3KO mice.
In oesophagi precontracted with 1 μM carbachol, (−)-noradrenaline caused relaxation mainly through β1-and β3-adrenoceptors. (−)-CGP 12177 (2 μM) relaxed oesophagi from WT by 61.4±5.1% and β3KO by 67.3±10.1% of the (−)-isoprenaline-evoked relaxation, consistent with mediation through putative β4-adrenoceptors.
In ureter, (−)-CGP 12177 (2 μM) reduced pacemaker activity by 31.1±2.3% in WT and 31.3±7.5% in β3KO, consistent with mediation through putative β4-adrenoceptors.
Relaxation of mouse colon and oesophagus by catecholamines are mediated through β1- and β3-adrenoceptors in WT. The putative β4-adrenoceptor, which presumably is an atypical state of the β1-adrenoceptor, mediates the effects of (−)-CGP 12177 in colon, oesophagus and ureter.
Keywords: Putative β4-adrenoceptor, β1- and β2-adrenoceptors, β3-adrenoceptor knockout mouse, colon, oesophagus, ureter, (−)-CGP 12177, (−)-[3H]-CGP 12177, (−)-noradrenaline, (−)-adrenaline
Introduction
β3-Adrenoceptors mediate relaxation of gastrointestinal smooth muscle (reviewed by Arch & Kaumann, 1993). In rat colon, the endogenous catecholamine, (−)-noradrenaline, selective β3-adrenoceptor agonists as well as (−)-CGP 12177 and (±)-cyanopindolol caused relaxant responses which were resistant to blockade by the β1- and β2-adrenoceptor antagonist (−)-propranolol (Kaumann & Molenaar, 1996). Both (−)-CGP 12177 and (±)-cyanopindolol were partial agonists which blocked the relaxant effects of the β3-adrenoceptor selective agonists and therefore appeared to cause their effects through a common receptor. We used the selective β3-adrenoceptor antagonist SR 59230A (Manara et al., 1995) and showed that it competitively blocked the effects of (−)-noradrenaline, β3-selective agonists and (−)-CGP 12177 (Kaumann & Molenaar, 1996). However, SR 59230A blocked the relaxant responses of (−)-CGP 12177 considerably less than those to (−)-noradrenaline and β3-selective agonists, suggesting involvement of a receptor distinct from β1-, β2- and β3-adrenoceptors, conceivably the putative β4-adrenoceptor. The pharmacology of the putative β4-adrenoceptor has been described in the heart of several species (Kaumann 1989; 1997; Lowe et al., 1998; 1999), including man (Kaumann 1996; Kaumann & Molenaar, 1997; Molenaar et al., 1997b) and mouse (Kaumann et al., 1998), and in murine and human adipocytes (Galitzky et al., 1997; Preitner et al., 1998). In cardiac muscle, stimulant effects are observed to a series of compounds described as non-conventional partial agonists which include (−)-CGP 12177. Non-conventional partial agonists cause blockade of responses mediated by stimulation of β1- and β2-adrenoceptors but higher concentrations cause stimulant effects which are resistant to blockade by antagonists such as (−)-propranolol, but can be blocked with moderate affinity by (−)-bupranolol. In this respect the pharmacology of the putative β4-adrenoceptor is similar to that of the β3-adrenoceptor, but the receptors are distinct because putative β4-adrenoceptor function is preserved in atrium (Kaumann et al., 1998) and fat cells of mice (Preitner et al., 1998) with targeted disruption of the β3-adrenoceptor gene.
The purpose of this study was to investigate the role of putative β4-adrenoceptors in smooth muscle and compare it to the function of β1-, β2- and β3-adrenoceptors. We used the mouse colon, oesophagus and ureter for which the relative role of β-adrenoceptor subtypes is unknown. β3-adrenoceptors and to a lesser extent β2-adrenoceptors mediate relaxation of rat oesophagus (de Boer et al., 1993). In canine ureter, CGP 12177 caused relaxation, an effect attributed to stimulation of β3-adrenoceptors (Tomiyama et al., 1998), however the contribution of the putative β4-adrenoceptor to CGP 12177 mediated relaxation in canine ureter was not investigated.
β1- and β3-adrenoceptors were activated with selective agonists and antagonists. To identify functional putative β4-adrenoceptors we used (−)-CGP 12177 but it can also stimulate β3-adrenoceptors (Kaumann & Molenaar, 1996; Molenaar et al., 1997a; Sennitt et al., 1998). To provide unambiguous evidence for the existence of a (−)-CGP 12177 activated receptor that is distinct from the β3-adrenoceptor, genetically engineered mice which had targeted disruption of the β3-adrenoceptor were used. This approach was previously used to show that the receptor activated by (−)-CGP 12177 (previously called putative β4-adrenoceptor) was pharmacologically distinct from the β3-adrenoceptor and remained functional in cardiac (Kaumann et al., 1998) and brown adipose tissues (Preitner et al., 1998).
Our results show that (−)-noradrenaline-evoked relaxation of mouse colon and oesophagus is mediated through β1- and β3-adrenoceptors. In the colon from β3KO mice, β1-adrenoceptors compensate for the lack of β3-adrenoceptors by mediating increased relaxation. Relaxation of colon, oesophagus and reduction of ureter pacemaker activity by (−)-CGP 12177 is mediated through putative β4-adrenoceptors.
Methods
129 Sv mice (male, 2–4 months) were obtained from the Animal Resources Centre, Canning Vale, Western Australia. Balb/c mice (either sex, 7–10 weeks) were bred at the Babraham Institute. 129 Sv×C57BL/6 wildtype (WT) and β3KO mice were off-springs of the previously described founders (Revelli et al., 1997). 4–5 month old female homozygous WT and β3KO mice were used.
Southern blot genotyping
The identification of the WT or homozygous β3KO mice was performed as previously described (Revelli et al., 1997; Kaumann et al., 1998).
Northern blots
Total RNA was isolated from colon fragments using RNeasy Mini Kit (QIAGEN, Basel, Switzerland). Twelve μg of total RNA was electrophoresed on a 1% agarose gel containing formaldehyde according to standard protocols and transferred to Electran Nylon blotting membranes (BDH Laboratory Supplies, Poole, U.K.) by vacuum blotting. Hybridizations were performed with radiolabelled probes using Quikhyb™ hybridization solution (Stratagene, La Jolla, CA, U.S.A.) as previously described (Revelli et al., 1997). The β1-adrenoceptor probe (size: 756 bp, between positions 297 and 1053 on the mouse sequence GenBank Accession L10084) was obtained by reverse transcription-polymerase chain reaction (RT-PCR) on mouse heart polyA+ RNA. The β2-adrenoceptor probe (size: 488 bp, between positions 641 and 1129 on the rat sequence GenBank Accession J03024) was obtained by polymerase chain reaction (PCR) on a rat β2-adrenoceptor cDNA. RNA size estimates for the RNA species were established by comparison with an RNA ladder (Gibco, BRL). Films were exposed for 3–4 days at −80°C. RNA levels were quantified by scanning photodensitometry of the autoradiograms. Subsequent hybridization of the blots with a γ−32P-ATP labelled synthetic oligonucleotide specific for the 18S rRNA subunit was used to correct for the differences in the amounts of RNA loaded onto the gels.
Longitudinal muscle of the colon
A partially depolarized colon preparation (30 mM K+) essentially as described previously (Kaumann & Molenaar, 1996) was used. Sv 129, WT and β3KO mice (either sex, aged 6–12 weeks) were killed either by dislocation of the neck or rapid exsanguination from the carotid arteries. One ‘whole' 2.5 cm (approximately) segment of colon was dissected free and placed immediately into continuously oxygenated (95% O2/5% CO2) modified Krebs' solution (mM): Na+ 125, K+ 5, Ca2+ 2.25, Mg2+ 0.5, Cl− 98.5, SO42− 0.5, HCO3− 32, HPO42− 1, EDTA 0.04, ascorbic acid 0.2 at room temperature. Tissues were mounted longitudinally in pairs in 50 ml organ baths, with care taken not to occlude the lumen, attached to Swema 4–45 strain gauge transducers and given 10 mN force. The incubation medium was exchanged with modified Krebs solution containing in addition (mM): Na+ 15, fumarate 5, pyruvate 5, L-glutamate 5 and glucose 10, together with 30 μM corticosterone (to block extraneuronal uptake of amines), 3 μM cocaine (to block neuronal uptake of amines) and 1 μM phentolamine (to block α-adrenoceptors) at 37°C. Tissues were allowed to equilibrate for 30 min, some in the presence of β-adrenoceptor antagonists before the addition of 30 mM KCl. Tone was maintained by KCl for 30 min, the tissues washed twice and allowed to equilibrate for a further 60 min before the re-addition of KCl. Antagonists were re-added after each wash. Agonists were added, either cumulatively in half log increments or as a bolus at maximally effective concentration. To terminate the experiment a maximally effective concentration of (−)-isoprenaline (200–600 μM) was added. Relaxant responses were expressed as a percentage of the effect of (−)-isoprenaline.
