Abstract
The vanilloid receptor (VR1) is a ligand-gated ion channel, which plays an important role in nociceptive processing. Therefore, a pharmacological characterization of the recently cloned rat VR1 (rVR1) was undertaken.
HEK293 cells stable expressing rVR1 (rVR1-HEK293) were loaded with Fluo-3AM and then incubated at 25°C for 30 min with or without various antagonists or signal transduction modifying agents. Then intracellular calcium concentrations ([Ca2+]i) were monitored using FLIPR, before and after the addition of various agonists.
The rank order of potency of agonists (resiniferatoxin (RTX)>capsaicin>olvanil>PPAHV) was as expected, and all were full agonists. The potencies of capsaicin and olvanil, but not RTX or PPAHV, were enhanced at pH 6.4 (pEC50 values of 7.47±0.06, 7.16±0.06, 8.19±0.06 and 6.02±0.03 respectively at pH 7.4 vs 7.71±0.05, 7.58±0.14, 8.10±0.05 and 6.04±0.08 at pH 6.4).
Capsazepine, isovelleral and ruthenium red all inhibited the capsaicin (100 nM)-induced Ca2+ response in rVR1-HEK293 cells, with pKB values of 7.52±0.08, 6.92±0.11 and 8.09±0.12 respectively (n=6 each). The response to RTX and olvanil were also inhibited by these compounds. None displayed any agonist-like activity.
The removal of extracellular Ca2+ abolished, whilst inhibition of protein kinase C with chelerythrine chloride (10 μM) partially (∼20%) inhibited, the capsaicin (10 μM)-induced Ca2+ response. However, tetrodotoxin (3 μM), nimodipine (10 μM), ω-GVIA conotoxin (1 μM), thapsigargin (1 μM), U73122 (3 μM) or H-89 (3 μM) had no effect on the capsaicin (100 nM)-induced response.
In conclusion, the recombinant rVR1 stably expressed in HEK293 cells acts as a ligand-gated Ca2+ channel with the appropriate agonist and antagonist pharmacology, and therefore is a suitable model for studying the effects of drugs at this receptor.
Keywords: Vanilloid, capsaicin, calcium, ligand-gated ion channel, FLIPR, nociception
Introduction
Improving the treatment of acute, and more especially chronic, pain is a major challenge for the pharmaceutical industry. Most current treatments either block prostaglandin production or activate opioid receptors, but both of these mechanisms are associated with significant side effects (Kress & Zeilhofer, 1999). A more promising alternative approach would be to block the activity of nociceptor-specific receptors. The term nociceptor is used to describe the afferent C- and A-δ fibres, which are activated by a wide range of noxious chemical, mechanical and thermal stimuli and are responsible for the first order transmission of nociceptive signalling from the periphery to the central nervous system (Dray, 1995; Furst, 1999).
One characteristic of nociceptors is that they are activated by capsaicin, a pungent chemical present in hot peppers (Bevan & Szolcsanyi, 1990; Szallasi & Blumberg, 1999). Moreover, this activation is specific to the nociceptors, long-lasting and, in mammals, results in a sensation of burning pain (Simone et al., 1987; Park et al., 1995). As capsaicin is not endogenous to mammals it presumably mimics the actions of an endogenous ligand or another physiological stimulus (Dray, 1995; Tominaga et al., 1998). Studies using 3H-labelled resiniferatoxin (RTX), another plant-derived noxious vanilloid, and the competitive antagonist capsazepine have demonstrated that nociceptors express a specific capsaicin-binding site, referred to as the vanilloid receptor (Bevan et al., 1992; Rinder et al., 1996; Szallasi & Blumberg, 1999). This receptor has subsequently been further characterized using a range of vanilloid ligands including olvanil, PPAHV, isovelleral and ruthenium red (Jung et al., 1998; Szallasi & Blumberg, 1999). The vanilloid receptor acts as a ligand-gated ion channel, allowing Na2+ and Ca2+ influx into the sensory neurones (Bevan & Szolcsanyi, 1990).
More recently a major breakthrough in the study of vanilloid receptor pharmacology occurred when the rat vanilloid receptor (rVR1) was cloned from the dorsal root ganglion (DRG) (Caterina et al., 1997). rVR1 consists of 838 amino acids, with six putative transmembrane domains, and is most closely related to the transient receptor potential channel family (Caterina et al., 1997; Tominaga et al., 1998). The molecular mass (95 kDa) of rVR1, and its distribution are consistent with that of the native vanilloid receptor (Caterina et al., 1997; Tominaga et al., 1998). Furthermore, when expressed in Xenopus oocytes (Caterina et al., 1997) or HEK293 cells (Tominaga et al., 1998) rVR1 acts as a non-selective cation channel, with similar electrophysiological properties to the native vanilloid-gated ion channel. Indeed, rVR1 was not only activated by capsaicin, but also by noxious heat and low pH (Caterina et al., 1997; Tominaga et al., 1998). Furthermore, the capsaicin-induced response was enhanced at pH 6.3 (Caterina 1997; Tominaga et al., 1998). Taken collectively these data confirm the important integratory role vVR1 plays for multiple pain-producing stimuli (Tominaga et al., 1998; Kress & Zeilhofer, 1999). However, there were some discrepancies between the pharmacology of rVR1 and the native receptor, most notably the low relative potency of RTX compared to capsaicin at rVR1 (Szallasi & Blumberg 1999). Moreover, as both studies of rVR1 only used a very limited range of vanilloid ligands (Caterina et al., 1997; Tominaga et al., 1998), they did not address the issue of potential receptor subtypes raised by the various different agonist-dependent responses reported in native tissues (Kress & Zeilhof, 1999; Szallasi & Blumberg 1999).
