Abstract
Apoptotic death has now been recognized in a number of common and threatening vascular diseases, including atherosclerosis. Interest in apoptosis research relates to the fact that apoptosis, in contrast to oncosis, is a highly regulated process of cell death which raises the hope for the development of specific therapeutic strategies to alter disease progression. This review summarizes the mechanisms involved in vascular endothelial and smooth muscle cell survival/apoptosis, and the potential roles of apoptotic death in atherosclerosis and restenosis. The potential effects of modulation of apoptosis in these diseases are also discussed.
Keywords: Apoptosis, atherosclerosis, caspases, endothelial cell, macrophage, smooth muscle cell, thrombosis
Introduction
Vascular pathologists have long been intrigued by the observation of cell death within the arterial wall in atherosclerosis (Thomas et al., 1976; Virchow, 1858) or during normal aging (Cliff, 1970) in the absence of overt necrosis. Rational explanations for these observations may now be at hand after the description by Kerr and colleagues in the early 1970s of a novel form of cell death distinct from necrosis, which they designated “apoptosis” (from the Greek word for “falling”) (Kerr et al., 1972). It has since been increasingly recognized that apoptosis may be involved in a number of critical events occurring during normal development and may play a key role in a wide variety of diseases (Hetts, 1998).
In contrast to the cellular and organelle swelling, and to the blebbing and membrane rupture associated with the classic form of induced cell death or “oncosis”, apoptosis was initially described in cells that are programmed to die on the basis of characteristic morphology (Kerr et al., 1972). Cells undergoing apoptosis show cell shrinkage, chromatin margination and condensation with subsequent internucleosomal fragmentation of DNA, membrane redistribution of phospholipids (Martin et al., 1995) and budding (emission of pseudopodia), with maintenance of membrane integrity. The process tends to break off into apoptotic bodies that are recognized and rapidly engulfed by professional macrophages or adjacent neighbouring cells without inducing an inflammatory response. However, cell corpses that escape phagocytosis and remain free undergo secondary necrosis (Majno & Joris, 1995).
Molecular mechanisms of apoptosis
Apoptosis can be initiated by intrinsic signals, during normal development for example. This occurs when cells activate an internal program of self-destruction (cell suicide, programmed cell death) in response to an internal clock, withdrawal of survival factors, changes in haemodynamic parameters or loss of contact. Every cell already contains all the components of the suicide machinery and is ready to engage in self-destruction unless it is actively signalled not to do so.
On the other hand, apoptosis can be initiated by a wide variety of extrinsic signals such as cytokines, hormones, oxidized lipids, chemotherapeutic, ionizing or viral agents (Haunstetter & Izumo, 1998). In contrast to the more chronic and progressive loss of cells that occurs during normal development, apoptosis triggered by extrinsic signals is generally more acute and massive. Therefore, the capacity for removal of apoptotic cells may be overcome and secondary necrosis of unremoved apoptotic cells is frequent. This may lead to chronic accumulation of cellular debris with the potential for inducing inflammatory and/or auto-immune responses (Casciola-Rosen et al., 1994; Rosen et al., 1995; Tan, 1994).
Apoptosis is controlled by an ordered, stereotyped cascade of cellular events. Some death-commitment pathways are ubiquitous (for example, those involved in radiation-induced DNA damage) while others are present only in specific cells, therefore ensuring their effective removal without the elimination of other cell types adjacent to them. It is the relative abundance of pro-apoptotic and anti-apoptotic molecules within the cell at a given time that will determine the cell's fate.
The programmed cell death cascade can be divided into at least four main functionally distinct stages. The apoptosis signalling pathways are described in more detailed reviews (Golstein, 1997; Green & Reed, 1998; Kroemer et al., 1997), and will be summarized below (Figure 1):
The initiation or signalling phase during which cells receive the death-inducing signal. This may be accomplished by attachment of death-promoting molecules (e.g., Tumour Necrosis Factor (TNF)-α, Fas ligand) to death receptors (TNFR1, Fas) on the cell surface with subsequent recruitment of death domain proteins (FADD, TRADD, RIP) required for caspase-8 activation (which initiates the lethal proteolytic cascade) (Ashkenazi & Dixit, 1998).
-
The control and effector phase during which activation of caspases occurs with loss of mitochondrial membrane potential (Green & Kroemer, 1998). Caspases, the mammalian CED-3 homologues, are a family of cysteine proteases with aspartate specificity that have been implicated in the transduction and execution of the apoptotic programme (Thornberry & Lazebnik, 1998). Caspases are present as inactive pro-enzymes, most of which are activated by proteolytic cleavage. They are responsible for the deliberate disassembly of a cell into apoptotic bodies. Caspase-8, caspase-9 and caspase-3 are situated at pivotal junctions in apoptotic pathways (Figures 1 and 2).
The execution phase is controlled by the Bcl-2 family members (Kroemer, 1997) acting upstream from the caspases, through inhibition of cytochrome c and/or apoptosis inducing factor (AIF) release from the mitochondrion or through binding and sequestration of Apaf-1 away from caspase-3. The Bcl-2 family of proteins contains both inhibitors (Bcl-2, Bcl-xL) and inducers (Bcl-xS, Bax, Bid, Bad, Bak) of apoptosis acting as homodimers and heterodimers. The balance between anti-apoptotic and pro-apoptotic Bcl-2 family members is critical to determining if a cell undergoes apoptosis. After an appropriate signal, Bax or Bak, for example, undergo a conformational change and move to the mitochondrial membrane where they cause release of cytochrome c into the cytosol (Goping et al., 1998; Griffiths et al., 1999).
The structural alterations and DNA degradation phase (Figure 2), where caspase activation leads to the cleaving of lamin, the intermediate filament of the nuclear envelope, of poly(ADP)ribose polymerase (PARP) (Nicholson et al., 1995) and of the inhibitor of caspase-activated deoxyribonuclease (ICAD/DFF45) (Enari et al., 1998; Liu et al., 1997; Sakahira et al., 1998). Fragmentation and degradation of genomic DNA results in an irreversible loss of viability.
The phase of recognition of apoptotic cells and removal of apoptotic bodies. After the completion of the apoptotic processes, dead cells are removed from the tissue as a result of specific recognition and phagocytosis by adjacent professional and/or non-professional cells through a variety of mechanisms that implicate phosphatidylserine (PS), thrombospondin/CD36 binding site, the vitronectin receptor or CD14, for example (Devitt et al., 1998; Fadok et al., 1992; 1998; Savill et al., 1993). Loss of membrane phospholipid asymmetry and consequent exposure of PS in the exoplasmic leaflet of the plasma membrane is one of the early hallmarks of cells undergoing apoptosis, and is a critical event in their recognition by some macrophage populations (Fadok et al., 1992, 1998). In addition cells undergoing apoptosis generate reactive oxygen species that may induce membrane peroxidation (Ashkenazi & Dixit, 1998). Indeed, apoptotic cells present oxidatively modified moieties on their surface that are structurally analogous to the surface of the oxidized LDL (oxLDL) particle (Chang et al., 1999). These oxidation-specific epitopes, including oxidized phospholipids, serve as ligands for recognition and phagocytosis by macrophages through their scavenger receptors, including SR-A, CD36, CD68, SR-B1 (CLA-I) and LOX-1 (Bird et al., 1999; Chang et al., 1999). Complement activation seems to be required for efficient uptake of apoptotic cells within the systemic circulation (Mevorach et al., 1998).
The transverse redistribution of plasma membrane PS is followed by the shedding of membrane particles which is a common feature of apoptotic cell death (Aupeix et al., 1997). Annexin V which has a strong affinity for PS and exerts an external membrane constraint is able to reduce the degree of proteolytic activation of caspase-3 and totally blocks the release of shed membrane microparticles (Gidon-Jeangirard et al., 1999).
Figure 1.

Schematic representation of main apoptotic pathways. Activation of caspase-8 (and subsequent activation of the caspase cascade) occurs following ligation of death-signal-transmitting receptors. Caspase-9 activates cell disassembly in response to agents or insults that trigger release of cytochrome c from the mitochondria, and is activated when complexed with dATP, Apaf-1, and extramitochondrial cytochrome c to form the multi-protein “apoptosome” ensemble. Caspase-8 and caspase-9 can activate caspase-3 by proteolytic cleavage, and caspase-3 amplifies caspase-8 and caspase-9 signals into fully-fledged commitment to disassembly, therefore propagating the caspase cascade. Ligation of the death-signal-transmitting receptor TNFR may also generate signal transduction pathways leading to activation of the nuclear transcription factor NF-κB and to the induction of cytoprotective genes.
Figure 2.

Schematic representation of the structural alterations and DNA fragmentation phase. DFF is composed of two subunits, DFF45 (ICAD) and DFF40 (CAD). Cleavage of DFF45 by caspase-3 activates the nuclease activity of DFF40. Other substrates for caspases include PARP and lamin.
Apoptosis of endothelial cells
Vascular endothelial cells form the inner lining of all blood vessels. During both normal development and pathology, the formation of new vessels and the regression of preexisting ones are dependent on the balance between endothelial cell proliferation and endothelial cell apoptosis. In mature vessels, endothelial cell turnover is also under the control of these tightly regulated phenomena. Since the vascular endothelium is involved in various physiological processes, endothelial cell apoptosis (and dysfunction) may constitute an initial step in a variety of pathological situations such as atherosclerosis and hypertension (see below). As for other cell types, it has been hypothesized that interactions of endothelial cells with their microenvironment may be critical for their survival.
Survival pathways
Main survival pathways in endothelial cells are summarized in Table 1 and represented in Figure 3.
Table 1.
Endothelial cell survival factors

Figure 3.

Schematic drawing that shows the importance of cell-to-cell and cell-to-matrix-interactions in endothelial cell survival. The survival effect of growth factors like VEGF is dependent on cell-to-cell contact (involving VE-cadherin pathway). VE-cadherins can be upregulated by shear stress. Akt phosphorylation is central to many anti-apoptotic signalling pathways and modulates the balance between anti-apoptotic and pro-apoptotic bcl-2 family members. Pro-inflammatory stimuli, including TNF-α and IFN-γ, may interfere with matrix-dependent cell survival pathways.
Cell–cell and cell–matrix interactions
Cell-to-cell and cell-to-matrix contacts are necessary for the maintenance of survival of anchorage-dependent cells such as endothelial cells (Brooks et al., 1994; Levkau et al., 1998; Meredith et al., 1993; Re et al., 1994; Scatena et al., 1998). Loss of cell-to-cell contact leads to the activation of a default death programme in a variety of cell types.
The extracellular matrix may generate survival signals aiming at the suppression of a p53-regulated cell death pathway. For example, survival signals from fibronectin are transduced by the focal adhesion kinase (FAK) whose phosphorylation leads to p53 inactivation and maintenance of cell survival (Ilic et al., 1998). Interaction with extracellular matrix proteins via the integrin αvβ3 inhibits p53 activity, decreases the expression of p21 and Bax (Stromblad et al., 1996), and activates NF-κB (Scatena et al., 1998), therefore promoting cell survival. This is particularly important during migration of endothelial cells in angiogenesis as well as in the maintenance of tumour vasculature (Rüegg et al., 1998).
The extracellular matrix may also generate survival signals aiming at the upregulation of an anti-apoptotic pathway mainly by promoting the anti-apoptotic activity of the Bcl-2 family of proteins. Vascular endothelial cadherin, VE-cadherin, mediates adhesion between endothelial cells and affect endothelial cell survival. Deficiency or truncation of VE-cadherin induces endothelial cell apoptosis and abolishes transmission of the endothelial survival signal by VEGF-A to Akt kinase and Bcl2 via reduced complex formation with VEGF receptor-2, β-catenin, and phosphoinositide 3 (PI3)-kinase (Carmeliet et al., 1999). Growth factor deprivation of endothelial cells induces apoptosis, and this correlates with cleavage and disassembly of intracellular and extracellular components of adherens junctions. Caspase-3 appears to initiate proteolytic processing, leading to the cleavage of FAK (Levkau et al., 1998), β-catenin and plakoglobin and to the shedding of VE-cadherin (Herren et al., 1998), actively interrupting extracellular signals required for endothelial cell survival.
