Abstract
Functional experiments have been conducted to assess the effects of acetylcholine and carbachol, and the receptors on which they act to facilitate neurotransmission to the stromal smooth muscle of the prostate gland of the guinea-pig.
Acetylcholine and carbachol (0.1 μM–0.1 mM) enhanced contractions evoked by trains of electrical field stimulation (20 pulses of 0.5 ms at 10 Hz every 50 s with a dial setting of 60 V) of nerve terminals within the guinea-pig isolated prostate. In these concentrations they had negligible effects on prostatic smooth muscle tone. The facilitatory effects of acetylcholine, but not those of carbachol, were further enhanced in the presence of physostigmine (10 μM).
The facilitatory effects of carbachol were unaffected by the neuropeptide Y Y1 receptor antagonist BIBP 3226 ((R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide) (0.3 μM, n=3) or suramin (100 μM, n=5). Prazosin (0.1 μM, n=5) and guanethidine (10 μM, n=5) alone and in combination (n=4), reduced responses to field stimulation and produced rightward shifts of the log concentration-response curves to carbachol.
The rank orders of potency of subtype-preferring muscarinic receptor antagonists in inhibiting the facilitatory actions of acetylcholine and carbachol were: pirenzepine > HHSiD (hexahydrosiladifenidol) > pF-HHSiD (para-fluoro-hexahydrosiladifenidol)⩾ 5 himbacine, and pirenzepine > HHSiD > himbacine⩾ 5 pF-HHSiD, respectively. These profiles suggest that muscarinic receptors of the M1-subtype mediate the facilitatory effects of acetylcholine and carbachol on neurotransmission to the smooth muscle of the guinea-pig prostate.
Keywords: Neurotransmission, smooth muscle contractility, guinea-pig, prostate gland, muscarinic receptor subtypes, pirenzepine, himbacine, hexahydrosiladifenidol, para-fluoro-hexahydrosiladifenidol
Introduction
The prostate of the guinea-pig, like that of man and in contrast to that of the rat, has a substantial stromal component, comprising a high proportion of smooth muscle cells (Ricciardelli et al., 1989). With age, the guinea-pig prostate develops a stromal hyperplasia histologically indistinguishable from that observed in ageing men (Horsfall et al., 1994). For these and other reasons including the fact that the guinea-pig prostate exhibits gonadal steroid sensitivity similar to that of the human (Maini et al., 1997), the guinea-pig prostate may provide a useful animal model for studies in the human. This possibility is reinforced by the finding that neuromuscular transmission to the smooth muscle of the guinea-pig prostate, as to that in the human prostate, is predominantly sympathetic and noradrenergic in nature (Ohkawa 1983; Haynes & Hill, 1997; Najbar-Kaszkiel et al., 1997; Lau et al., 1998).
The role(s) of cholinergic nerves within the prostate gland remain uncertain, but participation of acetylcholine in prostatic secretory processes (Farrell & Lyman, 1937; Farnsworth & Lawrence, 1965), and of muscarinic receptors in mitogenic effects (Luthin et al., 1997; Rayford et al., 1997; McVary et al., 1998) have been proposed. The guinea-pig prostate gland, like that in the human (see Dail, 1993 for a review), contains acetylcholinesterase-positive nerve fibres (Lau et al., 1998), which densely supplies the stroma as well as the acini of the gland. Acetylcholinesterase-positive staining is, however, not a definitive marker for cholinergic nerves. Moreover, early radioligand receptor autoradiographic studies suggested that muscarinic receptors in the human prostate, while prominent on the secretory epithelium (Lepor & Kuhar, 1984; Hedlund et al., 1985; James et al., 1989), were present in much lower density in the stroma, consistent with a role of cholinergic nerves in secretory processes. For these reasons, and since muscarinic cholinoceptor agonists have been reported not to be particularly effective in causing contraction of the human prostate (Caine et al., 1975; Hedlund et al., 1985), the possible role of endogenous acetylcholine in neurotransmission to the prostate stroma has not been extensively further examined. However, it has been proposed by Dail (1993), that cholinergic neurones, probably of sympathetic origin, may also contribute to nerve stimulation-induced contractions of prostatic smooth muscle.
Preliminary experiments in this laboratory (Lau & Pennefather, 1995) indicated that the smooth muscle of the guinea-pig prostate, like that of the human prostate, and in contrast to that of the rat (Lau & Pennefather, 1998), is not particularly responsive to contractile effects of cholinoceptor agonists. However, in our laboratory and in other recent studies, it has been found that atropine reduced field stimulation-induced contractions of the guinea-pig prostate, (Haynes & Hill, 1997; Najbar-Kaszkiel et al., 1997; Lau et al., 1998). In addition, we have observed that anticholinesterases produced a marked atropine-sensitive but hexamethonium-insensitive enhancement of field stimulation-induced contractions of prostate smooth muscle in this species (Lau et al., 1998). These observations provide evidence for the proposal that acetylcholine plays a role in neurotransmission to the prostate smooth muscle.