Therefore, the present study was undertaken to address these issues. rVR1 was cloned, stably expressed in HEK293 cells, and a detailed pharmacological characterization conducted using the Ca2+-sensitive dye, Fluo-3AM in a fluorometric imaging plate reader (FLIPR). The pharmacology of the recombinant rVR1 was confirmed and extended, with some agonist-dependent aspects of the rVR1-mediated response being identified for the first time in a homogenous system. This suggests that the pharmacology of rVR1 can account for some, but not all, of the putative vanilloid receptor subtypes identified pharmacologically in native tissues.
Methods
Cloning and expression of rVR1 receptors in HEK293 cells
A rat VR1 mammalian expression construct was prepared by amplifying cDNA from reverse transcribed adult rat DRG mRNA, using forward and reverse primers incorporating the restriction sites shown, RVR1F-HindIII (5′-cataagcttgccgccatggaacaacgggctagcttagactcagagg) and RVR1R-XbaI (5′-cattctagaccattatttctcccctgggaccatgg). Reaction products were cloned into pBSSKII+ (Stratagene), confirmed by sequencing and then subcloned into the HindIII-XbaI sites of pcDNA3.1 (Invitrogen). HEK 293 cells were transfected with rVR1.pcDNA3.1 using Lipofectamine Plus (Life Technologies), according to the manufacturer's instructions, followed by selection in 400 μg ml−1 geneticin (Life Technologies) and colony cloning. Stable clones were chosen for further analysis on the basis of rVR1 mRNA expression levels and functional expression of rVR1.
Cell culture
rVR1-HEK293 cells were routinely grown as monolayers in minimum essential medium (MEM) supplemented with non-essential amino acids, 10% foetal calf serum, and 0.2 mM L-glutamine, and maintained under 95%/5% O2/CO2 at 37°C. Cells were passaged every 3–4 days and the highest passage number used was 20.
Measurement of [Ca2+]i using the FLIPR
rVR1-HEK293 cells were seeded into black walled clear-base 96 well plates (Costar U.K.) at a density of 25,000 cells per well in MEM, supplemented as above, and cultured overnight. The cells were then incubated with MEM containing the cytoplasmic calcium indicator, Fluo-3AM (4 μM; Teflabs, Austin, Texas, U.S.A.) at 25°C for 120 min. The cells were washed four times with, and finally resuspended in, Tyrode's medium, before being incubated for 30 min at 25°C with either buffer alone (control) or buffer containing various antagonists or signal transduction modifying agents. In some studies calcium was omitted from the buffer. The plates were then placed into a FLIPR (Molecular Devices, U.K.) to monitor cell fluorescence λex=488 nM, λEM=540 nM) (Sullivan et al., 1999) before and after the addition of various agonists.
Data analysis
Responses were measured as peak fluorescence intensity (FI) minus basal FI, and where appropriate were expressed as a percentage of a maximum capsaicin-induced response. Data are expressed as mean±s.e.mean unless otherwise stated. Curve-fitting and parameter estimation were carried out using Graph Pad Prism 3.00 (GraphPad Software Inc., California, U.S.A.). Statistical comparisons were made where appropriate using Student's t-test.
Materials
PPAHV, RTX and isovelleral were obtained from Alexis Biochemicals (Nottingham U.K.). Olvanil was purchased from Tocris (Bristol, U.K.) and all other ligands were obtained from RBI (Natick, MA, U.S.A.). All signal transduction modifying agents were purchased from Calbiochem (Nottingham, U.K.). All cell culture media were obtained from Life Technologies (Paisley, U.K.).
Results
Capsaicin caused an increase in [Ca2+]i in rVR1-HEK293 cells, which was typified by an initially rapid then slowing onset (peak ∼30 s), followed by a gradually declining secondary phase (Figure 1). PPAHV and olvanil also increased [Ca2+]i with similar kinetics (data not shown). However, the RTX-induced Ca2+ response had different kinetics, with a more gradual onset (peak ∼90 s) followed by a similar slowly declining secondary phase (Figure 1).