Growth factors
In vitro, VEGF inhibits endothelial cell apoptosis in response to a variety of stimuli such as hyperoxia (Alon et al., 1995), growth factor withdrawal, disruption of extracellular matrix (Watanabe & Dvorak, 1997), ionizing radiation (Katoh et al., 1995), peroxynitrite and stimulation with TNF-α (Karsan, 1998). This is accomplished in part through interaction with adherens junctions (Carmeliet et al., 1999), stimulation of protein kinase C or PI3K-Akt signalling pathway (Karsan, 1998), upregulation of the anti-apoptotic proteins Bcl-2 and A1 (Gerber et al., 1998), or those of the inhibitors of apoptosis family of anti-apoptotic proteins, i.e. survivin and XIAP (Tran et al., 1999).
Angiopoietin-1 ligation to its receptor Tie-2 has recently been shown to provide survival signals for the endothelial cell (Fujikawa et al., 1999; Hayes et al., 1999; Kwak et al., 1999). Growth-deprived endothelial cells are rescued from apoptosis by addition of angiopoietin-1. Angiopoietin-1 induces Tie-2 autophosphorylation leading to PI3K-dependent activation of Akt. The anti-apoptotic effect of angiopoietin-1, as that of VEGF, is mediated through interaction with the extracellular matrix since the anti-apoptotic effect of these growth factors is lost in suspended cells (Fujikawa et al., 1999). Angiopoietin-1 expression is associated with tumour neovascularization and tumour growth in vivo (Holash et al., 1999).
Basic fibroblast growth factor (FGF-2) is another intravascular survival factor for endothelial cells in vivo. Intravenous administration of FGF-2 in a murine model of endotoxic shock blocks LPS-induced ceramide generation and endothelial cell apoptosis. Consequently, animal mortality is reduced despite persistent elevation in serum TNF-α (Haimovitz-Friedman et al., 1997). In vitro studies have shown that FGF-2 specifically induces Bcl-2, but not other members of the Bcl-2 family, and inhibits serum-deprivation-induced endothelial cell death (Karsan et al., 1997). However, other Bcl-2-independent mechanisms, such as tyrosine phosphorylation, may also account for the inhibition of endothelial apoptosis by FGF-2 (Karsan et al., 1997).
Shear stress
Vascular endothelial cells are continuously exposed to a range of haemodynamic forces which have a great impact on their cellular structure and function. Variations in blood flow play an important role in vessel growth or regression, and in the focal development of atherosclerosis. A link between mechanical stimulation and cell survival or death has been therefore suggested by several groups. Indeed, human umbilical vein endothelial cells (HUVEC) cultured under static conditions undergo a basal level of apoptosis (Kaiser et al., 1997). The empty space left between cells induces proliferation of adjacent cells so that an equilibrium state is reached with low levels of apoptosis and proliferation. Exposure to flow in a perfusion chamber or in an ex vivo organ culture directly inhibits the apoptotic process and indirectly suppresses proliferation, leading to a “truly” quiescent monolayer (Kaiser et al., 1997).
This anti-apoptotic pathway involves the shear-induced phosphorylation of Akt/PKB (Dimmeler et al., 1998), and the subsequent phosphorylation of the endothelial NO synthase (Dimmeler et al., 1999a). NO, released in response to shear stress, inhibits caspase-3 activation and prevents endothelial cell apoptosis (Dimmeler et al., 1997b).
The suppression of both endothelial cell apoptosis and caspase-3 activation by physiological levels of shear stress in other models (growth factor withdrawal or cytokine-induced apoptosis) may be prevented by pharmacological inhibition of glutathione biosynthesis or nitric oxide synthase (Dimmeler et al., 1996; Hermann et al., 1997). Shear stress-dependent upregulation of Cu/Zn SOD and NO synthase prevents activation of the caspase cascade in response to apoptotic stimuli, including oxygen free radicals, oxLDL or TNFα (Dimmeler & Zeiher, 1999). This suggests that the reduction in oxidative flux by shear stress may be essential to prevent the activation of caspases and to inhibit endothelial cell apoptosis.
The modulation of endothelial cell apoptosis by shear stress plays a major role in normal and pathological vascular remodelling in vivo (see below).
Apoptotic pathways
A number of extra- or intracellular agents can induce apoptosis in endothelial cells as a result of loss of survival factors (see above), modulation of survival pathways or induction of pro-apoptotic pathways.
Modulation of survival pathways
Some potentially important pro-apoptotic factors act essentially by inhibiting survival pathways. For example, exposure of human endothelial cells to TNF-α and IFN-γ results in suppression of endothelial cell αvβ3 activity leading to a decreased αvβ3-dependent endothelial cell adhesion and survival (Rüegg et al., 1998). In vivo, TNF-α and IFN-γ interact with αvβ3 to cause selective disruption of the tumour vasculature in patients with melanoma (Rüegg et al., 1998).
Stimulation of human endothelial cells with TNF-α leads to a specific degradation of Bcl-2, a process that is inhibited by specific proteasome inhibitors (Dimmeler et al., 1999b).
Finally, angiostatin-induced endothelial cell apoptosis (Claesson-Welsh et al., 1998; Lucas et al., 1998) paradoxically involves FAK activation (Claesson-Welsh et al., 1998). FAK activation by angiostatin is not associated with Src coactivation and does not involve integrin signalling. In fact, angiostatin probably causes a flawed, integrin-independent activation of FAK that perturbs the ordered turnover of focal adhesion contacts induced by VEGF and FGF-2. Angiostatin-induced endothelial apoptosis may be an important mechanism by which angiostatin modulates the angiogenic process.
Induction of pro-apoptotic pathways
Inflammatory mediators Endothelial dysfunction is central to many inflammatory diseases affecting the vessel wall (atherosclerosis, for example) and several other organs (septic shock). Also, inflammatory cytokines such as TNF-α are increased in the circulating blood of patients with terminal heart failure and may participate to the vascular dysfunction observed in this disease. Incubation of HUVEC with TNF-α markedly increases endothelial cell apoptosis via activation of caspase-3, a process that can be completely abrogated by inhibitors of caspases or by shear stress (Dimmeler et al., 1996).
Peripheral blood mononuclear monocytes (PBMCs) preactivated by bacterial lipopolysaccharide (LPS) or by ionizing radiation induce apoptotic death in cultured HUVECs (Lindner et al., 1997). This process is dependent at least in part on TNF-α since addition of an anti-TNF-α monoclonal antibody blocks the cell-to-cell contact-mediated apoptosis. Interestingly, the anti-inflammatory cytokine IL-10 has anti-apoptotic effects in this model, suggesting that the balance between endothelial cell survival and death may depend on the balance between pro- and anti-inflammatory cytokines. Incubation of HUVEC with LPS in culture induces apoptosis and secondary necrosis in a time-dependent manner. In this setting, apoptosis is independent of TNF-α release but is associated with the induction of Bax and is prevented by incubation with antioxidants (that enhance Bcl-2 and decrease Bax) (Haendeler et al., 1996). It is noteworthy that TNF-α, like other inflammatory cytokines, is capable of inducing human A1, a human Bcl-2 homologue (Karsan et al., 1996) and may also activate the NF-κB pathway (Van Antwerp et al., 1996). TNF-α may therefore initiate both pro-apoptotic and anti-apoptotic pathways.
Systemic administration of LPS induces the endotoxic shock syndrome with associated systemic inflammation, multiple organ damage, circulatory collapse and death. This process is mediated by the release of TNF-α and other cytokines. Since TNF-α and LPS can cause apoptotic death of endothelial cells, it has been hypothesized that the primary tissue target in septic shock may be the endothelium. Indeed, it was demonstrated that injection of LPS and TNF-α in mice induces massive apoptosis in the endothelium of several organs before any other form of tissue damage (Haimovitz-Friedman et al., 1997). Unlike studies performed in vitro, LPS-induced endothelial apoptosis in vivo is dependent on TNF-α since it is blocked by the TNF-binding protein. This latter molecule also prevents LPS-induced ceramide generation, suggesting that systemic TNF is required for both responses (Haimovitz-Friedman et al., 1997). The indispensable role of ceramide generation in disseminated endothelial apoptosis is demonstrated by the observation that acid sphingomyelinase knockout mice are protected from endothelial cell apoptosis and from death despite the normal increase in serum TNF-α in response to LPS (Haimovitz-Friedman et al., 1997). It would be interesting to examine whether the beneficial effects of IL-10 in reducing mortality from septic shock in animals (Howard et al., 1993) are related to its potential anti-apoptotic properties.
In the context of endothelial inflammation, it is particularly important to point out that the antiapoptotic proteins Bcl-2 and Bcl-XL are able to downregulate endothelial cell activation through specific inhibition of NF-κB at a level upstream of IκBα degradation (Badrichani et al., 1999). Bcl-2 and Bcl-XL may therefore be cytoprotective in endothelial cells by counteracting both proapoptotic and proinflammatory insults.
Oxidized lipoproteins The evidence available from several groups suggests that oxLDL play a central role in atherogenesis beginning in the earliest phases of this threatening process. In vitro studies had already shown that oxLDL exhibit cytotoxic effects on cultured endothelial cells, but the type of cell death elicited remained unknown (Hessler et al., 1979). However, several authors recently reported increased apoptotic cell death of bovine aortic endothelial cells exposed to cholesterol oxides in culture (Lizard et al., 1996) and increased apoptosis (and possibly secondary necrosis) of HUVEC after exposure to oxLDL (Dimmeler et al., 1997a; Escargueil-Blanc et al., 1997). OxLDL-induced apoptosis is subsequent to a sustained and delayed peak in cytosolic calcium and is prevented by chelating extracellular calcium or by inhibiting calcium influx (Escargueil-Blanc et al., 1997). Furthermore, ceramide generation by acid sphingomyelinase and activation of caspase-3 are indispensable for oxLDL-induced apoptosis (Harada-Shiba et al., 1998). OxLDL are known to increase the generation of reactive oxygen species which are implicated in the apoptotic process in various cell types. Addition of antioxidants (vitamins C and E) completely prevents the activation of caspase-3 by oxLDL and inhibits the apoptotic process (Dimmeler et al., 1997a). Although vascular endothelial cells are normally resistant to Fas-mediated cell apoptosis (Richardson et al., 1994), apoptosis induced by OxLDL in cultured endothelial cells and endothelium of arterial explants appears to be inhibited by Fas ligand neutralizing antibodies, suggesting that OxLDL may promote Fas-mediated endothelial cell apoptosis (Sata & Walsh, 1998).
Apoptosis of vascular smooth muscle cells
After decades of intensive research on the mechanisms of vascular smooth muscle cell (VSMC) proliferation, recent studies have been undertaken to understand the mechanisms and roles of smooth muscle cell survival/apoptosis in normal vessel development and pathology. VSMC growth is now viewed as the result of the opposing effects of cell proliferation and apoptosis.
Survival pathways
Cell–cell and cell–matrix interactions
As for endothelial cells, cell–matrix interactions are necessary for vascular smooth muscle cell homeostasis through the generation of signals involved in cell survival, migration and/or proliferation. Fibronectin and αvβ3 play important roles in the maintenance of these transduction pathways (Coleman et al., 1999; Dufourcq et al., 1998; Mason et al., 1999; Zheng & Clemmons, 1998). The matrix glycoprotein tenascin-C has also been shown to modulate smooth muscle cell survival and proliferation. Tenascin-C interactions with αvβ3 integrins upon upregulation by denatured type I collagen modify VSMC shape, and epidermal growth factor (EGF)-dependent growth by inducing a clustering of the EGF-receptors (Jones et al., 1997a). These extracellular matrix-dependent survival pathways have been shown to be involved in the progression of pulmonary vascular diseases (Jones et al., 1997b) and are likely to play an important role in VSMC survival within the atherosclerotic plaque.
