Abstract
Nanomolar concentrations of Cu2+ induce a slowly reversible block of GABAA receptor-mediated currents which can be removed by chelating substances.
The possible interaction of Cu2+ with the Zn2+ binding site on the GABAA receptor complex was studied in acutely isolated Purkinje cells using whole-cell recording and a fast drug application system.
When Zn2+ was applied together with 2 μM GABA, the Zn2+-induced block of GABA-mediated currents was not additive to the Cu2+-induced block. In the presence of 0.1 μM Cu2+ in the bath solution the degree of inhibition of GABA-mediated responses by Zn2+ was strongly attenuated.
Preapplication of 100 μM Zn2+ during 10 s, terminated 1 s before exposure to 2 μM GABA did not affect the GABA current in Cu2+-free solution, but relieved its block by 0.1 μM Cu2+. This effect of Zn2+ was concentration-dependent with an EC50 of 72 μM.
When the Cu2+-induced block was removed by histidine, preapplication of Zn2+ did not increase the GABA current, indicating that the relief of Cu2+ block by Zn2+ is the result of its ability to actively remove Cu2+ from the GABA receptor complex.
It is proposed that the inhibitory effects of Zn2+ and Cu2+ on GABA-induced currents result from an action of these metal ions at distinct, but conformationally linked sites on the GABAA receptor protein. Under physiological conditions Zn2+ would liberate Cu2+ from the GABAA receptor, thus facilitating Cu2+ turnover and its binding by other endogenous chelating molecules.
Keywords: Cu2+, Zn2+, GABA, cerebellum, histidine, patch-clamp, Wilson disease
Introduction
Cu2+ and Zn2+ are essential nutrients with close physiological interactions. High dietary levels of Zn2+ depress, Zn2+-deficiency increases Cu2+ absorption (Cousins, 1985). Both Zn2+ and Cu2+ are cofactors in the metalloenzyme Cu2+, Zn2+-superoxide dismutase. Zn2+ competes with Cu2+ for the binding site, it decreases the ability of Cu2+ to transfer electrons in certain conditions. Both metals are present in remarkably high levels in the brain, largely bound to proteins; the exact levels of the free ions in the extracellular space are not known. They occur in presynaptic terminals and are released with synaptic activity (Assaf & Chung, 1984; Frederickson, 1989; Hartter & Barnea, 1988; Kardos et al., 1989). Zn2+ ions can modulate a number of ligand- and voltage-gated ion channels (Harrison & Gibbons, 1994; Xie & Smart, 1991). Both Zn2+ and Cu2+ block GABAA receptor-mediated currents. The mechanisms of Zn2+ and Cu2+ modulation of GABA-mediated responses have been analysed separately in a number of preparations (Celentano et al., 1991; Draguhn et al., 1990; Kilic et al., 1993; Ma & Narahashi, 1993; Smart & Constanti, 1990; Smart et al., 1991; Trombley & Shepherd, 1996; White & Gurley, 1995; Yakushiji et al., 1987). Cu2+ and Zn2+ had a very similar action on GABA-mediated responses when studied in dorsal root ganglion cells. In this preparation Cu2+ antagonized the blocking action of Zn2+, suggesting that Cu2+ and Zn2+ may share a common site on the GABAA receptor.
We have recently found copper blocking GABA-induced currents on Purkinje cells with very high affinity (Sharonova et al., 1998). The block appeared at Cu2+ concentrations of about 10 nM, half-maximal inhibition (IC50) at 35 nM. It developed within about 1 min but washout took several minutes. We have now analysed the interaction between these two trace metals and suggest closely related but not identical sites of action at the GABAA receptor.
Methods
Preparation of Purkinje cells
Neurons were isolated from cerebella of 2–3 week-old male Wistar rats (Versuchstieranstalt Heinrich-Heine-Universität, Düsseldorf). Saggital slices were cut by vibratome perpendicularly to the cerebellar cortex surface and were incubated at room temperature for 1–8 h on a mesh near the bottom of a 150 ml beaker. The incubation solution was continuously bubbled with carbogene (5% CO2+95% O2). The solution had a following composition (in mM): NaCl 125; KCl 5; CaCl2 1.5; MgCl2 1.5; NaH2PO4 1.28; NaHCO3 25; glucose 10; phenol red 0.01%. One at a time, slices were transferred to the recording chamber and neurons were dissociated using a vibrating tip of a fused glass pipette (Vorobjev, 1991). The solution for dissociation and recording had the following composition (in mM): NaCl 150; KCl 5; CaCl2 2.7; MgCl2 2.0; HEPES 10, pH adjusted to 7.4 with NaOH. In most experiments 100 nM Cu2SO4 was present in the solution.