Figure 1.
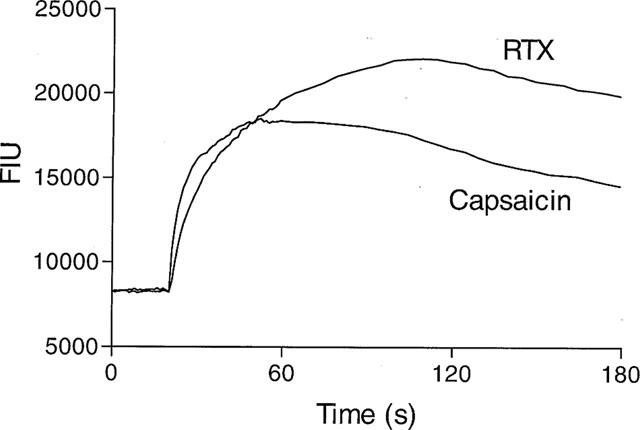
RTX- and capsaicin-induced Ca2+ responses have different kinetics in rVR1-HEK293 cells. [Ca2+]i (as fluorescent intensity units) was monitored using Fluo-3AM in HEK293 cells stably expressing rVR1 before and after the addition of RTX (100 nM) or capsaicin (100 nM). Data shown are representative traces, typical of at least n=100.
All agonist-induced responses were concentration-dependent (Figure 2), and were full agonists compared to capsaicin, although the RTX-induced response obtained a greater maximum than the rest (Figure 2). Nevertheless, the rank order of potency obtained (RTX>capsaicin>olvanil>PPAHV) was consistent with the established pharmacology of the VR1 (Table 1). Interestingly, capsaicin and RTX had a Hill coefficient of ∼2 (Table 1).
Figure 2.
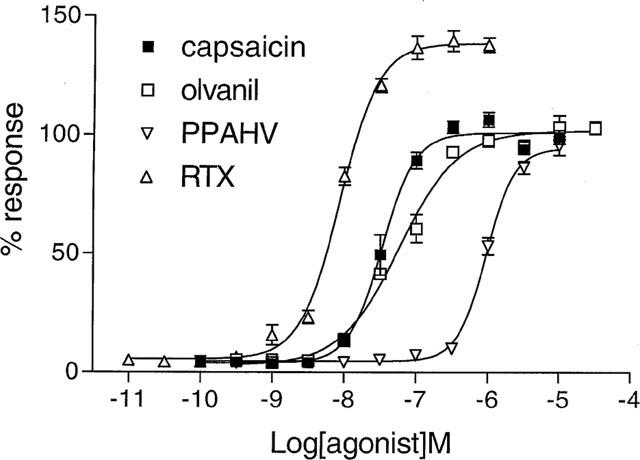
The agonist-induced Ca2+ responses are concentration-dependent. [Ca2+] was monitored using Fluo-3AM in rVR1-HEK293 cells before and after the addition of RTX (10 pM–1 μM), capsaicin (100 pM–10 μM), olvanil (300 pM–30 μM), or PPAHV (100 pM–10 μM). Responses were measured as peak increase in fluorescence minus basal, expressed relative to the maximum capsaicin response and are given as mean±s.e.mean, where n=6–8.
Table 1.
Acidification enhances the potency of some VR1 agonists

Lowering the pH to 6.4 enhanced (2–3 fold) the potencies of capsaicin and olvanil, but not RTX or PPAHV (Table 1), yet had no effect on the Hill coefficients (Table 1). The potency of carbachol at the endogenous muscarinic M3 receptor was unaffected at pH 6.4 (data not shown). Lowering the pH further (5.5) directly increased [Ca2+]i in both rVR1 transfected and wild type HEK293 cells, although the pH-induced Ca2+ response was prolonged in the transfected cells (Figure 3). Preincubation of the cells at pH 6.7 for 30 min abolished the response to pH 5.5 in the wild type HEK cells but had no effect on the response in the VR1 expressing cells (Figure 3). Using these conditions the pH-induced activation of VR1 was concentration-dependent, with an EC50 of 5.49 (Figure 3). Capsazepine (100 nM) had no effect on the pH-induced Ca2+ response in the non-transfected HEK293 cells, but abolished the response in the rVR1 transfected cells (data not shown).
Figure 3.
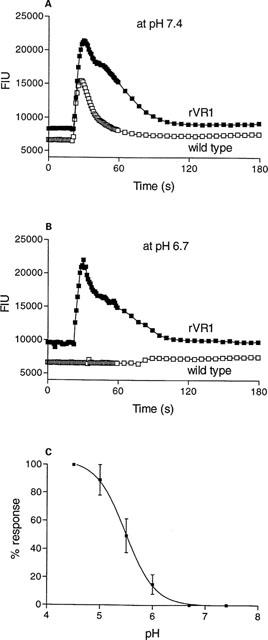
Acidic conditions cause a rVR1-mediated Ca2+ response. [Ca2+]i was measured (as fluorescent intensity units) using Fluo-3AM in wild type or rVR1-transfected HEK293 cells before and after the addition at 20 s of HCl (4.2 mM, A or 2.1 mM, B), and the associated change in pH from 7.4 to 5.5 (A) and 6.7 to 5.5 (B). Traces shown are typical of at least n=20. (C) Depicts the pH-dependency of the response in rVR1-expressing cells. Data are mean±s.e.mean, where n=5.