Longitudinal muscle of the oesophagus
Mice (Balb/c, WT, β3KO) were killed by dislocation of the neck (Balb/c) in accordance with Home Office (U.K.) procedures or rapid exsanguination (WT, β3KO) and the oesophagus immediately taken out and placed in equilibrated oxygenated (95% O2/5% CO2) solution at room temperature containing (mM): Na+ 146, K+ 5.2, Ca2+ 1.8, Mg2+ 1, Cl− 122.6, HCO3− 25, H2PO42− 1.2, glucose 10, pyruvate 2, EDTA 0.04 and ascorbic acid 0.2. Oesophagi were cut open longitudinally and set up in pairs at 37°C in a 50 ml organ bath containing the above solution. Tissues were attached to Swema 4–45 strain gauge transducers and force was recorded on a 12 channel Watanabe polygraph. Tissues had slight spontaneous activity and were stretched to 2 mN force. Tissues were incubated with 3 μM cocaine, 10 μM corticosterone and 1 μM phentolamine.
Before commencement of experiments, strips were washed once and allowed to equilibrate for 30 min. After obtaining a steady state contraction to carbachol (1 μM), cumulative concentration-relaxation curves to (−)-noradrenaline, CL 316,243 and (−)-CGP 12177 were constructed. Antagonists were present for 60 min (30 min before and 30 min during stabilization of the carbachol response) prior to the beginning of each curve. At the end of each concentration-effect curve (±)-isoprenaline (600 μM) was added to obtain maximal relaxation.
Concentration-effect curves to (−)-noradrenaline were constructed in the absence or presence of ICI 118,551 (fold selectivity: 125 β2 vs β1, Bilski et al., 1983; >6000 β2 vs β3, putative β4, Kaumann & Molenaar, 1996); CGP 20712A (fold selectivity: 16,000 β1 vs β2, Lemoine & Kaumann, 1991; >12,000 β1 vs β3, Kaumann & Molenaar, 1996; 1600 β1 vs putative β4, Kaumann & Molenaar, 1996); SR 59230A (fold selectivity: 125 β3 vs putative β4, Kaumann & Molenaar 1996) and (−)-propranolol (fold selectivity: >100 β1, β2 vs β3, Kaumann & Molenaar 1996; >600 β1, β2 vs putative β4, Kaumann & Molenaar 1996) either in combination or alone.
Curves to CL 316,243 and (−)-CGP 12177 were all constructed in the presence of 200 nM (−)-propranolol in the absence or presence of 10 μM SR 59230A. In some experiments carbachol was omitted and (−)-CGP 12177 added as a bolus concentration (2 μM).
Concentration-effect curves of CL 316,243 and (−)-CGP 12177 were expressed as a percentage reduction of the (1 μM) carbachol-induced contraction. Curves to (−)-noradrenaline were expressed as absolute values in mN relaxation to clearly show differences in the extent of contraction caused by carbachol and to show the extent of relaxation.
Ureter
β3KO and WT mice were rapidly exsanguinated as above, the left and right ureters excised and placed in oxygenated modified Krebs solution (composition as for colon experiments) at room temperature. Two preparations (2 mm in length) from the middle portion of each ureter were mounted on 40 μm stainless steel wires in Mulvany-style small vessel myographs (J.P. Trading, Aarhus, Denmark) for isometric recording of circular smooth muscle activity. The incubation medium was exchanged with modified Krebs solution containing in addition (mM): Na+ 15, fumarate 5, pyruvate 5, L-glutamate 5 and glucose 10 together with 30 μM corticosterone, 3 μM cocaine, 1 μM phentolamine and 1 μM prazosin. After a 30 min equilibration period at 37°C, the preparations were stretched to a passive force of 2.5 mN and allowed to equilibrate for a further 30 min. During this time baseline tension gradually stabilized at approximately 1 mN. Spontaneous beating was initiated in these otherwise quiescent preparations by increasing the KCl concentration to 25 mM in the modified Krebs solution and by adding the thromboxane A2 mimetic U46619 (100–300 nM). After beating commenced, preparations were allowed to stabilize for 10 min before agonists were applied. In other experiments using tissues from Balb/c mice, time control experiments demonstrated that these preparations maintained a steady rate of beating for up to 40 min.
Statistics and calculations
All data are expressed as mean±standard error. Differences between groups were assessed and tested for significance with Student's t-test at P⩽0.05.
The concentration ratio (CR) caused by an antagonist B, was determined by use of log forms, pD2 - pD2,B where (−log EC50=pD2, −log EC50 in the presence of antagonist=pD2,B).
The error of log CR was calculated from (s.e.mean2pD2+s.e.mean2pD2,B)1/2 (Kaumann, 1990).
The blockade by SR 59230A of the effects of CL 316,243 was analysed with a Schild-plot (Arunlakshana & Schild, 1959).
Drugs used
(−)-CGP 12177 was a gift from Dr Jonathan Arch (Smith Kline Beecham Pharmaceuticals, Harlow, Essex, U.K.), (−)-bupranolol was a gift from Dr Klaus Sandrock (Sanol-Schwarz, Monheim, Germany), SR 59230A was a gift from Dr Luciano Manara (Sanofi, Milan, Italy), CL 316,243 was a gift from Dr Kurt Steiner, (Wyeth-Ayerst Research Princeton, NJ, U.S.A.), ICI 118,551 from Zeneca (Wilmslow, Cheshire, U.K.), CGP 20712A from Novartis (Basel, Switzerland), U46619 from Sapphire Bioscience (Sydney, Australia), (−)-propranolol hydrochloride; (−)-isoprenaline bitartrate, (±)-isoprenaline bitartrate, corticosterone, phentolamine methanesulphonate, carbachol (carbamylcholine chloride) from Sigma (St Louis, MO, U.S.A.), cocaine HCl from SmithKline Beecham Pharmaceuticals (Harlow, U.K.) or Victorian Hospitals Association (Mulgrave, Australia), prazosin HCl from RBI (Nattick, Massachusetts, U.S.A.), (−)-noradrenaline bitartrate from RBI (Nattick, Massachusetts, U.S.A.) or Sigma (St Louis, MO, U.S.A.).
Results
Contribution of β1-, β2-, β3- and putative β4-adrenoceptors to relaxation of mouse (SV 129) colon
(−)-Noradrenaline caused concentration-dependent relaxation of the longitudinal muscle of 30 mM KCl-precontracted mouse colon (Figure 1a,b, Table 1). The effects were blocked by the β1-adrenoceptor-selective antagonist CGP 20712A (300 nM) but not by the β2-adrenoceptor-selective antagonist ICI 118,551 (50 nM, P=0.07) (Figure 1a, Table 1). However, the shift of the (−)-noradrenaline concentration-effect curve by CGP 20712A was 2-log units less than expected from its corresponding affinity for β1-adrenoceptors (Table 1). The simultaneous addition of CGP 20712A and ICI 118,551 did not cause significantly greater blockade than CGP 20712A alone. (−)-Propranolol (200 nM) caused similar blockade to CGP 20712A but again it was less than expected from its affinity for β1- and β2-adrenoceptors. The selective β3-adrenoceptor antagonist SR 59230A at a concentration of 3 μM caused a 1.0-log unit rightward shift of the concentration-effect curve to (−)-noradrenaline (Figure 1b, Table 1). When combined with 200 nM (−)-propranolol to block β1- and β2-adrenoceptors, 3 μM SR 59230A caused an additional rightward shift of the concentration-effect curve (Figure 1b, Table 1).
Figure 1.
