Abstract
Using in vivo microdialysis in the frontal cortex of the freely moving rat we evaluated the effects of chronic treatment with the serotonin specific reuptake inhibitor (SSRI) fluoxetine in the presence and absence of the 5-HT1A/β-adrenergic antagonist (±)pindolol.
Chronic vehicle treated animals produced no significant response to a challenge with fluoxetine (10 mg kg−1) on day 8 and 15. Alternatively, a significant (P<0.05) decrease in extracellular 5-HT was observed in control animals upon challenge with the 5-HT1A agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT; 0.03 and 0.1 mg kg−1).
Conversely, animals treated with fluoxetine (10 mg kg−1 o.d.) for 7 and 14 days produced a significant (P<0.05) 2 fold increase in extracellular 5-HT when challenged with fluoxetine (10 mg kg−1) on day 8 and 15. Moreover, no significant decrease in extracellular 5-HT was observed upon challenge with either dose of 8-OH-DPAT.
Animals chronically treated with (±)pindolol (10 or 20 mg kg−1 b.i.d.) produced a significant dose-related increase in extracellular 5-HT upon challenge with fluoxetine on day 15 only. Furthermore, both doses produced a significantly blunted response to the low dose challenge of 8-OH-DPAT (0.03 mg kg−1). In addition, 20 mg kg−1 (±)pindolol treated animals also had no response to the higher 0.1 mg kg−1 dose of 8-OH-DPAT.
Animals treated for 14 days with a combination of (±)pindolol (10 or 20 mg kg−1) and fluoxetine were not significantly different from vehicle treated animals when challenged with fluoxetine or 8-OH-DPAT.
Taken together it would therefore appear that although (±)pindolol alone has sufficient intrinsic activity to produce a desensitization of the 5-HT1A receptor, when given in combination with fluoxetine it is able to prevent the desensitization induced by not only fluoxetine but also itself. This may suggest that the clinical augmentation of antidepressant action by pindolol, when co-administered with a SSRI, is via antagonism of the 5-HT1A receptor.
Keywords: 5-Hydroxytryptamine (5-HT, serotonin); microdialysis; frontal cortex; (±)pindolol; fluoxetine; 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT)
Introduction
The use of serotonin specific reuptake inhibitors (SSRI) in the treatment of depression is now common place and has become a relatively effective pharmacotherapy for many depressed patients. A major draw back of SSRI treatment is that many patients experience a 2–4 week delay in effective therapeutic antidepressant (AD) response (Blier & de Montigny, 1994; Gardier et al., 1996). This delay is believed to be due to the time required for adaptive changes in serotonergic neurotransmission to occur. One such adaptation which is thought to play a key role in this mechanism is the desensitization of somatodendritic 5-HT1A autoreceptors. This hypothesis has been supported by preclinical studies using electrophysiology (Chaput et al., 1986; Blier et al., 1987) and in vivo microdialysis techniques (Rutter et al., 1994; Invernizzi et al., 1996; Hjorth et al., 1996; Dawson et al., 1998) which demonstrate that the 5-HT1A autoreceptor undergoes adaptation in response to various SSRI treatments.
Clinical investigations, based upon this rationale, have demonstrated an enhancement of SSRI action by the addition of pindolol, a 5-HT1A/β-adrenergic (β-AR) antagonist, to the treatment regime. The initial clinical report from Artigas et al. (1994) and later by Blier & Bergeron (1995) demonstrated that the combination of (±)pindolol with a SSRI shortened the onset of antidepressant action to a period of 3–7 days in contrast to 2–4 weeks. These initial observations have been confirmed in double blind placebo-controlled studies (Bordet et al., 1998; Zanardi et al., 1997, 1998). However, other studies have yielded conflicting results, demonstrating (±)pindolol to produce, at best, only meager enhancements of SSRI-induced AD activity (Berman et al., 1997; Perez et al., 1997; Tome et al., 1997).
Preclinical evidence has also demonstrated pindolol to potentiate SSRI-induced increases in extracellular serotonin (Dreshfield et al., 1996; Romero et al., 1996a; Hjorth, 1996; Hjorth & Auerbach, 1996; Dawson & Nguyen, 2000) in a comparable manner to more selective 5-HT1A antagonists such as WAY100635 (Romero et al., 1996a,1996b; Hjorth, 1993; Hjorth et al., 1997; Dawson & Nguyen, 1998). However, pindolol in addition to its 5-HT1A activity also has significant affinity for the 5-HT1B autoreceptor and 5-HT1B/D antagonists have been demonstrated to produce similar augmentations of SSRIs (Gobert et al., 1997; Sharp et al., 1997; Dawson & Nguyen, 2000). Moreover, recent data (Assie & Koek, 1996; Bourin, 1998; Dawson & Nguyen, 2000) has suggested that, at least acutely, (±)pindolol produces much of its augmentation of a SSRI via its action at the 5-HT1B receptor. This is further complicated by the fact (±)pindolol has been shown to be a partial 5-HT1A agonist (Hjorth & Carlsson, 1985, 1986; Clifford et al., 1998) and molecules with intrinsic activity do not appear to be as effective at acutely augmenting the increase in extracellular 5-HT induced by a SSRI (Dawson & Nguyen, 1998). Taken together these acute preclinical observations highlight the uncertainty concerning the clinical mechanism of augmentation of a SSRI by (±)pindolol.
The present studies were undertaken to assess the functional neurochemical consequences of prolonged treatment with fluoxetine both alone and in the presence of (±)pindolol using in vivo microdialysis in the freely moving rat. Following chronic treatment with various combinations animals were challenged with either fluoxetine or 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) to assess the status of the 5-HT1A receptor and 5-HT transporter.
Methods
Materials
All chemicals used were analytical grade and were purchased from Aldrich & Sigma chemicals (Milwaukee, WI, U.S.A.). (±) Pindolol, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) and fluoxetine were purchased from Research Biochemicals International (Natick, MA, U.S.A.).
Animals
Male Sprague-Dawley rats (Charles River; 280–350 g) were used in all experiments. Animals were group housed with food and water available ad libitum and maintained on a 12-h light/dark cycle with all work performed during the light phase. Following surgery, the animals were housed separately in plexiglass cages (45×45×30 cm) with free access to food and water.
Chronic dosing procedure
Rats were administered with either vehicle (water), fluoxetine (10 mg kg−1; ip; od), (±)pindolol (10 or 20 mg kg−1, ip, bid) or (±)pindolol+fluoxetine for 7 and 14 days prior to microdialysis studies. All microdialysis studies were initiated 24 h following the last administered dose.
