Abstract
Taurine is a sulphonic aminoacid present in high amounts in various tissues including cardiac and skeletal muscles showing different properties such as antioxidative, antimyotonic and anti-schaemic effects. The cellular mechanism of action of taurine is under investigation and appears to involve the interaction of the sulphonic aminoacid with several ion channels.
Using the patch-clamp technique we studied the effects of taurine in rat skeletal muscle fibres on ATP-sensitive K+ channel (KATP) immediately after excision and on channels that underwent rundown.
The cytoplasmic application of 20 mM of taurine reduced the KATP current; this effect was reverted by washout of the drug solution. In this experimental condition the IC50 was 20.1 mM. After rundown, taurine inhibited the KATP current with similar efficacy. Competition experiments showed that taurine shifted the dose-response inhibition curve of glybenclamide to the left on the log-dose axis without significantly affecting those of ATP or Ca2+ ion.
Single channel recording revealed that taurine affects the close state of the channel prolonging it and reducing the bursts duration.
Our data indicate that taurine inhibits the muscular KATP channel interfering with the glybenclamide site on the sulphonylurea receptor of the channel or on the site allosterically coupled to it. During ischaemia and hypoxia, the skeletal and heart muscles undergo several changes; for example, the activation of KATP channels and loss of the intracellular taurine content. The depletion of taurine during ischaemia would contribute to the early activation of KATP channels and salvage the intracellular ATP content.
Keywords: Taurine, skeletal muscle, ATP-sensitive K+ channels
Introduction
Taurine is a sulphonic aminoacid widely distributed in different tissues involved in many physiological processes such as osmoregulation, antioxidant action, and control of Ca2+ homeostasis (Huxtable & Sebring, 1986).
There is emerging evidence that the mechanism of action responsible for the physiological effects of taurine involving two factors is complex: first, the combination of the actions of the sulphonic aminoacid on several types of ion channels, transporters and enzymes; second, the environmental conditions. For example, in cardiac cells the effects of taurine are Ca2+ dependent, being an agonist of the Ca2+ current and delayed rectifier K+ current at low internal Ca2+ concentration (10−8 M) in turn promoting the shortening of the action potential duration (APD) with an inotropic effect (Satoh & Horie, 1997; Satoh, 1998). Conversely, at high Ca2+ ion concentration (10−6 M), taurine inhibits both types of currents resulting in the prolongation of the APD. This phenomenon in conjuction with the inhibition of the cardiac fast Na+ current contributes to the observed anti-ischaemic effect of the sulphonic aminoacid (Schanne & Dumaine, 1992; Satoh, 1998). Thus taurine might be expected to exert inotropic effects or anti-ischaemic effects depending on the internal concentration of the Ca2+ ion (Satoh, 1998). The anti-ischaemic effects of taurine are also supported by the fact that during cardiac ischaemia the intracellular content of the sulphonic aminoacid decreases (Kramer et al., 1981; Saransari & Oja, 1998). It has been proposed that, in cardiac fibres, this phenomenon would promote activation of a class of K+ channel the ATP-sensitive K+ channel (KATP) that is inhibited by taurine in a millimolar concentration range (Satoh, 1996). This is of certain importance, since the sarcolemma KATP channel opens in response to ischaemia insults leading to the repolarization of the fibres, reduction of the influx of Ca2+ ion, thus saving the intracellular ATP content, and in turn promoting cytoprotective effects (McPherson et al., 1993; Hearse, 1995). This idea is supported by the fact that KATP channel-deficiency COS-7 cells, which are vulnerable to chemical hypoxia-reoxygenation injury, when co-transfected with the cardiac KATP channel subunits and in the presence of agonists (KCO), gain resistance to hypoxia-reoxygenation injury (Jovanovic et al., 1998). A cytoprotective role of the KATP channel against ischaemia has also been proposed in the skeletal muscle, in which the openings of the muscular KATP channel by agonists appear to be involved in the anti-infarction effect of the ischaemic preconditioning (Pang et al., 1997).
However, to date the effects of taurine on skeletal muscle KATP channels are not known. Our previous work showed that taurine in skeletal muscle plays a fundamental role in the electrical stabilization of the sarcolemma through an increase of the macroscopic chloride conductance (GCI) (Conte Camerino et al., 1987). This is more clearly demonstrated by the reduction of GCI and by the shift to negative potentials of the voltage threshold for mechanical contraction consequent to experimentally induced taurine depletion (De Luca et al., 1996). In addition, taurine can counteract the low GCI in some forms of pharmacologically induced myotonias in rats (Conte Camerino et al., 1989) and in the age-dependent decrease of GCI (Pierno et al., 1998).