Growth factors
Many known growth factors for VSMCs (IGF-1, PDGF-BB, FGF-2 and TGF-β1) have been shown to act as survival factors rather than true mitogens. These growth factors partially prevent apoptosis of human or rat VSMCs cultured under low serum conditions (Bai et al., 1999; Bennett et al., 1995a; Fox & Shanley, 1996; Pollman et al., 1999a) in part by favouring the anti-apoptotic potential of the Bcl-2 family of proteins. For example, IGF-I stimulates increased expression of the inactive, phosphorylated form of Bad by a PI3-kinase-dependent pathway (Bai et al., 1999). These growth factors and their corresponding receptors are expressed in many vascular diseases and may prevent VSMC death within the arterial wall.
Apoptotic pathways
Many pro-apoptotic factors for VSMCs have been identified, some with potential implications in many vascular diseases such as atherosclerosis, restenosis or hypertension.
Mechanical factors
Balloon angioplasty induces rapid death of VSMCs within the normal arterial wall (Pollman et al., 1999b). VSMC apoptosis is associated with the induction of a redox-sensitive signalling pathway leading to the activation of the stress-activated protein kinase (SAPK). Administration of anti-oxidants inhibits SAPK activation and VSMC apoptosis. This signalling pathway could not be activated in neointimal smooth muscle cells due to the upregulation of the antiapoptotic mediator bcl-xL in these cells (Pollman et al., 1999b).
Oxidized LDL
Oxidized LDL (Joringe et al., 1997) and oxysterols (Nishio & Watanabe, 1996) have been shown to promote VSMC apoptosis in culture, in part by downregulation of Bcl-2 and activation of caspase-3 (Nishio & Watanabe, 1996).
Vasoactive substances
Local production of high levels of NO within the arterial wall inhibits cell growth and neointimal formation following balloon injury (von der Leyen et al., 1995), while forced local generation of Ang II in the vessel wall (after overexpression of the angiotensin-converting enzyme, ACE) promotes VSMC growth and wall thickening (Morishita et al., 1994). These effects on vascular remodelling are likely the result of a modulation of the apoptotic process in VSMCs. NO-induced apoptosis in VSMCs is prevented by the inhibition of the cGMP-dependent protein kinase Iα, as well as by the addition of Ang II (acting via the angiotensin receptor type 1), emphasizing the importance of the balance between NO and Ang II in the modulation of VSMC growth (Pollman et al., 1996). It is noteworthy that the pro-apoptotic effects of NO occur at supraphysiological (pathological or inflammatory) levels, while its anti-apoptotic effects, through the prevention of caspase activation, are essentially observed at physiological (endothelial) concentrations (Dimmeler et al., 1997b; Kim et al., 1999). Similarly, Ang II may have dual effects on VSMC apoptosis, preventing cell death via AT1 receptor stimulation, but promoting apoptosis via AT2 receptor stimulation (Yamada et al., 1998). Yet, the AT2 receptor is expressed abundantly in foetus, but scantily in adult tissues. Therefore it might play a major role in vascular remodelling during development but its role in adult remains to be evaluated.
Inflammatory mediators
Production of high levels of NO via the inducible nitric oxide synthase (iNOS) accounts for the pro-apoptotic effects of pro-inflammatory cytokines (TNF-α, IFN-γ and IL-1) in rat but not human VSMCs (Geng et al., 1996). Stimulation of VSMCs in culture by pro-inflammatory cytokines also induces the expression of Fas (and increases their sensitivity to apoptosis) (Geng et al., 1997), suggesting that the production of such cytokines by immune cells in atherosclerosis may promote VSMC death through this pathway. Inflammatory cells, particularly macrophages, have the potential of inducing VSMC apoptosis in culture. This process is dependent on cell-to-cell contact and on TNFα and FasL interactions with their corresponding receptors TNFR-I and II, and Fas (Boyle et al., 1998).
Oncogenes and tumour suppressor genes
Deregulated expression of the c-myc oncogene and stable infection with the adenovirus E1A induce apoptosis in rat VSMCs under low serum conditions (Bennett et al., 1994, 1995a). Moreover, overexpression of p53 induces apoptosis in rat VSMCs infected with c-myc and E1A, but not in normal cells (Bennett et al., 1995a).
These observations are important for several reasons: the c-myc oncogene is overexpressed in VSMCs from human atherosclerotic plaques and these cells display a lower growth rate in culture in comparison with VSMCs obtained from normal arteries, especially under low serum conditions (Parkes et al., 1991). As expected, this intrinsic defect in growth has recently been shown to be, at least in part, the result of increased spontaneous cell death by apoptosis (Bennett et al., 1995b). The characteristics of plaque VSMCs may also explain in part their hypersensitivity to p53-mediated apoptosis (Bennett et al., 1997). p53 activation in cells derived from atherosclerotic plaque transiently increases surface Fas expression by transport from the Golgi complex, and induces Fas-FADD binding, sensitizing cells to Fas-induced apoptosis (Bennett et al., 1998b). However, basal p53 activity is similar in plaque and normal VSMCs and suppression of basal p53 activity blocks growth arrest in both cell types, but does not prevent apoptosis suggesting that the mechanisms of p53-mediated apoptosis of plaque VSMCs may be distinct from those inducing growth arrest (Bennett et al., 1997). The slower rates of proliferation and earlier senescence of plaque VSMCs may be explained by the predominance of the hypophosphorylated form of the retinoblastoma gene product (RB) and the low E2F transcriptional activity in these cells (Bennett et al., 1998a). Both inactivation of RB and inhibition of p53 activity are required for plaque VSMCs to proliferate without apoptosis (Bennett et al., 1998a).
VSMC apoptosis may be induced in vitro by many factors including inhibitors of protein kinase C, calcium channel blockers, angiotensin-converting enzyme inhibitors, stimulators of cyclic AMP-dependent protein kinase (Leszczynski et al., 1994), and tissue inhibitor of metalloproteinase type 3 (Baker et al., 1998). However, the clinical relevance of the pro-apoptotic effects of these factors remains to be established.
Role of apoptosis in normal vessel development
Physiological remodelling of blood vessels before and after birth has been shown to be the result of a balance between apoptosis and cell proliferation. Developmentally programmed capillary regression is initiated by macrophage-mediated endothelial cell apoptosis (Lang & Bishop, 1993). The dying cells are projected into the capillary lumen causing temporary or permanent block to blood flow. Following cessation of flow, secondary synchronous apoptosis of vascular endothelial cells occurs, leading to capillary regression (Meeson et al., 1996). It can be hypothesized that during development, the selection of capillary beds is under the control of blood flow-dependent endothelial cell survival/apoptosis.
Apoptosis of smooth muscle cells occurs during the development of the human ductus arteriosus (Slomp et al., 1997). The role of apoptosis has also been investigated in vessel remodelling that occurs as arteries adapt to changes in cardiovascular function after birth. The abdominal aorta of neonatal lambs undergoes excessive remodelling in association with a profound decrease in blood flow after birth. However, DNA accumulation in this vessel is far below that predicted from proliferation rates if all cells were assumed to survive. Cho et al. (1995) have demonstrated that apoptosis significantly contributes to postpartum arterial remodelling and that changes in cell death rates alone may be sufficient to induce profound changes in the vessel wall mass (Cho et al., 1995).
Role of apoptosis in vascular pathology
Apoptosis appears to play a significant role in many vascular or vascular-related diseases. In this review, a particular emphasis is put on the potential role of apoptosis in atherosclerosis and restenosis. Reviews on the occurrence and role of apoptosis in hypertensive diseases have been recently published (Diez et al., 1998; Hamet et al., 1996).
Apoptosis in atherosclerosis
Observations that cell death occurs in atherosclerosis have already been made by Virchow (1858), and experimental studies in cholesterol-fed swines undertaken by Thomas et al. (1976) more than 20 years ago showed that cell death is a major event occurring during atherosclerotic plaque development and may be associated with cell proliferation. More recently, Parkes et al. observed that plaque-derived smooth muscle cells (p-VSMC) are characterized by c-myc overexpression and have a growth disadvantage in culture in comparison with VSMCs from normal arteries, especially under low serum conditions (Parkes et al., 1991). At that time, cancer researchers have shown that overexpression of c-myc induced cell death by apoptosis in the absence of serum and that cells can be rescued by overexpression of bcl-2 (Evan et al., 1992). On the basis of these results, it has been suggested that cell death by apoptosis may occur in human atherosclerosis. Bennett et al. have subsequently demonstrated that deregulated expression of c-myc in VSMCs induces apoptosis (Bennett et al., 1994) and that human p-SMCs have a markedly elevated rate of apoptosis in vitro, a process that can be reversed by expression of bcl-2 or by other survival factors such as IGF-1 and PDGF (Bennett et al., 1995b). This was the first indication that programmed cell death (apoptosis) may occur in human atherosclerosis.
In situ distribution of apoptosis
Several studies have shown evidence for in situ apoptotic cell death in animal and human atherosclerotic plaques (Björkerud & Björkerud, 1996; Cai et al., 1997; Geng & Libby, 1995; Han et al., 1995; Hegyi et al., 1996; Isner et al., 1995; Kockx et al., 1996; Mallat et al., 1997). Apoptosis, which is almost absent in normal arteries, becomes barely detectable in fatty streaks and is more abundant in advanced plaques. All cell types are involved, including SMCs, macrophages, T-lymphocytes (Björkerud & Björkerud, 1996; Cai et al., 1997; Geng & Libby, 1995; Han et al., 1995; Hegyi et al., 1996; Isner et al., 1995; Kockx et al., 1996; Mallat et al., 1997) and luminal endothelial cells (Tricot et al., 2000). Removal of apoptotic cells in human plaques may be inefficient, as a significant percentage of secondary necrosis is observed. Foam cell apoptosis (and secondary necrosis) is thought to contribute to the formation of the acellular lipid core (Cai et al., 1997), whereas VSMCs die within their cages of thickened basal lamina in the hypocellular fibrous cap and are split into a myriad of membrane vesicles (Kockx et al., 1998). The distribution of apoptosis is heterogeneous within the plaque, being more frequent in regions with a high density in macrophages suggesting that these cells may be participating in the induction of apoptosis. In addition, we have recently observed that luminal endothelial apoptosis is directly related to the blood flow direction, being more prevalent in the post-stenotic area where low flow and low shear stress prevail (Tricot et al., 2000). This in vivo finding in human atherosclerotic plaques confirms the in vitro observation of a strong modulation of endothelial cell survival by shear stress as indicated above.
Pro- and anti-apoptotic factors in atherosclerotic plaques
Many factors with in vitro pro- and anti-apoptotic activities in macrophages, smooth muscle cells and endothelial cells have been shown to be expressed in human atherosclerosis (Table 2). Pro-inflammatory cytokines with pro-apoptotic potential including IL-1, TNF-α, and IFN-γ are present in the human plaque and may be involved in the local induction of the apoptotic process, although no studies have been performed showing direct correlation between their expression and the occurrence of apoptosis. However, one of their mediators, inflammatory NO, produced as the result of upregulation of iNOS, may exert potent cell cytotoxicity. We have recently found a positive association between iNOS expression and TUNEL labelling for apoptotic cells in advanced human atherosclerosis (Mallat et al., 1999a).
Table 2.
Pro- and Anti-apoptotic factors in human atherosclerotic plaques
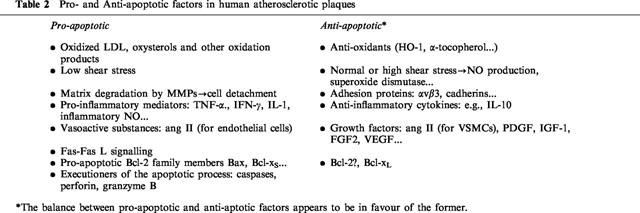
On the other hand, high levels of expression of the anti-inflammatory cytokine IL-10 are associated with significantly reduced levels of iNOS expression and apoptotic cell death (Mallat et al., 1999a), suggesting that apoptosis may result from an excessive inflammatory reaction and may be modulated by interfering with the inflammatory response.