Surgical procedure
Following induction of anaesthesia with gaseous administration of halothane (2%) (Fluothane, Zeneca, Cheshire, U.K.) the animals were secured in a stereotaxic frame with ear and incisor bars. Anaesthesia was maintained by continuous administration of halothane (1–2%). A microdialysis probe guide cannula (CMA/Microdialysis, Stockholm, Sweden) was implanted into the frontal cortex. Coordinates for the frontal cortex were taken according to Paxinos & Watson (1986): RC +3.2, L −3.5, (reference point taken from bregma), V −1.5 from the skull. A subcutaneous cannula (s.c.) was also implanted at this time between the animal's shoulders. Both cannula were secured to the skull using dental acrylic (Plastics One, Roanoke, VA, U.S.A.). The wound was sutured and the animals left to recover for 24 h in their home cages with free access to food and water.
Microdialysis
A pre-equilibrated (perfused over night in aCSF) microdialysis probe (OD 0.5 mm, membrane length 2 mm; CMA/Microdialysis, Sweden) was implanted, via the guide cannula, into the frontal cortex of the unrestrained rat 24 h post surgery. The probe was perfused with artificial cerebrospinal fluid (aCSF) (mM: NaCl 125, KCl 3.0, MgSO4 0.75 and CaCl2 1.2 phosphate buffer 1.75, pH 7.4) at a flow rate of 1 μl min−1. A 3 h stabilization period was allowed following probe implantation after which time microdialysis sampling was carried out by a modification of the method of Dawson & Routledge (1996). Four control samples were taken prior to drug injection to achieve a steady baseline. These four samples were averaged and all subsequent values were expressed as a percentage of this preinjection value. Vehicle or fluoxetine (10 mg kg−1) were injected, via the s.c. cannula following preinjection baseline determination. 8-OH-DPAT (0.03 mg kg−1) was injected following baseline determination and again, at the higher doses of 0.1 mg kg−1, at t=180 min or two vehicle injections. A 20 min sampling regime was used throughout the experimental period. At the end of the experiment probe placement was verified histologically and data from animals with incorrect probe placement were discarded.
Analysis of dialysates
5-Hydroxytryptamine (5-HT; serotonin) was separated by reverse phase high performance liquid chromatography (HPLC) (C18 ODS3 column, 150×3.0 mm, Metachem, Torrance, CA, U.S.A.) and detected using an ANTEC electrochemical detector (ANTEC, Netherlands) set at a potential of 0.65 V vs a Ag/AgCl reference electrode. Mobile phase was delivered by a Jasco PU980 HPLC pump (Jasco Ltd, Essex, U.K.) at 0.5 ml min−1 and contained 0.15 M NaH2PO4 buffer at pH 5.2, 0.25 mM EDTA, 315 μM 1-octane sodium sulphonic acid and 10% methanol/2% isopropanol. Data was acquired using the XChrom software package (VG data systems, Altringham, U.K.).
Data analysis
The fmol perfusate values of transmitters for the first four baseline samples were averaged and this value denoted as 100%. Subsequent sample values were expressed as a percentage of this preinjection control value. All results were analysed by 2-way analysis of variance (ANOVA) with repeated measures followed by pairwise comparisons using Bonferroni adjustment for multiple comparisons using the Statview software application (Abacus Concepts Inc., Berkeley, CA 1996) for the PC.
Results
Effects of chronic treatments on basal extracellular concentrations of 5-HT within the rat frontal cortex
Basal extracellular concentrations of 5-HT in animals treated for 7 and 14 days with either fluoxetine (10 mg kg−1 o.d.), (±)pindolol (10 mg kg−1 b.i.d.) or (±)pindolol+fluoxetine were not significantly different from vehicle treated groups (Table 1). In contrast, extracellular levels of 5-HT were significantly (P<0.05) elevated in animals treated chronically for 14 days with either (±)pindolol (20 mg kg−1 b.i.d.) or (±)pindolol (20 mg kg−1 b.i.d.)+fluoxetine (Table 1).
Table 1.
Effects of chronic treatments on basal extracellular concentrations of 5-HT within the rat frontal cortex
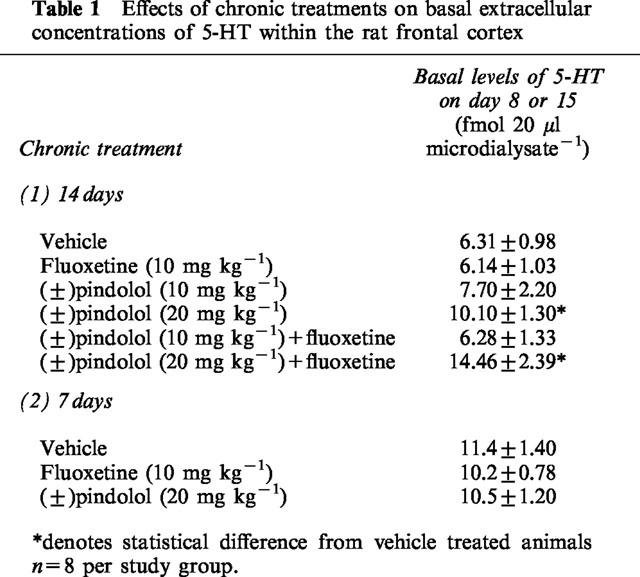
Effects of acute fluoxetine (10 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1) or fluoxetine (10 mg kg−1)
Chronic vehicle treated animals produced no significant change in extracellular 5-HT levels in response to an acute challenge with either fluoxetine (10 mg kg−1 s.c.) or vehicle (Figure 1) on day 15. In contrast, a significant increase in extracellular 5-HT was observed in 14 day chronic fluoxetine treated animals when acutely challenged with fluoxetine on day 15, with a maximum value of 197.1±37.3% of preinjection concentrations (Figure 1). Similarly, animals treated chronically with (±)pindolol (10 or 20 mg kg−1 b.i.d.) produced a dose-related increase in extracellular 5-HT upon challenged with fluoxetine reaching a maximum value of 204.7±23.5% of preinjection concentrations (Figure 1).
Figure 1.
Effects of acute fluoxetine (10 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1) or fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute challenge received. Arrow denotes fluoxetine or vehicle injection point. (P<0.05) denotes groups which attained statistical significance vs chronic vehicle–fluoxetine treated animals.