Furthermore, the mechanism by which taurine affects the KATP channel has not been extensively investigated. For example, it is not known whether the aminoacid affects the KATP channel by a mechanism which is dependent on the functional state of the channel complex (e.g. before and after rundown), since the effects of the KATP channel modulators depend on the functional state of the channel (Terzic, 1995; Tricarico et al., 1998a). This is further complicated by the fact that splice variants of the sulphonylurea receptor (SUR), when assembled with the inward rectifier K+ channel (Kir) forming the KATP channel complex (Yokoshiki et al., 1998), are expressed in the skeletal muscle contributing to the variability of the responses of the channel to different modulators (Chutkov et al., 1999).
In the present work we evaluated the effect of taurine on skeletal muscle KATP channels on macropatches immediately after excision or several minutes (on rundown channels) after patch excision. In order to study the interaction of taurine with KATP channels, we performed competition experiments constructing dose-response curves of specific blockers of these channels such as ATP and glybenclamide, and of channel modulator, the Ca2+ ion, in the presence of taurine. Single channel recordings were also performed to evaluate the possible effects of taurine on the channel gating.
Methods
Isolation of single fibres
Single fibres were prepared from flexor digitorum brevis (FDB) muscles of male adult Wistar rats of 5–6 months of age by enzymatic treatment as previously described (Tricarico & Conte Camerino, 1994).
Electrophysiology
Experiments were performed on inside-out membrane patches using standard patch-clamp techniques. Recordings of KATP current (I) were performed on macropatches during voltage steps from 0 mV (holding potential) to −60 mV with 150 mM KCl on both sides of the membrane patches, at 20°C. The spontaneous time-dependent decay of the current was observed at a constant voltage of −60 mV. The currents, sampled at 5 kHz and filtered at 0.5 kHz, were video taped by using a VCR system and played back later for computer analysis (Tricarico et al., 1998a). Macropatch currents were recorded by using Axon hardware and pClamp software (Tricarico & Conte Camerino, 1994). The effects on channels under similar conditions were determined, at −60 mV, immediately after excision, 6±2 min (n=21) (early stage of rundown) and 11±3 min (n=21) (late stage of rundown) after patch excision and in the absence of ATP.
Competition experiments were performed constructing dose-response curves of different antagonists of the KATP channel in the presence of taurine. In particular, the effects of taurine (10 mM) on macropatch currents were evaluated immediately after excision in the absence or in the presence of internal increasing concentrations of ATP (1–500 μM), glybenclamide (0.2–100 nM) or Ca2+ ion (0.2–32 μM).
Pipettes were prepared as previously described (Tricarico & Conte Camerino, 1994). Macropipettes having an average tip opening area of 4.9±0.1 μm2 (n=44) were used to measure the current sustained by multiple channels and the pharmacological properties of the KATP channel.
The single channel conductance and kinetics were measured by using micropipettes having a tip opening area of 0.85±0.03 μm2 (n=21). Having used this type of pipette, no more than 2–3 open channels were observed in the patches. Few patches contained a single active channel.
Analysis
The current (I) flowing through the macropatches was calculated by subtracting the baseline level of the current (defined as the closed state of the channels and measured in the presence of ATP) from the open channel level. The concentration–response relationships could be described by the following equation:
where I drug/I control is the ratio between the current measured in the presence and in the absence of taurine; IC50 is the concentration of taurine needed to reduce the current by 50%; n is the Hill coefficient of the curves; [Drug] is the taurine concentration. The algorithms of the fitting procedures used were based on the Marquardt least-squares fitting routine.
The single channel conductance and the kinetic parameters, the open probability (Popen), the burst durations and the close intervals between bursts were calculated as previously described (Tricarico et al., 1998a,1998b). The overall open probability (Popen) was measured as the ratio between the time spent in the open state and the total time of recording. Kinetic analysis was performed within the bursts of opening when single channel was active in the patches (Tricarico et al., 1998a). The open and close time distributions within the bursts before and after rundown were fitted with the sum of two exponential functions as previously described (Tricarico et al., 1998a).
Significant differences between individual pairs of means were determined by using the paired Student t-test. The data is expressed as mean±s.e.
Drugs and solutions
The solutions had the following composition (in mM): Pipette, KCl 150, CaCl2 2, 3-(N-morpholino)propanesulphonic acid (MOPS) 10, pH=7.2; Bath, normal Ringer NaCl 145, KCl 5.5, MgCl2 1, CaCl2 0.5, glucose 5, MOPS 10, pH=7.2; symmetrical K+ KCl 150, ethylene glycol-bis(b-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA) 0.5, MOPS 10, pH=7.2. Stock solutions (5 mM) of adenosine-triphosphate sodium salt (Na2ATP), and taurine (400 mM) were prepared by dissolving the chemicals in the symmetrical K+ solution. Glybenclamide was first dissolved in dimethylsulphoxide at a concentration of 1 mg ml−1. Aliquots of these solutions were added to the bath solution to give the concentrations required. Free Ca2+ ion concentrations in the bath solution ranging between 0.2 and 32 μM were prepared as previously described (Tricarico et al., 1997). The possible influence of the osmolarity on the KATP channel activity was evaluated by applying a bath solution enriched with saccarose to the internal side of the patches. As previously shown (Tricarico et al., 1997), no significant effects were observed after the addition to the bath of a solution containing 20 and 60 mM sucrose.