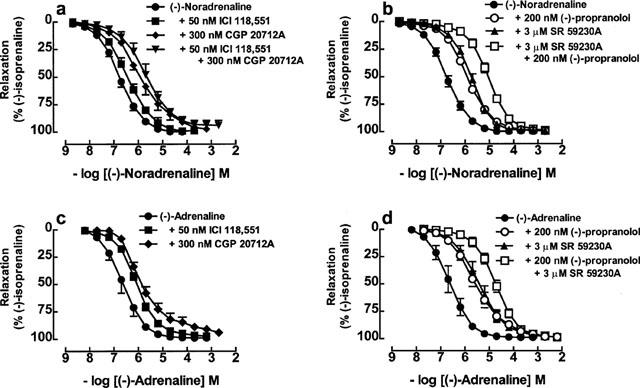
Relaxation of 129 Sv mouse colon by (−)-noradrenaline (a,b) and (−)-adrenaline (c,d) in the absence or presence of 50 nM ICI 118,551, 300 nM CGP 20712A, 50 nM ICI 118,551+300 nM CGP 20712A, 3 μM SR 59230A, 200 nM (−)-propranolol or 3 μM SR 59230A+200 nM (−)-propranolol. Values shown are mean±s.e.mean (vertical lines, where larger than symbol size), n=4–6 tissues for each curve.
Table 1.
Effects of antagonists on the relaxant effects of (−)-noradrenaline, (−)-adrenaline, CL 316,243 and (−)-CGP 12177 in colon of 129 Sv mice
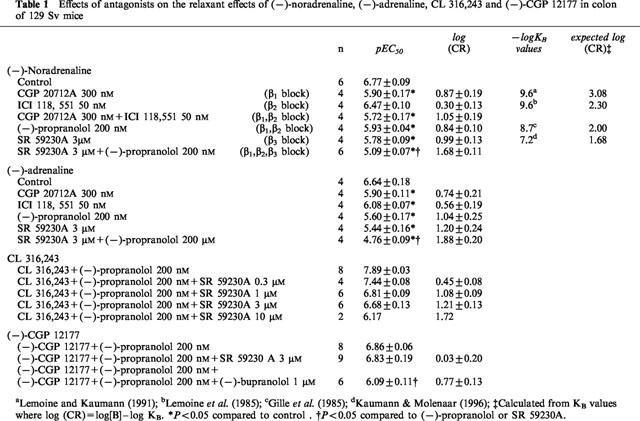
(−)-Adrenaline caused concentration dependent relaxant responses in colon (Figure 1c,d, Table 1). As for (−)-noradrenaline, the relaxant effects of (−)-adrenaline were partially resistant to blockade with CGP 20712A (Figure 1c, Table 1). ICI 118,551 (50 nM) caused a greater rightward shift of the concentration effect curve to (−)-adrenaline (0.56-log units, P=0.03) than to (−)-noradrenaline (0.30-log units, not significant). (−)-Propranolol at a concentration of 200 nM and SR 59230A (3 μM) each caused 1-log unit rightward shifts of the concentration-effect curves to (−)-adrenaline (Figure 1d, Table 1). The combination of (−)-propranolol and SR 59230A caused a further shift to the right (Figure 1d, Table 1).
The presence of the β3-adrenoceptor in colon was confirmed with the selective β3-adrenoceptor agonist CL 316,243 which was a partial agonist with respect to (−)-isoprenaline. In the presence of 200 nM (−)-propranolol, the concentration-effect curve to CL 316,243 was shifted to the right in a concentration dependent manner by SR 59230A (Figure 2a, Table 1). The pKB value for SR 59230A, (determined from the Schild plot with slope constrained to 1), was 6.81 (Figure 2b).
Figure 2.
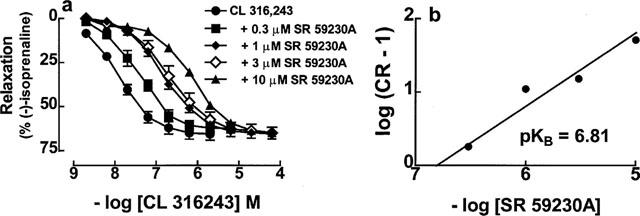
Relaxant responses in 129 Sv mouse colon to CL 316,243 and blockade by SR 59230A. Responses are to CL 316,243 in the absence or presence of SR 59230A. All experiments were carried out in the presence of 200 nM (−)-propranolol. Values shown are mean±s.e.mean (vertical lines, where larger than symbol size for experiments with n⩾4), n=2–8 tissues for each curve. The corresponding Schild-plot is shown in (b). The slope of the Schild-plot was not significantly different from 1.0 (slope 0.90±0.16) and was therefore constrained to unity.
In contrast, 3.3 μM SR 59230A did not cause a rightward shift of the concentration-response curve to (−)-CGP 12177 in the presence of 200 nM (−)-propranolol. (−)-Bupranolol (1 μM) however, caused an 0.77-log unit rightward shift (Figure 3, Table 1).
Figure 3.
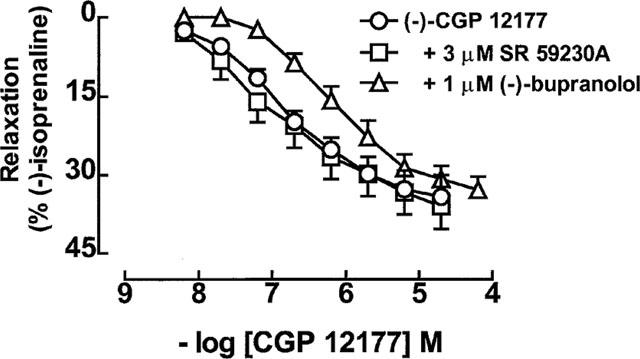
Relaxant responses in 129 Sv mouse colon of (−)-CGP 12177 in the absence or presence of 3 μM SR 59230A or 1 μM (−)-bupranolol. All experiments were carried out in the presence of 200 nM (−)-propranolol. Values shown are mean±s.e.mean (vertical lines, where larger than symbol size), n=6–9 tissues for each curve.
Contribution of β1-, β2- and putative β4-adrenoceptors to relaxation in β3KO mouse colon
There was no difference in the 30 mM KCl induced increase in tone in colon from WT (6.9±0.3 mN, n=156) and β3KO mice (6.0±0.6 mN, n=20, P=0.3). (−)-Noradrenaline relaxed colon from WT and β3KO mice with similar potencies (Figure 4a, Table 2). CGP 20712A (300 nM) caused a greater shift of the concentration-effect curve to (−)-noradrenaline in β3KO mice compared to WT mice (Figure 4a, Table 2). The addition of 50 nM ICI 118,551 to 300 nM CGP 20712A did not cause further blockade in WT or β3KO mice (Figure 4a, Table 2).
Figure 4.
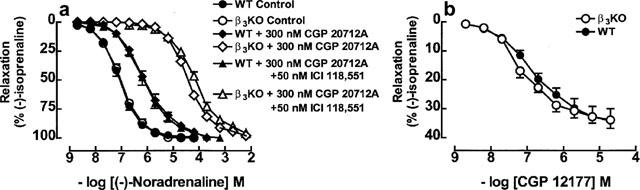
(a) Relaxant responses to (−)-noradrenaline in WT and β3KO mouse colon in the absence or presence of 300 nM CGP 20712A or 300 nM CGP 20712A+50 nM ICI 118,551. (b) Relaxant responses to (−)-CGP 12177 in the presence of 200 nM (−)-propranolol in β3KO and Sv 129 (from Figure 3 for comparison). Values shown are mean±s.e.mean (vertical lines, where larger than symbol size), n=4–8 tissues for each curve. Note that for WT, concentration–effect curves to (−)-noradrenaline in the presence of CGP 20712A alone or CGP 20712A+ICI 118,551 were nearly superimposable.
Table 2.
Effects of antagonists on the relaxant effects of (−)-noradrenaline in colon from wildtype (WT) and β3KO mice
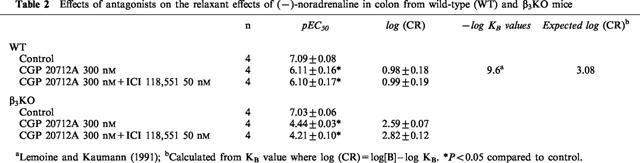
In the presence of 200 nM (−)-propranolol, a single concentration of CL 316,243, 2 μM caused 81.1±1.9% relaxation with respect to 600 μM (−)-isoprenaline (n=8) of colon from WT mice but had no effect in colon from β3KO mice, n=4 (Figure 5). In the presence of CL 316,243, the addition of 2 μM (−)-CGP 12177 caused 21.9±0.3% relaxation with respect to 600 μM (−)-isoprenaline in β3KO mice (n=4). A representative experiment is shown in Figure 5. In other colon from β3KO mice, (−)-CGP 12177 caused concentration dependent relaxation (pEC50, 7.19±0.04, n=4, Figure 4b).