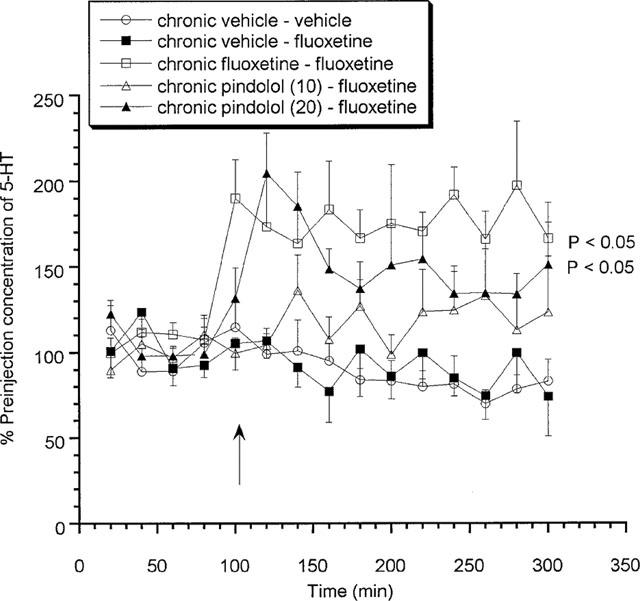
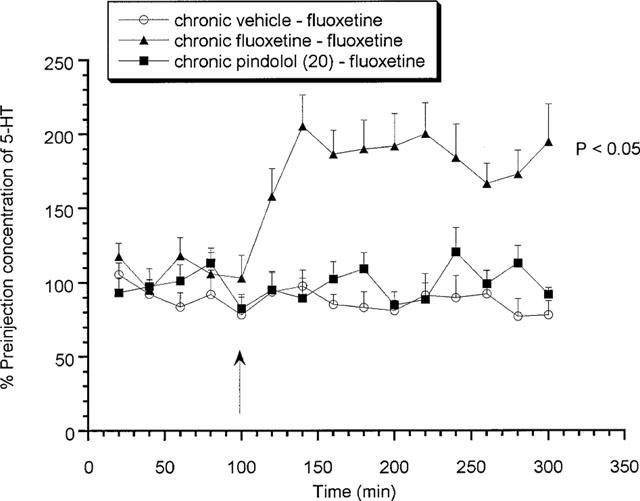
Effects of acute 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1) or fluoxetine (10 mg kg−1)
Chronic vehicle treated animals produced a significant (P<0.05) decrease in extracellular 5-HT concentrations in response to an acute challenge with increasing doses of 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on day 15 (Figure 2). Alternatively, neither dose of 8-OH-DPAT produced any effect on extracellular 5-HT levels in those animals that received 14 days fluoxetine treatment (Figure 2). Animals treated with 10 mg kg−1 (±)pindolol produced a somewhat reduced response to the low dose of 8-OH-DPAT (0.03 mg kg−1) but produced a significant (P<0.05) decrease in response to the higher dose of 0.1 mg kg−1 (Figure 2). Furthermore, animals which received chronic 20 mg kg−1 (±)pindolol produced no response to either the low or high dose of 8-OH-DPAT (Figure 2).
Figure 2.
Effects of acute 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1) or fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute challenge received (i.e. either low followed by a high dose of 8-OH-DPAT or two vehicle injections). Arrows denote 8-OH-DPAT or vehicle injection point. (P<0.05) denotes groups which attained statistical significance vs chronic vehicle–vehicle treated animals.
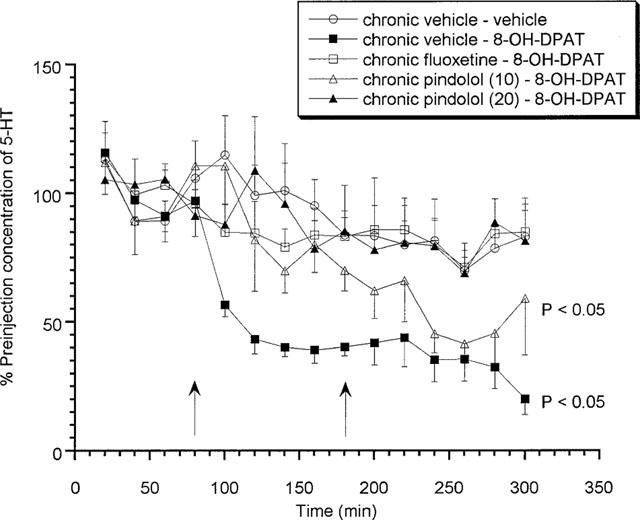
Effects of acute fluoxetine (10 mg kg−1 s.c.) or 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1)+fluoxetine (10 mg kg−1)
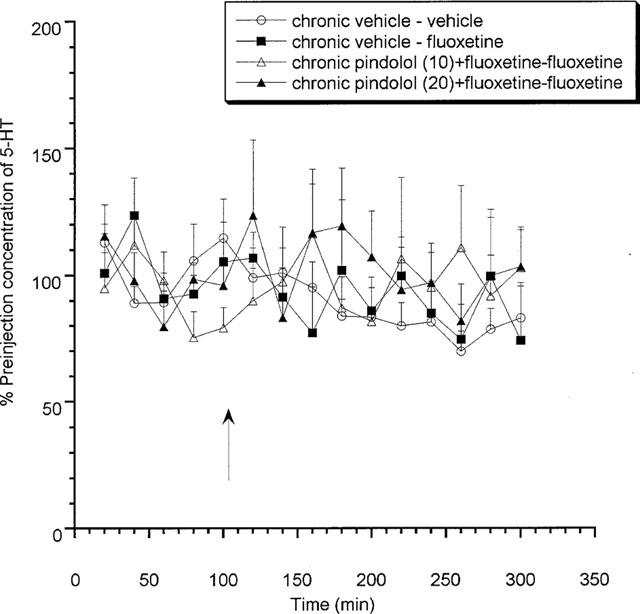
Animals treated chronically with either dose of (±)pindolol (10 or 20 mg kg−1) together with fluoxetine (10 mg kg−1) produced no response to the acute fluoxetine challenge (Figure 3) on day 15. In contrast, both (±)pindolol (10 mg kg−1)+fluoxetine and or (±)pindolol (20 mg kg−1)+fluoxetine treated animals produced a significant (P<0.05) decrease in extracellular 5-HT in response to 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) and this effect was not significantly different from that observed in vehicle treated groups (Figure 4). A comparative summary of all the 14 day chronic treatments can be seen in Table 2.