Results
Effect of taurine on KATP channel immediately after excision
A large KATP current was elicited immediately by excision of the macropatches (pipette area=4.9±0.1 μm2) from the fibres into an ATP free solution. The internal application of ATP abolished the current confirming that KATP channels sustained it. The effects of taurine were examined in the time frames of 20 s–3 min from patch excision called stage 1 (Figure 1). Indeed, as previously shown (Tricarico et al., 1998a), after 4±1.7 min from excision the KATP current started to rundown (Figure 1). At −60 mV (Vm), in the presence of 150 mM KCl on both sides of the membrane and in the absence of ATP (100 μM) the current had an average amplitude of −252.3±32 pA (n patches=22) per patch area. We found that the application of taurine (1–100 mM) to the patches induced a dose-dependent reduction of the KATP current that was restored after washout of the drug solution (Figure 2A). The concentration of taurine needed to produce the half inhibition of the current was 20.1±6 mM (slope=0.51). At the highest concentration tested (100 mM) taurine did not completely reduce the KATP current (Figure 2B). Experiments were performed to evaluate the possible interaction of taurine with the binding sites of classical channel blockers such as ATP and glybenclamide. In the absence of taurine, ATP inhibited KATP currents dose-dependently with an IC50 of 8.1±0.3 μM (slope=0.65) (Figure 3A) (n patches=7). In the presence of taurine (10 mM) the IC50 value for ATP was 5.9±0.5 μM (slope=0.45) (Figure 3A) (n patches=9). Under these experimental conditions washout restored the current.
Figure 1.
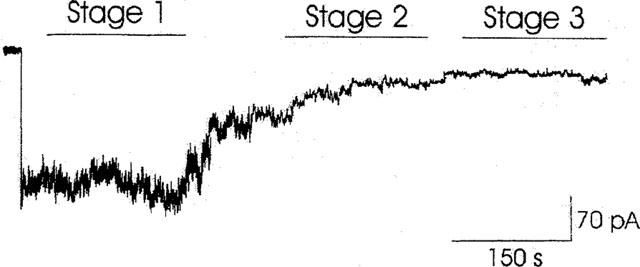
Time-dependent decay of the KATP current in an excised macropatch having a pipette area of 6.1 μm2. Sample trace of continuous recording of KATP current performed, at −60 mV (Vm), on inside-out patch with 150 mM KCl on both sides of the membrane. The background current was subtracted. The decay of the current can be described by three stages of different amplitude. Upon excision the current remained stable for 4 min (stage 1, time frames 20 s–4 min) then slowly decayed reaching a new level of lower amplitude (stage 2, time frames 7 min–10 min). After 12 min the patch became almost silent (stage 3).
Figure 2.
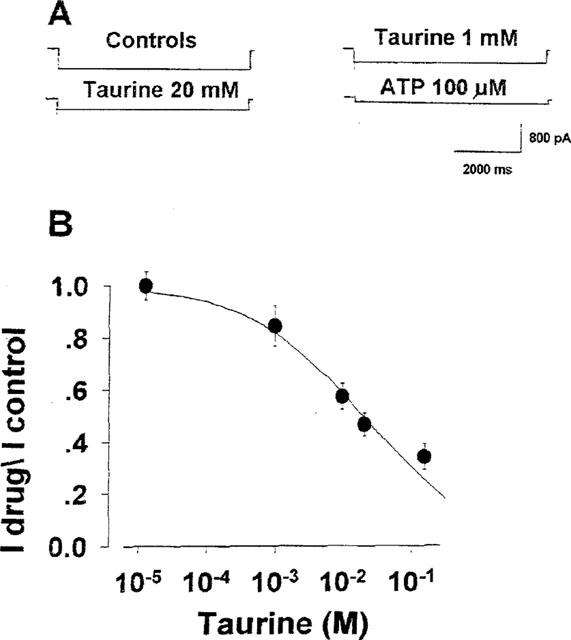
Effects of taurine on KATP currents of rat skeletal muscle recorded immediately after excision (stage 1). Digital average of KATP current traces from 21 patches recorded at −60 mV (Vm), in inside-out configuration, immediately after patch excision. The background currents were subtracted. (A) KATP currents of −566±29 pA of amplitude recorded in the presence of 150 mM KCl on both sides of the membranes. In the presence of 1 mM concentration of taurine the current amplitude was −388±27 pA. At 20 mM concentration the sulphonic aminoacid reduced the current at −311±12 pA of amplitude. The internal application of ATP, at 100 μM concentration, fully reduced the current at −111±12 pA of amplitude confirming that it was sustained by KATP channels. (B) Dose–response curve of the KATP current of rat skeletal muscle fibres versus taurine concentrations. The ordinate represents the ratio between the KATP currents in the presence of taurine and the currents in the absence of the drug (I compound/I control). The abscissa represents the concentrations of taurine. The sulphonic aminoacid inhibited the currents dose-dependently. Each experimental point represents the mean±s.e. of a minimum of five and a maximum of six macropatches.