Whole-cell recording
Voltage-clamp recording was obtained using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Glass recording patch pipettes were prepared from filament-containing borosilicate tubes using a two-stage puller. The electrodes, having resistances of 4–5 MΩ, were filled with recording solution of the following composition (in mM): KCl 140; CaCl2 0.5, MgCl2 4; HEPES 10; EGTA 5; ATP-Na 3 (pH adjusted to 7.3 with KOH). When high concentrations of GABA (10–50 μM) were used, recordings were performed with an intracellular solution containing low Cl− concentration resulting in a reduced amplitude of agonist induced current, in order to minimize the error due to the voltage drop through the uncompensated series resistance. 125 mM KCl in the intracellular solution were replaced by potassium methanesulphonate. Recordings were carried out at room temperature (22–26°C) using an EPC-9 patch-clamp amplifier. Currents were filtered at 2 kHz, sampled at 1 kHz, and stored on a computer disk. Data were collected with commercially available software (TIDA for Windows). The holding potential was maintained at −70 mV.
Drug application
A fast perfusion technique was used to apply GABA and drugs (Vorobjev et al., 1996). Isolated Purkinje cells were first patch clamped and then lifted into the outflow of the control bath solution. The substances were applied through two different glass capillaries, 0.1 mm in diameter, and, for continuous perfusion, they were added to the bath solution. The delivery ports of the capillaries were positioned within 0.4 mm from the cell under study. For exposure the application system was moved so as to place the cell in the solution stream leaving one of the application capillaries. One capillary was used to apply GABA or GABA together with substances (coapplication). Another capillary was used for perfusion of the cell with substances applied without GABA. The flow through each tube was gravity-driven. For activation of GABAA channels, in most experiments 2 μM GABA was applied for periods of 1 s, at 30 s intervals. Cu2+ (Cu2SO4) and Zn2+ (ZnCl2) were dissolved in the perfusate and applied extracellularly. All reagents were obtained from Sigma, Deisenhofen, Germany.
Data analysis
To quantify the inhibitory effects of Zn2+ upon the current induced by a constant GABA concentration the following equation was used:
where Imax is the maximal degree of block of the GABA-mediated response achieved by the tested ligand, IC50 is the concentration of the ligand producing a half-maximal block of GABA-mediated responses, and n is the Hill coefficient. The removal of Cu2+-induced block of GABA responses by preapplication of Zn2+ was fitted by the equation
where ICu is the amplitude of GABA responses blocked by Cu2+ and I0 the amplitude of the control GABA response and EC50 is the concentration of Zn2+ producing a half-maximal restoration of the GABA response. Other values have the meaning noted above. Data values are presented as mean±s.e.mean.
Results
Blocking actions of Cu2+ and Zn2+
We studied the effect of Zn2+ on GABA-induced current in control and in the presence of Cu2+ in the bathing medium. In the absence of copper in the outer solutions, Zn2+, applied together with GABA, suppressed the responses to GABA in a concentration-dependent manner (Figure 1A). The Zn2+ induced block was fitted by the logistic equation with the following parameters: IC50 36±5 μM with an Imax of 0.66±0.05 and a Hill slope of 1±0.2 (n=5). The presence of 100 nM Cu2+ in the bath solution – which itself reduced GABA induced currents to about 40% of control – markedly changed the concentration-response curve for inhibition of GABA induced currents by Zn2+ (Figure 1B). Figure 1C presents the analysis of six experiments carried out in Cu2+-free solutions and in the presence of 0.1 μM Cu2+ in the bath solution. Inhibition of GABA-mediated responses by Zn2+ was strongly attenuated in the presence of Cu2+. Moreover, at Zn2+ concentrations of 10 and 30 μM the amplitude of GABA-mediated currents was larger than in the presence of Cu2+ alone. Therefore, the dose-response curve for Zn2+ in the presence of Cu2+ was biphasic. Although the complex nature of the dose-response curve makes a quantitative analysis difficult, inspection of Figure 1C shows that the apparent IC50 for inhibition of the GABA mediated response by Zn2+ is increased in the presence of Cu2+. Besides this, after coapplication of GABA and 100 μM Zn2+ in the presence of 0.1 μM Cu2+ the subsequent response to GABA was slightly enhanced (Figure 1B).