Cytokine-induced apoptosis may be mediated in part by the Fas receptor. In vitro studies have shown that pro-inflammatory cytokines induce upregulation of Fas in human VSMCs and this fits well with the more prominent expression of Fas in the human plaque intima than in the media (Geng et al., 1997). Moreover, cytokine-primed VSMCs, but not unprimed cells, are sensitive to anti-Fas stimulation in vitro and undergo apoptosis. This may explain the association between Fas-positive cells and apoptotic VSMCs in regions of plaques that are rich in activated T-lymphocytes and macrophages (Geng et al., 1997). Fas-mediated apoptotic cytotoxicity was also reported in human transplant coronary artery disease (Dong et al., 1996). Interestingly, decreased Fas expression in a subset of plaque-SMCs has been reported, which could lead to resistance to apoptosis via the Fas system (Han et al., 1996).
Smooth muscle cells die within the human plaque despite the presence of survival factors such as IGF-1 and PDGF (Bennett et al., 1995b). Deregulated expression of c-myc in the absence of significant Bcl-2 expression (Bennett et al., 1994), and increased sensitivity to p53 (Bennett et al., 1997) might offer some explanations. Recent data have also shown increased expression of the pro-apoptotic protein Bax in human fatty streaks and advanced plaques. Bax was undetectable in normal arteries where expression of the anti-apoptotic protein Bcl-xL predominated (Kockx et al., 1998). Therefore, the balance between pro- and anti-apoptotic proteins in atherosclerosis is in favour of the former, suggesting that plaque-SMCs are programmed to die and undergo apoptosis when additional pro-apoptotic stimuli are present (inflammatory cells and cytokines).
Cell survival within human atherosclerotic plaques may also depend on cell interactions with the extracellular matrix. Matrix metalloproteinases (MMPs) are overexpressed in human plaques and play an important role in matrix degradation (Galis et al., 1994). This may result in cell detachment from the matrix and in induction of apoptosis. This hypothesis is supported by the finding that overexpression of the tissue inhibitor of metalloproteinases (TIMP-1) in the arterial wall of ApoE Knockout mice is associated with an increased density of smooth muscle cells within the atheromatous plaque (Rouis et al., 1999). On the other hand, nonfibrillar or monomeric collagen (present in atherosclerotic plaques) allows VSMCs to undergo proliferation in response to mitogens in culture, while fibrillar collagen (present in the normal media of arteries) inhibits cell proliferation by up-regulating specific inhibitors of the cell cycle (Koyama et al., 1996). Thus, the matrix that surrounds the cells is not neutral and may determine whether they remain quiescent, survive or multiply in response to growth factors (Ross, 1999).
The execution phase of apoptosis is caspase-dependent. In accordance with this concept, we and others (Geng & Libby, 1995; Mallat et al., 1997) have shown that caspases, especially caspase-3, colocalized with apoptotic cells, particularly macrophages, in advanced human atherosclerotic plaques. However, caspase-3 positive, but TUNEL-negative, T-lymphocytes may also be observed. This is consistent with the observation that caspase-3 cleavage in T-cells may occur without the induction of apoptosis (Miossec et al., 1997).
Potential roles of apoptosis in human atherosclerosis (Table 3)
Table 3.
Potential roles of apoptosis in atherosclerosis

Much interest has focused on the regulation of apoptosis, but only speculations have been raised about the potential roles, beneficial or harmful, of this process of cell death in atherosclerosis. Death of VSMCs by apoptosis may be viewed as harmful because it may weaken the fibrous cap by decreasing the synthesis of extracellular matrix and therefore may lead to plaque rupture. However, others may argue that plaque stabilization observed with anti-β3 integrin antibodies (Topol et al., 1994) may involve selective apoptosis of intimal smooth muscle cells that express αvβ3 integrin and whose attachment to the extracellular matrix are disrupted.
Death of T-lymphocytes and macrophages by apoptosis may be viewed as beneficial if apoptosis is not accompanied by an inflammatory reaction. Indeed, removal of these cells from the plaque could attenuate the inflammatory response, decrease the synthesis of MMPs and the consequent breakdown of the extracellular matrix, therefore favouring plaque stabilization. It may also be argued that apoptotic death of any cell type in the plaque may favour plaque regression over plaque progression. However, the rate of apoptotic cell death in the plaque (2–10%) (Bauriedel et al., 1998; Kockx et al., 1996; Mallat et al., 1997) may, in many instances, exceed the reported rate of cell proliferation (<1%) (Brandl et al., 1997; Gordon et al., 1990). In the light of observations that human atherosclerotic plaques are generally more prone to progression rather than regression, it can be suggested that apoptosis is actually implicated in natural plaque progression (rather than regresssion), through development of the acellular lipid “necrotic” core. Indeed, macrophage apoptosis has frequently been identified at the edges of the lipid core, suggesting that it may actively contribute to its formation. Moreover, the heterogeneous distribution of apoptosis implies that some regions of the plaque may show substantially higher levels of cell death, possibly predisposing to plaque rupture (and vessel occlusion or plaque progression). Removal of apoptotic cells from the atherosclerotic tissue may not be efficient because apoptotic cells may compete with oxidized lipids for removal by macrophages (Chang et al., 1999). This would lead to the persistence of apoptotic bodies with potentially high immunogenic properties (Levine & Koh, 1999). Moreover, unremoved apoptotic cells are prone to undergo secondary necrosis and this may lead to accumulation of extracellular lipids and to perpetuation of the inflammatory response.
We believe that one of the major roles of apoptosis in atherosclerosis is related to its procoagulant potential. PS exposure on the outer cell surface, a hallmark of apoptosis, greatly enhances tissue factor (TF) activity and is therefore highly thrombogenic (Bach et al., 1986). This procoagulant potential of apoptotic cells may be deleterious in atherosclerosis since it may be involved in determining plaque thrombogenicity following plaque rupture and may therefore favour the occurrence of acute ischaemic events and infarction. Indeed, Flynn et al. (1997) have shown that thrombin generation is increased at the contact of plaque-derived smooth cells undergoing apoptosis in culture. Moreover, we have recently observed significant extracellular TF expression in and around apoptotic cells in some regions of human atherosclerotic plaques suggesting that TF may be shed from apoptotic cells via apoptotic microparticles (Mallat et al., 1999b). To examine whether apoptosis may be involved in TF activity in vivo, we examined advanced human atherosclerotic plaques for the presence of shed membrane apoptotic microparticles (captured by biotinylated annexin V insolubilized onto streptavidin-coated microtitration plaques) and determined the procoagulant potential of these microparticles (Mallat et al., 1999b). We found that high levels of shed membrane microparticles of monocytic and lymphocytic origins are produced in the atherosclerotic plaques and are associated with increased TF activity. Interestingly, removing the microparticles from the supernatants resulted in a 97% reduction in TF activity, indicating that almost all of the TF activity of plaque extracts is associated with the shed membrane microparticles (Mallat et al., 1999b). These experiments provide direct evidence that apoptosis is involved in the increased thrombogenic potential of human atherosclerotic plaques and may be an important determinant of plaque thrombosis upon plaque rupture. In addition, we have recently found that a significant percentage of luminal endothelial cells may undergo apoptosis in human atherosclerotic plaques (Tricot et al., 2000). We believe that these apoptotic endothelial cells may initiate plaque erosion and promote local platelet aggregation and thrombosis in vivo, being therefore responsible for acute ischaemic syndromes. Finally, experiments in our laboratory show that plasma levels of procoagulant microparticles are increased in patients with acute coronary syndromes and may be associated with coronary reocclusion (Mallat et al., 2000).
Therapeutic modulation of apoptosis in atherosclerosis
Given the importance of apoptosis in plaque development and stability, interventions designed to modulate the apoptotic process are warranted and may profoundly affect disease evolution.
Induction of apoptosis to promote plaque regression (Table 4)
Table 4.
Potential effects of induction of apoptosis in atherosclerosis

Pollman et al. (1998) have reported regression of atherosclerotic plaques of cholesterol-fed rabbits after inhibition of neointimal cell Bcl-x expression and induction of neointimal cell apoptosis. Wang et al. (1999) have also observed regression of rabbit atheromatous lesions following in vivo administration of L-arginine to induce macrophage apoptosis via NO release. However, it is still unknown whether human atherosclerotic plaques will behave in the same manner. Rabbit atherosclerotic plaques do not rupture whereas human plaques are prone to rupture. In addition, others did not confirm this finding by using a different approach to induce intimal cell apoptosis (overexpression of FADD) (Schaub et al., 1998). Rather, vascular inflammation followed the induction of the apoptotic process and led to neointima progression (Schaub et al., 1998). On the other hand, it could be hazardous to induce massive apoptotic cell death in human atherosclerotic plaques without ensuring high anti-coagulation levels given the increased procoagulant potential of apoptotic cells (Bombeli et al., 1997; Flynn et al., 1997; Mallat et al., 1999b).
Plaque neovascularization may be involved in plaque development and progression (Moulton et al., 1999). Therefore, induction of endothelial cell apoptosis within plaque neovessels, using angiostatin or endostatin for example, could be a potential approach to limit plaque progression. However, such therapy would also induce apoptotic death in luminal endothelial cells with the potential of inducing a procoagulant state at the interface between the plaque and the circulating blood.
Inhibition of apoptosis to prevent plaque rupture/erosion and thrombosis (Table 5)
Table 5.
Potential effects of inhibition of apoptosis in atherosclerosis

We have shown that apoptosis occurs in the different cell types present in human atherosclerotic plaques, including endothelial cells, and may therefore initiate plaque disruption and thrombosis (Mallat et al., 1999b; Tricot et al., 2000). We believe that strategies aimed at the inhibition of apoptosis may limit plaque erosion, thrombosis and progression. Nitric oxide is a powerful anti-apoptotic molecule for endothelial cells (Dimmeler et al., 1997b) and accounts, at least in part, for the anti-apoptotic effects of laminar shear stress on endothelial cells in vitro. In vivo, the apoptotic luminal endothelial cells present in human atherosclerotic plaques are preferentially located in the low shear stress post-stenotic area (Tricot et al., 2000) where NO production can be expected to be reduced. Therefore, in vivo delivery of NO, via supplementation with L-arginine or transfer of the NOS III gene for example, or in vivo delivery of endothelial survival factors such as VEGF or FGF-2, may limit luminal endothelial cell apoptosis and thrombosis, and alter plaque progression.
In vitro studies have shown that angiotensin II induces endothelial cell apoptosis (Dimmeler et al., 1997c; Li et al., 1999a,1999b). The apoptotic process is prevented by addition of NO donors or angiotensin II antagonists (Dimmeler et al., 1997c; Li et al., 1999a,1999b). One could be argued that in vivo inhibition of angiotensin II production or signalling may prevent endothelial cell apoptosis. Recently, angiotensin-converting enzyme inhibitors have been shown to significantly reduce the occurrence of myocardial infarction in humans (Yusuf et al., 2000). We speculate that a reduction in endothelial cell apoptosis and thrombosis is one of the potential mechanisms responsible for this beneficial effect.
Oestrogen has been shown to be anti-atherogenic and to exert beneficial effects in the regulation of vascular tone in atherosclerotic arteries. Oestradiol treatment of HUVEC in culture results in a dose-dependent, receptor-mediated inhibition of TNF-α-induced apoptosis and activation of the caspase-1 pathway (Spyridipoulos et al., 1997). Moreover, oestrogen administration increases the release of bioactive nitric oxide by inhibiting superoxide anion production (Arnal et al., 1996). These data suggest that the beneficial atheroprotective effects of oestradiol may result, at least in part, from the preservation of endothelial integrity and prevention of vessel thrombosis. This may also be the case of vitamins with anti-oxidant properties which inhibit oxidized LDL-induced endothelial cell apoptosis in vitro (Dimmeler et al., 1997a).