Figure 3.
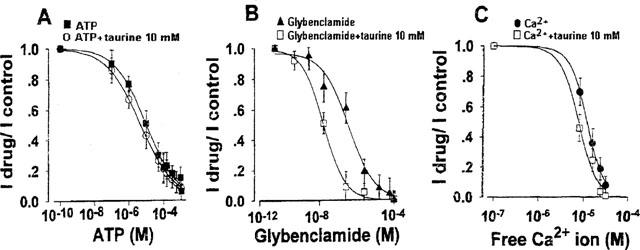
Dose–response curves of KATP current of rat skeletal muscle fibres versus ATP (A) glybenclamide (B) and Ca2+ ion (C) concentrations in the absence or in the presence of taurine (10 mM). The ordinate represents the ratio between the KATP currents in the presence of the blocker and the currents in the absence of the blocker (I compound/I control). The abscissa represents the concentrations of the blocker. The KATP current was recorded at −60 mV (Vm), in inside-out configuration, immediately after patch excision. Taurine shifted the dose-response curve of glybenclamide to the left on the log–dose axis without significantly affecting those of ATP and Ca2+ ion. Each experimental point is the mean±s.e. of a minimum of six and a maximum of nine macropatches.
As expected, the internal application of glybenclamide inhibited the KATP current dose-dependently. Taurine caused a leftward and significant shift of the dose–response curve of glybenclamide (Figure 3B). The IC50 was 50±6 nM (slope=0.41) for gybenclamide alone (n patches=6) and 20±3 nM (slope=0.68) for glybenclamide in the presence of taurine (10 mM), respectively (P>0.001) (n patches=9).
The internal application of different concentrations of free Ca2+ ions to the macropatches reduced the KATP currents, with an IC50 of 11.8±2 μM (slope=2.17) (n patches=7). In the presence of taurine (10 mM), the concentration–response curve of the KATP currents versus Ca2+ concentrations was not significantly modified showing an IC50 of 8.95±3 μM (slope=2.07) (n patches=9) (Figure 3C).
Single channel current was recorded by micropatches (pipette area=0.85±0.03 μm2), at −60 mV (Vm), in the absence and in the presence of 30 mM concentration of taurine. The sulphonic aminoacid reduced the channel open probability by 55.7%. In particular, the Popen decreased from 0.411±0.02 in the controls to 0.182±0.04 (n patches=3) in the presence of taurine. This effect was mediated by the reduction of the mean burst duration which was 48.1±6 ms in the controls and 22.2±5 ms (n patches=3) in the presence of taurine (Figure 4A). The sulphonic aminoacid also prolonged the close time intervals separating the bursts of openings, increasing it from 71±12 ms in the controls to 151±29 ms in the presence of taurine (n patches=3) (Figure 4A).
Figure 4.
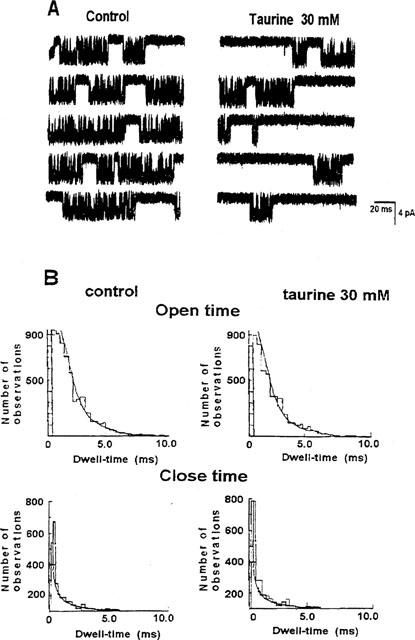
Effects of taurine on a single KATP channel immediately after excision. Downward deflections in the current record indicate inward current. (A) Continuous recording of channel activity was performed few seconds after excision, at −60 mV (Vm), with 150 mM KCl on both sides of the membrane in the absence (control) and in the presence of 30 mM concentration of taurine. The sulphonic aminoacid decreased the burst duration and prolonged the close time intervals within the bursts. (B) Kinetic analysis of the channel traces in A, performed within the bursts of openings. As shown, the open and close time distributions were fitted by two exponential functions not modified by taurine.