Figure 1.
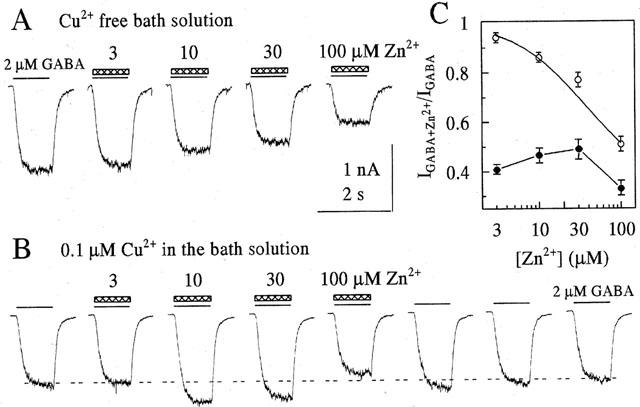
Copper and zinc block GABA mediated currents. (A) illustrates the block of currents evoked by sequential application of 2 μM GABA+0, 3, 10, 30, 100 μM Zn2+ in the absence of Cu2+ in the bath solution. (B) shows results from another cell to which the same set of solutions was applied, however in the presence of 100 nM Cu2+ in the bath solution. Applications were made every 30 s and are marked by thin lines (GABA) and hatched bars (Zn2+). The response following trace 5 (coapplication of GABA and 100 μM Zn2+) displays still a partial removal of Cu2+-block by the preceding Zn2+-application. (C) In Cu2+-free bath solution Zn2+ blocks GABA mediated currents in a concentration-dependent manner (open circles). Data points are fitted in equation 1 with Imax=0.66±0.05, IC50=36±5 μM; n=1±02. In the presence of 100 nM Cu2+ the concentration-response curve is biphasic (filled circles). Data points are normalized to the response elicited by 2 μM GABA in Cu2+-free solution. Each data point averages the responses of six cells.
Relief of Cu2+ inhibition by Zn2+
The increase of GABA current during coapplication with Zn2+ suggests a partial rescue from Cu2+ block by Zn2+. We therefore examined the effect of Zn2+-preapplication on Cu2+-induced inhibition of GABA responses. Preapplication of Zn2+ for 10 s was terminated 1 s before GABA exposure. Under control conditions (Cu2+-free solution) preapplication of Zn2+ (10–300 μM) for 10 s terminated 1 s before GABA exposure did not affect the GABA current (data not shown). The one second (1 s) interval between applications of Zn2+ and GABA was essential to minimize the direct blocking effect of Zn2+ on GABA responses (Figure 2B). When GABA currents were suppressed in the presence of 0.1 μM Cu2+ in the bath solution preapplication of 100 μM Zn2+ increased the amplitude of GABA-mediated response, in contrast to the inhibitory effect of high Zn2+ concentrations applied together with GABA (Figure 3B). In this set of experiments Zn2+ containing solutions were Cu2+-free.
Figure 2.
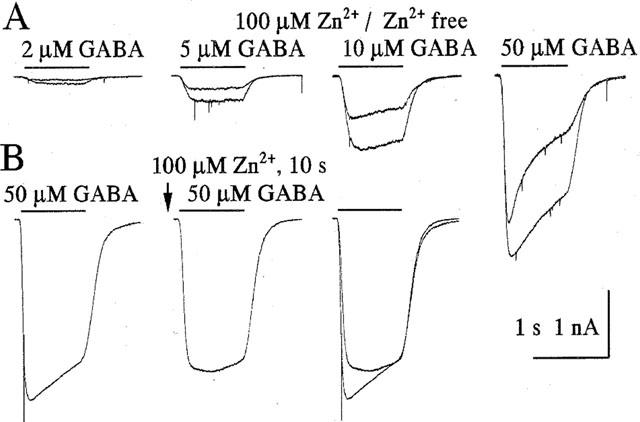
Properties of zinc inhibition of GABA-induced currents in Purkinje cells. (A) The inhibitory effect of Zn2+ does not depend on GABA concentration. Responses to application of different concentrations of GABA and GABA+100 μM Zn2+ obtained from a single cell. (B) Rapid reversal of Zn2+ inhibition. Responses to 50 μM GABA in control, after preapplication of 100 μM Zn2+ and superposition of two traces. Interval between GABA applications was 90 s, duration of GABA exposure 1 s, zinc application was for 10 s (arrow) terminating at the beginning of trace 2. Recovery from the zinc effect occurs within less than 1 s.