Another powerful anti-oxidant and anti-apoptotic product for endothelial cells is the product of the haeme oxygenase (HO)-1 gene (Ferris et al., 1999; Hancock et al., 1998; Yachie et al., 1999). Deficiency in HO-1 is associated with severe endothelial damage (Ferris et al., 1999; Hancock et al., 1998), while overexpression of HO-1 is associated with expression of various cytoprotective genes, such as Bcl-2, and prevents from transplant arteriosclerosis (Hancock et al., 1998). Moreover, endothelial integrity in this setting is associated with the expression of anti-inflammatory cytokines including IL-10 (Bach et al., 1997).
Finally, occurrence of apoptosis within human atherosclerotic plaques is highly dependent on the inflammatory balance (Mallat et al., 1999a). Expression of the anti-inflammatory cytokine IL-10 within the plaque is associated with decreased signs of inflammation and decreased apoptosis (Mallat et al., 1999a). Moreover, IL-10 plays an important protective role against the development of atherosclerosis in mice (Mallat et al., 1999c), and displays potential anti-thrombotic effects in vivo (Downing et al., 1998; Ernofsson et al., 1996). Therefore, in vivo administration of IL-10 might be a sound strategy to deactivate inflammatory cells, reduce apoptotic death and its pro-thrombogenic properties, leading to plaque stabilization. These effects may be beneficial in both atherosclerosis and restenosis.
Apoptosis in restenosis
Animal models of neointima formation following balloon injury have shown that apoptotic cell death occurs at different points in time and at variable levels during the repair process. Apoptotic death of smooth muscle cells is observed as early as 30 min after arterial injury and is associated with decreased expression of the anti-apoptotic protein Bcl-x (Perlman et al., 1997). After then, a second window of apoptosis is observed and is associated with increased smooth muscle cell proliferation (Han et al., 1995). Persistent apoptotic cell death at later stages, in the absence of cell proliferation, has been implicated in the regulation of intimal thickening (Bochaton-Piallat et al., 1995).
Studies available in human restenotic plaques are somewhat contradictory. Isner et al. observed an increased apoptotic rate in restenotic versus primary coronary plaques (Isner et al., 1995). However, this finding was recently challenged by Bauriedel et al. (1998) who observed decreased levels of apoptosis in restenotic versus primary plaques with no differences in necrosis levels. High cellularity is a key finding in late human restenosis and the findings of Bauriedel et al. (1998) are more consistent with the paradigm that lower rates of apoptosis result in hyperplasia. This also fits well with the finding of decreased p53 activity (and potentially p53-mediated apoptosis) in restenotic material from human coronary lesions (Speir et al., 1994).
The observation that apoptosis may participate in cellularity regulation in animal models of neointima formation and in human restenotic lesions prompted researchers to examine the effects of pro-apoptotic strategies on this process. Steg et al. (1997) used suicide gene therapy by transfer of a replication-defective adenoviral vector expressing the thymidine kinase gene combined with ganciclovir treatment. In vitro transfection and treatment of SMCs induced their death by apoptosis, and this mechanism was presumably responsible for the reduction of restenosis after angioplasty in transfected and treated atheromatous rabbit arteries. Pollman et al. (1998) used another approach interfering with the anti-apoptotic genes expressed in the neointima, which consisted in the local application of antisense oligonucleotides encoding a sequence complementary to the coding region for the anti-apoptotic bcl-x gene. Using this strategy, they were able to decrease neointimal expression of Bcl-x, inducing selective apoptosis of neointimal cells and regression of neointimal hyperplasia. Other investigators have used a direct pro-apoptotic strategy delivering Fas ligand into the vessel wall and inducing apoptosis of Fas-bearing VSMCs (Sata et al., 1998). This also led to significant inhibition of neointima formation adding to the evidence that apoptosis is a key determinant of neointimal thickening evolution after arterial injury. However, it remains to be determined whether such impressive results may be achieved in humans without serious side effects, namely vessel inflammation and thrombosis.
Conclusion
The observation that cell death and cell proliferation may occur independently and the growing understanding of the molecular basis for these phenomena will stimulate the development of interventions selectively aimed at cell death to prevent the progression of a variety of vessel diseases.
Abbreviations
- ACE
angiotensin-converting enzyme
- AIF
apoptosis inducing factor
- Aktinein/PKB
protein kinase B
- Anginein II
angiotensin II
- APAFinein-1
apoptotic protease-activating factor 1
- CAD
caspase-activated deoxyribonuclease
- Caspases
cysteine aspartate-specific proteases
- CDKI
cyclin-dependent kinase inhibitor
- CIDE
cell death-inducing DFF45-like effector
- CLAinein-1
CD36 and LIMPII analogous-1
- DFF
DNA fragmentation factor
- EC
endothelial cell
- EGF
epidermal growth factor
- FADD
Fas-associated death domain
- FAK
focal adhesion kinase
- FasL
Fas ligand
- FGFinein-2
basic fibroblast growth factor
- HUVEC
human umbilical vein endothelial cell
- ICAD
inhibitor of caspase-activated deoxyribonuclease
- IFN
interferon
- IGF
insulin-like growth factor
- IL
interleukin
- LDL
low density lipoprotein
- LOXinein-1
lectin-like oxLDL receptor-1
- LPS
lipopolysaccharide
- MMP
matrix metalloproteinases
- NO
nitric oxide
- oxLDL
oxidized low density lipoprotein
- PARP
poly(ADP)-ribose polymerase
- PBMC
peripheral blood mononuclear monocyte
- PDGF
platelet-derived growth factor
- PI3K
phosphoinositide 3 kinase
- PS
phosphatidylserine
- RIP
receptor-interacting protein
- SAPK
stress-activated protein kinase
- SOD
superoxide dismutase
- SR
scavenger receptor
- TF
tissue factor
- TIMPinein-1
tissue inhibitor of metalloproteinases-1
- TNF
tumour necrosis factor
- TNFR1
tumour necrosis factor receptor 1
- TRADD
tumour necrosis factor receptor-associated death domain
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cell
- XIAP
X-linked inhibitor of apoptosis
References
- ALON T., HEMO I., ITIN A., PE'ER J., STONE J., KESHET E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat. Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- ALVAREZ R.J., GIPS S.J., MOLDOVAN N., WILHIDE C.C., MILLIKEN E.E., HOANG A.T., HRUBAN R.H., SILVERMAN H.S., DANG C.V., GOLDSCHMIDT-CLERMONT P.J. 17β-estradiol inhibits apoptosis of endothelial cells. Biochem. Biophys. Res. Commun. 1997;237:372–381. doi: 10.1006/bbrc.1997.7085. [DOI] [PubMed] [Google Scholar]
- ARNAL J.F., CLAMENS S., PECHET C., NEGRESALVAYRE A., ALLERA C., GIROLAMI J.P., SALVAYRE R., BAYARD F. Ethinylestradiol does not enhance the expression of nitric oxide synthase in bovine endothelial cells but increases the release of bioactive nitric oxide by inhibiting superoxide anion production. Proc. Natl. Acad. Sci. U.S.A. 1996;93:4108–4113. doi: 10.1073/pnas.93.9.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHKENAZI A., DIXIT V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- AUPEIX K., HUGEL B., MARTIN T., BISCHOFF P., LILL H., PASQUALI J.L., FREYSSINET J.M. The significance of shed membrane particles during programmed cell death in vitro, and in vivo, in HIV-1 infection. J. Clin. Invest. 1997;99:1546–1554. doi: 10.1172/JCI119317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACH F.H., FERRAN C., HECHENLEITNER P., MARK W., KOYAMADA N., MIYATAKE T., WINKLER H., BADRICHANI A., CANDINAS D., HANCOCK W.W. Accommodation of vascularized xenografts: Expression of “protective genes” by donor endothelial cells in a host Th2 cytokine environment. Nature Med. 1997;3:196–204. doi: 10.1038/nm0297-196. [DOI] [PubMed] [Google Scholar]
- BACH R., GENTRY R., NEMERSON Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986;25:4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- BADRICHANI A.Z., STROKA D.M., BILBAO G., CURIEL D.T., BACH F.H., FERRAN C. Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-κB. J. Clin. Invest. 1999;103:543–553. doi: 10.1172/JCI2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAI H., POLLMAN M.J., INISHI Y., GIBBONS G.H. Regulation of vascular smooth muscle cell apoptosis. Modulation of bad by a phosphatidylinositol 3-kinase-dependent pathway. Circ. Res. 1999;85:229–237. doi: 10.1161/01.res.85.3.229. [DOI] [PubMed] [Google Scholar]
- BAKER A.H., ZALTSMAN A.B., GEORGE S.J., NEWBY A.C. Divergent effects of tissue inhibitor of metalloproteinase-1, -2 or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. J. Clin. Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAURIEDEL G., SCHLUCKEBIER S., HUTTER R., WELSCH U., KANDOLF R., LÜDERITZ B., PRESCOTT M.F. Apoptosis in restenosis versus stable-angina atherosclerosis. Implications for the pathogenesis of restenosis. Arterioscler. Thromb. Vasc. Biol. 1998;18:1132–1139. doi: 10.1161/01.atv.18.7.1132. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R., EVAN G.I., NEWBY A.C. Deregulated expression of the c-myc oncogene abolishes inhibition of proliferation of rat vascular smooth muscle cells by serum reduction, interferon-γ, heparin, and cyclic nucleotide analogues and induces apoptosis. Circ. Res. 1994;74:525–536. doi: 10.1161/01.res.74.3.525. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R., EVAN G.I., SCHWARTZ S.M. Apoptosis of rat vascular smooth muscle cells is regulated by p53-dependent and -independent pathways. Circ. Res. 1995a;77:266–273. doi: 10.1161/01.res.77.2.266. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R., EVAN G.I., SCHWARTZ S.M. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J. Clin. Invest. 1995b;95:2266–2274. doi: 10.1172/JCI117917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENNETT M.R., LITTLEWOOD T.D., SCHWARTZ S.M., WEISSBERG P.L. Increased sensitivity of human vascular smooth muscle cells from atherosclerotic plaques to p53-mediated apoptosis. Circ. Res. 1997;81:591–599. doi: 10.1161/01.res.81.4.591. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R., MACDONALD K., CHAN S.W., BOYLE J.J., WEISSBERG P.L. Cooperative interactions between RB and p53 regulate cell proliferation, cell senescence, and apoptosis in human vascular smooth muscle cells from atherosclerotic plaques. Circ. Res. 1998a;82:704–712. doi: 10.1161/01.res.82.6.704. [DOI] [PubMed] [Google Scholar]