In the present investigation we focus on the role of acetylcholine in neurotransmission to the stromal smooth muscle of the guinea-pig prostate and, in particular, on the muscarinic receptor subtypes mediating the facilitatory effects of muscarinic agonists. There are five subtypes of muscarinic cholinoceptor gene products, four of these correspond to pharmacologically defined receptors (M1–M4; see reviews by Eglen et al., 1996; Caulfield & Birdsall, 1998). The predominant muscarinic receptor subtype present in the prostate gland shows species variation; for example, M3 in the rat (Latifpour et al., 1991; Yazawa & Honda, 1993, Lau & Pennefather, 1998; Pontari et al., 1998) and M2 in the dog (Fernandez et al., 1998). The predominant subtype present in the human prostate is, in contrast, M1 (Ruggieri et al., 1995; Luthin et al., 1997). The subtype(s) present in the guinea-pig prostate have not been investigated.
Preliminary accounts of this study have been communicated to The Australasian Society of Clinical and Experimental Pharmacologists and Physiologists (Lau & Pennefather, 1995) and the International Society for Autonomic Neuroscience (Lau et al., 1997).
Methods
Animals
Adult male Dunkin-Hartley guinea-pigs (420–700 g) were housed in open runs at 22°C with a 12 h : 12 h light : dark cycle. Rodent chow, fruits, vegetables and water were provided ad libitum. Prior approval for animal experimentation was obtained from the Monash University Standing Committee on Ethics in Animal Experimentation (SCEAE Approval No. 95/086 and 95/141).
Organ bath studies
Ventral prostates were removed from guinea-pigs immediately after death by cervical dislocation and exsanguination. As described by us previously (Lau et al., 1998), three to four preparations, weighing 51.4±5.1 mg (from a sample of 20 preparations), from each animal were set up under a resting force of 0.5 g in 5- or 10-ml organ baths containing Krebs-Henseleit solution (maintained at 37°C and bubbled with 5% CO2 in O2) of the following composition (mM): NaCl, 118.1; KCl, 4.87; KH2PO4, 1.2; NaHCO3, 25.0; glucose, 11.7; MgSO4.7H2O, 0.5; CaCl2.2H2O, 2.5. After equilibration for 30 min, the preparations were electrically field stimulated with trains of 20 pulses of 0.5 ms at 10 Hz every 50 s with a dial setting of 60 V (supramaximal) via two parallel electrodes (≈0.5 cm apart) incorporated into the tissue holder, and connected to a Grass S88 stimulator. Isometric contractions were recorded with Grass FT03C force-displacement transducers connected to a MacLab data acquisition system (Chart 3.3) interfaced with a Macintosh LC575 computer. The stimulation parameters employed produce tetrodotoxin- and guanethidine-sensitive contractions indicating activation of sympathetic nerve terminals supplying the prostate smooth muscle (Lau et al., 1998).
Effects of cholinoceptor agonists
Field stimulated preparations of the guinea-pig prostate were allowed to equilibrate for 30 min before drug addition. Log concentration-response curves to the cholinoceptor agonists, DMPP (1,1-dimethyl-4-phenyl-piperazinium iodide; 1 μM–0.1 mM), carbachol (0.1 μM–1 mM), acetylcholine (0.1 μM–1 mM) and McN-A-343 ((4-hydroxy-2-butynyl)-1-trimethylammonium-m-chlorocarbanilate chloride; 0.1 μM–0.1 mM) were constructed cumulatively using one log unit concentration increments. Each concentration was added when responses to the previous concentration reached a plateau within 5–10 min after agonist exposure. The effect of nicotine (10 μM) was also investigated.
Experiments were also undertaken to examine the effect of physostigmine (10 μM) on acetylcholine- and carbachol-induced facilitation of field stimulation-induced contractions of the guinea-pig prostatic smooth muscle. The first concentration-response curve to each agonist was constructed in the absence of physostigmine and subsequent curves, at 60 min intervals, were constructed 5–10 min after exposure to physostigmine (10 μM). To determine whether acetylcholine contracted prostatic smooth muscle or enhanced the responses to noradrenaline, discrete log concentration-response curves to noradrenaline were constructed before and 10 min after exposure to acetylcholine (10 μM) in the presence of physostigmine (10 μM). These concentration-response curves to noradrenaline (0.1 μM–1 mM) were constructed with an exposure period of 60 s on a 10 min dose-cycle.
Effects of neurotransmitter antagonists on responses to acetylcholine and/or carbachol
To determine possible sites of action of the cholinoceptor agonists, log concentration-response curves to acetylcholine and/or carbachol were examined upon field stimulated preparations, before and 30 min after exposure to the ganglion blocking agent hexamethonium (0.1 mM), the P2 purinoceptor antagonist suramin (100 μM), the neuropeptide Y Y1 receptor antagonist BIBP 3226 (0.3 μM), the α1-adrenoceptor antagonist prazosin (0.1 μM), and the noradrenergic neurone blocking drug, guanethidine (10 μM).