The potential for cooperativity between agonists was also examined, by measuring the responses to sub-maximal concentrations of RTX (30 nM), PPAHV (1 μM), capsaicin (100 nM) and olvanil (300 nM), alone or in combination. All four agonists on their own produced sub-maximal Ca2+ responses, and in combination were simply additive (Figure 4).
Figure 4.
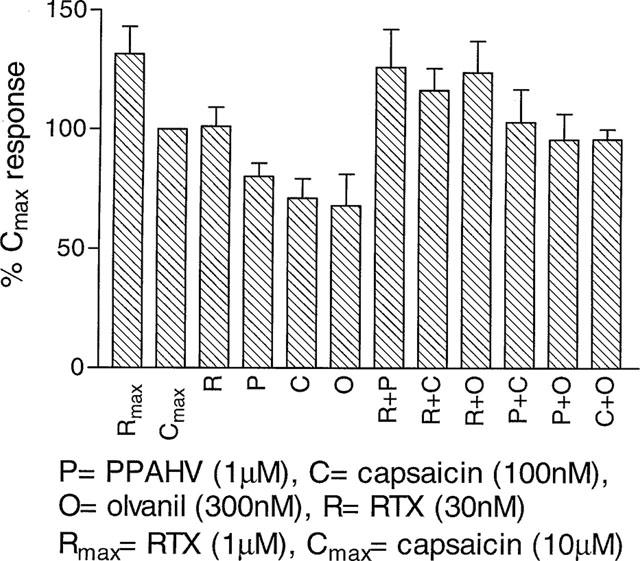
rVR1 agonists do not display cooperativity. [Ca2+]i was monitored using Fluo-3AM in rVR1-HEK293 cells before and after the addition of RTX (30 nM or 1 μM), capsaicin (100 nM or 10 μM), olvanil (300 nM), or PPAHV (1 μM), alone or in combination. Responses were measured as peak increase in fluorescence minus basal, expressed relative to the maximum capsaicin response and are given as mean±s.e.mean, where n=5.
Capsazepine (0.1 nM–10 μM), isovelleral (1 nM–10 μM) and ruthenium red (0.1 nM–10 μM) inhibited the capsaicin (100 nM)-induced Ca2+ responses in rVR1-HEK293 cells in a concentration-dependent manner (Figure 5). Capsazepine, isovelleral and ruthenium red also antagonized the RTX (30 nM)– and olvanil (300 nM)-induced responses (Table 2). None of these antagonists displayed any agonist-like activity (Table 1 and data not shown). The rank order of affinities obtained was ruthenium red>capsazepine>isovelleral (Table 2). Isovelleral caused concentration-dependent, parallel rightward shifts of the capsaicin concentration-response curve (Figure 5), indicating it was acting as a competitive antagonist. Capsazepine (0.1 μM) also caused a parallel, rightward shift of the capsaicin concentration-response curve (Figure 5).
Figure 5.
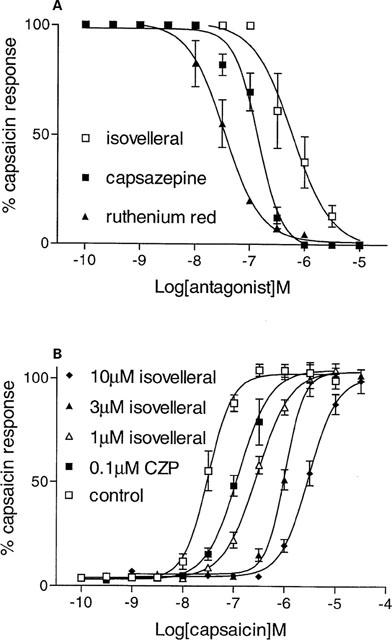
VR1 antagonists inhibit the capsaicin-induced Ca2+ response. In (A) Fluo-3AM-loaded rVR1-HEK293 cells were preincubated with buffer (control), capsazepine (0.1 nM–10 μM), isovelleral (1 nM–10 μM) or ruthenium red (0.1 nM–10 μM) for 30 min at 37°C, and then [Ca2+]i was monitored before and after the addition of capsaicin (100 nM). In (B), the concentration-response relationship for capsaicin (0.1 nM–30 μM) was assessed in the presence or absence of capsazepine (CZP, 0.1 μM) or isovelleral (1–10 μM). Responses were measured as peak increase in fluorescence minus basal, expressed relative to the control capsaicin response and are given as mean±s.e.mean, where n=6.