Figure 3.
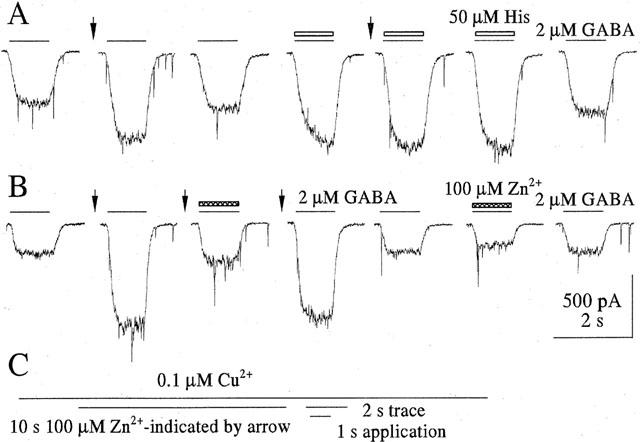
Brief Zn2+ exposure reduces copper induced block of GABA mediated currents. (C) Real-time-scheme of drug exposures: 100 nM Cu2+ was present throughout, the times of exposure to Zn2+, GABA, GABA+histidine and GABA+Zn2+ are indicated. The time for the two second traces illustrated in A and B is also given. The 10 s preapplication of 100 μM Zn2+ (at vertical arrows in A and B) which was terminated 1 s before GABA application removed the Cu2+ block. Two μM GABA (lines above traces) was applied for 1 s with 30 s intervals. (A) Sequential traces illustrate that the Zn2+ preapplication enhances the amplitude of GABA-induced responses. This effect is occluded by 50 μM histidine (open bars) applied together with GABA. (B) Illustrates sequential traces in another cell. Zn2+ preapplication enhanced the GABA response. Zn2+ applied together with GABA (filled bar above trace) markedly reduced this enhancement (third trace). Without Zn2+ preapplication Zn2+ did not alter the response significantly (sixth trace).
The copper-induced block of GABA current can be easily and reversibly removed by short exposure to a metal chelator such as histidine (Sharonova et al., 1998). In order to determine whether Zn2+ augments the GABA responses by removing Cu2+ from the receptor molecule, we compared the effects of Zn2+ and histidine on GABA responses suppressed by Cu2+. To avoid the formation of histidine Zn2+ was preapplied during 10 s as before. Figure 3A demonstrates that preapplication of 100 μM Zn2+ (at vertical arrows) increases the GABA current suppressed by Cu2+. However, when the Cu2+ block was removed by coapplication of GABA with 50 μM histidine, preapplication of Zn2+ failed to further augment the response to GABA. Thus the potentiating effect of Zn2+ is occluded by histidine-chelation of Cu2+. Figure 3B illustrates that a GABA response relieved from copper block by preapplied Zn2+ can be blocked by Zn2+ when coapplied with GABA. The further traces are sequential GABA responses showing that, without Zn2+ preapplication (without removal of copper block), the GABA response is only slightly reduced by 100 μM Zn2+. Thus, the effect of Zn2+ on the GABA response in the presence of 0.1 μM Cu2+ depends on the way of Zn2+ administration. Preapplication of Zn2+ during 10 s increased subsequent responses to GABA in a dose-dependent way with an EC50 of 72±13 μM and a Hill slope of 1.35±0.3 (five cells, Figure 4).
Figure 4.
