Abstract
Vasoactive Intestinal Polypeptide (VIP) interacts with a high affinity to two subclasses of G protein coupled receptors named VPAC1 and VPAC2, and has a 3–10 fold preference for VPAC1 over VPAC2 receptors. Selective ligands for each receptor subclass were recently described. [R16]-PACAP (1–23) and [L22]-VIP are two selective VPAC1 agonists.
Chimaeric human VPAC2-VPAC1 recombinant receptors expressed in CHO cells were used to identify the receptor domains implicated in these two selective ligands recognition.
The VPAC2 preference for [R16]-PACAP (1–27) over [R16]-PACAP (1–23) did not require the receptor's NH2-terminus domain but involved the whole transmembrane domain.
In contrast, the selectivity of [L22]-VIP depended only on the presence of the NH2 terminus and EC2 domains of the VPAC1 receptor.
The present data support the idea that in the GPCR-B family of receptors the different selective ligands require different domains for their selectivity, and that the peptides carboxyl terminal sequence (amino acids 24–27) folds back on the transmembrane receptor domain, close to the peptides, aminoterminus.
Keywords: Vasoactive intestinal peptide, VPAC1 receptors, VPAC2 receptors, VPAC1-selective agonists, peptide binding domains
Introduction
Vasoactive intestinal polypeptide (VIP) interacts with two receptor subtypes, previously named VIP1 and VIP2 and currently known as VPAC1 and VPAC2 (Harmar et al., 1998), that have been cloned in rat (Lutz et al., 1993; Ishihara et al., 1992), human (Couvineau et al., 1994; Svoboda et al., 1994) and mouse (Inagaki et al., 1994). These receptors belong to the class B of the G-protein coupled receptors that includes the calcitonin-, the corticotrophin releasing factor-, an insect diuretic hormone-, the gastric inhibitory peptide-, the glucagon-, the glucagon-like peptide I-, the growth hormone releasing factor-, the parathyroid hormone-, the pituitary adenylate cyclase activating polypeptide (PACAP)-, the secretin-, the VPAC1- and VPAC2- and also orphan receptors (Donnelly, 1997).
All these receptors have significant sequence homology (especially in the transmembrane domains) and possess a large extracellular NH2-terminal domain with six conserved cysteine residues. They are all positively coupled to adenylate cyclase but some of them may also be coupled to other effectors (Van Rampelbergh et al., 1997; Spengler et al., 1993).
In situ hybridization reveals that both VIP receptor subtypes are transcribed – and thus probably expressed – in different tissues or cells (Usdin et al., 1994). This is particularly obvious in brain where their localization in many areas appear mutually exclusive (Vertongen et al., 1997). The development of agonists and antagonists selective for each receptor subclass is therefore of great pharmacological importance.
The neuropeptides VIP and PACAP recognize both receptor subtypes with a high affinity (Couvineau et al., 1996; Svoboda et al., 1994). The peptide with a NH2-terminal histidine and a COOH-terminal isoleucine amide (PHI) or its longer form with a COOH-terminal valine amide (PHV) – that are co-synthesized and released with VIP – have a higher affinity for the human VPAC2 than for the human VPAC1 receptor (Couvineau et al., 1996; Gourlet et al., 1998), which is not the case for the rat receptors (Couvineau et al., 1996).
Several synthetic ligands, selective for each receptor subtype have been described recently (Gourlet et al., 1997a,1997b; 1998; Xia et al., 1997). Amongst them, [R16]-PACAP (1–23) (as example of truncated peptide) and [L22]-VIP (as example of single change in the peptide sequence) are selective VPAC1 receptor agonists (this study and Gourlet et al., 1998).
Little is known to date about the receptor determinants that are responsible for the different selectivity profiles observed for these related receptors.
In the present study, we constructed and stably expressed in Chinese hamster ovary (CHO) cells chimaeric receptors made by the replacement of parts of the human VPAC1 receptor sequence by the corresponding sequence of the human VPAC2 receptor to identify the receptor domains involved in this selectivity. The shortened original PACAP (1–27) derivative, [R16]-PACAP (1–23) and a mono-substituted VIP analogue [L22]-VIP were used in this study as VPAC1 selective ligands and were compared to [R16]-PACAP (1–27) and VIP respectively.
Methods
Wild-type receptors
The human VPAC2 receptor cDNA was previously cloned and characterized in our laboratory (Svoboda et al., 1994). The human VPAC1 receptor cDNA was cloned by PCR according to the sequence previously reported by Couvineau et al. (1994) using specific primers. Chimaeric receptor constructs The receptors studied in this work represented eight chimaeric constructs of wild type human VPAC2 and VPAC1 receptors, four of which (chimaeras C1, C4, C7 and C8) have been described previously (Juarranz et al., 1999a,1999b). Chimaeras were designed to replace portions of one wild type receptor cDNA with the corresponding portions of the other receptor. The following constructs were prepared (where the amino acids are numbered according to the human VPAC1 and VPAC2 receptor sequences): VPAC1, human wild-type VPAC1 receptor (1–457); VPAC2, human wild-type VPAC2 receptor (1–438); C1 (N-VPAC2-VPAC1), VPAC2 receptor (1–127) and VPAC1 receptor (144–457); C2 (N→IC1 VPAC2-VPAC1), VPAC2 receptor (1–158) and VPAC1 receptor (175–457); C3 (N→TMD-2 VPAC2-VPAC1), VPAC2 receptor (1–178) and VPAC1 receptor (195–457); C4 (N→EC1 VPAC2-VPAC1), VPAC2 receptor (1–203) and VPAC1 receptor (217–457); C5 (N→TMD-3 VPAC2-VPAC1), VPAC2 receptor (1–227) and VPAC1 receptor (241–457); C6 (N→TMD-4 VPAC2-VPAC1), VPAC2 receptor (1–261) and VPAC1 receptor (275–457); C7 (N→EC2 VPAC2-VPAC1), VPAC2 receptor (1–279) and VPAC1 receptor (294–457); C8 (N→EC3 VPAC2-VPAC1), VPAC2 receptor (1–360) and VPAC1 receptor (374–457).