Table 2.
Antagonist affinities at recombinant rVR1 expressed in HEK cells

Consistent with rVR1 being a ligand-gated ion channel, removal of extracellular calcium abolished the capsaicin (100 nM)-induced response in rVR1-HEK293 cells (Figure 6). Pretreatment with tetrodotoxin (3 μM), nimodipine (10 μM), ω-GVIA conotoxin (1 μM), thapsigargin (1 μM), U73122 (3 μM) or H-89 (3 μM) had no effect on the capsaicin (100 nM)-induced response. However, the PKC inhibitor, chelerythrine chloride (10 μM) partially (∼20%) inhibited the peak response and increased the rate of decline of the secondary phase (Figure 6).
Figure 6.
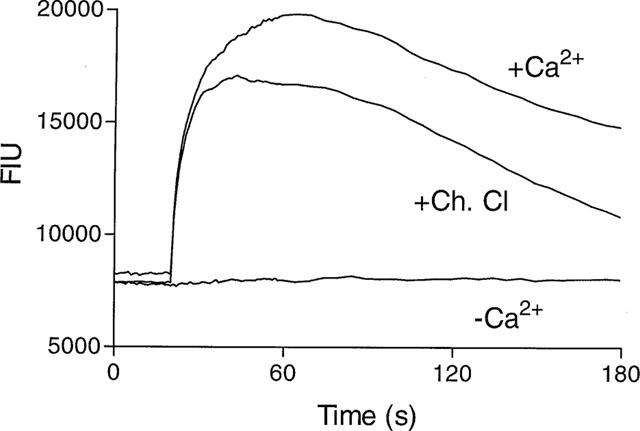
The role of extracellular Ca2+ and PKC in the rVR1-mediated Ca2+ response. [Ca2+]i was monitored (as fluorescent intensity units) using Fluo-3AM in rVR1-HEK293 cells, incubated in calcium-containing or calcium-free buffer, in the presence or absence of chelerythrine chloride (Ch. Cl, 10 μM), before and after a capsaicin (1 μM) challenge at 20 s. Data are representative traces, typical of at least n=5.
Discussion
Vanilloid receptors are important potential targets for the treatment of pain (Dray, 1995; Kress & Zeilhofer, 1999). The recent cloning of VR1 from rat DRG neurones (Caterina et al., 1997) was a major breakthrough, but only a limited pharmacological characterization of this receptor was undertaken (Caterina et al., 1997; Tominaga et al., 1998). The present study has confirmed and expanded this pharmacology, identifying for the first time in a homogenous recombinant system, apparent agonist-dependent aspects of the rVR1-mediated response. Furthermore, these data indicate that the pharmacology of rVR1 can account for some, but not all, of the putative vanilloid receptor subtypes previously identified pharmacologically in native tissues (Szallasi & Blumberg, 1999).
In the present study capsaicin increased [Ca2+]i in rVR1 expressing HEK293 cells, and this response was typified by an initial rapid onset which peaked at ∼30 s, followed by a gradual decline towards basal values. Such kinetics are consistent with those previously reported for the endogenous vanilloid receptor (Kress & Zeilhofer, 1999; Szallasi & Blumberg, 1999). Olvanil and PPAHV also evoked similar responses, again consistent with previous reports from native tissues (Szallasi & Blumberg, 1999). However, the RTX-induced Ca2+ response displayed different kinetics, with a markedly slower onset, peaking at ∼90 s, followed by a subsequent maintained phase. Similar differences in kinetics between RTX- and capsaicin-induced responses have been previously demonstrated in native tissues (Petersen et al., 1996) and Xenopus oocytes expressing recombinant rVR1 (Caterina et al., 1997). Furthermore, the magnitude of the maximum Ca2+ response induced by RTX was ∼20% greater than that induced by capsaicin, again in agreement with previous reports (Petersen et al., 1996; Caterina et al., 1997). Surprisingly, isovelleral, which has been reported to be a vanilloid receptor agonist in native tissues (Szallasi et al., 1996), had no agonist effect at any concentration tested.