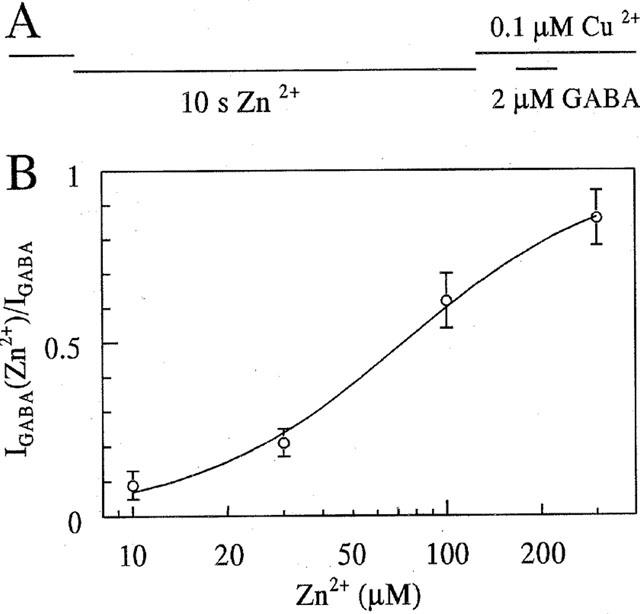
Zn2+ reduces Cu2+-block in a concentration dependent manner. (A) Real-time-scheme of experiments; upper line: Cu2+, lower lines Zn 10−300 nM for 10 s and GABA 2 μM for 1 s. data points in the dose response curve are normalized to control GABA responses and represent the mean responses of five cells. The data are fitted by equation 2 with EC50=72±13 μM, n=1.35±0.3.
Zn-induced block at different GABA concentrations
We have previously shown that an increasing GABA concentration decreased the blocking effect of Cu2+ (Sharonova et al., 1998). In contrast, the Zn2+-induced block of GABA responses could not be surmounted by increasing the GABA concentration (Figure 2A). The block induced by 100 μM Zn2+ was about the same at 2 μM GABA (to 0.55±0.05) and at 50 μM GABA (to 0.49±0.07) (n=4 cells). Thus, Zn2+ antagonizes GABA responses in cerebellar Purkinje cells in a non-competitive way.
Discussion
Do Cu2+ and Zn2+ bind to a common site?
The inhibitory action of Zn2+ on GABA-activated current is well documented (Celentano et al., 1991; Draguhn et al., 1990; Kilic et al., 1993; Smart et al., 1991; White & Gurley, 1995) as a direct interaction of Zn2+ with the GABAA receptor/ion channel complex. Studies on recombinant GABAA receptors have demonstrated that the action of Zn2+ requires presence of the γ subunit. A Zn2+ binding site seems to be present on channels that contain only α1 or β2 or a combination of these subunits. Zn2+ can block the current evoked by GABA in these channels with high potency (IC50 about 1 μM). When either the α or the α and β subunits were co-expressed with the γ subunit, GABAA receptors were rather insensitive to Zn2+ (Draguhn et al., 1990; Smart et al., 1991). Purkinje cells seem to contain only α1, β2, β3 and γ2 subunit mRNAs (Laurie et al., 1992; Persohn et al., 1992). In spite of the presence of the γ2 subunit in GABAA receptors on Purkinje cells, their sensitivity to Zn2+ was not as low (IC50 36 μM) as might have been expected from studies on recombinant GABAA receptors (Draguhn et al., 1990; White & Gurley, 1995). This may result from the influence of some other factors determining the sensitivity of the GABAA receptor to Zn2+ (Berger et al., 1998).
The mechanism of Zn2+ antagonism varies in different cell types. Zn2+ noncompetitively inhibits GABA-activated current from neurons of rat superior cervical ganglion (Smart & Constanti, 1990), hippocampus (Legendre & Westbrook, 1991; Mayer & Vyklicky, 1989), cerebellar granule cells (Kilic et al., 1993), from dorsal root ganglion neurons (Ma & Narahashi, 1993) and from recombinant GABAA receptors composed from α and β subunits (Draguhn et al., 1990; Smart et al., 1991). Zn2+ inhibition of some recombinant GABAA receptors is surmountable by GABA, indicating competitive antagonism (White & Gurley, 1995). In spinal cord neurons the Zn2+ block was partially surmountable when the agonist concentration was raised (Celentano et al., 1991). A general model of Zn2+ inhibition of GABAA receptors proposes that Zn2+ binds to a single site that allosterically induces two unconducting states; the site affinity is state dependent and controlled by the γ-subunit (Gingrich & Burkat, 1998). The inability to overcome Zn2+ inhibition at high GABA concentration in our study is consistent with a noncompetitive mechanism.