Kinetic analysis performed within the bursts of openings revealed that taurine did not alter the open and close time distributions. The open dwell-time distributions was well fitted by the sum of two exponential functions showing τ1 and τ2 values of 0.62±0.06 and 2.15±0.7 ms (n patches=3), respectively, in the controls, and 0.59±0.05 and 2.21±0.09 ms, respectively, in the presence of 30 mM concentration of taurine (Figure 4B). The close dwell-time distribution was well fitted by the sum of two exponential functions showing τ1 and τ2 of 0.34±0.04 and 0.21±0.02 ms, respectively, in the controls, and 0.33±0.06 and 0.19±0.04 ms, respectively, in the presence of the sulphonic aminoacid (Figure 4B).
Taurine did not affect the unitary conductance of the KATP channel. The slope conductance measured in the negative range of potentials was 72±4 pS (n patches=3) in the controls and 69±6 pS in the presence of 30 mM concentration of taurine, whereas in the positive range of potentials it was 40±6 pS (n patches=3) in the controls and 38±8 pS in the presence of the sulphonic aminoacid.
Effect of taurine on KATP channel after rundown
The effects of taurine were also evaluated on the KATP current in the time frames of 7–12 min from patch excision in stages 2 and 3 (Figure 1). After about 7 min from excision, the currents, at −60 mV (Vm), in the absence of taurine, reached a new value of 75±8 pA (n=20) being also sensitive to the stimulatory effect of the nucleoside di-phosphates. In this experimental condition, the application of 30 mM concentration of taurine to the macropatches reduced the currents by 57% (Figure 5) (n patches=4), an effect similar to that observed on channels immediately after excision (Figure 2E).
Figure 5.
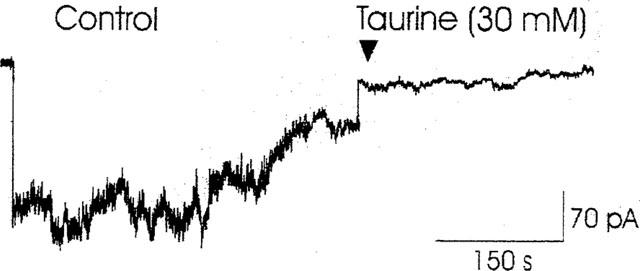
Effects of taurine on KATP currents during rundown (stage 2). The current was recorded by a macropatch having a tip opening area of 6 μm2, at −60 mV (Vm), in the presence of 150 mM KCl on both sides of the membrane. The background current was subtracted. The internal application of 30 mM concentration of taurine to the patch reduced the current by about 60% with respect to that measured in the absence of the sulphonic aminoacid and after rundown.
Single channel recordings were performed, at −60 mV (Vm), after 8 min from excision in the absence and in the presence of 30 mM concentration of taurine. The application of taurine to the excised patches caused a reduction of the channel open probability by 54%, being 0.081±0.03 (n patches=4) in the controls and 0.037±0.012 in the presence of the sulphonic aminoacid. In the same patches, the sulphonic aminoacid reduced the mean burst duration from 18.1±7 ms in the controls to 8.1±5 ms (n patches=4) in the presence of taurine. The sulphonic aminoacid also increased the close time intervals separating the bursts of openings by 53%, being 1520±421 ms in the controls and 3211±511 ms in the presence of taurine (n patches=4) (Figure 6A). No significant change was observed in the open and close time distributions after rundown in the presence of taurine. The τ1 and τ2 of the open dwell-time distributions were 0.48±0.07 ms and 1.71±0.09 ms, respectively, in the controls (n patches=4), and were 0.46±0.05 ms and 1.88±0.08 ms, respectively, in the presence of taurine (Figure 6B). The τ1 and τ2 of the close dwell-time distribution were 0.29±0.05 and 0.19±0.04 ms, respectively, in the controls (n patches=4), and 0.27±0.06 and 0.18±0.06 ms, respectively, in the presence of taurine (Figure 6B).
Figure 6.
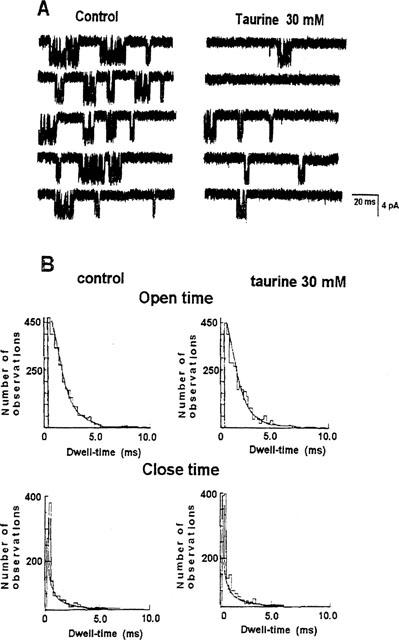
Effects of taurine on a single KATP channel after rundown. Downward deflections in the current record indicate inward current. (A) Continuous recording of channel activity was performed after 8 min from excision, at −60 mV (Vm), with 150 mM KCl on both sides of the membrane in the absence (control) and in the presence of 30 mM concentration of taurine. The sulphonic aminoacid decreased the burst duration and prolonged the close time intervals within the bursts. (B) Kinetic analysis of the channel traces in A, performed within the bursts of openings. As shown, the open and close time distributions were fitted by two exponential functions not modified by taurine.