Figure 3.
Effects of acute fluoxetine (10 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1)+fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute challenge received. Arrow denotes fluoxetine or vehicle injection point.
Figure 4.
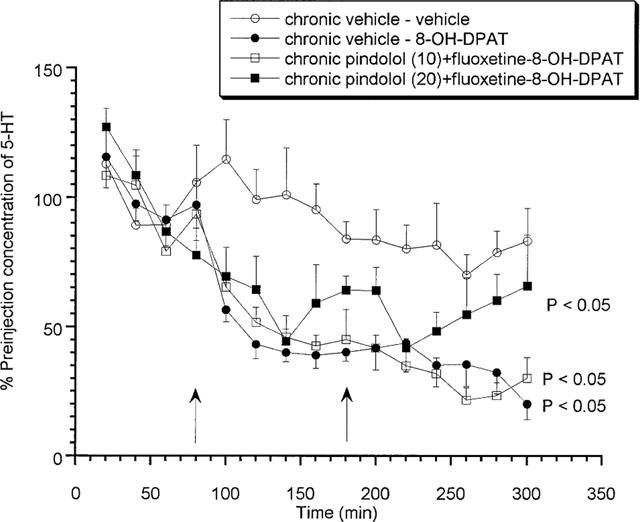
Effects of acute 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 14 days with (±)pindolol (10 or 20 mg kg−1)+fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute challenge received (i.e. either low followed by a high dose of 8-OH-DPAT or two vehicle injections). Arrows denote 8-OH-DPAT or vehicle injection point. (P<0.05) denotes groups which attained statistical significance vs chronic vehicle–vehicle treated animals.
Table 2.
Comparative summary of the effects of acute fluoxetine and 8-OH-DPAT on frontal cortex 5-HT following 7 and 14 day chronic treatment
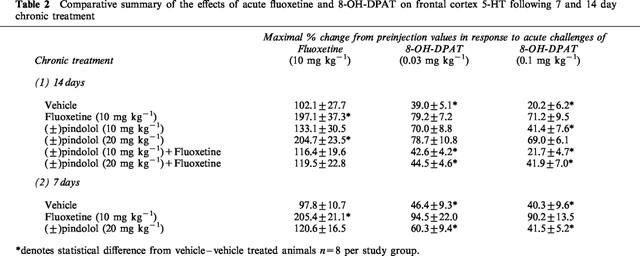
Effects of acute fluoxetine (10 mg kg−1 s.c.) or 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 7 days with (±)pindolol (20 mg kg−1) or fluoxetine (10 mg kg−1)
Animals chronically treated for 7 days with vehicle or 20 mg kg−1 (±)pindolol produced no significant increase in extracellular 5-HT in response to an acute challenge with fluoxetine (Figure 5) on day 8. However, both vehicle and (±)pindolol treated animals produced a significant (P<0.05) 8-OH-DPAT-induced decrease in extracellular 5-HT (Figure 6). Alternatively, 7 days chronic fluoxetine treatment produced a significant (P<0.05) increase in extracellular 5-HT in response to the acute fluoxetine challenge, with a maximum value of 205.4±21.1% of preinjection concentrations (Figure 5), but no response to 8-OH-DPAT (Figure 6).
Figure 5.
Effects of acute fluoxetine (10 mg kg−1 s.c.) on rats treated chronically for 7 days with (±)pindolol (20 mg kg−1) or fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute challenge received. Arrow denotes fluoxetine or vehicle injection point. (P<0.05) denotes groups which attained statistical significance vs chronic vehicle–fluoxetine treated animals.
Figure 6.
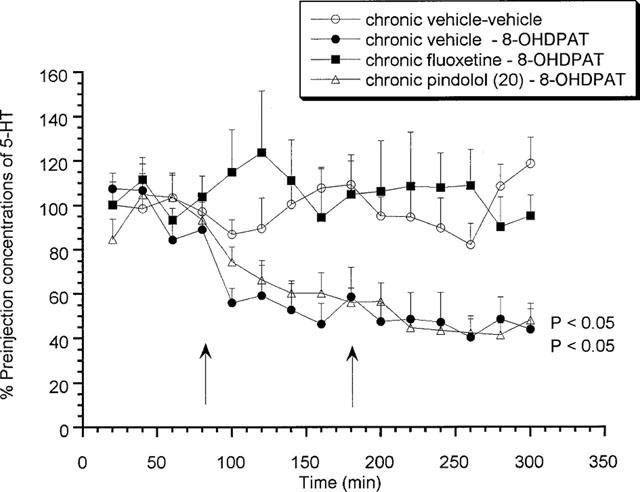
Effects of acute 8-OH-DPAT (0.03 and 0.1 mg kg−1 s.c.) on rats treated chronically for 7 days with (±)pindolol (20 mg kg−1) or fluoxetine (10 mg kg−1). Data expressed as mean±s.e.mean, n=8 per group. Figure legends denote chronic treatment received followed by acute received (i.e. either low followed by a high dose of 8-OH-DPAT or two vehicle injections). Arrows denote 8-OH-DPAT or vehicle injection point. (P<0.05) denotes groups which attained statistical significance vs chronic vehicle–vehicle treated animals.
Discussion
The addition of 5-HT1A/β-adrenergic antagonist (±)pindolol to a SSRI as an augmentation/acceleration strategy in the treatment of depression has received significant interest. To date, five of seven placebo-controlled studies have demonstrated an acceleration in onset of antidepressant activity and three of six claimed an augmentation in efficacy (for reviews see McAskill et al., 1998; Schechter et al., 1999). These effects have been ascribed to the 5-HT1A antagonist activities of (±)pindolol preventing the initial reduction in serotonergic firing (Blier et al., 1987; Gartside et al., 1995) associated with SSRI treatment. This hypothesis has received experimental support from preclinical studies showing that 5-HT1A antagonists can augment a SSRI-mediated increase in extracellular 5-HT (Hjorth, 1993; Hjorth et al., 1997; Ivernizzi et al., 1996; Romero et al., 1996b; Dawson & Nguyen, 1998). However, (±)pindolol has a variety of activities which may be affecting/contributing to its activity: these include partial 5-HT1A agonism, 5-HT1B antagonism and β-adrenergic antagonism. In addition, much of the preclinical evidence in support of pindolol's potentiation mechanism has come from acute treatment studies. Since the therapeutic utility of this compound, when given with a SSRI, for depression has only been observed with prolonged administration we have evaluated the possible mechanisms of action of (±)pindolol when given chronically and in combination with the SSRI, fluoxetine.