Figure 5.
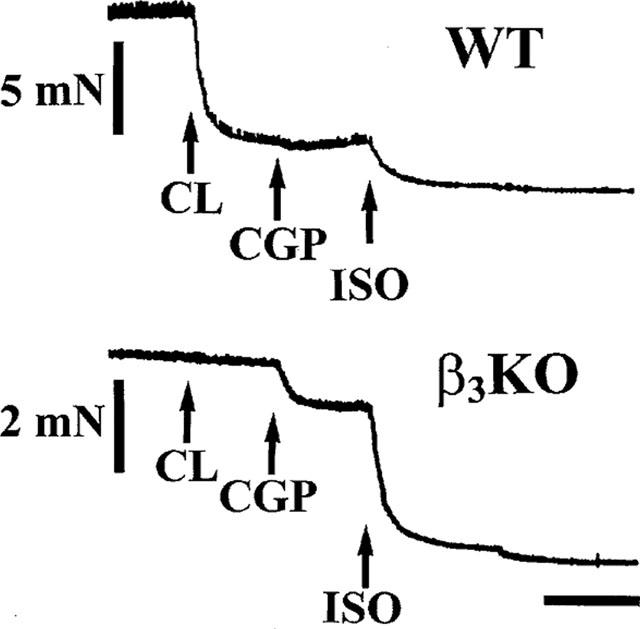
Original traces obtained from WT and β3KO mouse colonic tissues which were mounted in the same tissue bath. Tissues were pre-contracted with 30 mM KCl and then exposed to 2 μM CL 316,243 (CL), followed by 2 μM (−)-CGP 12177 (CGP) and finally 600 μM (−)-isoprenaline (ISO). Arrows indicate administration of drug. (−)-CGP 12177 caused relaxation in colon from β3KO mouse. Note the lack of effect of CL 316,243 in the same colon. Horizontal bar=5 min.
Quantitative determination of β1- and β2-adrenoceptor mRNA in WT and β3KO colon
β1- and β2-adrenoceptor mRNA was quantified in WT and β3KO mouse colon. β1- and β2-adrenoceptor transcripts had sizes of 3.1 kb and 2.3 kb respectively (Figure 6) which corresponded to those observed in white and brown adipose tissue (Revelli et al., 1991; 1997). The targeted disruption of the β3-adrenoceptor did not change the level of β1- or β2-adrenoceptor mRNA.
Figure 6.
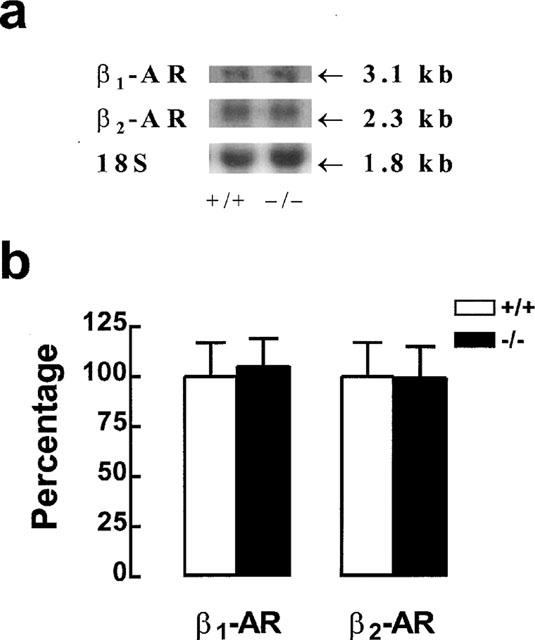
Photodensitometric comparisons of autoradiogram signals obtained from WT (+/+) or β3-adrenoceptor deficient (−/−) 2–3-month-old female mouse colon total RNA hybridized with β1- and β2-adrenoceptor (AR) probes. Representative autoradiogram signals obtained in colon with β1- and β2-ARs and 18S rRNA (18S) probes are shown in (a). The results are expressed as percentage±s.e. of the mean value in +/+ mice taken as 100%; n=20 experiments (b). The signals were quantified by scanning photodensitometry and normalized using the corresponding 18S rRNA values. Only signals obtained on the same Northern blot were compared. The sizes of the RNAs were 3.1 kb for the β1-AR and 2.3 kb for the β2-AR.
Contribution of β1-, β2-, β3- and putative β4-adrenoceptors to relaxation of mouse (Balb/c) oesophagus
The mouse oesophagus had slight spontaneous activity (Figure 7). Carbachol (1 μM) contracted the oesophagus (Figure 7a) and the contraction was enhanced by pre-incubation with ICI 118,551 (50 nM) (P=0.005) (Figure 8b) or (−)-propranolol (200 nM) (P=0.0009) (Figure 8d) but not CGP 20712A (300 nM) (P=0.26) (Figures 7a and 8a) or SR 59230A (10 μM) (P=0.19) (Figures 7a and 8c). The combination of both CGP 20712A (300 nM) and SR 59230A (10 μM) enhanced carbachol-evoked contractions (P=0.04, Figure 8e). (−)-Propranolol (200 nM) also caused an increase in basal tone (Figure 7b), as did a combination of both CGP 20712A (300 nM) and ICI 118,551 (50 nM) (Figure 7a), while CGP 20712A (Figure 7a), SR 59230A (Figure 7a) or ICI 118,551 (data not shown) alone had no effect.
Figure 7.
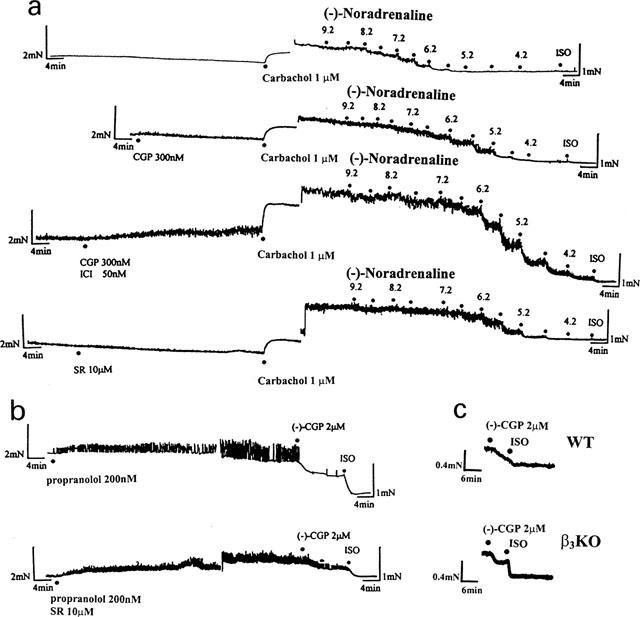
Representative recordings of mouse oesophageal muscle. In (a) concentration response curves to (−)-noradrenaline were constructed in the absence or presence of CGP 20712A (CGP 300 nM), a combination of both CGP 20712A (CGP 300 nM) and ICI 118,551 (ICI 50 nM) and SR 59230A (SR 10 μM) respectively. Half log unit increments in (−)-noradrenaline concentration were added. Maximal relaxation was tested by the addition of (±)-isoprenaline (ISO) (600 μM). In (b) a single concentration of (−)-CGP12177 ((−)-CGP 2 μM) caused a decrease of basal tone, either in the presence of (−)-propranolol or a combination of both (−)-propranolol and SR 59230A (10 μM). Maximal relaxation was tested by the addition of (±)-isoprenaline (ISO 600 μM). In (c) oesophagi from WT and β3KO were mounted in the same bath, incubated with 200 nM (−)-propranolol, exposed to (−)-CGP 12177 ((−)-CGP 2 μM) and then 600 μM (−)-isoprenaline (ISO). Note doubling of gain and readjustment of pen position in (b) and after equilibrium of carbachol response in (a).
Figure 8.