Taurine did not affect the unitary conductance of the KATP channel. The slope conductance measured in the negative range of potentials was 68±6 pS (n patches=4) in the controls and 67±8 pS in the presence of 30 mM concentration of taurine, whereas in the positive range of potentials it was 38±6 pS (n patches=4) in the controls and 41±8 pS in the presence of the sulphonic aminoacid.
Discussion
We found that taurine behaves as an inhibitor of skeletal muscle KATP channels showing an IC50 of 20 mM. This is not surprising considering that the effects of taurine on several ion channels and transporters are significant in the millimolar concentrations, and that in most tissues the intracellular content of the sulphonic aminoacid ranges between 10 and 60 mM (Pasantes-Morales et al., 1998). In cardiac tissue taurine inhibited the KATP channel with an IC50 of 13.5 mM (Han et al., 1996; Satoh, 1996) which is lower as compared to that calculated in the skeletal muscle in our experiments. At least two mechanisms may explain the different responses of the skeletal and cardiac muscle KATP channels to taurine. First, splice variants of the SUR subunit possibly showing a different pharmacological profile are expressed in the cardiac and skeletal muscles contributing to the variability of the responses of the channels to taurine (Chutkow et al., 1999). Second, in our experiments the dose–response curves were constructed in the presence of multi-channel preparations, whereas in the cardiac cells the experiments were performed on a single unit (Han et al., 1996; Satoh, 1996). This appears to be an important factor in patch clamp experiments in which channel–channel interactions induce a negative cooperativity phenomenon reducing the sensitivity of the channel to the blockers (Tricarico et al., 1998a).
Attempts were made to investigate the mechanism of action of taurine on the muscular KATP channel. The fact that the dose-response curves of the KATP current versus ATP and Ca2+ ion concentrations did not significantly differ in respect to that constructed in the presence of taurine alone, indicates that the sulphonic aminoacid does not interfere with the binding sites for ATP and Ca2+ ion and therefore its effects are not ATP and Ca2+ dependent. Chimera construct studies locate the ATP site on the carboxyterminus of the Kir subunit of the KATP channel complex (Drain et al., 1998), which also appears to be the site for the Ca2+ ion that would bind to the interface between the cytosolic loops containing the ATP site of the Kir (Trap et al., 1997; Drain et al., 1998) and the membrane phospholipids forcing the pore into the non conductive state (Fan & Makielski 1997). In contrast, we found that the sulphonic aminoacid potentiated the inhibitory effect of glybenclamide on the KATP channel. This can be due to an interaction of taurine with a site located on SUR proteins, or alternatively, taurine may affect the KATP channel by interacting with a site allosterically coupled to the SUR. Our data support the hypothesis that taurine allosterically modifies the KATP channel by binding to the polar phase of the membrane phospholipids on sites closely related to the SUR protein, in turn affecting the channel gating. We come to this conclusion considering that in our experiments the mechanism of action of the sulphonic aminoacid appears to be independent of the functional state of the channel being indeed dependent on the channel gating. This is demonstrated by the fact that the effects of taurine on both the macroscopic current and single channel current, measured immediately after patch excision as well as after rundown, do not differ significantly. Whereas, taurine affects the close state of the channel before and after rundown. The fact that the effects of taurine are independent from the functional state of the KATP channel appears to be a unique property of the sulphonic aminoacid; in fact, it is known that several agonists and antagonists of the KATP channel, having specific binding sites on the protein phase of SUR, also show effects which are dependent on the functional state of the channel (Schwanstecher et al., 1998). For example, the action of the sulphonylureas on the KATP channel depends on the operative condition of the channel, being strong inhibitors of cardiac channel at the beginning of ischaemia-poisoning and losing the potency during channel rundown (Findlay, 1992; Brady et al., 1998). Similarly we have found that mexiletine a well known anti-arrhythmic drug of Ib class, blocks skeletal muscle KATP channels by a mechanism of action that depends on the functional state of the channel being more effective on channels immediately after excision and losing the potency after rundown (Tricarico et al., 1998a). It is a common idea that the different responses that the KATP channel shows to the same molecule in various experimental conditions are not related to the chemical structure of the compound, but it depends on the structure and function of the KATP channel complex (Terzic et al., 1995).
In conclusion, the physiological effect of taurine in skeletal muscle appears to be a combination of multiple effects. For example, the activation of Cl− channels in skeletal muscle, and the inhibition of Na+ and Ca2+ channels in heart muscles caused by taurine, can allow a rapid fibre repolarization and savage the energy store of the cells. In the ischaemic and working muscle, these effects can also be sustained by the early openings of the KATP channel provoked by the decrease of the intracellular taurine content (Kramer et al., 1981; Saransari & Oja, 1998).