The strategy was based on the use of PCR overlap extension. Briefly : (i) using human VPAC2 and VPAC1 receptor cDNA as templates and appropriate chimaeric primers, we first generated cDNA fragments overlapping at their 5′ or 3′ extremity; (ii) after purification of these fragments, using High Pure PCR Product Purification Kit (Boehringer, Mannheim), they were used in a round of PCR overlap extension. The use of a phosphorylated forward primer surrounding the ATG initiation codon produced a 5′ hemi-phosphorylated cDNA fragment. This particularity combined with the presence of a 3′-A overhang resulting from the terminal transferase activity of Taq polymerase, allowed the unidirectional cloning of the chimaeric receptors in pCR3.1-Uni (Invitrogen) that was suitable for both prokaryotic and eukaryotic expressions. All PCR reactions were performed using Expand Long Template system (Boehringer, Mannheim) in the Geneamp 2400 thermocycler (Perkin-Elmer). Successful construction of chimaeras was confirmed by nucleotide sequence determination. Receptor expression-stable transfection Recombinant plasmids were transfected into CHO cells by electroporation using a gene pulser (Electroporator II, Invitrogen, San Diego, CA, U.S.A.). Briefly, 4.106 cells were preincubated on ice for 10 min with 20 μg of plasmid DNA in 0.25 ml of F12 nutrient mixture without serum (Gibco Life Technologies, Gent, Belgium). Electroporation was performed at 330 V and 1000 μF. After electroporation, cells were kept on ice for 10 min and then transferred into Petri dishes containing 10 ml of complete culture medium (Dulbecco-F12 supplemented with 10% foetal bovine serum, 2 mM L-glutamine, 100 μg ml−1 penicillin and 100 μg ml−1 streptomycin). After 48 h, cells were selected by addition of geneticin (400 μg ml−1) for 2 weeks. Resistant cells were cloned and the final selection was made on the basis of their ability to express VIP stimulated adenylate cyclase activity. The CHO cells were routinely grown in Dulbecco-F12 medium enriched with 10% foetal bovine serum and geneticin was maintained in the stock cultures only. Peptide synthesis All the non-cyclic peptides were synthesized as carboxyl-terminal amides by solid phase methodology using the Fmoc (9-fluorenylmethoxy carbonyl) strategy on an Applied Biosystems Apparatus 431A (Foster City, CA, U.S.A.). The cleavage and the purification of the peptides have already been described (Robberecht et al., 1992). The purity of the material was at least 95% as judged by capillary electrophoresis and analytical reverse-phase chromatography and the peptide conformity was assessed by electrospray mass spectrometry and, when possible, by sequencing.
Membrane preparation and receptor identification
Stably transfected CHO cells were harvested with a rubber policeman and pelleted by low speed centrifugation; the supernatant was discarded and the cells lysed in 1 mM NaHCO3 solution and immediately frozen in liquid nitrogen. After thawing, the lysate was first centrifuged at 4°C for 10 min at 400×g and the supernatant was further centrifuged at 20,000×g for 10 min. The pellet, resuspended in 1 mM NaHCO3 was used immediately as a crude membrane fraction. Binding was performed as described (Ciccarelli et al., 1994; Vertongen et al., 1997). In all cases, non-specific binding was defined as the residual binding in the presence of 1 μM VIP. Binding was performed at 37°C in a 20 mM (pH 7.4) Tris-maleate buffer with 2 mM MgCl2, 0.1 mg ml−1 bacitracin and 1% bovine serum albumin. Bound radioactivity was separated from free by filtration through glass-fibre GF/C filters pre-soaked for 24 h in 0.01% polyethyleneimine and rinsed three times with a 20 mM (pH 7.4) sodium phosphate buffer containing 1% bovine serum albumin.
Tracer choice
We confirmed in the present work that 125I-RO 25-1553 had a 3 fold higher affinity than 125I-VIP for the VPAC2 receptor (Vertongen et al., 1997), as well as for chimaeras C7 and C8. Consequently, the results obtained with the former tracer were technically more satisfactory (higher total over non-specific binding ratio). We therefore used [125I]-VIP for characterization of the VPAC1, C1 and C4 receptors, and [125I]-RO 25-1553 (Ac1-[Glu8, Lys12, Nle17, Ala19, Asp25, Leu6, Lys27,28, Gly29,30, Thr31]-NH2(cyclo 21–25)) for characterization of the VPAC2, C7 and C8 receptors. At the tracer concentrations used in these experiments (0.2–0.3 fold their Kd values), the unlabelled peptides IC50 values are very close to their inhibition constants, Ki, and should therefore be independent of the nature of the tracer used. We verified that the unlabelled peptides IC50 values were indeed independent of the tracer (125I-RO 25-1553 or 125I-VIP) chosen (not shown).
Specific tracer binding is proportional to the Bmax over KD ratio: it should theoretically be possible to compensate for low tracer affinities by increasing the membrane (and receptor) concentration. Unfortunately, non-specific 125I-RO 25-1553 or 125I-VIP binding also increased with increasing protein concentrations. We were therefore unable to obtain usable competition curves on CHO cell membranes expressing chimaeras C2, C3, C5 and C6, probably because their affinities for 125I-VIP and 125I-RO 25-1553 were too low.