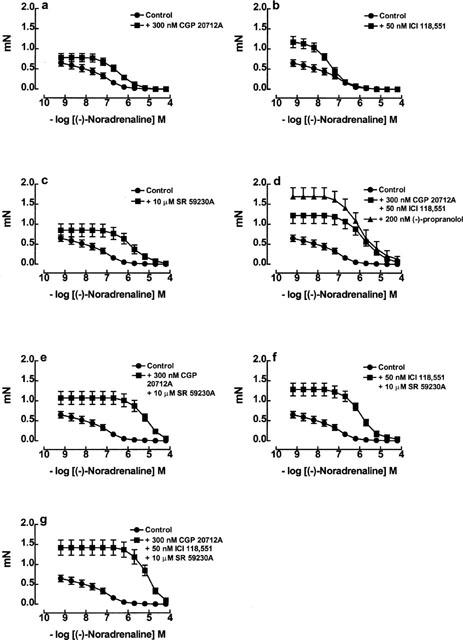
Relaxant responses to (−)-noradrenaline in mouse oesophageal muscle, in the absence or presence of (a) CGP 20712A 300 nM, (b) ICI 118,551 50 nM, (c) SR 59230A 10 μM, (d) (−)-propranolol 200 nM and a combination of both CGP 20712A 300 nM and ICI 118,551 50 nM, (e) a combination of both CGP 20712A 300 nM and SR 59230A 10 μM, (f) a combination of ICI 118,551 50 nM and SR 59230A 10 μM and (g) a combination of CGP 20712A 300 nM, ICI 118,551 50 nM and SR 59230A 10 μM. n=8−17 tissues for each group (see Table 3).
(−)-Noradrenaline caused concentration-dependent relaxation of the mouse oesophagus (Figure 7a and 8). The effects of (−)-noradrenaline were blocked surmountably by CGP 20712A (300 nM), SR 59230A (10 μM) or (−)-propranolol (200 nM), but not ICI 118,551 (50 nM) (Figure 8a–d, Table 3). The combination of both CGP 20712A (300 nM) and ICI 118,551 (50 nM) caused a greater shift of the concentration effect curve of (−)-noradrenaline compared to CGP 20712A alone (Figures 7a and 8a,d), as did the combination of both CGP 20712A (300 nM) and SR 59230A (10 μM) (Figure 8e). ICI 118,551 (50 nM) caused no additional blockade when combined with SR 59230A (10 μM) (Figures 8c,f, Table 3) or with the combination of CGP 20712A (300 nM) and SR 59230A (10 μM) (Figure 8g, Table 3). When shifts of concentration-effect curves were analysed at the 0.35 mN level of tone, ICI 118,551 alone or in combination with other antagonists caused small right-ward shifts (Table 3).
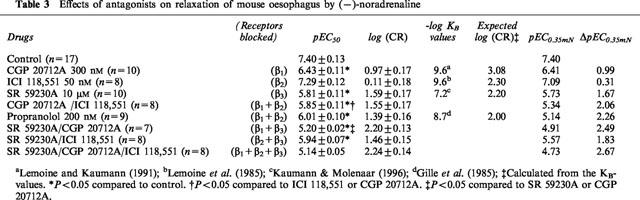
Table 3.
Effects of antagonists on relaxation of mouse oesophagus by (−)-noradrenaline
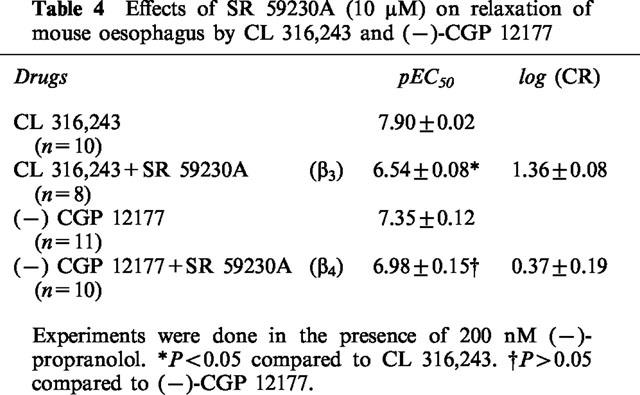
Both CL 316,243 and (−)-CGP 12177 caused concentration dependent relaxation of the mouse oesophagus in the presence of (−)-propranolol (200 nM). SR 59230A (10 μM) caused a 1.36-log unit rightward shift of the relaxant effects of CL 316,243 (Figure 9a, Table 4) but did not cause significant blockade of the relaxant effects of (−)-CGP 12177 (p=0.07, Figure 9b, Table 4).
Figure 9.
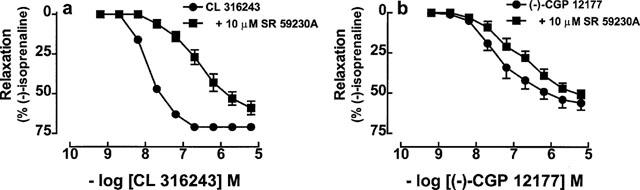
Relaxant responses in mouse oesophageal muscle of (a) CL 316,243 and (b) (−)-CGP 12177 in the absence or presence of 10 μM SR 59230A. All experiments were carried out in the presence of 200 nM (−)-propranolol. Values shown are mean±s.e.mean (vertical lines, where larger than symbol size), n=8–11 tissues for each curve.
Table 4.
Effects of SR 59230A (10 μM) on relaxation of mouse oesophagus by CL 316,243 and (−)-CGP 12177
In the presence of 200 nM (−)-propranolol and absence of carbachol, a single concentration of (−)-CGP12177 (2 μM) reduced basal tone by 67±6% (relaxation expressed as a percentage of the effect of 600 μM (±)-isoprenaline, n=6) which was unaffected by 10 μM SR 59230A (68±4%, Figure 7b).
(−)-CGP 12177 mediates relaxation of oesophagus from WT and β3KO mice
In the presence of 200 nM (−)-propranolol, 2 μM (−)-CGP 12177 caused similar relaxation (expressed as a percentage of the relaxation to 600 μM (−)-isoprenaline) of oesophagi from WT (61.4±5.1%, n=4) and β3KO (67.3±10.1%, n=4) mice (Figure 7c).
Reduction in pacemaker activity of ureter from WT and β3KO mice
CL 316,243 (2 μM) reduced KCl- and U46619-induced pacemaker activity in ureter from WT (per cent of control 80.5±2.8, n=4, P=0.006) but not β3KO (per cent of control 97.4±2.1, n=3, P=0.3) mice. In the presence of CL 316,243, 2 μM (−)-CGP 12177 caused a reduction of pacemaker activity in ureter from both WT (per cent of control 68.9±2.3, n=4, P=0.0009) and β3KO (per cent of control 68.7±7.5, n=3, P=0.05) mice (Figure 10).
Figure 10.
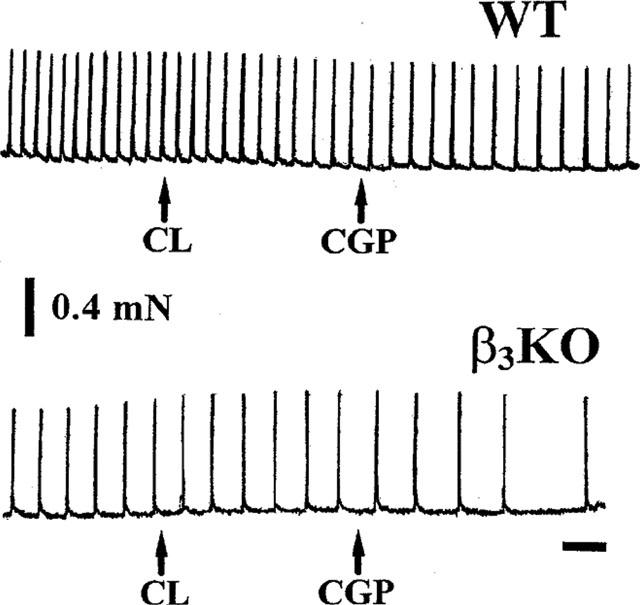
Original traces showing the effects of 2 μM CL 316,243 (CL) and 2 μM (−)-CGP 12177 (CGP) on KCl- and U46619-induced spontaneous contractions in WT and β3KO ureter. In this experiment the frequency of spontaneous contractions was lower in the ureter from the β3KO compared to the WT mouse. CL 316,243 reduced the frequency of spontaneous contractions in WT but not β3KO ureters. (−)-CGP 12177 reduced the frequency of contractions in β3KO. Time bar=1 mm
Discussion
The longitudinal smooth muscle of mouse colon and oesophagus is relaxed by stimulation of both β1- and β3-adrenoceptors. The effects of (−)-CGP 12177 were presumably mediated through the ‘putative β4-adrenoceptor' in colon, oesophagus and ureter, previously identified in heart (Kaumann, 1989; 1997; Kaumann & Molenaar, 1997; Molenaar et al., 1997b; Kaumann et al., 1998; Lowe et al., 1998; 1999) and adipose tissue (Galitzky et al., 1997; Preitner et al., 1998).