While the mechanisms of Zn2+ modulation of GABA mediated responses have been widely studied, information regarding the modulation of GABA-induced currents by Cu2+ is limited (Yakushiji et al., 1987; Trombley & Shepherd, 1996). Recently, we have described the properties of Cu2+-induced inhibition of GABA receptor currents in cerebellar Purkinje cells (Sharonova et al., 1998). The blocks of GABA responses by Zn2+ and Cu2+ ions in these neurons differ in several respects. Unlike Zn2+ inhibition, the effect of Cu2+ decreased with increasing GABA concentration, suggesting a competitive mode of interaction. In addition, Cu2+ acts with a higher apparent affinity than Zn2+ (IC50 0.035 μM for Cu2+ versus 36 μM for Zn2+). On the other hand, we have found an obvious interaction between the blocking effects of these metal ions. First, suppression of GABA responses by Zn2+ is diminished in the presence of Cu2+. Second, preapplication of Zn2+ can completely relief the block of GABAA receptors induced by Cu2+. When Cu2+-induced block was removed by histidine, preapplication of Zn2+ did not further increase the GABA-current, suggesting that the interaction of Zn2+ with the GABAA receptor results in the removal of Cu2+ from its binding site during this brief exposure. The decrease of the relative degree of Zn2+ inhibition in the presence of Cu2+ is explained by a model assuming that these two metal ions compete for the same binding site on the GABAA receptor. However, the relief of Cu2+ block by Zn2+ preapplication is not consistent with a passive substitution of Cu2+ by Zn2+ because, as we have found previously (Sharonova et al., 1998), Cu2+, in contrast to Zn2+ ions dissociate too slowly (τ⩽3 min) from the GABAA receptor to allow their unbinding from the protein just for the period of Zn2+ application (maximally 10 s); the copper binding sites remain occupied and unavailable for zinc during this time.
On the other hand, Cu2+-induced block can easily be removed by exposure to histidine for 10 s (Sharonova et al., 1998). To explain the fast recovery of the GABA response from Cu2+ block we have to assume that histidine chelates not only free Cu2+, but also Cu2+ bound on the protein, and removes it from this site. When we preapplied Zn2+, we observed a similar recovery from Cu2+ block, which was much faster than passive dissociation of Cu2+ from the GABAA receptor. Zn2+ seems to accelerate Cu2+ dissociation. The concentration response curve (Figure 4) shows that no removal of block occurs with only 10 μM Zn2+. The occupation of the Cu2+ binding site can be qualified as quasistationary; no turnover of Cu2+ occurs as the medium does not contain Cu2+ which could occupy vacant sites. The relief of Cu2+ block is only seen with higher Zn2+ concentrations. Since Zn2+ cannot interact with the binding site occupied by Cu2+, it has to bind to another site to facilitate Cu2+ dissociation. Thus, our results are consistent with the existence of different, but conformationally linked binding sites for Zn2+ and Cu2+ ions on the GABAA receptor molecule. We propose that the binding of Zn2+ lowers the affinity for Cu2+ and causes subsequent dissociation of Cu2+ from its binding site as a result of negative cooperativity between the binding sites for Cu2+ and Zn2+.
The existence of distinct sites for zinc and copper cations on the GABAA receptor is supported by a study (Fisher & Macdonald, 1998) demonstrating that the His residue located in the M2–M3 extracellular domain of the α6 subtype (rat α6 H273) plays an important role in determining the sensitivity of recombinant GABAA receptors to zinc, but not to copper. The α6-subtype confers a relatively low sensitivity to copper, and replacement of the H273 with Asn did not affect inhibition by copper. Studies on rat α1 and α6 chimeras also suggest that the extracellular N-terminal domain of the α1 subunit contributes to a regulatory site(s) for divalent cations, conferring high sensitivity to inhibition by copper and cadmium. These findings are in keeping with our suggestion that inhibitory effects of Cu2+ and Zn2+ on GABA-induced currents result from interaction of these metal ions with distinct, but allosterically connected binding sites on the GABAA-receptor.