Measurement of adenylate cyclase activity
Adenylate cyclase activity was determined according to the procedure of Salomon et al. (1974) on membranes from CHO cells stably transfected with the recombinant receptors. Membrane proteins (3–10 μg) were incubated in a total volume of 60 μl containing (mM): [α32P]-ATP 0.5, GTP 10, MgCl2 5, EGTA 0.5, cyclic AMP 1, theophylline 1, phospho(enol)pyruvate 10, pyruvate kinase 30 μg ml−1 and Tris-HCl 30 mM at a final pH of 7.8. The reaction was initiated by adding membranes and was terminated after a 15 min incubation at 37°C by adding 0.5 ml of a 0.5% sodium dodecyl-sulphate solution containing ATP 0.5 mM, cyclic AMP 0.5 mM and 20,000 c.p.m. [8-3H]-cyclic AMP. Cyclic AMP was separated from ATP by two successive chromatographies on Dowex 50 W×8 and neutral alumina.
Receptor density: absence of spare receptors?
The adenylate cyclase EC50 value may underestimate the full agonists Kact if the receptor is expressed at a very high density (spare receptors). The maximal responses to VIP and PACAP (1–27) were comparable for all the constructs studied in this work, suggesting that, like wild type receptors, the chimaeric receptors could be expressed at high levels by the CHO cells. It is difficult to estimate correctly the density of VPAC receptors, because our tracers (labelled agonists) discriminate at least two receptor states in transfected CHO cells (Busto et al., 1999). We therefore used functional criteria to ensure that the results were not affected by the presence of spare receptors. Whenever binding studies were possible (VPAC1, VPAC2, C1, C4, C7 and C8 receptors), we verified that the IC50 and EC50 values were in reasonable agreement (EC50⩾IC50). RO 25-1553 behaves as a good partial agonist on clones expressing low human VPAC1 receptor densities, and fully activates the adenylate cyclase at high VPAC1 receptor expression levels (Gourlet et al., 1997b). The observation that it behaved as a partial agonist on chimaeras C2 to C6 therefore supported the hypothesis that the clones selected for this study did not express spare receptors.
Data analysis
All competition- and dose-effect curves were analysed by a non-linear regression program (Graph Pad Prism). They were compatible with recognition of a single binding site. IC50 and EC50 values were the ligand concentrations required for half maximal inhibition of tracer binding and half maximal adenylate cyclase activation, respectively. In order to calculate the peptide-receptor interaction free energy, we used the van't Hoff relationship: ΔG0=−RT ln(EC50 or IC50), where R is the gas constant and T, the absolute temperature. ΔΔG0 represents the difference between the control and modified peptide binding or activation free energies. Differences between mean IC50, EC50 values were tested by Student's t-test. P<0.05 was accepted as being significant. The IC50 and EC50 standard deviations were always below 0.1 log units (typically, 0.05–0.08 log units). We therefore assumed that the standard deviations of the ΔΔG0 values (corresponding to an IC50 or EC50 ratios s.d. <0.2 log unit) were below 1.17 kJ mol−1 K−1.
Results
VIP, PACAP and analogues were tested on recombinant human VPAC1, VPAC2, and chimaeric receptors for their ability to bind to the receptors and to activate adenylate cyclase in membranes from transfected CHO cells. The ligands and a schematic representation of the chimaeric receptors are shown in Table 1 and in the Figures, respectively.
Table 1.
Comparative amino acid sequences of the peptide ligands
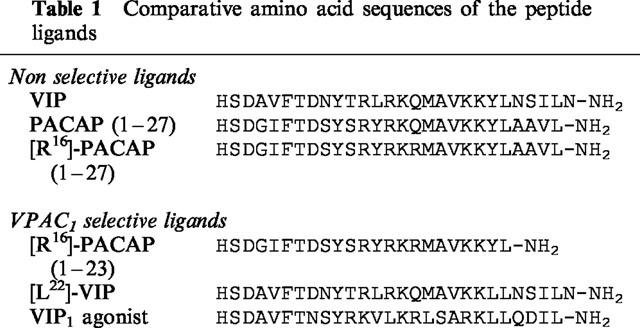
VIP, PACAP (1–27) and [R16]-PACAP (1–27) are considered as non-selective reference ligands. As previously published, the VPAC1 receptor had 5–10 fold lower IC50 and EC50 values for the three agonists than the VPAC2 receptor. Surprisingly, four chimaeric receptors (C2, C3, C5 and C6) showed much higher EC50 values for adenylate cyclase activation than the two wild-type receptors, and 125I-VIP or 125I-RO 25–1553 binding could not be valuably studied on these receptors (see Methods). If we consider only the chimaeric receptors on which binding studies were possible, the VPAC1 receptor sequence 294–373 (TMD-5 to EC3) seemed responsible for the higher affinity of the VPAC1 receptor for these three agonists.
In order to identify the receptor region(s) that interact with the C-terminal peptide sequences, we compared a mono-substituted VIP analogue ([L22]-VIP) with VIP, and the carboxyl-terminally truncated ligand ([R16]-PACAP (1–23)) with [R16]-PACAP (1–27).
[L22]-VIP had a 10 fold lower affinity than VIP for VPAC1 receptors, and a 120 fold lower affinity than VIP for VPAC2 receptors. The effect of the [Y]→[L22]-VIP substitution was 3– 4 fold greater on chimaeras C1 and C4 than on VPAC1 receptor, and 10 to 20 fold greater on chimaeras C7 and C8 (Figure 1 and Table 2). Representing the differences between the VIP-receptor interaction free energy and the [L22]-VIP-receptor interaction free energy (ΔΔG0) in the wild type and the different chimaeric receptors, we observed two ‘steps' in the binding affinity loss: one between VPAC1 and C1 and the other between C6 and C7 (Figure 2, top). This suggested that the VIP tyrosine 22 recognized the VPAC2 receptor N-terminal and EC2 region. Comparison of the VIP and [L22]-VIP EC50 values (adenylate cyclase activation) further supported this hypothesis.