Acknowledgments
The financial support of Cofin-M.U.R.S.T. 1998 is gratefully acknowledged.
Abbreviations
- APD
action potential duration
- EGTA
ethylene glycol-bis(b-aminoethyl ether)-N,N,N′,N′-tetra-acetic acid
- FDB
flexor digitorum brevis
- GCI
chloride conductance
- IC50
concentration of drug needed to reduce the current by 50%
- I control
current in the absence of drugs
- I drug
current in the presence of drugs
- KATP
ATP-sensitive K+ channels
- KCO
K+ channel agonists
- Kir
inward rectifier K+ channel
- MOPS
3-(N-morpholino)propanesulphonic acid
- n
Hill coefficient of the curves
- Na2ATP
adenosine-triphosphate sodium salt
- Popen
open probability
- SUR
sulfonylurea receptor
- VCR
video recorder
- Vm
voltage membrane
References
- BRADY P.A., ALEKSEEV A.E. , TERZIC A. Operative condition-dependent response of cardiac ATP-sensitive K+ channels toward sulfonylureas. Circ. Res. 1998;82:272–278. doi: 10.1161/01.res.82.2.272. [DOI] [PubMed] [Google Scholar]
- CHUTKOW W.A., MAKIELSKI J.C., NELSON D.J., BURAN C.F. , FAN Z. Alternative splicing of sur2 exon 17 regulates nucleotide sensitivity of the ATP-sensitive potassium channel. J. Biol. Chem. 1999;7/274:13656–13665. doi: 10.1074/jbc.274.19.13656. [DOI] [PubMed] [Google Scholar]
- CONTE CAMERINO D., DE LUCA A., MAMBRINI M., FERRANINI E., FRANCONI F., GIOTTI A. , BRYANT S.H. The effects of taurine on Pharmacologically induced myotonia. Muscle & Nerve. 1989;12:898–904. doi: 10.1002/mus.880121105. [DOI] [PubMed] [Google Scholar]
- CONTE CAMERINO D., FRANCONI F., MAMBRINI M., BENNARDINI F., FAILLI P., BRYANT S.H. , GIOTTI A. The action of taurine on chloride conductance and excitability characteristics of rat striated muscle fibers. Pharmacol. Res. Commun. 1987;19:685–701. doi: 10.1016/0031-6989(87)90099-3. [DOI] [PubMed] [Google Scholar]
- DE LUCA A., PIERNO S. , CONTE CAMERINO D. Effects of taurine depletion on excitation-contraction coupling and Cl− conductance of rat skeletal muscle. Eur. J. Pharmacol. 1996;296:215–222. doi: 10.1016/0014-2999(95)00702-4. [DOI] [PubMed] [Google Scholar]
- DRAIN P., LI L. , WANG J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc. Natl. Acad. Sci. 1998;95:13953–13958. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAN Z. , MAKIELSKI J.C. Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 1997;272/9:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- FINDLAY I. Inhibition of ATP-sensitive K+ channel in cardiac muscle by the sulphonylurea drug glibenclamide. J. Pharmacol. Exp. Ther. 1992;262:71–79. [PubMed] [Google Scholar]
- HAN J., EUIYONG K., WON-KYUNG H. , YUNG E.E. Blockade of the ATP-sensitive potassium channel by taurine in rabbit ventricular myocytes. J. Mol. Cell Cardiol. 1996;28:2043–2050. doi: 10.1006/jmcc.1996.0197. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J. Activation of ATP-sensitive potassium channels: a novel pharmacological approach to myocardial protection. Cardiovasc. Res. 1995;30:1–7. [PubMed] [Google Scholar]
- HUXTABLE R.J. , SEBRING L.J. Towards a unifying theory for the actions of taurine. Trends Pharmacol. Sci. 1986;7/12:481–485. [Google Scholar]
- JOVANOVIC A., JOVANOVIC S., LORENZ E. , TERZIC A. Recombinant Cardiac ATP-Sensitive K+ channel subunits confer resistance to chemical hypoxia-reoxygenation injury. Circulation. 1998;98:1548–1555. doi: 10.1161/01.cir.98.15.1548. [DOI] [PubMed] [Google Scholar]
- KRAMER J.H., CHOVAN J.P. , SCHAFFER S.W. Effect of taurine on calcium paradox and ischemic hearth failure. Am. J. Physiol. 1981;240/2:H238–246. doi: 10.1152/ajpheart.1981.240.2.H238. [DOI] [PubMed] [Google Scholar]
- MCPHERSON C.D., PIERCE G.N. , COLE W.C. Ischemic cardioprotection by ATP-sensitive K+ channel involves high-energy phosphate preservation. Am. J. Physiol. 1993;265:H1809–H1818. doi: 10.1152/ajpheart.1993.265.5.H1809. [DOI] [PubMed] [Google Scholar]
