Abstract
Cholinergic neurons were identified in rat striatal slices by their size, membrane properties, sensitivity to the NK1 receptor agonist (Sar9, Met(O2)11) Substance P, and expression of choline acetyltransferase mRNA. A1 receptor mRNA was detected in 60% of the neurons analysed, and A2A receptor mRNA in 67% (n=15).
The A1 receptor agonist R-N6-(2-phenylisopropyl)adenosine (R-PIA) hyperpolarized cholinergic neurons in a concentration dependent manner sensitive to the A1 antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 100 nM).
In dual stimulus experiments, the A2A receptor antagonist 8-(3-chlorostyryl)caffeine (CSC, 500 nM) decreased release of [3H]-acetylcholine from striatal slices (S2/S1 0.78±0.07 versus 0.95±0.05 in control), as did adenosine deaminase (S2/S1 ratio 0.69±0.05), whereas the A1 receptor antagonist DPCPX (100 nM) had no effect (S2/S1 1.05±0.14).
In the presence of adenosine deaminase the adenosine A2A receptor agonist 2-p-((carboxyethyl)phenylethylamino)-5′-N-ethylcarboxamidoadenosine (CGS21680, 10 nM) increased release (S2/S1 ratio 1.03±0.05 versus 0.88±0.05 in control), an effect blocked by the antagonist CSC (500 nM, S2/S1 0.68±0.05, versus 0.73±0.08 with CSC alone). The combined superfusion of bicuculline (10 μM), saclofen (1 μM) and naloxone (10 μM) had no effect on the stimulation by CGS21680 (S2/S1 ratio 0.99±0.04).
The A1 receptor agonist R-PIA (100 nM) inhibited the release of [3H]-acetylcholine (S2/S1 ratio 0.70±0.03), an effect blocked by DPCPX (S2/S1 ratio 1.06±0.07).
It is concluded that both A1 and A2A receptors are expressed on striatal cholinergic neurons where they are functionally active.
Keywords: Adenosine receptor, A2A receptor, striatum, acetylcholine
Introduction
Adenosine A2A receptor antagonists represent a class of non dopamine related drugs which are being tested for their efficacy in the treatment of Parkinson's Disease (Richardson et al., 1997). However the mechanism of action of such drugs is unclear, and in particular the possible involvement of such receptors on striatal cholinergic interneurons is debatable (Fredholm & Svenningsson, 1998; Richardson et al., 1998). Indeed, there are a number of discrepancies in the literature relating to the expression and function of adenosine A1 and A2A receptors in these neurons.
The adenosine A1 receptor has been shown to inhibit ACh release from striatal cholinergic nerve terminals (Brown et al., 1990), and the adenosine A2A receptor has been shown to stimulate acetylcholine release from striatal synaptosomes (Brown et al., 1990; Kurokawa et al., 1994; Kirk & Richardson, 1994; Gubitz et al., 1996). However, A2A receptor agonists have also been reported to have no effect on acetylcholine release from striatal slices, other than in the presence of dopamine (Jin et al., 1993; Jin & Fredholm, 1997). In addition few molecular studies have confirmed the expression of A1 receptor mRNA in these cells (Reppert et al., 1991; Rivkees et al., 1995; Dixon et al., 1996), and the majority of attempts to detect A2A receptor mRNA expression in striatal cholinergic neurons have failed (Schiffmann et al., 1991; Schiffmann & Vanderhaeghen, 1993; Fink et al., 1992; Svenningsson et al., 1997). Only one in situ hybridization study has shown mRNA encoding the A2A receptor to be expressed in some large (presumably cholinergic) striatal neurons at low abundance (Dixon et al., 1996).
Since the discrepancies concerning adenosine receptor mRNA expression in striatal cholinergic neurons may have been due to differing sensitivities of the techniques used, we have employed a highly sensitive single cell RT–PCR method to examine A1 and A2A receptor expression in these cells. In addition it is possible that the debate over the potential functional relevance of the A2A receptor in these cells was a consequence of the different in vitro preparations (synaptosomes and slices) used. We have therefore also examined the ability of A1 and A2A receptor agonists to regulate acetylcholine release from rat brain striatal slices.