Effects of subtype-preferring muscarinic receptor antagonists
Log concentration-response curves to acetylcholine in the presence of physostigmine or to carbachol were constructed in the absence and presence of the following subtype-preferring muscarinic receptor antagonists: pirenzepine (M1; 0.1–1 μM), himbacine (M2; 1 μM), HHSiD, (M1/M3; 0.1–1 μM) or pF-HHSiD, (M3; 1 μM), with an antagonist incubation period of 30 min, and in a subset of experiments with pirenzepine (1 μM), an incubation period of 90 min. Only one antagonist concentration was tested in any one tissue preparation. Control experiments were conducted in parallel to correct for any tissue sensitivity changes due to time and/or vehicle (bath concentrations of up to 0.01% ethanol).
Measurement and analysis of data
The effects of agonists on the magnitude of responses to electrical field stimulation were expressed as percentage increases of mean basal field stimulation-induced responses. The mean peak force developed (in g) of four stimulation-induced responses was determined just prior to the initial agonist addition to estimate mean basal field stimulation-induced force. The corresponding estimates of mean peak force developed when the response to each concentration had reached a plateau (5–10 min after each dosage increment) were determined in the absence and presence of antagonists. Log concentration-response curves to acetylcholine and carbachol before and after exposure to antagonists and/or physostigmine were constructed by pooling data from individual curves.
The effects of acetylcholine on noradrenaline-induced contractions of the guinea-pig prostatic smooth muscle were determined by measuring the magnitude of contractile force developed (in g) in response to each concentration of noradrenaline in the absence and presence of acetylcholine.
Non-linear regression analyses of log concentration-response curves were undertaken using the GraphPad PRISM software program. To estimate the potencies of physostigmine in enhancing the effects of acetylcholine and of antagonists in inhibiting the effects of acetylcholine and carbachol, the slopes of the mean log concentration-response curves to the agonists in the absence and presence of these drugs were compared. When the slopes did not differ significantly, as indicated by overlap of the 95% confidence limits of these slopes, concentration ratios were determined. Mean estimates of apparent dissociation constants (KB) of the muscarinic receptor subtype-preferring antagonists, expressed as apparent pKB, for each concentration used were calculated using the equation: apparent KB=(antagonist concentration)/(concentration ratio −1) (Furchgott, 1972).
Data are presented as mean value±standard error of the mean (s.e.mean); n represents the number of experimental animals. Statistical evaluation of data was performed using one and two-way repeated measures analyses of variance (ANOVA), Student's paired or unpaired t-tests, where appropriate. In all cases, values of P<0.05 were considered significant.
Drugs
The following drugs were used: (−) arterenol (noradrenaline) bitartrate, acetylcholine chloride, carbachol (carbamylcholine chloride), 1,1-dimethyl-4-phenyl-piperazinium iodide (DMPP), physostigmine hemisulphate, prazosin (Sigma), guanethidine (Ciba-Geigy), hexamethonium tartrate (May & Baker), hexahydrosiladifenidol (HHSiD) hydrochloride, para-fluoro-hexahydrosiladifenidol (pF-HHSiD) hydrochloride, 4-hydroxy-2-butynyl-1-trimethylammonium-m - chlorocarbamilate chloride (McN-A-343) (Research Biochemicals International) and nicotine hydrogen (+)-tartrate (BDH). Pirenzepine dihydrochloride and himbacine hydrochloride were gifts from Dr K. Thomae, Boehringer Ingelheim and Prof W.C. Taylor of University of Sydney, Australia, respectively. BIBP 3226 ((R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide) was a gift from Dr Margaret Morris, University of Melbourne. Suramin was a gift from Bayer, Australia.
Noradrenaline was dissolved and diluted in a catecholamine diluent (mM): NaCl, 154.0; NaH2PO4, 1.2; ascorbic acid, 0.2. All other drugs except HHSiD and pF-HHSiD were dissolved in distilled water. Stock concentrations (1 mM) of HHSiD and pF-HHSiD were prepared in absolute ethanol. Dilutions to working concentrations were made in distilled water.
Results
Effects of agonists on prostatic smooth muscle tone
Cumulative addition of acetylcholine and carbachol (0.1 μM–0.1 mM), did not cause any appreciable effect on the guinea-pig prostatic smooth muscle tone. In some preparations, at higher concentrations ( > 0.1 mM), they produced small increases in the smooth muscle tone. Nicotine (10 μM), DMPP (1 μM–0.1 mM) and McN-A-343 (0.1 μM–0.1 mM) were without effect on the prostatic smooth muscle tone. Noradrenaline (0.1 μM–1 mM) produced concentration-dependent contractions of the guinea-pig prostatic smooth muscle; its effects were not modified in the presence of acetylcholine (10 μM) and physostigmine (10 μM) (Figure 1).
Figure 1.