All the vanilloid-induced Ca2+ responses were concentration-dependent, yielding affinities and a rank order of potency generally in keeping with the established pharmacology of the endogenous receptor (Szallasi & Blumberg, 1999). However, as previously reported for the recombinant rVR1 (Caterina et al., 1997), RTX was only a few fold more potent than capsaicin, contrary to the several thousand fold higher affinity RTX displayed in binding studies in native tissues (Szallasi, 1994; Szallasi & Blumberg, 1996). Nevertheless, different binding sites on the vanilloid receptor for RTX and capsaicin have been proposed (Szallasi, 1997), and there is some evidence which suggests that the RTX-binding site is relatively unimportant in the activation of the cation channel (Szallasi & Blumberg, 1999). Moreover, a recent study has identified both these binding sites on cells expressing recombinant rVR1 (Szallasi et al., 1999). It is worth noting however that RTX was more potent than capsaicin in a 45Ca2+ efflux assay in DRG cells as well (Acs et al., 1996). Another important feature of the vanilloid-induced responses in the present study was that all the agonists tested displayed Hill coefficients of ∼2, indicative of positive cooperativity. Similar Hill coefficients have been obtained electrophysiologically for RTX and capsaicin in both recombinant (Caterina et al., 1997) and endogenous (Szallasi et al., 1993; Oh et al., 1996) systems. Interestingly, in native tissues PPAHV does not display cooperativity, but does activate two distinct currents (Liu et al., 1998). This apparent discrepancy most likely reflects differences in the experimental conditions. Indeed, the kinetics of the PPAHV-induced response in the current investigation indicate that only one current was activated in rVR1-HEK293 cells.
It has been proposed that protons associated with low extracellular pH are the endogenous activators of the vanilloid receptor (for review see Kress & Zeilhofer, 1999), although others have suggested that they merely play a modulatory role (Szallasi & Blumberg, 1999). In keeping with this in the present study the potency of capsaicin was enhanced ∼3 fold by lowering the pH to 6.4. Similar findings have been reported for both the recombinant VR1 (Caterina et al., 1997; Tominaga et al., 1998) and endogenous vanilloid receptor (Petersen & LaMotte 1993; Baumann et al., 1996). The effect of pH was specific to the vanilloid receptor as the muscarinic receptor-mediated Ca2+ response in the same cells was unaffected. However, although the response to olvanil was also enhanced at pH 6.4, the responses to RTX and PPAHV were not, indicating that the modulatory effects of pH were agonist-dependent. Indeed, as PPAHV and RTX are structurally similar to each other (Appendino et al., 1996), and dissimilar to capsaicin and olvanil (Szallasi & Blumberg, 1999), this suggests that the structure-activity relationships of the vanilloid and pH-mediated sites are different. Moreover, and consistent with earlier studies of the cloned receptor (Tominaga et al., 1998), lowering the pH further, to 5.5, activated the cation channel and so increased [Ca2+]i in the rVR1-HEK293 cells, in the absence of capsaicin. However, more transient, Ca2+ responses of a similar magnitude were also found in the non-transfected HEK293 cells, which were not activated by capsaicin nor blocked by capsazepine, suggesting an endogenous acid-sensitive ion channel (ASIC) was present. Preincubation of the cells at pH 6.7 desensitized the ASIC, but did not effect the VR1-mediated response. Under these conditions the pH-induced response in the VR1-expressing cells was concentration-dependent, with a similar EC50 to that previously reported for VR1 by Tominaga & colleagues (1998), and capsazepine-sensitive.
As pH potentiated responses to some VR1 agonists, and others have suggested that different ligands may bind to different sites on the vanilloid receptor (Szallasi, 1997), the possibility of cooperativity between different agonists was tested. The responses to RTX, capsaicin, olvanil and PPAHV were simply additive, and the maximum response to a given agonist was unaltered in the presence of another agonist, demonstrating that the ligands tested were not cooperative. Similar findings have been reported for PPAHV and RTX in DRG neurones (Szallasi et al., 1996).
In the present study capsazepine inhibited the capsaicin-induced [Ca2+]i response in a concentration-dependent manner, with an affinity similar to that reported for both the recombinant (Caterina et al., 1997) and endogenous (Bevan et al., 1992; Acs et al., 1997) vanilloid receptors. Capsazepine also inhibited the RTX- and olvanil-induced Ca2+ responses in rVR1-HEK293 cells with similar affinities. This is in agreement with the affinity obtained for capsazepine versus RTX in sensory neurones (Bevan et al., 1992; Wardle et al., 1997), but is contrary to the report that capsazepine was ineffective versus olvanil (Davey et al., 1994). However, this apparent discrepancy may simply reflect non-vanilloid receptor mediated effects of olvanil (Beltramo & Piomelli, 1999). Capsazepine has also been reported to have agonist-like activity in rabbits (Wang & Hakanson, 1993), but had no such effects in rVR1-HEK293 cells. Ruthenium red also inhibited the capsaicin-induced Ca2+ response in the current investigation, with an affinity similar to that reported in native tissues (Acs et al., 1997). Others have previously reported ruthenium red inhibited the capsaicin-induced response in rVR1 expressing cells, but did not examine the concentration-dependency of this effect (Caterina et al., 1997; Tominaga et al., 1998). Surprisingly, isovelleral also acted as a competitive antagonist at rVR1, inhibiting capsaicin-, RTX- and olvanil-induced Ca2+ responses at sub-micromolar concentrations. This suggests that the capsaicin-like agonist effects of isovelleral in rat sensory neurones (Szallasi et al., 1996) may not be mediated by rVR1.