- BENNETT M.R., MACDONALD K., CHAN S.W., LUZIO J.P., SIMARI R., WEISSBERG P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998b;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- BIRD D.A., GILLOTTE K.L., HORKKO S., FRIEDMAN P., DENNIS E.A., WITZTUM J.L., STEINBERG D. Receptors for oxidized low-density lipoprotein on elicited mouse peritoneal macrophages can recognize both the modified lipid moieties and the modified protein moieties: implications with respect to macrophage recognition of apoptotic cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6347–6352. doi: 10.1073/pnas.96.11.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BJÖRKERUD S., BJÖRKERUD B. Apoptosis is abundant in human atherosclerotic lesions, especially in inflammatory cells (macrophages and T cells), and may contribute to the accumulation of gruel and plaque instability. Am. J. Pathol. 1996;149:367–380. [PMC free article] [PubMed] [Google Scholar]
- BOCHATON-PIALLAT M.L., GABBIANI F., REDARD M., DESMOULIÈRE A., GABBIANI G. Apoptosis participates in cellularity regulation during rat aortic intimal thickening. Am. J. Pathol. 1995;146:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- BOMBELI T., KARSAN A., TAIT J.F., HARLAN J.M. Apoptotic vascular endothelial cells become procoagulant. Blood. 1997;89:2429–2442. [PubMed] [Google Scholar]
- BOYLE J.J., BOWYER D.E., PROUDFOOT D., WEISSBERG P.L., BENNETT M.R. Human monocyte/macrophages induce human vascular smooth muscle cell apoptosis in culture Circulation 199898I-598Abstract [Google Scholar]
- BRANDL R., RICHTER T., HAUG K., WILHELM M.G., MAURER P.C., NATHRATH W. Topographic analysis of proliferative activity in carotid endarterectomy specimens by immunocytochemical detection of the cell cycle-related antigen Ki-67. Circulation. 1997;96:3360–3368. doi: 10.1161/01.cir.96.10.3360. [DOI] [PubMed] [Google Scholar]
- BROOKS P.C., MONTGOMERY A.M.P., ROSENFELD M., REISFELD R.A., HU T.H., KLIER G., CHERESH D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- CAI W., DEVAUX B., SCHAPER W., SCHAPER J. The role of Fas/APO 1 and apoptosis in the development of human atherosclerotic lesions. Atherosclerosis. 1997;131:177–186. doi: 10.1016/s0021-9150(97)06099-1. [DOI] [PubMed] [Google Scholar]
- CARMELIET P., LAMPUGNANI M.G., MOONS L., BREVIARIO F., COMPERNOLLE V., BONO F., BALCONI G., SPAGNUOLO R., OOSTUYSE B., DEWERCHIN M., ZANETTI A., ANGELLILO A., MATTOT V., NUYENS D., LUTGENS E., CLOTMAN F., DE RUITER M.C., GITTENBERGER-DE-GROOT A., POELMANN R., LUPU F., HERBERT J.M., COLLEN D., DEJANA E. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- CASCIOLA-ROSEN L.A., ANHALT G., ROSEN A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J. Exp. Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG M.K., BERGMARK C., LAURILA A., HORKKO S., HAN K.H., FRIEDMAN P., DENNIS E.A., WITZTUM J.L. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6353–6358. doi: 10.1073/pnas.96.11.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO A., COURTMAN D.W., LANGILLE B.L. Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ. Res. 1995;76:168–175. doi: 10.1161/01.res.76.2.168. [DOI] [PubMed] [Google Scholar]
- CLAESSON-WELSH L., WELSH M., ITO N., ANAND-APTE B., SOKER S., ZETTER B., O'REILLY M., FOLKMAN J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIFF W.J. The aortic tunica media in aging rats. Exp. Mol. Pathol. 1970;13:172–189. doi: 10.1016/0014-4800(70)90004-3. [DOI] [PubMed] [Google Scholar]
- COLEMAN K.R., BRADEN G.A., WILLINGHAM M.C., SANE D.C. Vitaxin, a humanized monoclonal antibody to the vitronectin receptor (αvβ3), reduces neointimal hyperplasia and total vessel area after balloon injury in hypercholesterolemic rabbits. Circ. Res. 1999;84:1268–1276. doi: 10.1161/01.res.84.11.1268. [DOI] [PubMed] [Google Scholar]
- DEVITT A., MOFFATT O.D., RAYKUNDALIA C., CAPRA J.D., SIMMONS D.L., GREGORY C.D. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature. 1998;392:505–509. doi: 10.1038/33169. [DOI] [PubMed] [Google Scholar]
- DIEZ J., FORTUNO M.A., RAVASSA S. Apoptosis in hypertensive heart disease. Curr. Opin. Cardiol. 1998;13:317–325. doi: 10.1097/00001573-199809000-00005. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., ASSMUS B., HERMANN C., HAENDELER J., ZEIHER A.M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ. Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., BREITSCHOPF K., HAENDELER J., ZEIHER A.M. Dephosphorylation targets Bcl-2 for ubiquitin-dependent degradation: a link between the apoptosome and the proteasome pathway. J. Exp. Med. 1999b;189:1815–1822. doi: 10.1084/jem.189.11.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIMMELER S., FLEMING I., FISSLTHALER B., HERMANN C., BUSSE R., ZEIHER A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999a;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., HAENDELER J., GALLE J., ZEIHER A.M. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases: A mechanistic clue to the “response to injury” hypothesis. Circulation. 1997a;95:1760–1763. doi: 10.1161/01.cir.95.7.1760. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., HAENDELER J., NEHLS M., ZEIHER A.M. Suppression of apoptosis by nitric oxide via inhibition of interleukin-1 beta-converting enzyme (ICE)-like and cysteine protease protein (CPP)-32-like proteases. J. Exp. Med. 1997b;185:601–607. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIMMELER S., HAENDELER J., RIPPMANN V., NEHLS M., ZEIHER A.M. Shear stress inhibits apoptosis of human endothelial cells. FEBS Lett. 1996;399:71–74. doi: 10.1016/s0014-5793(96)01289-6. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., RIPPMANN V., WEILAND U., HAENDELER J., ZEIHER A.M. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ. Res. 1997c;81:970–976. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., ZEIHER A.M. Nitric oxide–an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- DONG C., WILSON J.E., WINTERS G.L., MCMANUS B.M. Human transplant coronary artery disease: Pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft arteriopathy. Lab. Invest. 1996;74:921–931. [PubMed] [Google Scholar]
- DOWNING L.J., STRIETER R.M., KADELL A.M., WILKE C.A., AUSTIN J.C., HARE B.D., BURDICK M.D., GREENFIELD L.J., WAKEFIELD T.W. IL-10 regulates thrombus-induced vein wall inflammation and thrombosis. J. Immunol. 1998;161:1471–1476. [PubMed] [Google Scholar]
- DUFOURCQ P., LOUIS H., MOREAU C., DARET D., BOISSEAU M.R., LAMAZIERE J.M., BONNET J. Vitronectin expression and interaction with receptors in smooth muscle cells from human atheromatous plaque. Arterioscler. Thromb. Vasc. Biol. 1998;18:168–176. doi: 10.1161/01.atv.18.2.168. [DOI] [PubMed] [Google Scholar]
- ENARI M., SAKAHIRA H., YOKOYAMA H., OKAWA K., IWAMATSU A., NAGATA S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- ERNOFSSON M., TENNO T., SIEGBAHN A. Inhibition of tissue factor surface expression in human peripheral blood monocytes exposed to cytokines. Br. J. Haematol. 1996;95:249–257. doi: 10.1046/j.1365-2141.1996.d01-1893.x. [DOI] [PubMed] [Google Scholar]
- ESCARGUEIL-BLANC I., MEILHAC O., PIERAGGI M.-T., ARNAL J.-F., SALVAYRE R., NÈGRE-SALVAYRE A. Oxidized LDLs induce massive apoptosis of cultured human endothelial cells through a calcium-dependent pathway: prevention by aurintricarboxylic acid. Arterioscler. Thromb. Vasc. Biol. 1997;17:331–339. doi: 10.1161/01.atv.17.2.331. [DOI] [PubMed] [Google Scholar]
- EVAN G.I., WYLLIE A.H., GILBERT C.S., LITTLEWOOD T.D., LAND H., BROOKS M., WATERS C.M., PENN L.Z., HANCOCK D.C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- FADOK V.A., SAVILL J.S., HASLETT C., BRATTON D.L., DOHERTY D.E., CAMPBELL P.A., HENSON P.M. Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. J. Immunol. 1992;149:4029–4035. [PubMed] [Google Scholar]
- FADOK V.A., WARNER M.L., BRATTON D.L., HENSON P.M. CD36 is required for phagocytosis of apoptotic cells by human macrophages that use either a phosphatidylserine receptor or the vitronectin receptor (αvβ3) J. Immunol. 1998;161:6250–6257. [PubMed] [Google Scholar]
- FERRIS C.D., JAFFREY S.R., SAWA A., TAKAHASHI M., BRADY S.D., BARROW R.K., TYSOE S.A., WOLOSKER H., BARANANO D.E., DORE S., POSS K.D., SNYDER S.H. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell. Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- FLYNN P.D., BYRNE C.D., BAGLIN T.P., WEISSBERG P.L., BENNETT M.R. Thrombin generation by apoptotic vascular smooth muscle cells. Blood. 1997;89:4378–4384. [PubMed] [Google Scholar]
- FOX J.C., SHANLEY J.R. Antisense inhibition of basic fibroblast growth factor induces apoptosis in vascular smooth muscle cells. J. Biol. Chem. 1996;271:12578–12584. doi: 10.1074/jbc.271.21.12578. [DOI] [PubMed] [Google Scholar]
- FUJIKAWA K., DE AOS SCHERPENSEEL I., JAIN S.K., PRESMAN E., VARTICOVSKI L. Role of PI 3-kinase in angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells [In Process Citation] Exp. Cell. Res. 1999;253:663–672. doi: 10.1006/excr.1999.4693. [DOI] [PubMed] [Google Scholar]
- GALIS Z.S., SUKHOVA G.K., LARK M.W., LIBBY P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENG Y.J., HENDERSON L.E., LEVESQUE E.B., MUSZYNSKI M., LIBBY P. Fas is expressed in human atherosclerotic intima and promotes apoptosis of cytokine-primed human vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 1997;17:2200–2208. doi: 10.1161/01.atv.17.10.2200. [DOI] [PubMed] [Google Scholar]
- GENG Y.-J., LIBBY P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1β-converting enzyme. Am. J. Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- GENG Y.J., WU Q., MUSZYNSKI M., HANSSON G.K., LIBBY P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler. Thromb. Vasc. Biol. 1996;16:19–27. doi: 10.1161/01.atv.16.1.19. [DOI] [PubMed] [Google Scholar]
- GERBER H.P., DIXIT V., FERRARA N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- GIDON-JEANGIRARD C., HUGEL B., HOLL V., TOTI F., LAPLANCHE J.L., MEYER D., FREYSSINET J.M. Annexin V delays apoptosis while exerting an external constraint preventing the release of CD4+ and PrPc+ membrane particles in a human T lymphocyte model. J. Immunol. 1999;162:5712–5718. [PubMed] [Google Scholar]
- GOLSTEIN P. Controlling cell death. Science. 1997;275:1081–1082. doi: 10.1126/science.275.5303.1081. [DOI] [PubMed] [Google Scholar]
- GOPING I.S., GROSS A., LAVOIE J.N., NGUYEN M., JEMMERSON R., ROTH K., KORSMEYER S.J., SHORE G.C. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON D., REIDY M.A., BENDITT E.P., SCHWARTZ S.M. Cell proliferation in human coronary arteries. Proc. Natl. Acad. Sci. U.S.A. 1990;87:4600–4604. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D., KROEMER G. The central executioners of apoptosis: caspases or mitochondria. Trends Cell. Biol. 1998;8:267–271. doi: 10.1016/s0962-8924(98)01273-2. [DOI] [PubMed] [Google Scholar]
- GREEN D.R., REED J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1311. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS G.J., DUBREZ L., MORGAN C.P., JONES N.A., WHITEHOUSE J., CORFE B.M., DIVE C., HICKMAN J.A. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J. Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAENDELER J., ZEIHER A.M., DIMMELER S. Vitamin C and E prevent lipopolysaccharide-induced apoptosis in human endothelial cells by modulation of Bcl-2 and Bax. Eur. J. Pharmacol. 1996;317:407–411. doi: 10.1016/s0014-2999(96)00759-5. [DOI] [PubMed] [Google Scholar]