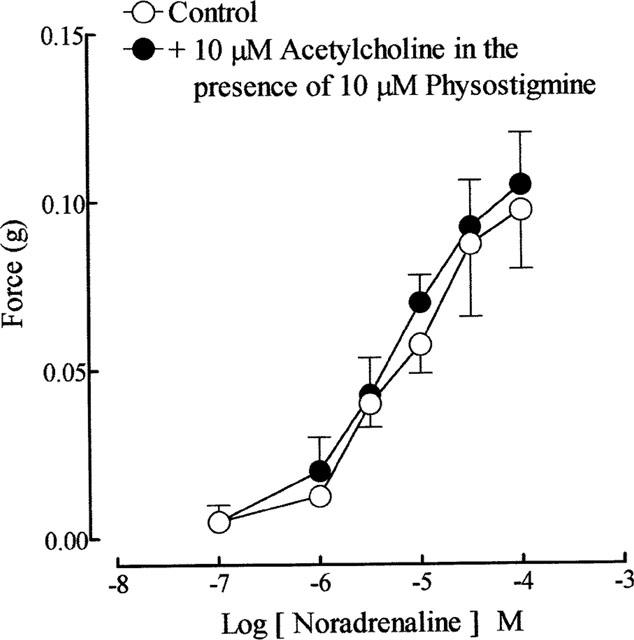
Mean log sequential concentration-response curves to noradrenaline on the guinea-pig prostatic preparations before and after exposure to acetylcholine (10 μM) in the presence of physostigmine (10 μM). Data are shown as mean values with vertical lines representing s.e.mean from four experiments.
Effects of agonists on contractile responses evoked by field stimulation
As reported previously (Lau et al., 1998), repetitive trains of stimuli (20 pulses of 0.5 ms at 10 Hz every 50 s) evoked reproducible contractions of the guinea-pig prostatic smooth muscle; the magnitudes (0.12±0.02 g; sample n=12) of these responses were consistent over the experimental period. Application of acetylcholine and carbachol caused concentration-related enhancement of the field stimulation-induced contractions (Figure 2). These effects were reversible after several washouts. As shown in Figure 3, in the absence of physostigmine, acetylcholine was less potent than carbachol in enhancing field stimulation-induced contractions. Control experiments indicated that the first and second log concentration-response curves to carbachol, constructed in the absence and presence of the ethanol vehicle at intervals of 60 min, were not significantly different (P > 0.05, two-way ANOVA; Figure 4), but there was a small rightward shift in the position of the third curve. The magnitudes of the basal field stimulation-induced contraction and the increase induced by the highest concentration (0.1 mM) of carbachol applied were similar in each of these curves.
Figure 2.
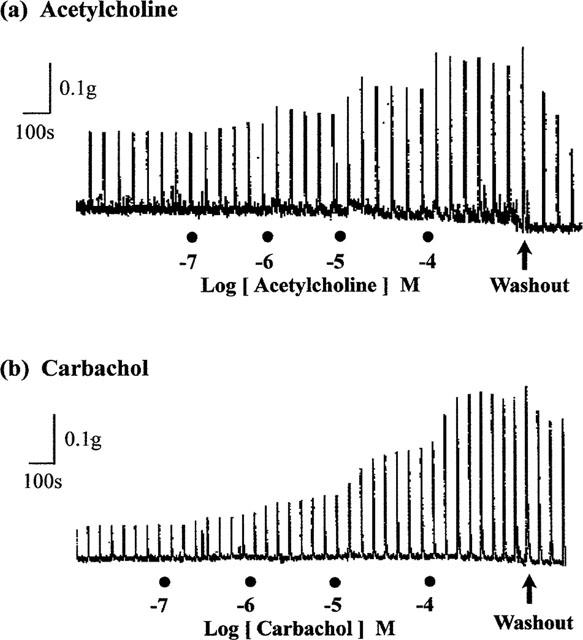
Representative traces showing the enhancing effect of increasing concentrations of (a) acetylcholine and (b) carbachol on field stimulation (trains of 20 pulses of 0.5 ms at 10 Hz every 50 s, 60 V)-induced contractions of the guinea-pig prostatic smooth muscle preparations.
Figure 3.
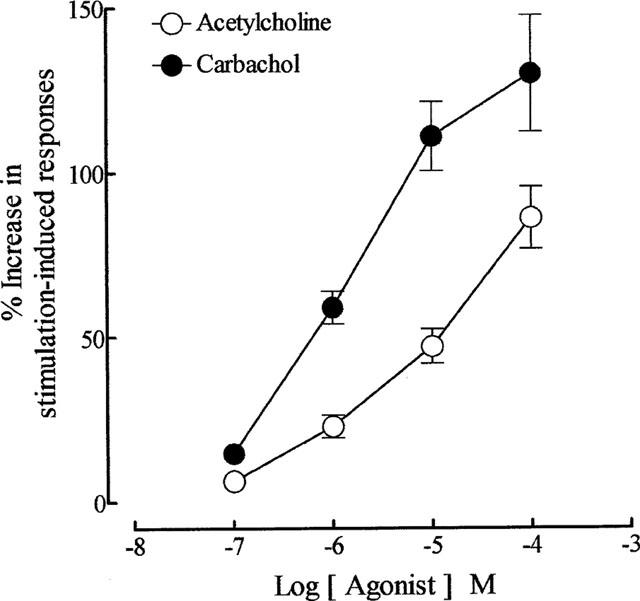
Mean log concentration-response curves for the enhancing effect of acetylcholine (in the absence of physostigmine) and carbachol on field stimulation (trains of 20 pulses of 0.5 ms at 10 Hz every 50 s, 60 V)-induced contractions of the guinea-pig prostatic preparations. Data are shown as mean values with vertical lines representing s.e.mean from 16 experiments.