These studies indicate that chronic treatment with vehicle for 7 and 14 days produced no response to a challenge with fluoxetine but produced a decrease in extracellular 5-HT in response to the 5-HT1A agonist 8-OH-DPAT. Acutely treated animals produce similar responses to both fluoxetine (Dawson & Nguyen, 1998) and 8-OH-DPAT (unpublished data) indicating that prolonged vehicle treatment produced no detectable changes. In contrast, animals treated for 7 and 14 days with fluoxetine produced a 2 fold increase in extracellular 5-HT in response to the fluoxetine challenge. Moreover, these animals showed no response to the 8-OH-DPAT challenge. Taken together these data suggest that an adaptive change in serotonergic transmission has occurred that appears to be mediated by a desensitization of the 5-HT1A receptor, illustrated by the blunted response to the agonist. Other groups have reported similar results with fluoxetine (Rutter et al., 1994; Le Poul et al., 1995; Invernizzi et al., 1996) and other SSRI's (Bel & Artigas, 1993; Invernizzi et al., 1994), although some investigators have failed to confirm these observations (Bosker et al., 1995; Hjorth & Auerbach, 1994; Gundlah et al., 1997). It is also interesting to note that no significant difference was observed between basal levels of 5-HT between the vehicle and fluoxetine groups which suggests that even after desensitization of the 5-HT1A receptors, there is little tonic activation of this system under these experimental conditions. Moreover, any SSRI still present following chronic administration (i.e. not washed out) is insufficient to significantly elevate basal extracellular 5-HT concentrations within the frontal cortex.
Animals treated for 14 days with either 10 or 20 mg kg−1 of (±)pindolol exhibited a dose-dependent increase in extracellular 5-HT in response to a fluoxetine challenge on day 15. The maximum increase was not significantly different from that observed with 7 and 14 days fluoxetine treatment. Furthermore, both doses produced a significantly blunted response to the low dose challenge of 8-OH-DPAT (0.03 mg kg−1), moreover the 20 mg kg−1 (±)pindolol treated animals also had no response to the higher 0.1 mg kg−1 dose. Conversely, 7 days chronic treatment produced responses to fluoxetine and 8-OH-DPAT, which were not significantly different from vehicle. Taken together these data demonstrate that (±)pindolol, when administered for 14 days, is able to desensitize the 5-HT1A receptor in a dose-dependent manner which is presumably due to (±)pindolol's partial agonist activity. It has been previously demonstrated by various investigators using both in vivo electrophysiology (Clifford et al., 1998; Fornal et al., 1999) and microdialysis (Clifford et al., 1998) that pindolol possesses significant intrinsic activity for the presynaptic 5-HT1A receptor. From these data it would appear that pindolol possesses sufficient activity, when given long term, to produce a similar functional desensitization of the 5-HT1A receptor as that induced by fluoxetine.
In contrast, animals treated with a combination of (±)pindolol (10 or 20 mg kg−1) and fluoxetine produced no response to the day 15 fluoxetine challenge. The decrease induced by 8-OH-DPAT was not significantly different from vehicle for animals receiving chronic 10 mg kg−1 (±)pindolol+fluoxetine. The agonist induced response for 20 mg kg−1 (±)pindolol+fluoxetine treatment was also of a similar magnitude but the duration of the agonist effect appeared to be somewhat shorter for these animals. It would therefore appear that (±)pindolol has sufficient intrinsic activity to produce a desensitization of the 5-HT1A receptor. However, when given in combination with fluoxetine pindolol is able to prevent the desensitization induced by either agent. The reason for this difference is presumably because of the difference in serotonergic tone in the presence of the SSRI, i.e. under lower tonic conditions (±)pindolol acts as an agonist due to the presynaptic receptor reserve, however under conditions of increased serotonergic tone (as would occur when the 5-HT transporter is blocked) the available receptor reserve is greatly reduced (due to increased endogenous agonist) so (±)pindolol is able to behave more as an antagonist. Similar results have been observed with the more selective 5-HT1A antagonist WAY100635 (Dawson et al., 1998) in that the desensitization induced by chronic fluoxetine treatment can be prevented by co-administration of the antagonist. Concurrently, no change in the sensitivity of the 5-HT1A receptor was detected following chronic treatment with the more selective and silent antagonist alone. One interesting difference is that 20 mg kg−1 (±)pindolol ± fluoxetine appears to have produced an increase in basal levels of 5-HT. The reason for this is not totally clear at this time although it may be that high doses of (±)pindolol may have produced some other adaptation response. It is unlikely that this increase is due simply to a desensitization of the 5-HT1A receptor since fluoxetine produced a similar effect but did not increase basal levels. It may also be significant that this occurred at the highest dose of (±)pindolol only, suggesting that this maybe a consequence of some non-selective action. Alternatively, (±)pindolol has significant β-adrenergic activity which has been shown to modulate other transmitter systems (Gobert & Millan, 1999), that may also undergo adaptation upon long term exposure to relatively high doses of pindolol and ultimately lead to alterations in serotonergic transmission within the frontal cortex.
The prolonged administration of both fluoxetine and (±)pindolol has produced a functional adaptation in serotonergic neurotransmission within the frontal cortex. These data demonstrate that this is mediated, at least in part, by the desensitization of the 5-HT1A receptors. The mechanism by which the 5-HT1A desensitization occurs and which sub-population of receptors are involved is not entirely clear. A number of investigators have demonstrated that there is no change in the presynaptic density of 5-HT1A receptors within the dorsal or median raphe neurones (MRN and DRN) following chronic fluoxetine treatment (Le Poul et al., 1995; Li et al., 1996; Raap et al., 1999; Hervas et al., 1999). Therefore, desensitization may be a function of either decreased G-protein coupling or a down regulation of selective G-protein subunits (Li et al., 1996; Raap et al., 1999). Furthermore, recent data has suggested that postsynaptic receptors are also involved in presynaptic regulation of 5-HT release within the prefrontal cortex (Ceci et al., 1994; Casanovas et al., 1999; Hajos et al., 1999) and postsynaptic alterations in various Gα subunits have been detected upon prolonged exposure to fluoxetine (Li et al., 1996). The role of pre vs postsynaptic functional regulation of frontal cortex 5-HT1A receptors cannot be isolated from these experiments and the observations reported may be a net consequence of both autoregulatory adaptation mechanisms. In addition, (±)pindolol also has significant affinity for the 5-HT1B receptor, an activity which has been shown to contribute to its acute augmentation of SSRI's (Assie & Koek, 1996; Bourin et al., 1998; Dawson & Nguyen, 2000) and for β-adrenergic receptors. Clearly the status of the 5-HT1B and β-AR receptors have not been evaluated in this study but alterations in these receptor subtype have been noted following these types of chronic treatment (Gobbi et al., 1997) and as such cannot be ruled out.