Functional significance
Release of Cu2+ and Zn2+ into the extracellular space from cortical and hypothalamic synaptosomes was observed upon depolarization (Hartter & Garnea, 1988; Kardos et al., 1989). According to the estimate made by Kardos et al. (1989), the concentration of Cu2+ in the synaptic cleft is in the range of 100–250 μM. Most of the extracellular Cu2+ in the brain is not free copper, it is bound to protein. But under reduced amounts of Cu-binding proteins such as caerulopasmin or the PrPc (Brown et al., 1997) increased free copper levels and their pathogenic consequences are to be expected. Synaptically released Zn2+ may, depending on the free copper level or the occupancy of proteins with copper, have potentiating or inhibiting effects on gabaergic transmission. In physiological conditions Zn2+ would liberate Cu2+ from GABAA receptors, thus facilitating Cu2+ turnover and its binding by other endogenous molecules which are more effective in chelating free Cu2+ than Cu2+ bound to protein. In this way many copper chelating substances may participate in the regulation of GABAergic transmission.
Furthermore, this mechanism may contribute to the beneficial effects of chelating agents and Zn2+ when they are used for treating Wilson's disease, an inherited disorder related with copper imbalance. Disturbances in copper metabolism result in its accumulation in the liver, the basal ganglia and cause hepatolenticular degeneration. Zinc is the treatment of choice for maintenance therapy because of its high efficacy and lack of toxicity, it blocks copper absorbtion (Brewer & Yuzbasiyan Gurkan, 1992). Our results raise the possibility that interaction with copper binding to neuronal proteins including the GABAA-receptor may be partially responsible for the therapeutic effect of zinc in Wilson's disease.
Acknowledgments
Supported by Deutsche Forschungsgemeinschaft (SFB 194, B13).
References
- ASSAF S.Y. , CHUNG S.H. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- BERGER T., SCHWARZ C., KRAUSHAAR U. , MONYER H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J. Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREWER G.J. , YUZBASIYAN GURKAN V. Wilson disease. Medicine Baltimore. 1992;71:139–164. doi: 10.1097/00005792-199205000-00004. [DOI] [PubMed] [Google Scholar]
- BROWN D.R., QIN K., HERMS J.W., MADLUNG A., MANSON J., STROME R., FRASER P.E., KRUCK T., VON BOHLEN A., SCHULZ SCHAEFFER W., GIESE A., WESTAWAY D. , KRETZSCHMAR H. The cellular prion protein binds copper in vivo. Nature. 1997;390:684–687. doi: 10.1038/37783. [DOI] [PubMed] [Google Scholar]
- CELENTANO J.J., GYENES M., GIBBS T.T. , FARB D.H. Negative modulation of the gamma-aminobutyric acid response by extracellular zinc. Mol. Pharmacol. 1991;40:766–773. [PubMed] [Google Scholar]
- COUSINS R.J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol. Rev. 1985;65:238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- DRAGUHN A., VERDORN T.A., EWERT M., SEEBURG P.H. , SAKMANN B. Functional and molecular distinction between recombinant rat GABAA receptors subtypes by Zn2+ Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- FISHER J.L. , MACDONALD R.L. The role of an alpha subtype M2–M3 His in regulating inhibition of GABAA receptor current by zinc and other divalent cations. J. Neurosci. 1998;18:2944–2953. doi: 10.1523/JNEUROSCI.18-08-02944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICKSON C.J. Neurobiology of zinc and zinc-containing neurons. Int. Rev. Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- GINGRICH K.J. , BURKAT P.M. Zn2+ inhibition of recombinant GABAA receptors: an allosteric, state-dependent mechanism determined by the gamma-subunit. J. Physiol. Lond. 1998;506:609–625. doi: 10.1111/j.1469-7793.1998.609bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B. , SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HARRISON N.L. , GIBBONS S.J. Zn2+: an endogenous modulator of ligand- and voltage-gated ion channels. Neuropharmacology. 1994;33:935–952. doi: 10.1016/0028-3908(94)90152-x. [DOI] [PubMed] [Google Scholar]