Figure 1.
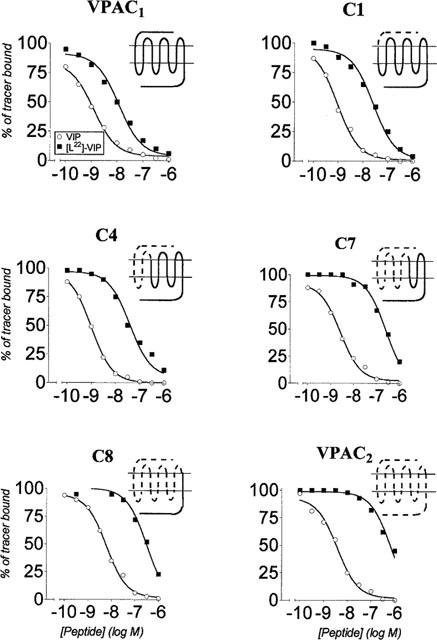
Effect of VIP and [L22]-VIP on tracer binding to membranes from CHO cells expressing the VPAC1, VPAC2 and VPAC2-VPAC1 chimaeric receptors C1, C4, C7 and C8. The values were the means of at least three experiments made in duplicate. The data are expressed as per cent of specific tracer binding in the absence of added ligand.
Table 2.
Interaction of the VIP and [L22]-VIP with the VPAC1, VPAC2 and VPAC2-VPAC1 chimaeric receptors (C1 to C8)
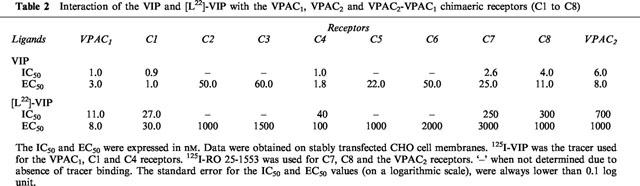
Figure 2.
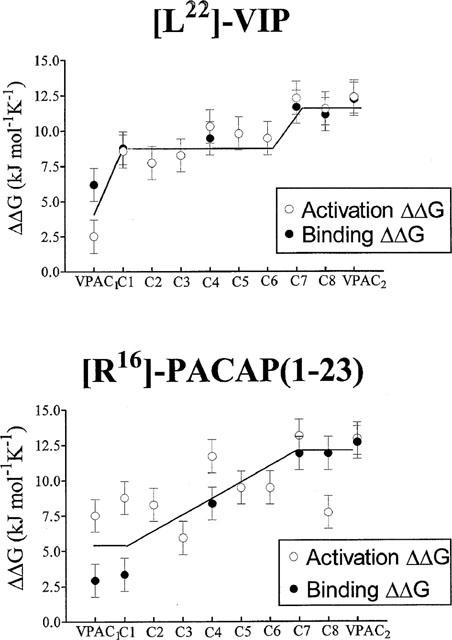
Top panel: The differences between the free energy of binding or activation (±s.d.) of the different constructs by VIP and [L22]-VIP were calculated from at least three experiments. The following binding ΔΔG0 differences were (≠) or were not (=) significantly different: (1): VPAC1, ≠Cl=C4≠C7=C8=VPAC2. Bottom panel: The differences between the free energy of binding or activation (±s.d.) of the different constructs by [R16]-PACAP (1–27) and [R16]-PACAP (1–23) were calculated from at least three experiments. The following binding ΔΔG0 differences were (≠) or were not (=) significantly different: VPAC1=C1≠C4≠;C7=C8=VPAC2.
The other selective ligand tested, [R16]-PACAP (1–23) had a similar (3 fold lower) affinity as the related non selective peptide [R16]-PACAP (1–27) for VPAC1 and C1 receptors, but had a 25 fold lower affinity for C4, and was 100–150 fold less potent on chimaeras C7, C8 and VPAC2 receptors (Table 3 and Figure 3 for binding data). It is possible to estimate the contribution of aminoacids 24–27 to [R16]-PACAP (1–27) recognition by calculating the difference between the binding free energy of [R16]-PACAP (1–27) and [R16]-PACAP (1–23) (ΔΔG°). As shown in Figure 2 (bottom), the receptor amino terminus did not contribute to the discrimination between [R16]-PACAP (1–23) and [R16]-PACAP (1–27) (VPAC1 → C1). The chimaeric receptor's ability to discriminate the two peptides increased progressively, from C1 to C8; the last chimaera behaved like VPAC2 receptors in this respect. Functional data (adenylate cyclase activation) on the eight chimaeric receptors did not allow us to pinpoint more accurately the receptor regions(s) involved in the C-terminal PACAP recognition.
Table 3.
Interaction of PACAP (1–27), [R16]-PACAP (1–27) and [R16]-PACAP (1–23) with the VPAC1, VPAC2 and VPAC2-VPAC1 chimaeric receptors (C1 to C10)
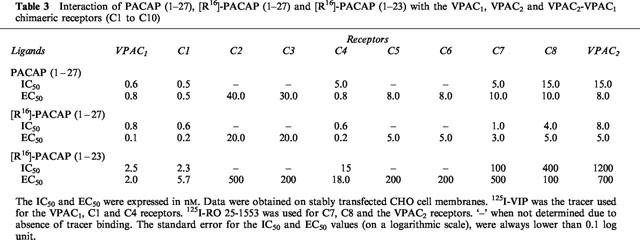
Figure 3.