The time course of the chronic administration and different pharmacokinetic profiles of these drugs is clearly an issue. Fluoxetine has a long half-life and remains in the system for long periods following administration, making it relatively easy to maintain chronic levels. However, the half-life of pindolol is much shorter so systemic levels will be much more phasic (even with multiple dosing of relatively high concentrations). It could therefore be argued that occupancy of the 5-HT1A receptor is not maintained throughout. However, from the data presented it is apparent that even this phasic dosing of (±)pindolol produces sufficient receptor occupancy to firstly, desensitize the 5-HT1A receptor when given alone and moreover, prevent any detectable fluoxetine-induced desensitization when administered with the SSRI. Alternatively, it could be argued that residual pindolol may remain within the system following chronic treatment. Of course the levels of pindolol remaining and the accumulation cannot be fully evaluated from this study. If significant concentrations of pindolol did remain within the brain and occupied the presynaptic 5-HT1A receptor, then administration of fluoxetine would produce an acute increase in extracellular 5-HT and block the 8-OH-DPAT response. Moreover, pindolol and fluoxetine are metabolized by the same pathway which would in theory further increase the circulating or brain bound concentrations of pindolol. Based on this, the animals which received both pindolol (20 mg kg−1)+fluoxetine are likely to have the highest concentration of pindolol following chronic dosing and thus more likely to produce an increase in response fluoxetine and blunted response to 8-OH-DPAT. Animals which receive this combination produced no fluoxetine-induced increase in 5-HT but showed a 8-OH-DPAT induced decrease, indicating that any residual pindolol present at the time of the acute challenges is insufficient to block the 5-HT1A receptor. In addition, 5-HT1A radioligand binding studies performed in parallel with the microdialysis indicated that Kd and Bmax of [3H]-8-OH-DPAT did not differ among the various treatment groups (unpublished data). This further suggests that at the time of the acute challenges, following chronic pindolol treatment, there was no detectable residual pindolol occupying the 5-HT1A receptor.
In summary, it would appear from these data that (±)pindolol has sufficient intrinsic agonist activity at the 5-HT1A receptor to induce a desensitization of this receptor upon chronic treatment, however, when given in combination with fluoxetine it behaves more like a 5-HT1A antagonist in preventing fluoxetine induced desensitization. This may therefore suggest that the clinical action of pindolol, when co-administered with a SSRI is via its antagonism of the 5-HT1A receptor.
Abbreviations
- AD
antidepressant
- β-AR
β-adrenergic receptor
- DRN
dorsal raphe neurones
- 5-HT
5-hydroxytryptamine (serotonin)
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- SSRI
serotonin specific reuptake inhibitor
References
- ARTIGAS F., PEREZ V. , ALVAREZ E. Pindolol induces a rapid improvement of patients with depression treated with serotonin reuptake inhibitors. Arch. Gen. Psychiatry. 1994;51:248–251. doi: 10.1001/archpsyc.1994.03950030084009. [DOI] [PubMed] [Google Scholar]
- ASSIE M-B. , KOEK W. (−)-Pindolol and (±)-tertatolol affect rat hippocampal 5-HT levels through mechanisms involving not only 5-HT1A, but also 5-HT1B receptors. Neuropharmacology. 1996;35:213–222. doi: 10.1016/0028-3908(95)00169-7. [DOI] [PubMed] [Google Scholar]
- BEL N. , ARTIGAS F. Chronic treatment with fluvoxamine increases extracellular serotonin in frontal cortex but not raphe nuckei. Synapse. 1993;15:243–245. doi: 10.1002/syn.890150310. [DOI] [PubMed] [Google Scholar]
- BERMAN R.M., DARNELL A.M., MILLER H.L., ANAND A. , CHARNEY D.S. Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: a double-blind, placebo-controlled trial. Am. J. Psychiatry. 1997;154:37–43. doi: 10.1176/ajp.154.1.37. [DOI] [PubMed] [Google Scholar]
- BLIER P. , BERGERON R. Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J. Clin. Psychopharmacol. 1995;15:217–222. doi: 10.1097/00004714-199506000-00011. [DOI] [PubMed] [Google Scholar]
- BLIER P. , DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. , CHAPUT Y. Modifications of the serotonin system by antidepressant treatments: implications for the therapeutic response in major depression. J. Clin. Psychopharmacol. 1987;7:24–35. [PubMed] [Google Scholar]
- BORDET R., THOMAS P. , DUPUIS B. Effect of pindolol on onset of action of paroxetine in the treatment of major depression: intermediate analysis of a double-blind, placebo-controlled trial. Am. J. Psychiatry. 1998;155:1346–1351. doi: 10.1176/ajp.155.10.1346. [DOI] [PubMed] [Google Scholar]
- BOSKER F.J., VAN ESSEVELDT K.E., KLOMPMAKERS A.A. , WESTENBERG H.G.M. Chronic treatment with fluvoxamine by osmotic minipumps fails to induce persistent functional changes in central 5-HT1A and 5-HT1B receptors, as measured by in vivo microdialysis in dorsal hippocampus of conscious rats. Psychopharmacology. 1995;117:358–363. doi: 10.1007/BF02246110. [DOI] [PubMed] [Google Scholar]
- BOURIN M., REDROBE J.P. , BAKER G.B. Pindolol does not act on 5-HT1A receptors in augmenting antidepressant activity in the mouse forced swimming test. Psychopharmacology. 1998;136:226–234. doi: 10.1007/s002130050560. [DOI] [PubMed] [Google Scholar]