Figure 4.
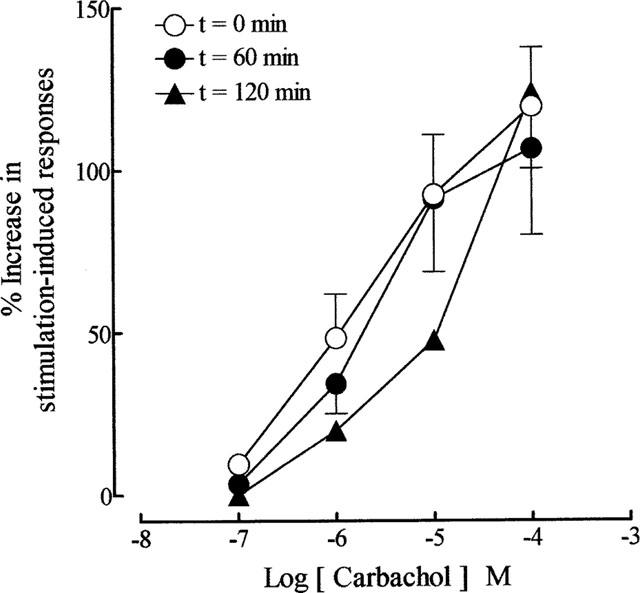
Mean log concentration-response curves to carbachol at t=0 min (in the absence of ethanol), t=60 min (in the presence of 0.01% ethanol) and t=120 min (in the presence of 0.01% ethanol) on field stimulation (trains of 20 pulses of 0.5 ms at 10 Hz every 50 s, 60 V)-induced contractions of the guinea-pig prostatic preparations. Data are shown as mean values with vertical lines representing s.e.mean from 4–5 experiments.
As reported previously (Lau et al., 1998), physostigmine (10 μM) potentiated the stimulation-induced contractions of the guinea-pig prostate. In the present experiments it produced a 66.7±7% (n=25) increase in the magnitude of the responses. In time control experiments, physostigmine-induced effects were maintained throughout the 20–25 min period needed for construction of concentration-response curves to acetylcholine. In its presence, the second mean log concentration-response curves to acetylcholine were shifted to the left in a parallel manner with a potency ratio of 14.8 (95% confidence limits: 11.9, 18.4; d.f.=62; n=8) (Figure 5). The position of the third curve to acetylcholine, constructed in the presence of physostigmine, was not significantly different from the second curve (P > 0.05, two-way ANOVA) (Figure 5). The enhancement of field stimulation-induced contractions induced by the highest concentration (0.1 mM) of acetylcholine applied was not significantly altered by physostigmine. Physostigmine was without effect on carbachol-induced facilitation (n=5; P > 0.05, two-way ANOVA).
Figure 5.
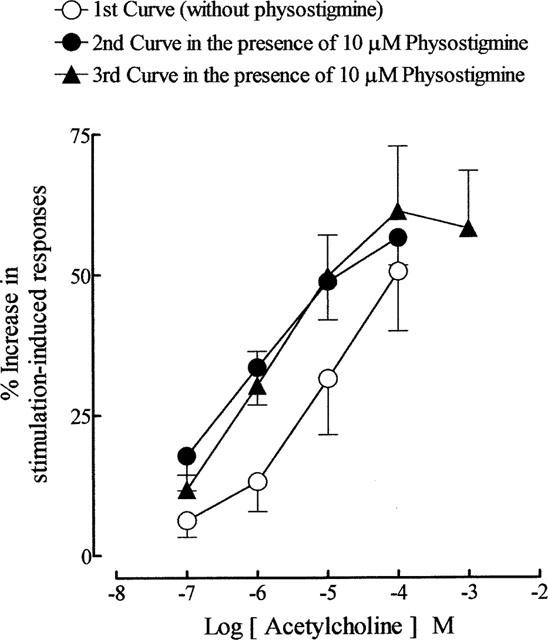
The effects of physostigmine (10 μM) on the mean log concentration-response curves for the enhancing effects of acetylcholine on field stimulation (trains of 20 pulses of 0.5 ms at 10 Hz every 50 s, 60 V)-induced contractions of the guinea-pig prostatic preparations. The first curve to acetylcholine was constructed in the absence of physostigmine. Physostigmine was added 5–10 min before construction of the second and third curves to acetylcholine. Data are shown as mean values with vertical lines representing s.e.mean from eight experiments.