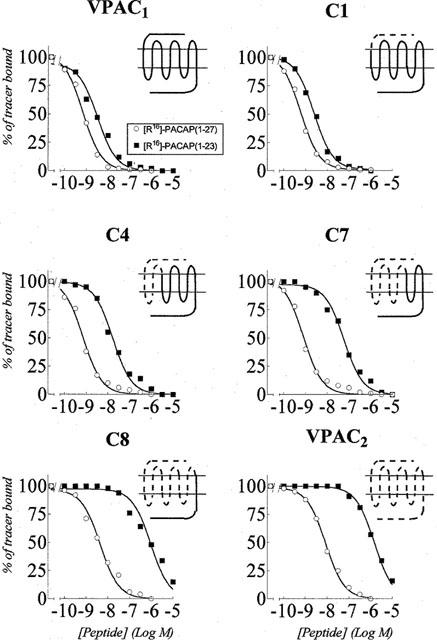
Effect of [R16]-PACAP (1–27) and [R16]-PACAP (1–23) on tracer binding to membranes from CHO cells expressing the VPAC1, VPAC2 and VPAC2-VPAC1 chimaeric receptors C1, C4, C7 and C8. The values were the means of at least three experiments made in duplicate. The data are expressed as per cent of specific tracer binding in the absence of added ligand.
Discussion
It has been established, before the cloning of the receptors, that VIP acts through at least two functionally distinct receptors: the ‘classical' VIP receptor, exemplified by the receptor of the intestinal epithelial cells, now known as the VPAC1 receptor and the helodermin-preferring VIP receptor, exemplified by the receptor of the lymphoblastic cell line SUP T1 and now named VPAC2 receptor.
The importance of the receptor NH2-terminus for selective ligand recognition has already been shown for other related receptors: chimaeric constructions between the rat secretin and the rat VPAC1 receptors, between the rat VPAC1 and rat PACAP (PAC1) receptors, and between human VPAC1 and VPAC2 receptors (Van Rampelbergh et al., 1996; Gourlet et al., 1996c,1996d; Vilardaga et al., 1996; Cao et al., 1995; Hashimoto et al., 1997; Holtmann et al., 1995; 1996a,1996b; Juarranz et al., 1999a,1999b) indicated that in all cases, the NH2-terminal domain is a major contributor to the ligand selectivity.
The ‘Ala-Scan' of VIP performed by O'Donnell et al. (1994) focused our attention on the importance of amino acid [Y22]. By substituting the tyrosine residue present in VIP by a leucine or an alanine residue (Gourlet et al., 1998), single mutated VIP analogues selective for the VPAC1 receptors were obtained. The search for the shortest VIP or PACAP sequence that binds to the VIP and PACAP receptors led to the discovery that the carboxyl-terminally shortened peptides also have a preference for the VPAC1 receptor (Gourlet et al., 1996b), and that PACAP (1–23) retained a relatively high affinity for the VPAC1 receptor but not for the VPAC2 receptor. Furthermore, the introduction in VIP and PACAP analogues of an arginine residue in position 16 increased their affinities for both VIP receptors (Gourlet et al., 1996a). [R16]-PACAP (1–23) was synthesized and behaved as a selective, high affinity VPAC1 receptor ligand (this study).
The aim of the present work was to identify the receptor region(s) recognized by the C-terminal ligand sequence. To achieve this goal, we measured the ability of chimaeric receptors to discriminate two selective ligands, [L22]-VIP and [R16]-PACAP (1–23) from the corresponding non-selective ligands VIP and [R16]-PACAP (1–27), respectively. Chimaeras C1, C4, C7 and C8 had a good affinity for VIP, PACAP (1–27) and [R16]-PACAP (1–27) in binding and functional studies. The chimaeric receptors C2, C3, C5 and C6, in contrast, had surprisingly low affinities for VIP and all the ligands tested: specific binding of the radioiodinated tracers was poor to undetectable, and the peptides potencies (EC50−1) for adenylate cyclase activation were very low (Tables 2 and 3).
The non selective peptides VIP, PACAP (1–27) and [R16]-PACAP (1–27) had a slightly weaker affinity and potency for VPAC2 receptor as compared to VPAC1 receptor. The binding determinant(s) involved appeared to belong to the receptor's transmembrane domain, between TMD-5 and EC3, as chimaeras C1, C4 and C7 had binding and functional properties equivalent to VPAC1, and chimaera C8 to the VPAC2 receptor. We are unfortunately not able to be more precise: the intermediate chimaeras, C5 and C6, had a very weak affinity for the three ligands, suggesting that one of their common anchoring points was unavailable in these constructions. This might be due to incompatibilities between the VPAC2 extracellular loops and VPAC1 transmembrane domain: the extracellular loops EC1 and EC2, that are probably involved in two or three disulphide bridges (Vilardaga et al., 1997), have different lengths and sequences in the two receptors.
[L22]-VIP, with a single (tyrosine to leucine) mutation in position 22, had an 11 fold lower affinity than VIP on VPAC1 receptors and 120 fold lower affinity than VIP on VPAC2 receptors. As shown in Table 2, the introduction of the N-terminal VPAC2 receptor domain was sufficient to decrease the [L22]-VIP affinity, as compared to VIP. This observation is in line with the results of Dong et al. (1999) indicating that the [L22] of secretin is close to the receptor amino-terminal tail in the secretin-receptor complex. A further decrease of [L22]-VIP affinity and potency was observed between chimaeras C4 and C7, the latter chimaeric receptor having an affinity ratio comparable to VPAC2 receptors: Leu22 probably interacted, in addition, with a region found between EC1 and TMD-5, that is, with TMD-3, TMD-4 or EC2. We made the assumption that, as suggested above, chimaeras C5 and C6 had lost a ‘common contact' with all the peptides (contributing approximately 6.3 kJ mol−1 to the peptide-receptor interaction). We calculated the [L22]-VIP over VIP EC50 ratios to estimate the additional contribution of Tyr22 to VIP recognition by the different chimaeras (Figure 2, top). Our results supported the hypothesis that Tyr22 was in contact with EC2 (C6→C7), rather than with TMD-3 or TMD-4.