Methods
Recording and analysis
Three hundred μm thick coronal slices (male Sprague Dawley rats, 14–28 days old) were prepared using a vibratome, and bathed in aCSF of the following composition (mM); NaCl 125, NaHCO3 25, glucose 10, KCl 2.5, NaH2PO4 1.25, CaCl2 2, MgCl2 1, bubbled with 95% O2 and 5% CO2 gas mixture, and viewed with a Zeiss Axioskop microscope (Carl Zeiss Ltd., Welwyn Garden City, U.K.) fitted with a x64 water-immersion objective as previously described (Lee et al., 1998). The electrode buffer contained (in mM): K gluconate 120, NaCl 10, MgCl2 2, EGTA 0.5, HEPES 10, Na2ATP 4, Na2GTP 0.3, pH adjusted to 7.2 with KOH. In addition 0.5 μg ml−1 glycogen (Boehringer) and RNase inhibitor (Pharmacia, 0.1 u μl−1 were included to facilitate the harvesting of RNA from the cells. All solutions were made up in diethyl pyrocarbonate treated water (to inactivate RNases). Borosilicate recording electrodes were baked (2 h, 250°C) before being pulled to a resistance of between 3 and 5 MΩ. Electrophysiological signals were detected using an Axopatch-1D patch-clamp amplifier in the current clamp configuration and were recorded onto digital audiotape for later production. Membrane signals were filtered at 1 kHz and were digitized at 5 kHz through a Digidata 1200A/D converter using pClamp 6.0 software (Axon Instruments Inc, CA, U.S.A.).
The cytoplasm from large cells (>30 μm in one dimension) was gently aspirated under visual control into a patch-clamp recording electrode until at least 40% of the somatic cytoplasm had been collected. The electrode was then withdrawn from the cell to form an outside-out patch, or a nucleated patch, to prevent contamination on subsequent withdrawal from the slice. The contents of the electrode were forced into a microtube and reverse transcribed, subjected to 3′ cDNA amplification (TPEA–PCR), and 6% of the product used in each gene specific PCR reaction as described in Dixon et al. (1998) with the following modifications. Four defined pentameric sequences were present at the 3′ ends of the second-strand cDNA primers, each primer contained the following heel sequence at the 5′ end: CTG CAT CTA TCT AAT GCT CC. The four arbitrary 3′ sequences were: CGAGA, CGACA, CGTAC and ATGCG. 2.5 pg of each second strand primer were annealed at 50°C to the first strand cDNA, and second strand cDNA synthesis was performed at 72°C for 8 min using Taq DNA polymerase (0.35 units, Perkin Elmer) at pH 8.3 using 4.5 mM MgCl2 and 0.5 mM dNTPs. Subsequently, 1 ng of the 5′ and 3′ heel primers were added in 5 μl of PCR buffer containing 67 mM Tris HCl (pH 8.3), 4.5 mM MgCl2 0.5 mM dNTPs and 0.16 units of Taq and the reaction subjected to 10 cycles of 92°C (2.5 min, denaturation), 60°C (annealing, 1.5 min) and 72°C (extension, 1 min), followed by a final 10 min extension. A further 10 ng of each heel primer were then added in 20 μl of PCR buffer and subjected to a further 40 rounds of PCR (conditions as before). The final reaction mixture was then diluted to 100 μl with 10 mM Tris/ 0.1 mM EDTA (pH 8.1), and 6 μl samples used for subsequent gene specific PCR. Gene specific PCR was performed as described (Dixon et al., 1998) using the following primers (accession number, base number): choline acetyltransferase: forward primer (sequence from Brice et al., 1989, 2134–2155 TACTAAGCTCTGTTCCCATCCC, reverse primer 2303–2285, ACCCAGGTTGCTTCCAAAC), adenosine A1 receptor (Y12519, forward primer 2507–2526; reverse primer 2637–2619), adenosine A2A receptor (L08102, forward primer 1817–1835; reverse primer 1980–1960), NK1 receptor (J05097, forward primer 2991–3010; reverse primer 3195–3176; preproenkephalin (S49491, forward primer 1033–1050; reverse primer 1180–1159); preprotachykinin (M34184, forward primer 717–735; reverse primer 877–85). The genomic primers recognized two polymorphic repeats: STS ET3 (forward primer: GCCTGCATTCATCTTCATCTGC, reverse primer: AAAGGTGGAACTCGCCCGTTT) and STS RR1023 (forward primer: AGCCTCATCGATGCTCCTGT, reverse primer: CCAAGAGCTACCTGCACTCC).