DMPP (1 μM–0.1 mM, n=3), nicotine (10 μM, n=4) and McN-A-343 (0.1 μM–0.1 mM, n=3) were without significant effect on the field stimulation-induced contractions (P > 0.05, one-way ANOVA) of the prostatic smooth muscle.
Effects of antagonists on responses to acetylcholine and/or carbachol
Neither hexamethonium (0.1 mM) nor the neuropeptide Y Y1 receptor antagonist BIBP 3226 (0.3 μM) modified the magnitude of stimulation-induced contractions or the facilitatory effects of acetylcholine and/or carbachol on stimulation-induced contractions of the guinea-pig prostatic smooth muscle (n=3–4; P > 0.05, two-way ANOVA). Exposure of the prostatic preparations to suramin (100 μM, n=5), prazosin (0.1 μM, n=5), guanethidine (10 μM, n=5) and prazosin and guanethidine in combination (n=4) caused 20±12%, 73±7%, 82±4% and 86±6% inhibition respectively, of the magnitude of stimulation-induced contractions. The facilitatory effects of carbachol were unaltered in the presence of suramin. Prazosin and guanethidine, alone and in combination produced small but significant decreases to the carbachol-induced responses (P<0.05, two-way ANOVA). Log concentration-response curves to carbachol were shifted 2.52-(95% confidence limits: 2.43, 2.60; d.f.=58), 3.92-(95% confidence limits: 3.26, 4.73; d.f.=61) and 3.66-(95% confidence limits: 3.62, 3.71; d.f.=48) fold to the right in the presence of prazosin, guanethidine, alone and in combination, respectively.
Effects of subtype-preferring muscarinic receptor antagonists
All the subtype-preferring muscarinic receptor antagonists used (pirenzepine 0.1–1 μM, HHSiD 0.1–1 μM and pF-HHSiD 1 μM) except himbacine (1 μM) caused slight but significant inhibition of the magnitude of stimulation-induced contractions of the guinea-pig prostatic smooth muscle preparations (P<0.05, Student's paired t-tests). The maximum extent of inhibition, of 33±11% (n=5) was seen with HHSiD (0.1 μM).
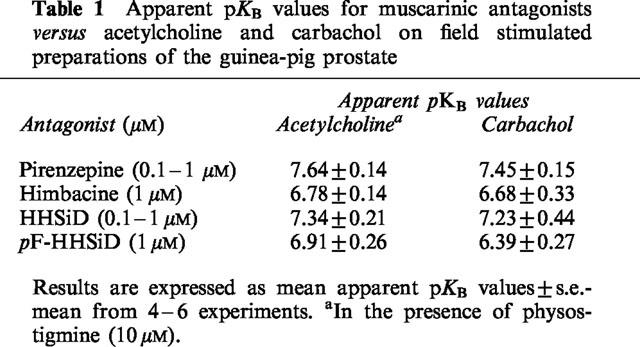
In the presence of these subtype-preferring antagonists, log concentration-response curves to acetylcholine in the presence of physostigmine and to carbachol were shifted to the right without significant suppression of the responses to maximal concentrations of agonist applied. Estimates of the affinities/potencies (apparent pKB values) of these antagonists in inhibiting responses to the agonists are shown in Table 1. The effect of extension of the incubation period for pirenzepine from 30 to 90 min was examined; there was no significant change in the apparent pKB values (n=5; P > 0.05, Student's unpaired t-tests).
Table 1.
Apparent pKB values for muscarinic antagonists versus acetylcholine and carbachol on field stimulated preparations of the guinea-pig prostate
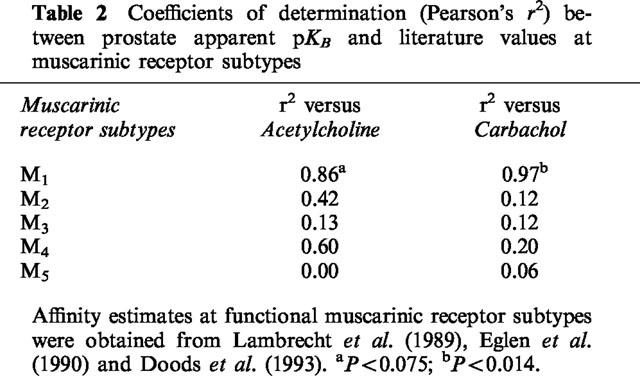
Correlation analysis between the apparent pKB values of antagonists in inhibiting the facilitatory effects of acetylcholine and carbachol on stimulation-mediated contractions of the guinea-pig prostate and published mean estimates of their affinities (pA2 or apparent pKB values) at muscarinic receptor systems showed a significant correlation with affinity for the muscarinic M1 receptor using carbachol as the agonist. Estimates of r2 and associated P values are listed in Table 2.
Table 2.
Coefficients of determination (Pearson's r2) between prostate apparent pKB and literature values at muscarinic receptor subtypes
Discussion
The aims of this study were: (1) to examine the effects of cholinoceptor agonists on smooth muscle tone and on neuromuscular transmission to the guinea-pig isolated prostate gland; and (2) to determine the muscarinic receptor subtype mediating the effects of agonists on the prostate of this species.