Both the recombinant rVR1 (Caterina et al., 1997) and the endogenous vanilloid receptor (Wood et al., 1988) have been reported to act as non-selective cation channels with a preference for Ca2+. Consistent with this, the removal of extracellular Ca2+ abolished the capsaicin-induced Ca2+ response in rVR1-HEK293 cells, whilst thapsigargin and U73122 were without effect, indicating that activation of VR1 promoted Ca2+ influx, but did not mobilise Ca2+ from intracellular stores (Berridge 1993). Furthermore, although some have suggested that TTX-sensitive sodium channels and/or voltage-sensitive calcium channels are involved in the effects of capsaicin in sensory neurones (Maggi et al., 1988), neither TTX, nimodipine nor ω-GVIA conotoxin had any effect on the capsaicin-induced response in the present study. Taken collectively these data conform rVR1 acts as a ligand-gated ion channel. Moreover, inhibition of PKC with chelerythrine chloride inhibited the capsaicin-induced Ca2+ response in rVR1-HEK293 cells, whilst inhibition of PKA with H-89 was without effect. This was somewhat surprising as both PKC and PKA modulate the capsaicin-induced response in sensory neurones (Sluka et al., 1997; Lopshire & Nicol, 1998), and rVR1 has three PKA phosphorylation sites (Caterina et al., 1997), but may reflect the differing tonic activity of these two kinases.
In conclusion, the present study has confirmed and extended the pharmacological characterization of rVR1, identifying for the first time in a homogenous, recombinant system, agonist-dependent aspects of the rVR1-mediated response. Furthermore, these data suggest the pharmacology of rVR1 can account for some, but not all, of the putative vanilloid receptor subtypes identified pharmacologically in native tissues.
Acknowledgments
The authors would like to thank Sarah Rushton for her technical support.
Abbreviations
- ASIC
acid-sensitive ion channel
- [Ca2+]i
intracellular calcium concentration
- DRG
dorsal root ganglion
- FI
fluorescence intensity
- FLIPR
fluorometric imaging plate reader
- MEM
minimum essential medium
- PKA
protein kinase A
- PKC
protein kinase C
- RTX
resiniferatoxin
- rVR1
rat VR1
- TTX
tetrodotoxin
- VR1
vanilloid receptor
References
- ACS G., BIRO T., ACS P., MODARRES D. , BLUMBERG P.M. Differential activation and desensitization of sensory neurons by resiniferatoxin. J. Neurosci. 1997;17:5622–5628. doi: 10.1523/JNEUROSCI.17-14-05622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACS G., LEE J., MARQUEZ V. , BLUMBERG P.M. Distinctstructure-activity relations for stimulation of 45Ca uptake and high affinity binding in cultured rat dorsal root ganglion neurons and dorsal root ganglion membranes. Mol. Brain Res. 1996;35:173–182. doi: 10.1016/0169-328x(95)00204-6. [DOI] [PubMed] [Google Scholar]
- APPENDINO G., CRAVOTTO G., PALMISANO G., ANNUNZIATA R. , SZALLASI A. Synthesis and evaluation of phoboid-20-homovanillates. Discovery of a class of ligand binding to the vanilloid receptor with different degrees of cooperativity. J. Med. Chem. 1996;39:3123–3131. doi: 10.1021/jm960063l. [DOI] [PubMed] [Google Scholar]
- BAUMANN T.K., BURCHIEL K.J., INGRAM S.L. , MARTENSON M.E. Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain. 1996;65:31–38. doi: 10.1016/0304-3959(95)00145-X. [DOI] [PubMed] [Google Scholar]
- BELTRAMO M. , PIOMELLI D. Anandamide transport inhibition by the vanilloid agonist olvanil. Eur. J. Pharmacol. 1999;364:75–78. doi: 10.1016/s0014-2999(98)00821-8. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Inositol triphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S.J. , YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S. , SZOLCSANYI J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol. Sci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D. , JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DAVEY P.T., HAMILTON T.C. , SANGER G.J. Divergent effects of olvanil in two acute models of nociception in the mouse. Br. J. Pharmacol. 1994;112:660P. [Google Scholar]
- DRAY A. Inflammatory mediators of pain. Br. J. Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- FURST S. Transmitters involved in antinociception in the spinal cord. Brain Res. Bull. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- JUNG Y-S., CHO T-S., MOON C-H. , SHIN H-S. Capsaicin-induced desensitization is prevented by capsazepine but not by ruthenium red in guinea pig bronchi. Eur. J. Pharmacol. 1998;362:193–198. doi: 10.1016/s0014-2999(98)00786-9. [DOI] [PubMed] [Google Scholar]
- KRESS M. , ZEILHOFER H.U. Capsaicin, protons and heat: new excitement about nociceptors. Trends Pharmacol. Sci. 1999;20:112–118. doi: 10.1016/s0165-6147(99)01294-8. [DOI] [PubMed] [Google Scholar]