All of the gene specific primers used had similar sensitivities, detecting cDNA derived from as little as 10 pg total whole brain RNA, while the genomic primers detected as little as 10 pg of rat genomic DNA.
Release experiments
Three hundred μm thick rat striatal slices, prepared as described above, were labelled with [3H]-choline chloride (0.2 μM, for 45 min, at 37°C) and perfused with aCSF at a rate of 0.5 ml min−1. The superfusion buffer contained hemicholinium-3 (10 μM) to inhibit choline re-uptake. After an equilibration period of 45 min, samples were collected at 2 min intervals and counted for radioactivity. Release was stimulated twice in each case by inclusion of 15 mM KCl in the superfusion buffer for 1.5 min. The evoked release for each stimulus was expressed as a percentage of the total amount of radiolabel present in the slice at the point at which release was evoked, and evoked release calculated by subtracting basal release from total release for each stimulus. Modulation of release was assessed by changes in the ratio of the [3H]-label release between two stimuli S1 and S2 (spaced 20 min apart). In control experiments S1 and S2 both contained 15 mM KCl, but in test experiments S1 contained KCl whereas S2 contained KCl plus agonist i.e. CGS 21680 (10 nM) or R-PIA (100 nM). The receptor antagonists CSC (500 nM), DPCPX (100 nM), bicuculline (10 μM), saclofen (1 mM) and naloxone (10 μM) were included in the superfusion buffer for 20 min prior to and during the second stimulus. In some experiments assessing the role of endogenous adenosine, adenosine deaminase was present in the superfusion buffer 20 min prior to and during the second stimulus. However in most experiments adenosine deaminase (I u ml−1) was present throughout the superfusion. The identity of the released [3H]-label during stimulation by 15 mM KCl (in the presence of eserine, 1 mM) was shown to be 60–70% acetylcholine by thin layer chromatography (Marchbanks, 1968), as previously found in this system (Kirkpatrick & Richardson, 1993; 1994; Preston et al., 1999). For thin layer chromatography 0.2 ml samples were collected during superfusion (both during stimulation and basal release), 0.1 μmol of choline and acetylcholine added as carrier, and applied to thin layer cellulose chromatography plates. The plates were developed using a mobile phase of butan-1-ol, acetic acid, water and ethanol (8 : 1 : 3 : 2), and the spots containing choline and acetylcholine visualized using a spray of 4.5% potassium iodide, 0.25% platinic chloride in aqueous ethanol (1 : 1 v v−1). The spots were excised and the radioactivity determined in a liquid scintillation counter. Means±s.e.mean were calculated and statistical significance assessed using Student's t-test.
Results
Cholinergic neurons in striatal slices were identified on the basis of their size (30 μm diameter), and by their resting electrophysiological properties on membrane rupture (Kawaguchi 1992; 1993). Thus these cells had a resting membrane potential of −58.6±1.4 mV, and were frequently found to fire spontaneous action potentials at a rate of 2–5 Hz. These action potentials were associated with a large after hyperpolarization (of 24.1±1.3 mV), as previously observed in interneurons of this type (Kawaguchi 1992; 1993). These cells also showed a decrease in apparent input resistance on injection of hyperpolarizing current as reported previously (Jiang & North, 1991), and also depolarized in response to bath application of the NK1 receptor agonist Sar9, Met(O2)11 Substance P, (Bell et al., 1998). After harvesting the cytoplasmic contents of such cells, reverse transcription and TPEA–PCR, the expression of a variety of genes was assessed in gene specific PCR assays. As shown in Figure 1, the coexpression of choline acetyltransferase with the A1 receptor was observed in 60%, and the adenosine A2A receptor in 67% (n=15) of the cells tested. In contrast PCR primers for preproenkephalin, preprotachykinin and the genomic sequences tested consistently failed to reveal a PCR product.