It is interesting to compare the present results with our previous work on the VIP1 agonist (Table 1) (Juarranz et al., 1999a). The VPAC1>VPAC2 selectivity of the agonist peptide was due for the most part to preferential recognition of the VPAC1 N-terminal and EC1 domains, with a (smaller) contribution of a more distal receptor domain (probably EC2: unpublished data). The Tyr→Leu replacement in position 22 explained the importance of EC2, and part of the contribution of the N-terminal domain for the VIP1-agonist selectivity. Further studies are needed to identify the additional positions that contribute to the VPAC1>VPAC2 selectivity of the VIP1-agonist.
In contrast with [L22]-VIP, [R16]-PACAP (1–23) had the same high affinities for the VPAC1 receptor and chimaera C1. It therefore seems likely that the 3-dimensional structure of [R16]-PACAP (1–23) was conserved, allowing the correct positioning of Tyr22 with respect to the N-terminal receptor domain. The region involved in the [R16]-PACAP (1–23) selectivity (Table 3 and Figure 2, bottom) was the TMD-1 to EC3 region. This result supported the hypothesis that the receptor core (TMD helices+EC loops) may participate to the recognition of the C-terminal sequence (amino acids 22–27 or 28) of the ligand.
The implication of both the NH2-terminal domain and of TMD-1 to EC2 in the selectivity of the VPAC1-preferring peptides studied in this work suggests that the ligands might be ‘sandwiched' between the amino-terminal domain and the upper extracellular part of the core of the receptor. Alternatively, it is also possible that the extracellular loops play an important role in the folding of the N-terminal domain and in its positioning with respect to the peptide ligand.
The present results, taken together with published experimental data, allowed us to propose a general model of the peptide-receptor interaction, as follows: (1) N-terminal peptide binding. VIP, PACAP and secretin have identical sequences in positions 1–3, 6 and 7. We previously suggested that the aspartic acid in position 3 of secretin recognizes highly conserved amino acids in the secretin receptor TMD-2 (Vilardaga et al., 1996), corresponding to Lysine 173 and arginine 188 in the VPAC1 receptor and to lysine 179 and arginine 172 in the VPAC2 receptor. The hypothesis that the VIP amino terminal region (like secretin's) interacts with the transmembrane receptor domain is further supported by the observation that acylation of Histidine1, that increases VIP's affinity for VPAC2 receptor but not VPAC1 receptors, did not increase the peptides' affinity for the VPAC2-VPAC1 chimaeric receptor (C1) but did increase its affinity for the ‘mirror image' chimaera (Juarranz et al., 1999a); (2) Aminoacids 8–15. We previously demonstrated, using a series of hybrid PACAP and secretin peptides and rat VPAC1 and secretin chimaeric receptors that the positions 8 to 15 of secretin and PACAP interacted preferentially with the secretin and VPAC1 receptors N-terminal domains, respectively (Gourlet et al., 1996b,1996c); (3) Aminoacids 22–27. Our present results suggest that the VIP carboxyl terminal region folds back towards the transmembrane region: Tyrosine 22 recognition was affected by the N-terminal domain and EC2, and the C-terminal PACAP sequence (Ala24, Ala25, Val26, Leu27), necessary for high affinity recognition of the VPAC2 transmembrane receptor domain (Gourlet et al., 1996b,1996d); (4) C-terminal extended peptides. The VPAC2-selective peptide, RO 25-1553, differs from [acetyl-His1]VIP in positions 9, 12, 19, 21 and 25–31. In contrast with [R16]-PACAP (1–23) recognition: the transmembrane receptor domain did not contribute to the selective RO 25-1553-VPAC2 receptor interaction (Juarranz et al., 1999b). This suggests that Ala12, Lys19 and the C-terminal extension of RO 25-1553, that markedly contribute to its selectivity interact with the extracellular N-terminal domain (in preparation).
Taken together, our results suggest that the different peptides that discriminate VPAC1 from VPAC2 receptors do so for different reasons, and can be used to visualise the relative receptor-ligand positioning. They are compatible with the hypothesis that the N-terminal peptide sequence forms a turn that is buried in the transmembrane domain; that amino acids 8–22 interact with the extracellular domain but fold back towards the extracellular loops, in order to allow amino acids 24–27 to interact with the transmembrane domain.
Acknowledgments
This work was supported by grants no 3.4507.98 from the F.R.S.M., by an ‘Action de Recherche Concertée' from the Communauté Française de Belgique and by a ‘Interuniversity Poles of Attraction Programme–Belgian State, Prime Minister's Office–Federal Office for Scientific, Technical and Cultural Affairs' and by the PAVE project of the European Community. Maria-Guillerma Juarranz was a post-doctoral fellow from the Marie Curie European Foundation. We thank Dr Philippe Gourlet for the synthesis of the peptides.