Acetylcholine and carbachol produced little or no direct effect on the smooth muscle tone of the guinea-pig isolated prostate. Cohen & Drey (1989) have previously reported that, in contrast to histamine and α-adrenoceptor agonists, carbachol produced minimal contractile activity in the prostatic smooth muscle preparations of the guinea-pig. Similarly, in the human prostate, although acetylcholine contracts the prostatic capsule it has minimal effect on the prostate stroma (Caine et al., 1975; Hedlund et al., 1985). Species variations in the responsiveness of the prostate to cholinoceptor agonists do occur as the contractile effect of carbachol is prominent in rat (Lau & Pennefather, 1998) and dog prostates (Fernandez et al., 1998).
Despite a lack of direct contractile effect, acetylcholine and carbachol both enhanced neurotransmission to the guinea-pig prostate gland in a concentration-dependent manner. Carbachol was the more potent. In the presence of the anticholinesterase, physostigmine, the facilitatory effects of acetylcholine, but not of carbachol, were significantly enhanced, confirming the presence of cholinesterase in the prostate from the guinea-pig. Since the effects of acetylcholine and carbachol were unaltered by the ganglion blocking agent, hexamethonium, and since DMPP and nicotine, were without effect on field stimulation-induced contractions of the prostatic smooth muscle, it can be assumed that nicotinic receptors are not involved in the responses to acetylcholine and carbachol, despite the possibility that ganglion cells may be located in close proximity to the prostate in this species (Lamano Carvalho et al., 1986). Thus the facilitatory effects of acetylcholine and carbachol, and those of the anticholinesterases examined in our previous study (Lau et al., 1998), are likely to be mediated by a muscarinic receptor.
The mechanism/s through which acetylcholine and carbachol act to facilitate neurotransmission to the prostate were examined indirectly. Noradrenaline produces contractions of guinea-pig prostate smooth muscle by activation of α1L-adrenoceptors (Pennefather et al., 1999). Acetylcholine, in the presence of physostigmine, did not enhance these contractions. This finding is in contrast to that reported for the guinea-pig vas deferens (Sjostrand, 1973; Sjostrand & Swedin, 1974), indicating an absence of synergism between the two neurotransmitters at postjunctional receptors in the prostate gland. Our findings that neither suramin nor the neuropeptide Y Y1 receptor antagonist BIBP 3226 (Rudolf et al., 1994), affected responses to carbachol indicated lack of an effect on the release or actions of either ATP or neuropeptide Y. Previous reports by Cohen & Drey (1989) indicated that neuropeptide Y was without effect on guinea-pig prostate smooth muscle. Similarly, although suramin caused some decrease in the response to field stimulation in the guinea-pig prostate in our present and previous (Lau et al., 1998) studies; surprisingly, ATP was without contractile effect on this tissue (Lau, 1998). Taken together, these observations raise the possibility that the cholinoceptor agonists may produce facilitation of neurotransmission to the guinea-pig prostate by enhancing noradrenaline overflow, but this possibility needs further examination.
Operational characterization of the four pharmacologically defined muscarinic receptor subtypes (M1–M4) is commonly achieved by obtaining potency profiles of a series of subtype-preferring muscarinic receptor antagonists (reviews by Caulfield, 1993; Eglen et al., 1996; Caulfield & Birdsall, 1998). Pirenzepine (M1), himbacine (M2/M4), HHSiD (M1/M3) and pF-HHSiD (M3) were employed in the present study. We have previously reported that atropine (1 μM) partially reduced field stimulation-induced contractions of guinea-pig prostate stroma (Lau et al., 1998). With the exception of himbacine, the subtype-preferring antagonists we used in this study also partially reduced responses to field stimulation. The effects of the antagonists were not established with sufficiently low concentrations to allow estimation of the relative potencies as a possible guide to the nature of the receptor subtype/s involved, as our major aim in this study was to determine the subtype of muscarinic receptors mediating the facilitatory effects of exogenously administered acetylcholine and carbachol on neurally-mediated contractions.
The potencies of the muscarinic receptor subtype-preferring antagonists in inhibiting the facilitatory effects of the choline esters are broadly consistent with agonist actions at muscarinic M1 receptors. The potency rank orders (with apparent pKB values in parenthesis) versus acetylcholine, namely pirenzepine (7.64) > HHSiD (7.34) > pF-HHSiD (6.91)⩾ 5 himbacine (6.78) and versus carbachol, namely pirenzepine (7.45) > HHSiD (7.23) > himbacine (6.68) ⩾ pF-HHSiD (6.39) were similar except for the order of himbacine and PF-HHSiD.