Figure 1.
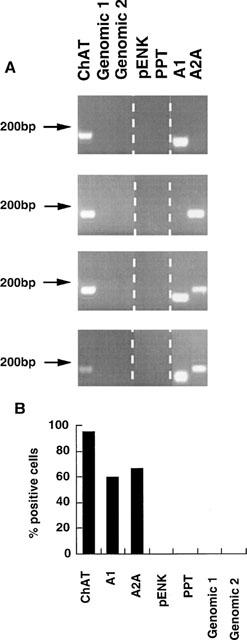
Expression of adenosine A1 and A2A receptors in striatal cholinergic neurons. Cytoplasmic samples from identified cholinergic neurons were subjected to TPEA–PCR followed by gene specific PCR. (A) Typical ethidium bromide stained agarose gels showing the expression of choline acetyltransferase (ChAT), and adenosine A1 and A2A receptors in four cholinergic neurons, but no detection of genomic DNA by genomic primers 1 and 2, nor of preproenkephalin (pENK) nor preprotachykinin (PPT) mRNA. (B) Bar plot showing the expression of these genes in striatal cholinergic neurons, expressed as per cent of the neurons in which expression of these genes was detected, n=25 (ChAT, genomic, preproenkephalin, preprotachykinin), n=15 (A1 and A2A receptors).
Bath application of the A1 receptor agonist R-PIA to the superfusion fluid caused a concentration dependent small hyperpolarization of the large cholinergic neurons. Thus 10 and 100 nM R-PIA had no discernible effect on the resting membrane properties of these neurons (n=3 for each), 1 μM R-PIA caused a 3.2±1.1 mV hyperpolarization (n=8 out of 11 cells), and 5 μM R-PIA produced a 3.8±0.5 mV hyperpolarization (n=5 out of seven cells). The effects of 1 μM R-PIA were retained in the presence of 1 μM TTX (n=4, 3.1±0.5 mV hyperpolarization) and were completely blocked by preincubation of the slice with the A1 receptor antagonist DPCPX (100 M, n=3, Figure 2).
Figure 2.

Whole cell current clamp recording illustrating the effect of R-PIA on the resting electrophysiological properties of a visually identified cholinergic interneuron. Bath application of 1 μM R-PIA produced a reversible hyperpolarization of the neuron which was associated with the cessation of action potential firing (upper panel). This effect of R-PIA is maintained in the presence of 1 μM TTX (middle panel) but is lost in the presence of 100 nM DPCPX (bottom panel. Note that approximately 20 min time interval is present between traces.
The effect of the A1 and A2A receptors on acetylcholine release from striatal slices evoked by a submaximal concentration of KCl (15 mM) was investigated. Inclusion of the A1 receptor antagonist (DPCPX, 100 nM) had no significant effect on the release of [3H]-acetylcholine. In contrast the A2A receptor antagonist CSC (500 nM) decreased the release (Figure 3), which suggested the action of endogenous adenosine within the slice. This was confirmed by inclusion of adenosine deaminase (1 u ml−1) after the first stimulus, which caused a reduction in the release of acetylcholine in the second stimulus (Figure 3).
Figure 3.

Adenosine receptor modulation of ACh release from striatal slices. 3H-ACh release was evoked by two 2 min pulses of 15 mM KCl spaced 20 min apart (S1 and S2) and the S2/S1 ratio are shown after subtraction of basal release. The agonists CGS21680 (10 nM) and R-PIA (100 nM) were administered during S2 only, while the antagonists CSC (500 nM), DPCPX (100 nM), bicuculline (10 μM), saclofen (1 μM) and naloxone (10 μM) were present 20 min before, and during, S2. Antags: combined administration of bicuculline, saclofen and naloxone. (A) Effect of endogenous adenosine. Results are means±s.e.mean of between four and eight experiments, each containing duplicates. AdA: adenosine deaminase present in the superfusion fluid 20 min before and during S2 (1 u ml−1); *indicates significantly different from control (P<0.05). (B) Effect of adenosine receptor ligands in the presence of adenosine deaminase. Adenosine deaminase was present throughout the superfusion of the slices. Results are means±s.e.mean of between four and eight experiments each containing duplicates or triplicates. Antagonists: simultaneous administration of bicuculline (10 μM), saclofen (1 μM) and naloxone (10 μM) *indicates significantly different from control (P<0.05), **indicates significantly different from agonist alone (P<0.05).