- PANG C.Y., NELIGAN P., XU H., HE W., ZHONG A., HOPPER R. , FORREST C.R. Role of ATP-sensitive K+ channels in ischemic preconditioning of skeletal muscle against infarction. Am. J. Physiol. 1997;42:H44–H51. doi: 10.1152/ajpheart.1997.273.1.H44. [DOI] [PubMed] [Google Scholar]
- PASANTES-MORALES H., QUESADA O. , MORAN J.Taurine: an osmolytein mammalian tissues Taurine 1998New York: Plenum Press; 209–215.eds Schaffer et al., pp [Google Scholar]
- PIERNO S., DE LUCA A., CAMERINO C., HUXTABLE R.J. , CONTE CAMERINO D. Chronic administration of taurine to aged rats improves the electrical and contractile properties of skeletal muscle fibers. J. Pharmacol. Exp. Ther. 1998;286:1183–1190. [PubMed] [Google Scholar]
- SARANSARI P. , OJA S.S. Mechanism of ischemia-induced taurine release in mouse hippocampal slices. Brain Research. 1998;807:118–124. doi: 10.1016/s0006-8993(98)00793-8. [DOI] [PubMed] [Google Scholar]
- SATOH H. Direct Inhibition by Taurine of the ATP-sensitive K+ Channel in Guinea Pig Ventricular Cardiomyocytes. Gen. Pharm. 1996;27/4:625–627. doi: 10.1016/0306-3623(95)02068-3. [DOI] [PubMed] [Google Scholar]
- SATOH H.Cardiac action of taurine as a modulator of the ion channels Taurine 1998New York: Plenum Press; 121–128.eds. Schaffer pp [DOI] [PubMed] [Google Scholar]
- SATOH H. , HORIE M. Actions of taurine on the L-type Ca++ channel current in guinea pig ventricular cardiomyocites. J. Cardiovasc. Pharmacol. 1997;30:711–716. doi: 10.1097/00005344-199712000-00002. [DOI] [PubMed] [Google Scholar]
- SCHANNE O.F. , DUMAINE R. Interaction of taurine with the fast Na-current in isolated rabbit myocytes. J. Pharm. Exp. Ther. 1992;263/3:1233–1240. [PubMed] [Google Scholar]
- SCHWANSTECHER M., SIEVERDING C., DORSCHNER H., GROSS I., AGUILAR-BRYAN L., SCHWANSTECHER C. , BRYAN J. Potassium channel openers require ATP to bind to and act through sulphonylurea receptors. EMBO J. 1998;17/9:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERZIC A., JAHANGIR A. , KURACHI Y. Cardiac ATP-sensitive K+ channel: regulation by intracellular nucleotides and K+ channel-opening drugs. Am. J. Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- TRAP S., TUCKER S.J. , ASHCROFT F.M. Activation and inhibition of K-ATP currents by guanine nucleotides is mediated by different channel subunits. Proc. Natl. Acad. Sci. 1997;94:8872–8877. doi: 10.1073/pnas.94.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRICARICO D. , CONTE CAMERINO D. ATP-sensitive K+ channels of skeletal muscle fibers from young adult and aged rats: possible involvement of thiol-dependent redox mechanisms in the age-related modifications of their biophysical and pharmacological properties. Mol. Pharmacol. 1994;46:754–761. [PubMed] [Google Scholar]
- TRICARICO D., BARBIERI M., FRANCHINI C., TORTORELLA V. , CONTE CAMERINO D. Effects of mexiletine on ATP sensitive K+ channel of rat skeletal muscle fibres: a state dependent mechanism of action. Br. J. Pharmacol. 1998a;125:858–864. doi: 10.1038/sj.bjp.0702117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRICARICO D., PETRUZZI R. , CONTE CAMERINO D. Changes of the biophysical properties of calcium-activated potassium channels of rat skeletal muscle fibres during aging. Pflug. Archiv. 1997;434:822–829. doi: 10.1007/s004240050471. [DOI] [PubMed] [Google Scholar]
- TRICARICO D., PIERNO S., MALLAMACI R., SIRO BRIGIANI G., CAPRIULO R., SANTORO G. , CONTE CAMERINO D. The biophysical and pharmacological characteristics of skeletal muscle ATP-sensitive K+ channels are modified in K+-depleted rat, an animal model of hypokalaemic periodic paralysis. Mol. Pharmacol. 1998b;54:197–206. doi: 10.1124/mol.54.1.197. [DOI] [PubMed] [Google Scholar]
- YOKOSHIKI H., SUNAGAWA M., SEKI T. , SPERELAKIS N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am. J. Physiol. 1998;274:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]