- LIU L., SZALLASI A. , SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13-acetate 20 homovanillate, reveals vanilloid receptor subtypes on rat trigeminal neurons. Neurosci. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- LOPSHIRE J.C. , NICOL G.D. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: whole cell and single channel studies. J. Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., PATACCHINI R., GIULIANI S., SANTICIOLI P. , MELI A. Evidence for two independent modes of activation of the ‘efferent' function of capsaicin-sensitive nerves. Eur. J. Pharmacol. 1988;156:367–373. doi: 10.1016/0014-2999(88)90282-8. [DOI] [PubMed] [Google Scholar]
- OH U., HWANG S.W. , KIM D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. J. Neurosci. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK K.M., MAX M.M., ROBINOVITZ E., GRACELY R.H. , BENNET G.J. Effects of intravenous ketamine, alfentanil, or placebo on pain, pinprick hyperalgesia, and allodynia produced by intradermal capsaicin in human subjects. Pain. 1995;63:163–172. doi: 10.1016/0304-3959(95)00029-R. [DOI] [PubMed] [Google Scholar]
- PETERSEN M. , LAMOTTE R.H. Effects of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- PETERSEN M., LAMOTTE R.H., KLUSCH A. , KNIFFKI K.D. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neurosci. 1996;75:495–505. doi: 10.1016/0306-4522(96)00259-x. [DOI] [PubMed] [Google Scholar]
- RINDER J., SZALLASI A. , LUNDBERG J.M. Capsaicin-, resiniferatoxin-, and lactic acid-evoked vascular effects in the pig nasal mucosa in vivo with reference to characterization of the vanilloid receptor. Pharmacol. Toxicol. 1996;78:327–335. doi: 10.1111/j.1600-0773.1996.tb01384.x. [DOI] [PubMed] [Google Scholar]
- SIMONE D.A., NGEOW J.Y.F., PUTTERMAN G.J. , LAMOTTE R.H. Hyperalgesia to heat after intradermal injection of capsaicin. Brain Res. 1987;418:201–203. doi: 10.1016/0006-8993(87)90982-6. [DOI] [PubMed] [Google Scholar]
- SLUKA K.A., REES H., CHEN P-S., TSURUOKA M. , WILLIS W.D. Capsaicin-induced sensitization of primate spinothalamic tract cells is prevented by a protein kinase C inhibitor. Brain Res. 1997;772:82–86. doi: 10.1016/s0006-8993(97)00876-7. [DOI] [PubMed] [Google Scholar]
- SULLIVAN E., TUCKER E.M. , DALE I.L.Measurement of [Ca2+]i using the fluometric imaging plate reader (FLIPR) Calcium Signaling Protocols 1999New Jersey: Humana Press; 125–136.ed Lambert, D.G. [Google Scholar]
- SZALLASI A. The vanilloid (capsaicin) receptor: receptor types and species differences. Gen. Pharmacol. 1994;25:223–243. doi: 10.1016/0306-3623(94)90049-3. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. Perspectives on vanilloids in clinical practice. Drug News Persp. 1997;10:522–527. [Google Scholar]
- SZALLASI A. , BLUMBERG P.M. Vanilloid receptors: new insights enhance potential as a therapeutic target. Pain. 1996;68:195–208. doi: 10.1016/s0304-3959(96)03202-2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. , BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M., ANNICELLI L.L., KRAUSE J.E. , CORTRIGHT D.N. The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 1999;56:581–587. doi: 10.1124/mol.56.3.581. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., CONTE B., GOSO C., BLUMBERG P.M. , MANZINI S. Characterization of a peripheral vanilloid (capsaicin) receptor in the rat urinary bladder. Life Sci. 1993;52:PL221–PL226. doi: 10.1016/0024-3205(93)90051-4. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., JONASSOHN M., ACS G., BIRO T., ACS P., BLUMBERG P.M. , STERNER O. The stimulation of capsaicin-sensitive neurones in a vanilloid receptor-mediated fashion by pungent terpenoids possessing an unsaturated 1,4-dialdehyde moiety. Br. J. Pharmacol. 1996;119:283–290. doi: 10.1111/j.1476-5381.1996.tb15983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I. , JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- WANG Z-Y. , HAKANSON R. Effects of ruthenium red and capsazepine on C-fibres in the rabbit iris. Br. J. Pharmacol. 1993;110:1073–1078. doi: 10.1111/j.1476-5381.1993.tb13923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLE K.A., RANSON J. , SANGER G.J. Pharmacological characterization of the vanilloid receptor in the rat dorsal horn. Br. J. Pharmacol. 1997;121:1012–1016. doi: 10.1038/sj.bjp.0701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD J.N., WINTER J., JAMES I.F., RANG H.P., YEATS J. , BEVAN S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 1988;8:3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]