In an attempt to reduce the influence of endogenous adenosine, adenosine deaminase was included throughout the superfusion in subsequent experiments. Under these conditions DPCPX had no significant effect on acetylcholine release, although the A1 receptor agonist R-PIA significantly inhibited the release in a DPCPX sensitive manner. In contrast, the selective agonist CGS 21680 (10 nM) significantly enhanced release in a CSC sensitive manner (Figure 3). Since the A2A receptor has been shown to regulate striatal GABA release from striatopallidal (enkephalin containing) neurons, the effect of GABA and opioid antagonists was investigated. The enhancement of acetylcholine release by CGS 21680 was slightly, but not significantly, reduced by the coadministration of bicuculline (10 μM), saclofen (1 μM) and naloxone (10 μM, Figure 3).
Discussion
Striatal cholinergic neurons were identified on the basis of their morphology, resting electrophysiological responses (Kawaguchi, 1992; 1993), response to the NK1 receptor agonist (Sar9, Met(O2)11) Substance P (Bell et al., 1998) and the expression of mRNAs encoding choline acetyltransferase. In these cells mRNA encoding the A1 receptor was detected in 60% of the neurons sampled and the A2A receptor mRNA in 67%. These cells were shown not only to express the receptor messages but also functional receptor protein, since the A1 receptor agonist R-PIA hyperpolarized these cells and inhibited acetylcholine release, while the A2A receptor agonist CGS21680 increased acetylcholine release. Although the majority of these cholinergic neurons express these receptors, those that were apparently negative may represent a distinct subpopulation which do not express these receptors, or alternatively that the mRNAs were below the level of detection in these cells.
The discrepancy between these results and those obtained by in situ hybridization is most probably a consequence of the previously recognised low abundance of A2A receptor mRNA in striatal cholinergic neurons (Dixon et al., 1996), and the high sensitivity of this PCR based technique. It is unlikely that the detection of the adenosine receptors was due to contamination by other cells or genomic DNA since mRNAs encoding preproenkephalin and preprotachykinin (two genes expressed in high abundance in 90% of striatal neurons, Graybiel, 1990) were not detected in our samples, nor were two genomic STS sequences. Expression of the A1 receptor is consistent with some previous in situ hybridization data (Dixon et al., 1996), and with the reported ability of A1 receptor agonists to inhibit acetylcholine release (Brown et al., 1990; Jin et al., 1993).
In an attempt to reconcile conflicting reports concerning the functional effects of adenosine receptors in these cells (Jin et al., 1993; Jin & Fredholm, 1997; Brown et al., 1990; Kirk & Richardson, 1994; Kurokawa et al., 1994) we examined the effect of selective ligands on acetylcholine release in striatal slices. Adenosine deaminase revealed a tonic endogenous adenosine tone which increased acetylcholine release, and was blocked by the presence of the A2A receptor selective antagonist CSC. The increase in release elicited by CGS21680 was also reversed by CSC, but not by a combination of opioid and GABA receptor antagonists, suggesting that the A2A receptor was not acting presynaptically on the GABA and enkephalin containing striatopallidal recurrent collatetral nerve terminals (Kirk & Richardson, 1994; Mori et al., 1996). Since these antagonists were also unable to mimic the effect of CSC on potassium stimulated release, it is probable the endogenous tone was also directly exerted on the cholinergic neuron.
The overall stimulatory action of adenosine contrasts with a tonic inhibitory effect seen in the slices of Jin et al. (1993) and Jin & Fredholm (1997). This difference in the action of endogenous adenosine between the two preparations may underlie the differing effects seen with selective agonists, and could perhaps be explained by different degrees of A2A receptor desensitization during slice preparation.
In conclusion both A1 and A2A receptors are expressed in striatal cholinergic neurons where they are functionally active.