β1- and β3-adrenoceptors mediate relaxation of smooth muscle
The relaxant effects of (−)-noradrenaline in colon and oesophagus were partially blocked by selective β1- (CGP 20712A) and β3- (SR 59230A) but not β2-adrenoceptor antagonists (ICI 118,551) indicating relaxation mediated by both β1- and β3-adrenoceptors. Assessment of the blockade caused by CGP 20712A and SR 59230A alone showed that both β1- and β3-adrenoceptors share to around the same extent the mediation of relaxation by both (−)-adrenaline and (−)-noradrenaline. In colon of β3KO mice, the 2.6-log unit rightward shift of the concentration-effect curve to (−)-noradrenaline by 300 nM CGP 20712A showed that β1-adrenoceptors were mostly responsible for relaxation. Previously we observed that 200 nM (−)-propranolol, the concentration used in this study, did not affect the potency of (−)-noradrenaline in rat colon indicating that (−)-noradrenaline mediates its effects essentially by stimulation of β3-adrenoceptors and that this concentration of (−)-propranolol does not block β3-adrenoceptors (Kaumann & Molenaar, 1996). However 200 nM (−)-propranolol caused a 0.8-log unit rightward shift in the concentration-effect curve to (−)-noradrenaline in mouse colon indicating a greater relative involvement of β1-adrenoceptors in mouse compared to rat colon.
The partial resistance of blockade by β1- and β2-adrenoceptors with (−)-propranolol or with the combination of CGP 20712A and ICI 118,551 together with blockade by the β3-selective antagonist SR 59230A of relaxant responses of the catecholamines in colon and oesophagus revealed the involvement of the β3-adrenoceptor. The presence of the β3-adrenoceptor in mouse colon and oesophagus was confirmed by the use of the selective β3-adrenoceptor agonist CL 316,243 and blockade of its relaxant effects by the antagonist SR 59230A. In mouse colon the affinity of SR 59230A, pKB=6.8 was similar to that observed in rat colon against the β3-adrenoceptor agonists ZD 2079 (7.0), SR 586118 (6.9) and BRL 37344 (6.9) at β3-adrenoceptors (Kaumann & Molenaar, 1996).
β2-adrenoceptors in colon and oesophagus
β2-adrenoceptors are present in colon and oesophagus but play a minor role in mediating relaxation. The small degree of blockade caused by 50 nM ICI 118,551 to the relaxant responses of (−)-adrenaline (but not (−)-noradrenaline) revealed a small contribution of β2-adrenoceptors to relaxation in colon. The presence of β2-adrenoceptors in colon was confirmed by the detection of β2-adrenoceptor mRNA. Higher concentrations of (−)-noradrenaline may cause relaxation of oesophagus by stimulation of β2-adrenoceptors. This was shown by the rightward shift of the concentration-effect curve to (−)-noradrenaline at the 0.35 mN level by the combination of CGP 20712A and ICI 118,551 (2.06-log units) which was 1-log unit greater than in the presence of CGP 20712A alone (0.99-log units) (Table 3).
In the oesophagus (−)-propranolol and a combination of both CGP 20712A and ICI 118,551 increased basal tone. (−)-Propranolol and ICI 118,551 also enhanced the carbachol evoked contraction. Blockade of either postsynaptic β1- or β3-adrenoceptors alone by CGP 20712A or SR 59230A respectively did not increase basal tone or the carbachol evoked contraction however the combination of CGP 20712A and SR 59230A enhanced the carbachol evoked contraction. These observations may be explained by the presence of prejunctional sympathetic nerve β2-adrenoceptors which facilitate release of (−)-noradrenaline in a number of tissues (Majewski, 1983; Göthert & Kollecker, 1986; Münch et al., 1996). In the mouse oesophagus, stretch in the organ bath could release endogenous noradrenaline, possibly facilitated by stimulation of prejunctional β2-adrenoceptors which in turn stimulates post-synaptic β1- and β3-adrenoceptors to reduce muscle tone. The increase of basal tone and carbachol evoked contraction observed after administration of ICI 118,551 and (−)-propranolol could be caused by blockade of pre-junctional β2-adrenoceptors, leading to a reduction of endogenous (−)-noradrenaline release. Interestingly ICI 118,551 on its own did not increase basal tone but did so in the presence of carbachol. Carbachol and other muscarinic receptor agonists can reduce endogenous (−)-noradrenaline release from sympathetic nerves by stimulating prejunctional muscarinic receptors (Muscholl, 1980) and when combined with ICI 118,551 to block prejunctional β2-adrenoceptors, a further reduction in transmitter release may occur. Blockade of both post-synaptic β1- and β3-adrenoceptors could prevent endogenously released (−)-noradrenaline from reducing muscle tone.
Evidence for the putative β4-adrenoceptor in smooth muscle
The selective β3-adrenoceptor antagonist SR 59230A in the presence of (−)-propranolol blocked the relaxant effects of CL 316,243 but not significantly the effects of (−)-CGP 12177 in both colon and oesophagus, indicating a lack of involvement of β1-, β2-and β3-adrenoceptors for (−)-CGP 12177 mediated relaxation. This together with the maintenance of (−)-CGP 12177-evoked relaxation in colon and oesophagus of β3KO mice, suggested an effect mediated by stimulation of putative β4-adrenoceptors. In the oesophagus a single concentration of (−)-CGP 12177 also reduced basal tone in the absence of carbachol. (−)-CGP 12177 is also an antagonist at both β1-and β2-adrenoceptors (Kaumann, 1983; Nanoff et al., 1987) and would have been expected to increase basal tone as observed for (−)-propranolol if not for its ability to stimulate the putative β4-adrenoceptor.
In the ureter the selective β3-adrenoceptor agonist CL 316,243 and (−)-CGP 12177 both mediated a reduction in pacemaker frequency suggesting the involvement of both β3- and possibly putative β4-adrenoceptors. The effect of (−)-CGP 12177 but not CL 316,243 was maintained in ureter from β3KO mice, consistent with mediation through the putative β4-adrenoceptor.
Compensatory increases in the function of β1- and putative β4-adrenoceptors in colon of β3KO mice
Interestingly, there was no difference in the potency of (−)-noradrenaline in colonic tissues taken from β3KO (pEC50 7.1) or WT mice (pEC50 7.0), suggesting that β1-adrenoceptors took over the relaxant function of β3-adrenoceptors. The compensatory hyperfunction of β1-adrenoceptors in β3KO is strongly supported by the considerably greater blockade of the relaxant effects of (−)-noradrenaline by CGP 20712A in colon from β3KO than WT mice. It is not yet known whether the compensatory hyperfunction of β1-adrenoceptors is due to an increase in receptor density of these receptors but the lack of change β1-adrenoceptor transcript level in colon from the two groups argues against an increase of receptor density. Instead, β1-adrenoceptors may be coupled more efficiently to the pathway leading to relaxation. The regulation of β1-adrenoceptor mRNA is tissue specific and is not necessarily predictable as no change was observed in white adipose tissue of β3KO mice, but a reduction in brown adipose tissue was observed in the same study (Revelli et al., 1997). (−)-CGP 12177 was 2 fold more potent in colon from β3KO (pEC50 7.2) than WT mice (pEC50 6.9, P=0.005). The markedly enhanced blocking potency of CGP 20712A of the relaxant effects of (−)-noradrenaline in β3KO colon suggests greater compensatory increases of β1-adrenoceptor function than putative β4-adrenoceptor function. Thus, the presence of the β3-adrenoceptor may have an inhibitory effect on coupling of both β1- and putative β4-adrenoceptors with β1-adrenoceptor>putative β4-adrenoceptor.