Abbreviations
- CHO
Chinese hamster ovary
- EC
extracellular loop
- EC50
concentration of agonist required for half maximal response
- GPCR
G-protein coupled receptor
- IC50
concentration of ligand required for 50% inhibition of tracer binding
- PACAP
pituitary adenylate cyclase activating polypeptide
- TMD
transmembrane domain
- VIP
vasoactive intestinal polypeptide
References
- BUSTO R., JUARRANZ M.G., DE MARIA S., ROBBERECHT P. , WAELBROECK M. Evidence for multiple rat VPAC1 receptor states with different affinities for agonists. Cell Signal. 1999;11:691–696. doi: 10.1016/s0898-6568(99)00041-8. [DOI] [PubMed] [Google Scholar]
- CAO Y.J., GIMPL G. , FAHRENHOLZ F. The amino-terminal fragment of the adenylate cyclase activating polypeptide (PACAP) receptor functions as a high affinity PACAP binding domain. Biochem. Biophys. Res. Commun. 1995;212:673–680. doi: 10.1006/bbrc.1995.2021. [DOI] [PubMed] [Google Scholar]
- CICCARELLI E., VILARDAGA J.P., DE NEEF P., DI PAOLO E., WAELBROECK M., BOLLEN A. , ROBBERECHT P. Properties of the VIP-PACAP type II receptor stably expressed in CHO cells. Regul. Pept. 1994;54:397–407. doi: 10.1016/0167-0115(94)90537-1. [DOI] [PubMed] [Google Scholar]
- COUVINEAU A., ROUYER FESSARD C., DARMOUL D., MAORET J.J., CARRERO I., OGIER D.E. , LABURTHE M. Human intestinal VIP receptor: cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem. Biophys. Res. Commun. 1994;200:769–776. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- COUVINEAU A., ROUYER FESSARD C., MAORET J.J., GAUDIN P., NICOLE P. , LABURTHE M. Vasoactive intestinal peptide (VIP)1 receptor. Three nonadjacent amino acids are responsible for species selectivity with respect to recognition of peptide histidine isoleucineamide. J. Biol. Chem. 1996;271:12795–12800. doi: 10.1074/jbc.271.22.12795. [DOI] [PubMed] [Google Scholar]
- DONG M., WANG Y., PINON D.I., HADAC E.M. , MILLER L.J. Demonstration of a direct interaction between residue 22 in the carboxyl-terminal half of secretin and the amino-terminal tail of the secretin receptor using photoaffinity labeling. J. Biol. Chem. 1999;274:903–909. doi: 10.1074/jbc.274.2.903. [DOI] [PubMed] [Google Scholar]
- DONNELLY D. The arrangement of the transmembrane helices in the secretin receptor family of G-protein-coupled receptors. FEBS Lett. 1997;409:431–436. doi: 10.1016/s0014-5793(97)00546-2. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VANDERMEERS A., VANDERMEERS PIRET M., DE NEEF P., WAELBROECK M. , ROBBERECHT P. Effect of introduction of an arginine 16 in VIP, PACAP and secretin on ligand affinity for the receptors. Biochim. Biophys. Acta. 1996a;1314:267–273. doi: 10.1016/s0167-4889(96)00106-1. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VANDERMEERS A., VANDERMEERS PIRET M., RATHE J., DE NEEF P. , ROBBERECHT P. C-terminally shortened pituitary adenylate cyclase-activating peptides (PACAP) discriminate PACAP I, PACAP II-VIP1 and PACAP II-VIP2 recombinant receptors. Regul. Pept. 1996b;62:125–130. doi: 10.1016/0167-0115(96)00010-9. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VANDERMEERS A., VERTONGEN P., RATHE J., DE NEEF P., CNUDDE J., WAELBROECK M. , ROBBERECHT P. Development of high affinity selective VIP1 receptor agonists. Peptides. 1997a;18:1539–1545. doi: 10.1016/s0196-9781(97)00228-3. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VANDERMEERS PIRET M., RATHE J., DE-NEEF P., CNUDDE J., ROBBERECHT P. , WAELBROECK M. Vasoactive intestinal peptide modification at position 22 allows discrimination between receptor subtypes. Eur. J. Pharmacol. 1998;348:95–99. doi: 10.1016/s0014-2999(98)00133-2. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VERTONGEN P., VANDERMEERS A., VANDERMEERS PIRET M., RATHE J., DE NEEF P., WAELBROECK M. , ROBBERECHT P. The long-acting vasoactive intestinal polypeptide agonist RO 25–1553 is highly selective of the VIP2 receptor subclass. Peptides. 1997b;18:403–408. doi: 10.1016/s0196-9781(96)00322-1. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VILARDAGA J.P., DE NEEF P., VANDERMEERS A., WAELBROECK M., BOLLEN A. , ROBBERECHT P. Interaction of amino acid residues at positions 8-15 of secretin with the N-terminal domain of the secretin receptor. Eur. J. Biochem. 1996c;239:349–355. doi: 10.1111/j.1432-1033.1996.0349u.x. [DOI] [PubMed] [Google Scholar]
- GOURLET P., VILARDAGA J.P., DE NEEF P., WAELBROECK M., VANDERMEERS A. , ROBBERECHT P. The C-terminus ends of secretin and VIP interact with the N-terminal domains of their receptors. Peptides. 1996d;17:825–829. doi: 10.1016/0196-9781(96)00107-6. [DOI] [PubMed] [Google Scholar]
- HARMAR A.J., ARIMURA A., GOZES I., JOURNOT L., LABURTHE M., PISEGNA J.R., RAWLINGS S.R., ROBBERECHT P., SAID S.I., SREEDHARAN S.P., WANK S.A. , WASCHEK J.A. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol. Rev. 1998;50:265–270. [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO H., OGAWA N., HAGIHARA N., YAMAMOTO K., IMANISHI K., NOGI H., NISHINO A., FUJITA T., MATSUDA T., NAGATA S. , BABA A. Vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide receptor chimeras reveal domains that determine specificity of vasoactive intestinal polypeptide binding and activation. Mol. Pharmacol. 1997;52:128–135. doi: 10.1124/mol.52.1.128. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., GANGULI S., HADAC E.M., DOLU V. , MILLER L.J. Multiple extracellular loop domains contribute critical determinants for agonist binding and activation of the secretin receptor. J. Biol. Chem. 1996a;271:14944–14949. doi: 10.1074/jbc.271.25.14944. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., HADAC E.M. , MILLER L.J. Critical contributions of amino-terminal extracellular domains in agonist binding and activation of secretin and vasoactive intestinal polypeptide receptors. Studies of chimeric receptors. J. Biol. Chem. 1995;270:14394–14398. doi: 10.1074/jbc.270.24.14394. [DOI] [PubMed] [Google Scholar]