Abbreviations
- CGS21680
2-p-((carboxyethyl)phenylethylamino)-5,-N-ethylcarboxy amidoadenosine
- CSC
8-(3-chlorostyryl)caffeine
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- TPEA PCR
3′ end amplification polymerase chain reaction
References
- BELL M.I., RICHARDSON P.J. , LEE K. Characterisation of the mechanism of action of tachykinins in rat striatal cholinergic interneurones. Neurosci. 1998;87:649–658. doi: 10.1016/s0306-4522(98)00187-0. [DOI] [PubMed] [Google Scholar]
- BRICE A., BERRARD S., RAYNAUD B., ANSIEAU S., COPPOLA T., WEBER M.J. , MALLET J. Complete sequence of a cDNA encoding an active rat choline acetyltransferase: a tool to investigate the plasticity of cholinergic phenotype expression. J. Neurosci. Res. 1989;23:266–273. doi: 10.1002/jnr.490230304. [DOI] [PubMed] [Google Scholar]
- BROWN S.J., JAMES S., REDDINGTON M. , RICHARDSON P.J. Both A1 & A2A purine receptors regulate striatal acetylcholine release. J. Neurochem. 1990;55:31–38. doi: 10.1111/j.1471-4159.1990.tb08817.x. [DOI] [PubMed] [Google Scholar]
- DIXON A.K., GUBITZ A.K., SIRINATHSINGHJI D.J.S., RICHARDSON P.J. , FREEMAN T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON A.K., RICHARDSON P.J., LEE K., CARTER N.P. , FREEMAN T.C. Expression profiling of single-cells using three prime end amplification (TPEA) PCR. Nucl. Acid Res. 1998;26:4426–4431. doi: 10.1093/nar/26.19.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINK J.S., WEAVER D.R., RIVKEES S.A., PETERFREUND R.A., POLLACK A.E., ADLER E.M. , REPPERT S.M. Molecular-cloning of the rat adenosine-A2 receptor - selective coexpression with D2-dopamine receptors in rat striatum. Mol. Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B. , SVENNINGSSON P. Striatal adenosine A2a receptors–where are they? What do they do. Trends in Pharmacol. Sci. 1998;19:46–47. doi: 10.1016/s0165-6147(97)01160-7. [DOI] [PubMed] [Google Scholar]
- GRAYBIEL A.M. Neurotransmitters & neuromodulators in the basal ganglia. Trends in Neurosci. 1990;13:244–254. doi: 10.1016/0166-2236(90)90104-i. [DOI] [PubMed] [Google Scholar]
- GUBITZ A.K., WIDDOWSON L., KUROKAWA M., KIRKPATRICK K.A. , RICHARDSON P.J. Dual signalling by the adenosine A2A receptor involves activation of both N- & P-type calcium channels by different G proteins & protein kinases in the same striatal nerve terminals. J. Neurochem. 1996;67:374–381. doi: 10.1046/j.1471-4159.1996.67010374.x. [DOI] [PubMed] [Google Scholar]
- JIANG Z.-G. , NORTH R.A. Membrane properties and synaptic responses of rat striatal neurons in vitro. J. Physiology. 1991;443:533–553. doi: 10.1113/jphysiol.1991.sp018850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN S.Y. , FREDHOLM B.B. Adenosine A2A receptor stimulation increases release of acetylcholine from rat hippocampus but not striatum & does not affect catecholamine release. Naun. Schmied. Arch. Pharmacol. 1997;355:48–56. doi: 10.1007/pl00004917. [DOI] [PubMed] [Google Scholar]
- JIN S.Y., JOHANSSON B. , FREDHOLM B.B. Effects of adenosine A1 & A2 receptor activation on electrically evoked dopamine & acetylcholine release from rat striatal slices. J. Pharmacol. Exp. Ther. 1993;267:801–808. [PubMed] [Google Scholar]
- KAWAGUCHI Y. Large aspiny cells in the matrix of the rat neostriatum in vitro – physiological identification, relation to the compartments & excitatory postsynaptic currents. J. Neurophysiol. 1992;67:1669–1682. doi: 10.1152/jn.1992.67.6.1669. [DOI] [PubMed] [Google Scholar]