- HARTTER D.E. , BARNEA A. Evidence for release of copper in the brain: depolarization-induced release of newly taken-up 67copper. Synapse. 1988;2:412–415. doi: 10.1002/syn.890020408. [DOI] [PubMed] [Google Scholar]
- KARDOS J., KOVACS I., HAJOS F., KALMAN M. , SIMONYI M. Nerve endings from rat brain tissue release copper upon depolarization. A possible role in regulating neuronal excitability. Neurosci. Lett. 1989;103:139–144. doi: 10.1016/0304-3940(89)90565-x. [DOI] [PubMed] [Google Scholar]
- KILIC G., MORAN O. , CHERUBINI E. Currents activated by GABA and their modulation by Zn2+ in cerebellar granule cells in culture. Eur. J. Neurosci. 1993;5:65–72. doi: 10.1111/j.1460-9568.1993.tb00206.x. [DOI] [PubMed] [Google Scholar]
- LAURIE D.J., WISDEN W. , SEEBURG P.H. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGENDRE P. , WESTBROOK G.L. Noncompetitive inhibition of gamma-aminobutyric acid A channels by Zn. Mol. Pharmacol. 1991;39:267–274. [PubMed] [Google Scholar]
- MA J.Y. , NARAHASHI T. Differential modulation of GABAA receptor-channel complex by polyvalent cations in rat dorsal root ganglion neurons. Brain Res. 1993;607:222–232. doi: 10.1016/0006-8993(93)91510-y. [DOI] [PubMed] [Google Scholar]
- MAYER M.L. , VYKLICKY L., JR The action of zinc on synaptic transmission and neuronal excitability in cultures of mouse hippocampus. J. Physiol. Lond. 1989;415:351–365. doi: 10.1113/jphysiol.1989.sp017725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERSOHN E., MALHERBE P. , RICHARDS J.G. Comparative molecular neuroanatomy of cloned GABAA receptor subunits in the rat CNS. J. Comp. Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- SHARONOVA I.N., VOROBJEV V.S. , HAAS H.L. High-affinity copper block of GABA(A) receptor-mediated currents in acutely isolated cerebellar Purkinje cells of the rat. Eur. J. Neurosci. 1998;10:522–528. doi: 10.1046/j.1460-9568.1998.00057.x. [DOI] [PubMed] [Google Scholar]
- SMART T.G. , CONSTANTI A. Differential effect of zinc on the vertebrate GABAA-receptor complex. Br. J. Pharmacol. 1990;99:643–654. doi: 10.1111/j.1476-5381.1990.tb12984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART T.G., MOSS S.J., XIE X. , HUGANIR R.L. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br. J. Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROMBLEY P.Q. , SHEPHERD G.M. Differential modulation by zinc and copper of amino acid receptors from rat olfactory bulb neurons. J. Neurophysiol. 1996;76:2536–2546. doi: 10.1152/jn.1996.76.4.2536. [DOI] [PubMed] [Google Scholar]
- VOROBJEV V.S. Vibrodissociation of sliced mammalian nervous tissue. J. Neurosci. Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]
- VOROBJEV V.S., SHARONOVA I.N. , HAAS H.L. A simple perfusion system for patch-clamp studies. J. Neurosci. Methods. 1996;68:303–307. doi: 10.1016/0165-0270(96)00097-0. [DOI] [PubMed] [Google Scholar]
- WHITE G. , GURLEY D.A. Alpha subunits influence Zn block of gamma 2 containing GABAA receptor currents. Neuroreport. 1995;6:461–464. doi: 10.1097/00001756-199502000-00014. [DOI] [PubMed] [Google Scholar]
- XIE X.M. , SMART T.G. A physiological role for endogenous zinc in rat hippocampal synaptic neurotransmission. Nature. 1991;349:521–524. doi: 10.1038/349521a0. [DOI] [PubMed] [Google Scholar]
- YAKUSHIJI T., TOKUTOMI N., AKAIKE N. , CARPENTER D.O. Antagonists of GABA responses, studied using internally perfused frog dorsal root ganglion neurons. Neuroscience. 1987;22:1123–1133. doi: 10.1016/0306-4522(87)92987-3. [DOI] [PubMed] [Google Scholar]