- HOLTMANN M.H., HADAC E.M., ULRICH C.D. , MILLER L.J. Molecular basis and species specificity of high affinity binding of vasoactive intestinal polypeptide by the rat secretin receptor. J. Pharmacol. Exp. Ther. 1996b;279:555–560. [PubMed] [Google Scholar]
- INAGAKI N., YOSHIDA H., MIZUTA M., MIZUNO N., FUJII Y., GONOI T., MIYAZAKI J. , SEINO S. Cloning and functional characterization of a third pituitary adenylate cyclase-activating polypeptide receptor subtype expressed in insulin-secreting cells. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2679–2683. doi: 10.1073/pnas.91.7.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIHARA T., SHIGEMOTO R., MORI K., TAKAHASHI K. , NAGATA S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- JUARRANZ M.G., VAN RAMPELBERGH J., GOURLET P., DE NEEF P., CNUDDE J., ROBBERECHT P. , WAELBROECK M. Different vasoactive intestinal polypeptide receptor domains are involved in the selective recognition of two VPAC2-selective ligands. Mol. Pharmacol. 1999a;56:1280–1287. doi: 10.1124/mol.56.6.1280. [DOI] [PubMed] [Google Scholar]
- JUARRANZ M.G., VAN RAMPELBERGH J., GOURLET P., DE NEEF P., CNUDDE J., ROBBERECHT P. , WAELBROECK M. Vasoactive intestinal polypeptide VPAC1 and VPAC2 receptor chimeras identify domains responsible for the specificity of ligand binding and activation. Eur. J. Biochem. 1999b;265:449–456. doi: 10.1046/j.1432-1327.1999.00769.x. [DOI] [PubMed] [Google Scholar]
- LUTZ E.M., SHEWARD W.J., WEST K.M., MORROW J.A., FINK G. , HARMAR A.J. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- O'DONNELL M., GARIPPA R.J., RINALDI N., SELIG W.M., SIMKO B., RENZETTI L., TANNU S.A., WASSERMAN M.A., WELTON A. , BOLIN D.R. Ro 25-1553: a novel, long-acting vasoactive intestinal peptide agonist. Part I: In vitro and in vivo bronchodilator studies. J. Pharmacol. Exp. Ther. 1994;270:1282–1288. [PubMed] [Google Scholar]
- ROBBERECHT P., GOURLET P., DE NEEF P., WOUSSEN C.M., VANDERMEERS PIRET M., VANDERMEERS A. , CHRISTOPHE J. Receptor occupancy and adenylate cyclase activation in AR 4-2J rat pancreatic acinar cell membranes by analogs of pituitary adenylate cyclase-activating peptides amino-terminally shortened or modified at position 1, 2, 3, 20, or 21. Mol. Pharmacol. 1992;42:347–355. [PubMed] [Google Scholar]
- SALOMON Y., LONDOS C. , RODBELL M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SPENGLER D., WAEBER C., PANTALONI C., HOLSBOER F., BOCKAERT J., SEEBURG P.H. , JOURNOT L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- SVOBODA M., TASTENOY M., VAN RAMPELBERGH J., GOOSSENS J.F., DE NEEF P., WAELBROECK M. , ROBBERECHT P. Molecular cloning and functional characterization of a human VIP receptor from SUP-T1 lymphoblasts. Biochem. Biophys. Res. Commun. 1994;205:1617–1624. doi: 10.1006/bbrc.1994.2852. [DOI] [PubMed] [Google Scholar]
- USDIN T.B., BONNER T.I. , MEZEY E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- VAN RAMPELBERGH J., GOURLET P., DE NEEF P., ROBBERECHT P. , WAELBROECK M. Properties of the pituitary adenylate cyclase-activating polypeptide I and II receptors, vasoactive intestinal peptide1, and chimeric amino-terminal pituitary adenylate cyclase-activating polypeptide-vasoactive intestinal peptide1 receptors: evidence for multiple receptor states. Mol. Pharmacol. 1996;50:1596–1604. [PubMed] [Google Scholar]
- VAN RAMPELBERGH J., POLOCZEK P., FRANCOYS I., DELPORTE C., WINAND J., ROBBERECHT P. , WAELBROECK M. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIP1) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim. Biophys. Acta. 1997;1357:249–255. doi: 10.1016/s0167-4889(97)00028-1. [DOI] [PubMed] [Google Scholar]
- VERTONGEN P., SCHIFFMANN S.N., GOURLET P. , ROBBERECHT P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides. 1997;18:1547–1554. doi: 10.1016/s0196-9781(97)00229-5. [DOI] [PubMed] [Google Scholar]
- VILARDAGA J.P., DI PAOLO E., BIALEK C., DE NEEF P., WAELBROECK M., BOLLEN A. , ROBBERECHT P. Mutational analysis of extracellular cysteine residues of rat secretin receptor shows that disulfide bridges are essential for receptor function. Eur. J. Biochem. 1997;246:173–180. doi: 10.1111/j.1432-1033.1997.00173.x. [DOI] [PubMed] [Google Scholar]
- VILARDAGA J.P., DI PAOLO E., DE NEEF P., WAELBROECK M., BOLLEN A. , ROBBERECHT P. Lysine 173 residue within the first exoloop of rat secretin receptor is involved in carboxylate moiety recognition of Asp 3 in secretin. Biochem. Biophys. Res. Commun. 1996;218:842–846. doi: 10.1006/bbrc.1996.0150. [DOI] [PubMed] [Google Scholar]
- XIA M., SREEDHARAN S.P., BOLIN D.R., GAUFO G.O. , GOETZL E.J. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J. Pharmacol. Exp. Ther. 1997;281:629–633. [PubMed] [Google Scholar]