The putative β4-adrenoceptor as an atypical state of the β1-adrenoceptor
As with other non-conventional partial agonists (Kaumann, 1989; 1997), (−)-CGP 12177 shows dissociation of antagonist (high affinity for β1- and β2-adrenoceptors) and agonist (low cardio-stimulant potency) properties. Interestingly, Pak & Fishman (1996) also observed a similar pattern of agonist and antagonist activity for (±)-CGP 12177 on rat and human recombinant β1- and β2-adrenoceptors expressed at high non-physiological densities in several cell lines. As found with native receptors (Sarsero et al., 1998b; 1999), Pak & Fishman (1996) detected a high and low affinity population for (−)-[3H]-CGP 12177. The effects of (±)-CGP 12177 were attributed to stimulation of a guanine nucleotide sensitive low-affinity form of the β1-adrenoceptor which comprised approximately 10% of the total population of β1-adrenoceptors (Pak & Fishman, 1996).
But can the effects ascribed to the putative β4-adrenoceptor be explained in terms of a low affinity conformation of the β1-adrenoceptor? In mouse (Kaumann et al., 1998), rat (Sarsero et al., 1998b; 1999) and human cardiac tissues (Sarsero et al., 1998a), (−)-3H-CGP 12177 labels a population of high affinity receptors (pK∼9) corresponding to β1- and β2-adrenoceptors and another distinct low affinity population (pK∼7) attributed to the putative β4-adrenoceptor. This population is not affected by guanine nucleotides unlike rat and human recombinant and transfected β1-adrenoceptors whose affinity for (−)-[3H-CGP 12177 is reduced (Sarsero et al., 1998b). Furthermore, the population attributed to putative β4-adrenoceptors in rat heart can be subdivided into a high-affinity form (β4H) and a low-affinity form (β4L, pK∼5) for several non-conventional partial agonists but not for (−)-CGP 12177 which has the same affinity for both forms (Sarsero et al., 1999). Because the affinity of several non-conventional partial agonists for the β4H form agrees with their corresponding inotropic and chronotropic potencies it has been suggested to mediate the positive inotropic and chronotropic effects of these ligands (Sarsero et al., 1999). The suggestion is also in line with the low binding affinity of (−)-propranolol for the putative β4-adrenoceptor (Sarsero et al., 1998b) which correlates with its failure to block the cardiostimulant effects of (−)-CGP 12177 and other non-conventional partial agonists (Kaumann, 1996; Kaumann & Molenaar, 1996). In contrast, (−)-propranolol is a potent antagonist of the cardiostimulant effects of catecholamines mediated through β1-adrenoceptors, suggesting that the putative β4-adrenoceptor is distinct from the β1-adrenoceptor or is a (−)-propranolol-resistant form of the β1-adrenoceptor.
The hypothesis that some agonist effects of (−)-CGP 12177 are mediated through an atypical state of the β1-adrenoceptor has recently received conclusive support. Cardiostimulation by (−)-CGP 12177 was observed in atria from mice lacking β2-adrenoceptors (β2-AR knockout) but not in atria from mice lacking both β1- and β2-adrenoceptors (double β1-/β2-AR knockout) (Kaumann et al., 2000). Furthermore, CGP 12177 stimulates adenylyl cyclase of mouse brown fat through a high and low affinity receptor population; the high-affinity and low-affinity effects were not observed in β1-AR knockout and β3-AR knockout, respectively (Konkar et al., 2000). Taken together, this evidence indicates that β1-adrenoceptors have an obligatory role for the mediation of at least part of the cardiostimulant effects and agonist effects of CGP 12177 in brown fat. It is therefore plausible, if not likely, that the smooth muscle relaxant effects of (−)-CGP 12177 are also mediated through an atypical state of the β1-adrenoceptor. In line with this contention is our observation that both the relaxant effects of (−)-noradrenaline, mediated through β1-adrenoceptors, and of (−)-CGP 12177 were found to be enhanced in smooth muscle from β3-AR knockout compared to wild-type mice.
(−)-CGP 12177 has recently been found to be 40 times more potent than (−)-isoprenaline in eliciting arrhythmic calcium transients in mouse ventricular myocytes (Freestone et al., 1999). This effect of (−)-CGP 12177 was also (−)-propranolol-resistant and its potency subnanomolar, in agreement with its high-affinity for β1-adrenoceptor, suggesting the existence of a high-affinity β1-adrenoceptor state which is distinct from the low affinity state that presumably mediates positive chronotropic, inotropic and lusitropic effects (Kaumann et al., 1998; Sarsero et al., 1999) and relaxation of smooth muscle (this work).
Conclusions
In mouse colon and oesophagus, activation of β1-and β3-adrenoceptors mediate relaxation. In β3KO, β1- and putative β4-adrenoceptors compensate for the lack of β3-adrenoceptor contribution to relaxation. In comparison, β2-adrenoceptors have a minor role. As recently demonstrated for cardiac and adipose tissues, the putative β4-adrenoceptor in smooth muscle may correspond to an atypical state of the β1-adrenoceptor.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (P. Molenaar), Swiss National Science Foundation (Grant 31-43405.95, J.P. Giacobino, F. Preitner, M. Jimenez), British Heart Foundation (A.J. Kaumann) and the Dr Saal van Zwanenberg Foundation, The Netherlands (J. Oostendorp).
Abbreviations
- β3KO
β3-adrenoceptor knockout
- Carbachol
carbamylcholine chloride
- (−)-CGP 12177
(−)-4-(3-tertiarybutylamino-2-hydroxypropoxy) benzimidazol-2-one
- CL 316,243
disodium (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl-amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate]
- CGP 20712A
2-hydroxy-5(2-((2-hydroxy-3-(4-((1-methyl-4-trifluoromethyl) 1H-imidazole-2-yl) -phenoxy) propyl) amino) ethoxy)-benzamide monomethane sulfonate
- CR
concentration-ratio
- ICI 118,551
(erythro-DL-1(7-methylindan-4-yloxy)-3-isopropylamino-butano-2-ol)
- SR 59230A
3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-2S-2-propanol oxalate
- WT
wildtype
References
- ARCH J.R.S. , KAUMANN A.J. β3 and atypical β-adrenoceptors (1993) Med. Res. Rev. 1993;13:663–729. doi: 10.1002/med.2610130604. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O. , SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILSKI A.J., HALLIDAY S.E., FITZGERALD J.D. , WALE J.L. The pharmacology of a β2-selective adrenoceptor antagonist (ICI 118,551) J. Cardiovasc. Pharmacol. 1983;5:430–437. doi: 10.1097/00005344-198305000-00013. [DOI] [PubMed] [Google Scholar]
- DE BOER R.E.P., BROUWER F. , ZAAGSMA J. The β-adrenoceptors mediating relaxation of rat oesophageal muscularis mucosae are predominantly of the β3-, but also of the β2-subtype. Br. J. Pharmacol. 1993;110:442–446. doi: 10.1111/j.1476-5381.1993.tb13830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESTONE N.S., HEUBACH J.F., WETTWER E., RAVENS U., BROWN D. , KAUMANN A.J. Putative β4-adrenoceptors are more effective than β1-adrenoceptors in mediating arrhythmic Ca2+ transients in mouse ventricular myocytes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:445–456. doi: 10.1007/s002109900075. [DOI] [PubMed] [Google Scholar]
- GALITZKY J., LANGIN D., VERWAERDE P., MONTASTRUC J.-L., LAFONTAN M. , BERLAN M. Lipolytic effects of conventional β3-adrenoceptor agonists and of CGP 12,177 in rat and human fat cells: preliminary pharmacological evidence for a putative β4-adrenoceptor. Br. J. Pharmacol. 1997;122:1244–1250. doi: 10.1038/sj.bjp.0701523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLE E., LEMOINE H., EHLE B. , KAUMANN A.J. The affinity of (−)-propranolol for β1- and β2-adrenoceptors of human heart. Differential antagonism of the positive inotropic effects and adenylate cyclase stimulation by (−)-noradrenaline and (−)-adrenaline. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;331:60–70. doi: 10.1007/BF00498852. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M. , KOLLECKER P. Subendothelial β2-adrenoceptors in the rat vena cava: facilitation of noradrenaline release via local stimulation of angiotensin II synthesis. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;334:156–165. doi: 10.1007/BF00505816. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Cardiac β-adrenoceptors. Experimental viewpoints. Z. Kardiol. 1983;72:63–82. [PubMed] [Google Scholar]