- HAIMOVITZ-FRIEDMAN A., CORDON-CARDO C., BAYOUMY S., GARZOTTO M., MCLOUGHLIN M., GALLILY R., EDWARDS C.K., III, SCHUCHMAN E.H., FUKS Z., KOLESNICK R. Lipopolysaccharide induces disseminated endothelial apoptosis requiring ceramide generation. J. Exp. Med. 1997;186:1831–1841. doi: 10.1084/jem.186.11.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMET P., MOREAU P., DAM T.V., ORLOV S.N., TEA B.S., DEBLOIS D., TREMBLAY J. The time window of apoptosis: A new component in the therapeutic strategy for cardiovascular remodeling. J. Hypertens. 1996;14:S65–S70. [PubMed] [Google Scholar]
- HAN D.K.M., HAUDENSCHILD C.C., HONG M.K., TINKLE B.T., LEON M.B., LIAU G. Evidence for apoptosis in human atherogenesis and in a rat vascular injury model. Am. J. Pathol. 1995;147:267–277. [PMC free article] [PubMed] [Google Scholar]
- HAN D.K.M., WRIGHT M.E., DIXIT V.M., PRUIT R., SON SOE M., LYNCH D.H., SCHWARTZ S.M. Evidence for escape of apoptosis by a loss of Fas in atherosclerotic plaque smooth muscle cells. Circulation. 1996;94 suppl I:I-397. [Google Scholar]
- HANCOCK W.W., BUELOW R., SAYEGH M.H., TURKA L.A. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat. Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- HARADA-SHIBA M., KINOSHITA M., KAMIDO H., SHIMOKADO K. Oxidized low density lipoprotein induces apoptosis in cultured human umbilical vein endothelial cells by common and unique mechanisms. J. Biol. Chem. 1998;273:9681–9687. doi: 10.1074/jbc.273.16.9681. [DOI] [PubMed] [Google Scholar]
- HAUNSTETTER A., IZUMO S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ. Res. 1998;82:1111–1129. doi: 10.1161/01.res.82.11.1111. [DOI] [PubMed] [Google Scholar]
- HAYES A.J., HUANG W.Q., MALLAH J., YANG D., LIPPMAN M.E., LI L.Y. Angiopoietin-1 and its receptor Tie-2 participate in the regulation of capillary-like tubule formation and survival of endothelial cells. Microvasc. Res. 1999;58:224–237. doi: 10.1006/mvre.1999.2179. [DOI] [PubMed] [Google Scholar]
- HEGYI L., SKEPPER J.N., CARY N.R., MITCHINSON M.J. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J. Pathol. 1996;180:423–442. doi: 10.1002/(SICI)1096-9896(199612)180:4<423::AID-PATH677>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- HERMANN C., ZEIHER A.M., DIMMELER S. Shear stress inhibits H2O2-induced apoptosis of human endothelial cells by modulation of the glutathione redox cycle and nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 1997;17:3588–3592. doi: 10.1161/01.atv.17.12.3588. [DOI] [PubMed] [Google Scholar]
- HERREN B., LEVKAU B., RAINES E.W., ROSS R. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol. Biol. Cell. 1998;9:1589–1601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESSLER J.L., ROBERTSON A.L., CHISOLM G.M. LDL-induced cytotoxicity and its inhibition by HDL in human vascular smooth muscle and endothelial cells in culture. Atherosclerosis. 1979;32:213–229. doi: 10.1016/0021-9150(79)90166-7. [DOI] [PubMed] [Google Scholar]
- HETTS S.W. To die or not to die: an overview of apoptosis and its role in disease. JAMA. 1998;279:300–307. doi: 10.1001/jama.279.4.300. [DOI] [PubMed] [Google Scholar]
- HOLASH J., MAISONPIERRE P.C., COMPTON D., BOLAND P., ALEXANDER C.R., ZAGZAG D., YANCOPOULOS G.D., WIEGAND S.J. Vessel cooption, regression, and growth in tumors mediated by angiopoiteins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- HOWARD M., MUCHAMEL T., ANDRADE S., MENON S. Interleukin 10 protects mice from lethal endotoxemia. J. Exp. Med. 1993;177:1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ILIC D., ALMEIDA E.A., SCHLAEPFER D.D., DAZIN P., AIZAWA S., DAMSKY C.H. Extracellular matrix survival signals transduced by focal adhesion kinase suppress p53-mediated apoptosis. J. Cell. Biol. 1998;143:547–560. doi: 10.1083/jcb.143.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISNER J.M., KEARNEY M., BORTMAN S., PASSERI J. Apoptosis in human atherosclerosis and restenosis. Circulation. 1995;91:2703–2711. doi: 10.1161/01.cir.91.11.2703. [DOI] [PubMed] [Google Scholar]
- JONES P.L., COWAN K.N., RABINOVITCH M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am. J. Pathol. 1997b;150:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- JONES P.L., CRACK J., RABINOVITCH M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the αvβ3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J. Cell Biol. 1997a;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORINGE S., CRISBY M., THYBERG J., NILSSON J. DNA fragmentation and ultrastructural changes of degenerating cells in atherosclerotic lesions and smooth muscle cells exposed to oxidized LDL in vitro. Arterioscler. Thromb. Vasc. Biol. 1997;17:2225–2231. doi: 10.1161/01.atv.17.10.2225. [DOI] [PubMed] [Google Scholar]
- KAISER D., FREYBERG M.A., FRIEDL P. Lack of hemodynamic forces triggers apoptosis in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1997;231:586–590. doi: 10.1006/bbrc.1997.6146. [DOI] [PubMed] [Google Scholar]
- KARSAN A. Tumor necrosis factor and endothelial cell death. Trends Cardiovasc. Med. 1998;8:19–24. doi: 10.1016/S1050-1738(97)00126-6. [DOI] [PubMed] [Google Scholar]
- KARSAN A., YEE E., KAUSHANSKY K., HARLAN J.M. Cloning of a human Bcl-2 homologue: Inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996;87:3089–3096. [PubMed] [Google Scholar]
- KARSAN A., YEE E., POIRIER G.G., ZHOU P., CRAIG R., HARLAN J.M. Fibroblast growth factor-2 inhibits endothelial cell apoptosis by Bcl-2 dependent and independent mechanisms. Am. J. Pathol. 1997;151:1775–1784. [PMC free article] [PubMed] [Google Scholar]
- KATOH O., TAUCHI H., KAWAISHI K., KIMURA A., SATOW Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res. 1995;55:5687–5692. [PubMed] [Google Scholar]
- KERR J.F.R., WYLLIE A.H., CURRIE A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y.M., BOMBECK C.A., BILLIAR T.R. Nitric oxide as a bifunctional regulator of apoptosis. Circ. Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- KOCKX M.M., DEMEYER G.R.Y., MUHRING J., BULT H., BULTINCK J., HERMAN A.G. Distribution of cell replication and apoptosis in atherosclerotic plaques of cholesterol-fed rabbits. Atherosclerosis. 1996;120:115–124. doi: 10.1016/0021-9150(95)05691-2. [DOI] [PubMed] [Google Scholar]
- KOCKX M.M., DE MEYER G.R.Y., MUHRING J., JACOB W., BULT H., HERMAN A.G. Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation. 1998;97:2307–2315. doi: 10.1161/01.cir.97.23.2307. [DOI] [PubMed] [Google Scholar]
- KOYAMA H., RAINES E.W., BORNFELDT K.E., ROBERTS J.M., ROSS R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- KROEMER G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat. Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- KROEMER G., ZAMZAMI N., SUSIN S.A. Mitochondrial control of apoptosis. Immunol. Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- KWAK H.J., SO J.N., LEE S.J., KIM I., KOH G.Y. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Lett. 1999;448:249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- LANG R.A., BISHOP J.M. Macrophages are required for cell death and tissue remodeling in the developing mouse eye. Cell. 1993;74:453–462. doi: 10.1016/0092-8674(93)80047-i. [DOI] [PubMed] [Google Scholar]
- LESZCZYNSKI D., ZHAO Y., LUOKKAMÄKI M., FOEGH M.L. Apoptosis of vascular smooth muscle cells. Protein kinase C and oncoprotein Bcl-2 are involved in regulation of apoptosis in non-transformed rat vascular smooth muscle cells. Am. J. Pathol. 1994;145:1265–1270. [PMC free article] [PubMed] [Google Scholar]
- LEVINE J.S., KOH J.S. The role of apoptosis in autoimmunity: immunogen, antigen, and accelerant. Semin. Nephrol. 1999;19:34–47. [PubMed] [Google Scholar]
- LEVKAU B., HERREN B., KOYAMA H., ROSS R., RAINES E.W. Caspase-mediated cleavage of focal adhesion kinase pp125FAK and disassembly of focal adhesions in human endothelial cell apoptosis. J. Exp. Med. 1998;187:579–586. doi: 10.1084/jem.187.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVKAU B., SCATENA M., GIACHELLI C.M., ROSS R., RAINES E.W. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-κB loop. Nat. Cell. Biol. 1999;1:227–233. doi: 10.1038/12050. [DOI] [PubMed] [Google Scholar]
- LI D., TOMSON K., YANG B., MEHTA P., CROKER B.P., MEHTA J.L. Modulation of constitutive nitric oxide synthase, bcl-2 and Fas expression in cultured human coronary endothelial cells exposed to anoxia-reoxygenation and angiotensin II: role of AT1 receptor activation. Cardiovasc. Res. 1999a;41:109–115. doi: 10.1016/s0008-6363(98)00196-5. [DOI] [PubMed] [Google Scholar]
- LI D., YANG B., PHILIPS M.I., MEHTA J.L. Proapoptotic effects of ANG II in human coronary artery endothelial cells: role of AT1 receptor and PKC activation. Am. J. Physiol. 1999b;276:H786–H792. doi: 10.1152/ajpheart.1999.276.3.H786. [DOI] [PubMed] [Google Scholar]
- LINDNER H., HOLLER E., ERTL B., MULTHOFF G., SCHREGLMANN M., KLAUKE I., SCHULTZ-HECTOR S., EISSNER G. Peripheral blood mononuclear cells induce programmed cell death in human endothelial cells and may prevent repair: role of cytokines. Blood. 1997;89:1931–1938. [PubMed] [Google Scholar]
- LIU X., ZOU H., SLAUGHTER C., WANG X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89:175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- LIZARD G., DECKERT V., DUBREZ L., MOISANT M., GAMBERT P., LAGROST L. Induction of apoptosis in endothelial cells treated with cholesterol oxides. Am. J. Pathol. 1996;148:1625–1638. [PMC free article] [PubMed] [Google Scholar]
- LUCAS R., HOLMGREN L., GARCIA I., JIMENEZ B., MANDRIOTA S.J., BORLAT F., SIM B.K., WU Z., GRAU G.E., SHING Y., SOFF G.A., BOUCK N., PEPPER M.S. Multiple forms of angiostatin induce apoptosis in endothelial cells. Blood. 1998;92:4730–4741. [PubMed] [Google Scholar]
- MAJNO G., JORIS I. Apoptosis, oncosis, and necrosis. Am. J. Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- MALLAT Z., BENAMER H., HUGEL B., BENESSIANO J., STEG P.G., FREYSSINET J.M., TEDGUI A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation. 2000;101:841–843. doi: 10.1161/01.cir.101.8.841. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., BESNARD S., DURIEZ M., DELEUZE V., EMMANUEL F., BUREAU M.F., SOUBRIER F., ESPOSITO B., DUEZ H., FIEVET C., STAELS B., DUVERGER N., SCHERMAN D., TEDGUI A. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 1999c;85:e17–e24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., HEYMES C., OHAN J., FAGGIN E., LESÈCHE G., TEDGUI A. Expression of interleukin-10 in advanced human atherosclerotic plaques. Relation to inducible nitric oxide synthase expression and cell death. Arterioscler. Thromb. Vasc. Biol. 1999a;19:611–616. doi: 10.1161/01.atv.19.3.611. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., HUGEL B., OHAN J., LESÈCHE G., FREYSSINET J.M., TEDGUI A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques. A role for apoptosis in plaque thrombogenicity. Circulation. 1999b;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., OHAN J., LESÈCHE G., TEDGUI A. Colocalization of CPP-32 with apoptotic cells in human atherosclerotic plaques. Circulation. 1997;96:424–428. doi: 10.1161/01.cir.96.2.424. [DOI] [PubMed] [Google Scholar]