- CASANOVAS J.P., HERVAS I. , ARTIGAS F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. Neuroreport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- CECI A., BASCHIROTTO A. , BORSINI F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–713. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- CHAPUT Y., BLIER P. , DE MONTIGNY C. Effects of a selective 5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: electrophysiological studies in the rat brain. Naunyn-Schmeideberg's Arch. Pharmacol. 1986;333:342–348. doi: 10.1007/BF00500007. [DOI] [PubMed] [Google Scholar]
- CLIFFORD E.M., GARTSIDE S.E., UMBERS V., COWEN P.J., HAJOS M. , SHARP T. Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autoreceptor in vivo. Br. J. Pharmacol. 1998;124:206–212. doi: 10.1038/sj.bjp.0701796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON L.A. , NGUYEN H.Q. Effects of 5-HT1A receptor antagonists on fluoxetine-induced changes in serotonin in rat frontal cortex. Eur. J. Pharmacol. 1998;345:41–46. doi: 10.1016/s0014-2999(97)01580-x. [DOI] [PubMed] [Google Scholar]
- DAWSON L.A. , NGUYEN H.Q. The role of 5-HT1A and 5-HT1B/1D receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: the mechanism of action of (±) pindolol. Neuropharmacology. 2000;39:1044–1052. doi: 10.1016/s0028-3908(99)00192-6. [DOI] [PubMed] [Google Scholar]
- DAWSON L.A. , ROUTLEDGE C. Differential effects of potassium channel blockers on extracellular levels of serotonin and dopamine in the striatum of conscious rats. Br. J. Pharmacol. 1996;116:3260–3264. doi: 10.1111/j.1476-5381.1995.tb15133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON L.A., NGUYEN H.Q., SMITH D.L. , SCHECHTER L.E. Effects of chronic fluoxetine treatment, in the presence and absence of 5-HT1A receptor blockade, on extracellular concentrations of 5-HT: a microdialysis study. Soc. Neurosci. Abstr. 1998;24:438.11. [Google Scholar]
- DRESHFIELD L.J., WONG D.T., PERRY K.W. , ENGLEMAN E.A. Enhancement of fluoxetine-dependent serotonin (5-HT) levels by (−) pindolol, an antagonist at 5-HT1A receptors. Neurochem. Res. 1996;21:557–562. doi: 10.1007/BF02527753. [DOI] [PubMed] [Google Scholar]
- FORNAL C.A., MARTIN F.J., METZLER C.W. , JACOBS B.L. Pindolol suppresses serotonergic neuronal activity and does not block the inhibition of serotonergic neurons produced by 8-hydroxy-2-(di-n-propylamino)tetralin in awake cats. J. Pharmacol. Exp. Ther. 1999;291:229–238. [PubMed] [Google Scholar]
- GARDIER A.M., MALAGIE I., TRILLAT A.C., JACQUOT C. , ARTIGAS F. Role of 5-HT1A autoreceptors in the mechanism of action of serotonergic antidepressant drugs: recent findings from in vivo microdialysis studies. Fundam. Clin. Pharmacol. 1996;10:16–27. doi: 10.1111/j.1472-8206.1996.tb00145.x. [DOI] [PubMed] [Google Scholar]
- GARTSIDE S.E., UMBERS V., HAJOS M. , SHARP T. Interaction between a selective 5-HT1A receptor antagonist and an SSRI in vivo: effects on 5-HT cell firing and extracellular 5-HT. Br. J. Pharmacol. 1995;115:1064–1070. doi: 10.1111/j.1476-5381.1995.tb15919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOBBI M., CRESPI D., FODDI M.C., FRACASSO C., MANCINI L., PAROTTI L. , MENNINI T. Effects of chronic treatment with fluoxetine and citalopram on 5-HT uptake, 5-HT1B autoreceptors, 5-HT3 and 5-HT4 receptors in rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:22–28. doi: 10.1007/pl00005024. [DOI] [PubMed] [Google Scholar]
- GOBERT A. , MILLAN M.J. Modulation of dialysate levels of dopamine, noradrenaline and serotonin (5-HT) in the frontal cortex of freely moving rats by (−)-pindolol alone and in association with 5-HT reuptake inhibitors: comparative roles of β-adrenergic, 5-HT1A and 5-HT1B receptors. Neuropsychopharmacology. 1999;21:268–284. doi: 10.1016/S0893-133X(99)00035-4. [DOI] [PubMed] [Google Scholar]
- GOBERT A., RIVET J.-M., CISTARELLI L. , MILLAN M.J. Potentation of the fluoxetine-induced increase in the frontal cortex of freely moving rats by combined blockade of 5-HT1A and 5-HT1B receptors with WAY100635 and GR127935. J. Neurochem. 1997;68:1159–1163. doi: 10.1046/j.1471-4159.1997.68031159.x. [DOI] [PubMed] [Google Scholar]
- GUNDLAH C., HJORTH S. , AUERBACH S.B. Autoreceptor antagonists enhance the effect of the reuptake inhibitor citalopram on extracellular 5-HT: the effect persists after repeated citalopram treatment. Neuropharmacology. 1997;36:475–482. doi: 10.1016/s0028-3908(97)00052-x. [DOI] [PubMed] [Google Scholar]
- HAJOS M., HAJOS-KORCSOK E. , SHARP T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br. J. Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERVAS I., VILARO M.T., MENGOD G. , ARTIGAS F. Modulation of the density and sensitivity of 5-HT1A autoreceptors by prolonged treatment with fluoxetine alone or with WAY100635. Soc. Neurosci. Abstr. 1999;24:687.12. [Google Scholar]
- HJORTH S. Serotonin 5-HT1A autoreceptor blockade potentiates the ability of the 5-HT reuptake inhibitor citalopram to increase nerve terminal output of 5-HT in vivo: a microdialysis study. J. Neurochem. 1993;60:776–779. doi: 10.1111/j.1471-4159.1993.tb03217.x. [DOI] [PubMed] [Google Scholar]
- HJORTH S. (−) Pindolol, but not buspirone, potentiates the citalopram-induced rise in extracellular 5-hydroxytryptamine. Eur. J. Pharmacol. 1996;303:183–186. doi: 10.1016/0014-2999(96)00185-9. [DOI] [PubMed] [Google Scholar]
- HJORTH S. , AUERBACH S.B. Lack of 5-HT1A autoreceptor desensitization following chronic citalopram treatment, as determined by in vivo microdialysis. Neuropharmacology. 1994;33:331–334. doi: 10.1016/0028-3908(94)90062-0. [DOI] [PubMed] [Google Scholar]