- KAUMANN A.J. Is there a third heart β-adrenoceptor. Trends Pharmacol. Sci. 1989;10:316–320. doi: 10.1016/0165-6147(89)90065-5. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. Piglet sinoatrial 5-HT receptors resemble human atrial 5-HT4-like receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:619–622. doi: 10.1007/BF00169055. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. (−)-CGP 12177- induced increase of human atrial contraction through a putative third β-adrenoceptor. Br. J. Pharmacol. 1996;117:619–622. doi: 10.1111/j.1476-5381.1996.tb15159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. Four β-adrenoceptor subtypes in mammalian heart. Trends Pharmacol. Sci. 1997;18:70–76. doi: 10.1016/s0165-6147(96)01033-4. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J. , MOLENAAR P. differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J. , MOLENAAR P. Modulation of human cardiac function through 4 β-adrenoceptor populations. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;335:667–681. doi: 10.1007/pl00004999. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., ENGELHARDT S., MOLENAAR P. , LOHSE M. (−)-CGP 12177-evoked cardiostimulation is abolished in double β1-/β2-adrenoceptor (AR) knockout mice. Proc. Austral. Soc. Clin. Exp. Pharmacol. Toxicol. 2000;7:70. [Google Scholar]
- KAUMANN A.J., PREITNER F., SARSERO D., MOLENAAR P., REVELLI J.-P. , GIACOBINO J.P. (−)-CGP 12177 causes cardiostimulation and binds to cardiac putative β4-adrenoceptors in both wild-type and β3-adrenoceptor knockout mice. Mol. Pharmacol. 1998;53:670–675. doi: 10.1124/mol.53.4.670. [DOI] [PubMed] [Google Scholar]
- KONKAR A.A., ZHAI Y. , GRANNEMAN J.G. β1-adrenergic receptors mediate β3-adrenergic-independent effects of CGP 12177 in brown adipose tissue. Mol. Pharmacol. 2000;57:252–258. [PubMed] [Google Scholar]
- LEMOINE H. , KAUMANN A.J. Regional differences of β1- and β2-adrenoceptor-mediated functions in feline heart. A β2-adrenoceptor-mediated positive inotropic effect possibly unrelated to cyclic AMP. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:56–69. doi: 10.1007/BF00167383. [DOI] [PubMed] [Google Scholar]
- LEMOINE H., EHLE B. , KAUMANN A.J. Direct labelling of β2-adrenoceptors: comparison of binding potency of 3H-ICI 118,551 and blocking potency of ICI 118,551. Naunyn-Schmiedeberg's Arch. Pharmacol. 1985;331:40–51. doi: 10.1007/BF00498850. [DOI] [PubMed] [Google Scholar]
- LOWE M.D., GRACE A.A. , KAUMANN A.J. Blockade of putative β4- and β1-adrenoceptors by carvedilol in ferret myocardium. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:400–403. doi: 10.1007/pl00005367. [DOI] [PubMed] [Google Scholar]
- LOWE M.D., GRACE A.A., VANDENBERG J.I. , KAUMANN A.J. Action potential shortening through the putative β4-adrenoceptor in ferret ventricle: comparison with β1- and β2-adrenoceptor-mediated effects. Br. J. Pharmacol. 1998;124:1341–1344. doi: 10.1038/sj.bjp.0702013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJEWSKI H. Modulation of noradrenaline release through activation of presynaptic β-adrenoceptors. J. Auton. Pharmacol. 1983;3:47–60. doi: 10.1111/j.1474-8673.1983.tb00496.x. [DOI] [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U. , LE FUR G. Aryloxypropanolaminotetralins are the first selective antagonists for atypical (β3) β-adrenoceptors. Pharmacol. Comm. 1995;6:253–258. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., SARSERO D., ARCH J.R.S., KELLY J., HENSON S.M. , KAUMANN A.J. Effects of (−)-RO363 at human atrial β-adrenoceptor subtypes, the human cloned β3-adrenoceptor and rodent intestinal β3-adrenoceptors. Br. J. Pharmacol. 1997a;120:165–176. doi: 10.1038/sj.bjp.0700850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLENAAR P., SARSERO D. , KAUMANN A.J. Proposal for the interaction of non-conventional partial agonists and catecholamines with the ‘putative β4-adrenoceptor' in mammalian heart. Clin. Exp. Pharmacol. Physiol. 1997b;24:647–656. doi: 10.1111/j.1440-1681.1997.tb02107.x. [DOI] [PubMed] [Google Scholar]
- MÜNCH G., KURZ T., URLBAUER T., SEYFARTH M. , RICHARDT G. Differential presynaptic modulation of noradrenaline release in human atrial tissue in normoxia and anoxia. Br. J. Pharmacol. 1996;118:1855–1861. doi: 10.1111/j.1476-5381.1996.tb15614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSCHOLL E. Peripheral muscarinic control of noradrenaline release in the cardiovascular system. Am. J. Physiol. 1980;239:H713–H720. doi: 10.1152/ajpheart.1980.239.6.H713. [DOI] [PubMed] [Google Scholar]
- NANOFF C., FREISSMUTH M. , SCHÜTZ W. The role of a low β1-adrenoceptor selectivity of (−)-[3H]-CGP 12177A for resolving subtype-selectivity of competitive ligands. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:519–525. doi: 10.1007/BF00169308. [DOI] [PubMed] [Google Scholar]
- PAK M.D. , FISHMAN P.H. Anomalous behavior of CGP 12177A on β1-adrenergic receptors. J. Recept. Signal. Tranduct. Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- PREITNER F., MUZZIN P., REVELLI J.-P., SEYDOUX J., GALITZKY J., BERLAN M., LAFONTAN M. , GIACOBINO J.-P. Metabolic response to various β-adrenoceptor agonists in β3-adrenoceptor knockout mice: Evidence for a new β-adrenergic receptor in brown adipose tissue. Br. J. Pharmacol. 1998;124:1684–1688. doi: 10.1038/sj.bjp.0702007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVELLI J.-P., PESCINI R., MUZZIN P., SEYDOUX J., FITZGERALD M.G., FRASER C.M. , GIACOBINO J.-P. Changes in beta 1- and beta 2-adrenergic receptor mRNA levels in brown adipose tissue and heart of hypothyroid rats. Biochem. J. 1991;277:625–629. doi: 10.1042/bj2770625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REVELLI J.P., PREITNER F., SAMEC S., MUNIESA P., KUEHNE F., BOSS O., VASSALLI J.D., DULLOO A., SEYDOUX J., GIACOBINO J.P., HUARTE J. , ODY C. Targeted gene disruption reveals a leptin-independent role for the mouse β3-adrenoceptor in the regulation of body composition. J. Clin. Invest. 1997;100:1098–1106. doi: 10.1172/JCI119620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., MOLENAAR P. , KAUMANN A.J. (−)-[3H]-CGP 12177 radiolabels β1-, β2-and putative β4-adrenoceptors in human atrium and ventricle. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998a;358:R629. [Google Scholar]
- SARSERO D., MOLENAAR P. , KAUMANN A.J. Validity of (−)-[3H]-CGP 12177A as a radioligand for the ‘putative β4-adrenoceptor' in rat atrium. Br. J. Pharmacol. 1998b;123:371–380. doi: 10.1038/sj.bjp.0701609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARSERO D., MOLENAAR P., KAUMANN A.J. , FREESTONE N.S. Putative β4-adrenoceptors in rat ventricle mediate increases in contractile force and cell Ca2+: comparison with atrial receptors and relationship to (−)-[3H]-CGP 12177 binding. Br. J. Pharmacol. 1999;128:1445–1460. doi: 10.1038/sj.bjp.0702936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENNITT M.V., KAUMANN A.J., MOLENAAR P., BEELEY L.J., YOUNG P.W., KELLY J., CHAPMAN H., HENSON S.M., BERGE J.M., DEAN D.K., KOTECHA N.R., MORGAN H.K.A., RAMI H.K., WARD R.W., THOMPSON M., WILSON S., SMITH S.A., CAWTHORNE M.A., STOCK M.J. , ARCH J.R.S. The contribution of classical (β1/2-) and atypical β-adrenoceptors to the stimulation of human white adipocyte lipolysis and right atrial appendage contraction by novel β3-adrenoceptor agonists of differing selectivities. J. Pharmacol. Exp. Ther. 1998;285:1084–1095. [PubMed] [Google Scholar]
- TOMIYAMA Y., HAYAKAWA K., SHINAGAWA K., AKAHANE M., JISAWA Y., PARK Y.-C. , KURITA T. β-Adrenoceptor subtypes in the ureteral smooth muscle of rats, rabbits and dogs. Eur. J. Pharmacol. 1998;352:269–278. doi: 10.1016/s0014-2999(98)00360-4. [DOI] [PubMed] [Google Scholar]