- KAWAGUCHI Y. Physiological, morphological & histochemical characterization of three classes of interneurons in rat neostriatum. J. Neurosci. 1993;13:4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRK I.P. , RICHARDSON P.J. Adenosine A2A receptor mediated modulation of striatal [3H]-GABA & [3H]-ACh release. J. Neurochem. 1994;62:960–966. doi: 10.1046/j.1471-4159.1994.62030960.x. [DOI] [PubMed] [Google Scholar]
- KIRKPATRICK K.A. , RICHARDSON P.J. Adenosine receptor mediated modulation of acetylcholine release from striatal synaptosories. Br. J. Pharmacol. 1993;110:949–954. doi: 10.1111/j.1476-5381.1993.tb13905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUROKAWA M., KIRK I.P., KIRKPATRICK K.A., KASE H. , RICHARDSON P.J. Inhibition by KF17837 of adenosine A2A receptor-mediated modulation of striatal GABA & ACh release. Br. J. Pharmacol. 1994;113:43–48. doi: 10.1111/j.1476-5381.1994.tb16171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE K., DIXON A.K., FREEMAN T.C. , RICHARDSON P.J. Identification of an ATP sensitive potassium channel current in rat striatal cholinergic interneurons. J. Physiol. 1998;510:441–453. doi: 10.1111/j.1469-7793.1998.441bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHBANKS R.M. The uptake of (14C) choline into synaptosomes in vitro. Biochem. J. 1968;110:533–541. doi: 10.1042/bj1100533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORI A., SHINDOU T., ICHIMURA M., NONAKA H. , KASE H. The role of adenosine A2A receptors in regulating GABAergic synaptic transmission in striatal medium spiny neurons. J. Neurosci. 1996;16:605–611. doi: 10.1523/JNEUROSCI.16-02-00605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESTON Z., LEE K., RICHARDSON P.J. , PINNOCK R.D. Tachykinins increase [3H]-acetylcholine release in mouse striatum through multiple receptor subtypes. Neuroscience. 1999;95:367–376. doi: 10.1016/s0306-4522(99)00440-6. [DOI] [PubMed] [Google Scholar]
- REPPERT S., WEAVER D., STEHLE J. , RIVKEES S. Molecular cloning & characterization of a rat A1-adenosine receptor that is widely expressed in brain & spinal cord. Mol. Endocrinology. 1991;5:1037–1048. doi: 10.1210/mend-5-8-1037. [DOI] [PubMed] [Google Scholar]
- RICHARDSON P.J., KASE H. , JENNER P.G. Adenosine A2a receptor antagonists as new agents for the treatment of Parkinson's disease. Trends in Pharmacol. Sci. 1997;18:338–344. doi: 10.1016/s0165-6147(97)01096-1. [DOI] [PubMed] [Google Scholar]
- RICHARDSON P.J., KASE H. , JENNER P.G. Mechanisms of action of striatal adenosine A2a antagonists. Trends in Pharmacol. Sci. 1998;19:47–48. [Google Scholar]
- RIVKEES S., PRICE S. , ZHOU F. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum & basal ganglia. Brain Res. 1995;677:193–203. doi: 10.1016/0006-8993(95)00062-u. [DOI] [PubMed] [Google Scholar]
- SCHIFFMANN S.N., JACOBS O. and , VANDERHAEGEN J.J. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin, but not by substance P neurons: an in situ hybridisation histochemistry study. J. Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- SCHIFFMANN S.N. and , VANDERHAEGHEN J.J. Adenosine A2 receptors regulate the gene expression of striatopallidal & striatonigral neurons. J. Neurosci. 1993;13:1080–1087. doi: 10.1523/JNEUROSCI.13-03-01080.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNINGSSON P., LE MOINE C., KULL B., SUNAHARA R., BLOCH B. , FREDHOLM B.B. Cellular expression of adenosine A2a receptor mRNA in the rat central nervous system with special reference to dopamine innervated areas. Neurosci. 1997;80:1171–1185. doi: 10.1016/s0306-4522(97)00180-2. [DOI] [PubMed] [Google Scholar]