- MARTIN S.J., REUTELINGSPERGER C.P.M., MCGAHON A.J., RADER J.A., VAN SCHIE R.C.A., LAFACE D.M., GREEN D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Ab1. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASON C.A., BIGRAS J.L., O'BLENES S.B., ZHOU B., MCINTYRE B., NAKAMURA N., KANEDA Y., RABINOVITCH M. Gene transfer in utero biologically engineers a patent ductus arteriosus in lambs by arresting fibronectin-dependent neointimal formation. Nat. Med. 1999;5:176–182. doi: 10.1038/5538. [DOI] [PubMed] [Google Scholar]
- MEESON A., PALMER M., CALFON M., LANG R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- MEREDITH J., FAZELI B., SCHWARTZ M. The extracellular matrix as a survival factor. Mol. Biol. Cell. 1993;4:953–961. doi: 10.1091/mbc.4.9.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEVORACH D., MASCARENHAS J.O., GERSHOV D., ELKON K.B. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIOSSEC C., DUTILLEUL V., FASSY F., DIUHERCEND A. Evidence for CPP32 activation in the absence of apoptosis during T lymphocyte stimulation. J. Biol. Chem. 1997;272:13459–13462. doi: 10.1074/jbc.272.21.13459. [DOI] [PubMed] [Google Scholar]
- MORISHITA R., GIBBONS G.H., ELLISON K.E., LEE W., ZHANG L.N., YU H., KANEDA Y., OGIHARA T., DAZU V.J. Evidence for direct local effect of angiotensin in vascular hypertrophy–in vivo gene transfer of angiotensin converting enzyme. J. Clin. Invest. 1994;94:978–984. doi: 10.1172/JCI117464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULTON K.S., HELLER E., KONERDING M.A., FLYNN E., PALINSKI W., FOLKMAN J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;90:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D.W., ALI A., THORNBERRY N.A., VAILLANCOURT J.P., DING C.K., GALLANT M., GAREAU Y., GRIFFIN P.R., LABELLE M., LAZEBNIK Y.A., MUNDAY N.A., RAJU S.M., SMULSON M.E., YAMIN T.-T., YU V.L., MILLER D.K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- NISHIO E., WATANABE Y. Oxysterols induced apoptosis in cultured smooth muscle cells through CPP32 protease activation and BCL-2 protein downregulation. Biochem. Biophys. Res. Commun. 1996;226:928–934. doi: 10.1006/bbrc.1996.1452. [DOI] [PubMed] [Google Scholar]
- NOBLE K.E., WICKREMASINGHE R.G., DECORNET C., PANAYIOTIDIS P., YONG K.L. Monocytes stimulate expression of the Bcl-2 family member, A1, in endothelial cells and confer protection against apoptosis. J. Immunol. 1999;162:1376–1383. [PubMed] [Google Scholar]
- PARKES J.L., CARDELL R.R., HUBBARD F.C.J., HUBBARD D., MELTZER A., PENN A. Cultured human atherosclerotic plaque smooth muscle cells retain transforming potential and display enhanced expression of the myc protooncogene. Am. J. Pathol. 1991;138:765–775. [PMC free article] [PubMed] [Google Scholar]
- PERLMAN H., MAILLARD L., KRASINSKI K., WALSH K. Evidence for the rapid onset of apoptosis in medial smooth muscle cells after balloon injury. Circulation. 1997;95:981–987. doi: 10.1161/01.cir.95.4.981. [DOI] [PubMed] [Google Scholar]
- POLLMAN M.J., HALL J.L., GIBBONS G.H. Determinants of vascular smooth muscle cell apoptosis after balloon angioplasty injury: influence of redox state and cell phenotype. Circ. Res. 1999b;84:113–121. doi: 10.1161/01.res.84.1.113. [DOI] [PubMed] [Google Scholar]
- POLLMAN M.J., HALL J.L., MANN M.J., ZHANG L., GIBBONS G.H. Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat. Med. 1998;4:222–227. doi: 10.1038/nm0298-222. [DOI] [PubMed] [Google Scholar]
- POLLMAN M.J., NAUMOVSKI L., GIBBONS G.H. Vascular cell apoptosis: cell type-specific modulation by transforming growth factor-β1 in endothelial cells versus smooth muscle cells. Circulation. 1999a;99:2019–2026. doi: 10.1161/01.cir.99.15.2019. [DOI] [PubMed] [Google Scholar]
- POLLMAN M.J., YAMADA T., HORIUCHI M., GIBBONS G.H. Vasoactive substances regulate vascular smooth muscle cell apoptosis. Countervailing influences of nitric oxide and angiotensin II. Circ. Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- RE F., ZANETTI A., SIRONI M., POLENTARUTTI N., LANFRANCONE L., DEJANA E., COLOTTA F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 1994;127:537–546. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON B.C., LALWANI N.D., JOHNSON K.J., MARKS R.M. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur. J. Immunol. 1994;24:2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- ROSEN A., CASCIOLA-ROSEN L., AHEARN J. Novel packages of viral and self-antigens are generated during apoptosis. J. Exp. Med. 1995;181:1557–1561. doi: 10.1084/jem.181.4.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSS R. Atherosclerosis an inflammatory disease. N. Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- ROUIS M., ADAMY C., DUVERGER N., LESNIK P., HORELLOU P., MOREAU M., EMMANUEL F., CAILLAUD J.M., LAPLAUD P.M., DACHET C., CHAPMAN M.J. Adenovirus-mediated overexpression of tissue inhibitor of metalloproteinase-1 reduces atherosclerotic lesions in apolipoprotein E-deficient mice. Circulation. 1999;100:533–540. doi: 10.1161/01.cir.100.5.533. [DOI] [PubMed] [Google Scholar]
- RÜEGG; C., YILMAZ A., BIELER G., BAMAT J., CHAUBERT P., LEJEUNE F.J. Evidence for the involvement of endothelial cell integrin αvβ3 in the disruption of the tumor vasculature induced by TNF and IFN-γ. Nat. Med. 1998;4:408–413. doi: 10.1038/nm0498-408. [DOI] [PubMed] [Google Scholar]
- SAKAHIRA H., ENARI M., NAGATA S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- SATA M., PERLMAN H., MURUVE D.A., SILVER M., IKEBE M., LIBERMANN T.A., OETTGEN P., WALSH K. Fas ligand gene transfer to the vessel wall inhibits neointima formation and overrides the adenovirus-mediated T cell response. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1213–1217. doi: 10.1073/pnas.95.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATA M., WALSH K. Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J. Clin. Invest. 1998;102:1682–1689. doi: 10.1172/JCI3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVILL J., FADOK V., HENSON P., HASLETT C. Phagocyte recognition of cells undergoing apoptosis. Immunol. Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- SCATENA M., ALMEIDA M., CHAISSON M.L., FAUSTO N., NICOSIA R.F., GIACHELLI C.M. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J. Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAUB F.J.W., COATS S.A., SEIFERT R.A., RAMACHANDRAN R.K., HAN D.K.M., SCHWARTZ S.M. Regulated overexpression of the Fas-associated death domain (FADD) protein in seeded vascular smooth muscle cells causes apoptosis followed by recruitment of macrophages Circulation 199898I-597Abstract [Google Scholar]
- SLOMP J., GITTENBERGERDEGROOT A.C., GLUKHOVA M.A., VANMUNSTEREN J.C., KOCKX M.M., SCHWARTZ S.M., KOTELIANSKY V.E. Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler. Thromb. Vascr. Biol. 1997;17:1003–1009. doi: 10.1161/01.atv.17.5.1003. [DOI] [PubMed] [Google Scholar]
- SPEIR E., MODALI R., HUANG E.S., LEON M.B., SHAWL F., FINKEL T., EPSTEIN S.E. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- SPYRIDOPOULOS I., SULLIVAN A.B., KEARNEY M., ISNER J.M., LOSORDO D.W. Estrogen-receptor-mediated inhibition of human endothelial cell apoptosis–Estradiol as a survival factor. Circulation. 1997;95:1505–1514. doi: 10.1161/01.cir.95.6.1505. [DOI] [PubMed] [Google Scholar]
- STEG P.G., TAHLIL O., AUBAILLY N., CAILLAUD J.M., DEDIEU J.F., BERTHELOT K., LE ROUX A., FELDMAN L., PERRICAUDET M., DENEFLE P., BRANELLEC D. Reduction of restenosis after angioplasty in an atheromatous rabbit model by suicide gene therapy. Circulation. 1997;96:408–411. doi: 10.1161/01.cir.96.2.408. [DOI] [PubMed] [Google Scholar]
- STROMBLAD S., BECKER J.C., YEBRA M., BROOKS P.C., CHERESH D.A. Suppression of p53 and p21 expression by vascular cell integrin αvβ3 during angiogenesis in vivo. J. Clin. Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAN E.M. Autoimmunity and apoptosis. J. Exp. Med. 1994;179:1083–1086. doi: 10.1084/jem.179.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS W.A., REINER J.M., FLORENTIN F.A., LEE K.T., LEE W.M. Population dynamics of arterial smooth muscle cells. V. Cell proliferation and cell death during initial three months in atherosclerotic lesions induced in swine by hypercholesterolemic diet and intimal trauma. Exp. Mol. Pathol. 1976;24:360–374. doi: 10.1016/0014-4800(76)90071-x. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., LAZEBNIK Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- TOPOL E.J., CALIFF R.M., WEISMAN H.F., ELLIS S.G., TCHENG J.E., WORLEY S., IVANHOE R., GEORGE B.S., FINTEL D., WESTON M., et al. Randomized trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. The EPIC Investigators. Lancet. 1994;343:881–886. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- TRAN J., RAK J., SHEEHAN C., SAIBIL S.D., LACASSE E., KORNELUK R.G., KERBEL R.S. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem. Biophys. Res. Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- TRICOT O., MALLAT Z., HEYMES C., LESÈCHE G., TEDGUI A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques Circulation 2000. in press [DOI] [PubMed]
- VAN ANTWERP D.J., MARTIN S.J., KAFRI T., GREEN D.R., VERMA I.M. Suppression of TNF-alpha-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- VIRCHOW R. Cellular Pathology as Based Upon Physiological and Pathological Histology 1858Birmingham, AL: Classics of Medicine Library; 361p [DOI] [PubMed] [Google Scholar]
- VON DER LEYEN H., GIBBONS G.H., MORISHITA R., LEWIS N.P., ZHANG L., KANEDA Y., COOKE J.P., DZAU V.J. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG B.Y., HO H.K., LIN P.S., SCHWARZACHER S.P., POLLMAN M.J., GIBBONS G.H., TSAO P.S., COOKE J.P. Regression of atherosclerosis: role of nitric oxide and apoptosis. Circulation. 1999;99:1236–1241. doi: 10.1161/01.cir.99.9.1236. [DOI] [PubMed] [Google Scholar]
- WATANABE Y., DVORAK H.F. Vascular permeability factor/vascular endothelial growth factor inhibits anchorage-disruption-induced apoptosis in microvessel endothelial cells by inducing scaffold formation. Exp. Cell. Res. 1997;233:340–349. doi: 10.1006/excr.1997.3583. [DOI] [PubMed] [Google Scholar]
- YACHIE A., NIIDA Y., WADA T., IGARASHI N., KANEDA H., TOMA T., OHTA K., KASAHARA Y., KOIZUMI S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMADA T., AKISHITA M., POLLMAN M.J., GIBBONS G.H., DZAU V.J., HORIUCHI M. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis and antagonizes angiotensin II type 1 receptor action: an in vitro gene transfer study. Life Sci. 1998;63:L289–L295. doi: 10.1016/s0024-3205(98)00448-2. [DOI] [PubMed] [Google Scholar]
- YUSUF S., SLEIGHT P., POGUE J., BOSCH J., DAVIES R., DAGENAIS G., The Heart Out comes Prevention Evaluation Study Investigators Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on death from cardiovascular causes, myocardial infarction, and stroke in high-risk patients N. Engl. J. Med. 2000342145–153.Published erratum appears in N. Engl. J. Med. 2000; 342: 74810639539 [Google Scholar]
- ZHENG B., CLEMMONS D.R. Blocking ligand occupancy of the αvβ3 integrin inhibits insulin-like growth factor I signaling in vacular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11217–11222. doi: 10.1073/pnas.95.19.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]