- HJORTH S. , AUERBACH S.B. 5-HT1A autoreceptors and the mode of action of selective serotonin reuptake inhibitors (SSRI) Behav. Brain Res. 1996;73:281–283. doi: 10.1016/0166-4328(96)00113-1. [DOI] [PubMed] [Google Scholar]
- HJORTH S., BENGTSSON H.J. , MILANO S. Raphe 5-HT1A autoreceptors, but not postsynaptic 5-HT1A receptors or β-adrenoceptors, restrain the citalopram-induced increase in extracellular 5-hydroxytryptamine in vivo. Eur. J. Pharmacol. 1996;316:43–47. doi: 10.1016/s0014-2999(96)00779-0. [DOI] [PubMed] [Google Scholar]
- HJORTH S. , CARLSSON A. (−)-Pindolol stereo specifically inhibits rat brain serotonin (5-HT) synthesis. Neuropharmacology. 1985;24:1143–1146. doi: 10.1016/0028-3908(85)90207-2. [DOI] [PubMed] [Google Scholar]
- HJORTH S. , CARLSSON A. Is pindolol a mixed agonist-antagonist at central serotonin (5-HT) receptors. Eur. J. Pharmacol. 1986;129:131–138. doi: 10.1016/0014-2999(86)90344-4. [DOI] [PubMed] [Google Scholar]
- HJORTH S. , WESTLIN D. , BENGTSSON H.J. WAY100635-induced augmentation of the 5-HT-elevating action of citalopram: relative importance of the dose of the 5-HT1A (auto) receptor blocker versus that of the 5-HT reuptake inhibitor. Neuropharmacology. 1997;36:461–465. doi: 10.1016/s0028-3908(97)00050-6. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M. , SAMANIN R. Chronic treatment with citalopram facilitates the effect of a challenge dose on cortical serotonin output: role of presynaptic 5-HT1A receptors. Eur. J. Pharmacol. 1994;260:243–246. doi: 10.1016/0014-2999(94)90344-1. [DOI] [PubMed] [Google Scholar]
- INVERNIZZI R., BRAMANTE M. , SAMANIN R. Role of 5-HT1A receptors in the effects of acute and chronic fluoxetine on extracellular serotonin in the frontal cortex. Pharmacol. Biochem. Behav. 1996;54:143–147. doi: 10.1016/0091-3057(95)02159-0. [DOI] [PubMed] [Google Scholar]
- LE POUL E., LAARIS N., DOUCET E., LAPORTE A.M., HAMON M. , LANFUMEY L. Early desensitization of somatodendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn-Schmeidebergs Arch. Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- LI Q., MUMA N.A. , VAN DE KAR L.D. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: reductions in hypothalamic and midbrain Gi and G(o) proteins and in neuroendocrine responses to a 5-HT1A agonist. J. Pharmacol. Exp. Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- MCASKILL R., MIR S. , TAYLOR D. Pindolol augmentation of antidepressant therapy. Br. J. Psychiatry. 1998;173:203–208. doi: 10.1192/bjp.173.3.203. [DOI] [PubMed] [Google Scholar]
- PAXINOS G. , WATSON C. Academic Press, New York; 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- PEREZ V., GILABERTE I., FARIES D., ALVAREZ E. , ARTIGAS F. Randomized, double-blind, placebo-controlled trial of pindolol in combination with fluoxetine antidepressant treatment. Lancet. 1997;349:1594–1597. doi: 10.1016/S0140-6736(96)08007-5. [DOI] [PubMed] [Google Scholar]
- RAAP D.K., EVANS S., GARCIA F., LI Q., MUMA N.A., WOLF W.A., BATTAGLIA G. , VAN DE KAR L.D. Daily injections of fluoxetine induce dose-dependent desensitization of hypothalamic 5-HT1A receptors: reductions in neuroendocrine responses to 8-OH-DPAT and in levels of Gz and Gi proteins. J. Pharmacol. Exp. Ther. 1999;288:98–106. [PubMed] [Google Scholar]
- ROMERO L., BEL N., ARTIGAS F., DE MONTIGNY C. , BLIER P. Effects of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: in vivo microdialysis and electrophysiological studies in the rat brain. Neuropsychopharmacology. 1996a;15:349–360. doi: 10.1016/0893-133X(95)00240-E. [DOI] [PubMed] [Google Scholar]
- ROMERO L., ILDEFONSO H. , ARTIGAS F. The 5-HT1A antagonist WAY100635 selectively potentiates the presynaptic effects of serotonergic antidepressants in rat brain. Neurosci. Letts. 1996b;219:123–126. doi: 10.1016/s0304-3940(96)13199-2. [DOI] [PubMed] [Google Scholar]
- RUTTER J.J., GUNDLAH C. , AUERBACH S.B. Increase in extracellular serotonin produced by uptake inhibitors is enhanced after chronic treatment with fluoxetine. Neurosci. Letts. 1994;171:183–186. doi: 10.1016/0304-3940(94)90635-1. [DOI] [PubMed] [Google Scholar]
- SCHECHTER L.E., MCGONIGLE P. , BARRETT J.E. Serotonergic antidepressants: Current and future perspectives. Curr. Opin. Cent. Periph. Nerv. Syst. Invest. Drugs. 1999;1:432–447. [Google Scholar]
- SHARP T., UMBERS V. , GARTSIDE S.E. Effect of a selective 5-HT reuptake inhibitor in combination with 5-HT1A and 5-HT1B receptor antagonists on extracellular 5-HT in rat frontal cortex in vivo. Br. J. Pharmacol. 1997;121:941–946. doi: 10.1038/sj.bjp.0701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOME M.B., ISAAC M.T., HARTE R. , HOLLAND C. Paroxetine and pindolol: a randomized trial of serotonergic autoreceptor blockade in the reduction of antidepressant latency. Int. Clin. Psychopharmacol. 1997;12:81–89. [PubMed] [Google Scholar]
- ZANARDI R., ARTIGAS F., FRANCHINI L., SFORZINI L., GASPERINI M., SMERALDI E. , PEREZ J. How long should pindolol be associated with paroxetine to improve the antidepressant response. J. Clin. Psychopharmacol. 1997;17:446–450. doi: 10.1097/00004714-199712000-00002. [DOI] [PubMed] [Google Scholar]
- ZANARDI R., FRANCHINI L., GASPERINI M., LUCCA A., SMERALDI E. , PEREZ J. Faster onset of action of fluvoxamine in combination with pindolol in the treatment of delusional depression: a controlled study. J. Clin. Psychopharmacol. 1998;18:441–446. doi: 10.1097/00004714-199812000-00004. [DOI] [PubMed] [Google Scholar]