The pKB estimates of pirenzepine (7.45–7.64) against both agonists are slightly lower than literature values obtained at other M1 systems (8.1–8.5, from reviews by Hulme et al., 1990; Caulfield, 1993; Eglen & Watson, 1996), but are too high to indicate actions at muscarinic M2 and M3 receptors (6.8 and 6.9, respectively from Table 2, Eglen & Watson, 1996). They are comparable with those reported for interactions with muscarinic M4 receptors (7.7–8.1, Caulfield, 1993). However, the relatively low pKB values (6.68–6.78) obtained for the muscarinic M2/M4-receptor preferring antagonist, himbacine, suggest that neither muscarinic M2 nor M4 receptors are important in mediating the facilitatory effects of acetylcholine and carbachol.
HHSiD (M1=M3 > M2) has a relatively high affinity for muscarinic M1 (pA2 value of 7.9 in rabbit vas deferens) and for M3 (pA2 value of 8.0 in guinea-pig ileum) receptors but a low potency at muscarinic M2 receptors (pA2 value of 6.5 in rat atria) (Lambrecht et al., 1989). Therefore it is a useful tool to distinguish muscarinic M1 or M3 receptors from M2 receptors. The relatively high pKB estimates obtained for HHSiD (7.23–7.34) versus acetylcholine and carbachol in facilitating neurotransmission to the prostate further substantiate the non-involvement of muscarinic M2 receptors in this effect. These estimates for this antagonist, are however, lower than those observed by Lambrecht et al. (1989) in M1 and M3 systems. It is unclear why lower estimates for HHSiD and for pirenzepine were obtained in the present study. It is unlikely that the incubation period is too short since other studies have used similar incubation periods, (Doods et al., 1993; Roffel et al., 1993; Kerr et al., 1995). Indeed extension of the incubation period for pirenzepine versus carbachol to 90 min was without effect on the potency estimate.
pF-HHSiD (M3 ⩾ M1 ⩾ M2) was included to differentiate between muscarinic M1 and M3 receptors because of its relatively greater affinity for the latter subtype. At muscarinic M1, M2 and M3 receptors, it exhibits pA2 values of 7.2–7.5, 6.0–6.9 and 7.8–7.9, respectively (Caulfield, 1993). The relatively low pKB estimates obtained for pF-HHSiD (6.39–6.91) in the present study clearly do not support the view that the neuromodulatory effect of the agonists is mediated by activation of muscarinic M3 receptors.
Taken together, the antagonist potency profiles obtained in this study are compatible with the possibility that the cholinoceptor agonists facilitate neurotransmission to the guinea-pig prostatic smooth muscle mainly via muscarinic M1 receptors. As shown in Table 2, the potencies of the subtype-preferring antagonists versus carbachol-induced facilitation of neurotransmission in the present study correlate well with those reported in the literature for muscarinic M1 receptors. In contrast, poor correlation with literature values reported for the other muscarinic receptor subtypes was observed. While muscarinic receptor heterogeneity cannot be excluded, there were only small (less than 5 fold) disparities between the present estimates for HHSID and pirenzepine and published values for the M1 receptor.
There is evidence for the presence of both facilitatory and inhibitory prejunctional muscarinic receptors of the M1 subtype on both sympathetic and/or parasympathetic neurones supplying other genito-urinary organs (Eltze, 1988; Somogyi & de Groat, 1990; Somogyi et al., 1994; 1996; 1997). In our study the putatively M1 receptor-selective agonist McN-A-343 was without effect on neurotransmission to the guinea-pig prostate smooth muscle. While this finding would suggest that M1 receptors may not mediate the effects of cholinoceptor agonists it should be noted that McN-A-343 is, however, a partial agonist at muscarinic M1–M4 receptors (Lazareno et al., 1993), and this may explain its lack of effect. Moreover its effects on neurotransmission to the male genito-urinary tract are species-related. Thus while it inhibits neurotransmission to the rabbit vas deferens by activation of a prejunctional M1 receptor (Eltze et al., 1988), it enhances that to the guinea-pig vas deferens by activation of an M2 receptor (Walsh et al., 1995).
In conclusion, this study establishes for the first time that acetylcholine and carbachol can facilitate neurotransmission to the smooth muscle of the prostate from the guinea-pig. While it is unknown whether similar facilitation occurs with the human prostate, it is of interest that muscarinic M1 receptors are likely to be involved in mediating these actions in the guinea-pig prostate. This is of particular interest since radioligand binding and immunocytochemical studies by Ruggieri et al. (1995) have indicated a preponderance of muscarinic receptors of the M1 subtype in the human prostate. Thus guinea-pig and human prostates differ from those in dog and rat firstly in that they do not contract in response to choline esters and secondly in that M1 receptors are prominent only in prostates from the former two species.
Abbreviations
- BIBP 3226
(R)-N2-(diphenylacetyl)-N-[(4-hydroxyphenyl)methyl]-argininamide
- DMPP
(1,1-dimethyl-4-phenyl-piperazinium iodide
- HHSiD
hexahydrosiladifenidol
- McN-A-343
(4-hydroxy-2-butynyl)-1-trimethylammonium-m-chlorocarbanilate chloride
- pF-HHSiD
para-fluoro-hexahydrosiladifenidol
References
- CAINE M., RAZ S., ZEIGLER M. Adrenergic and cholinergic receptors in the human prostate, prostatic capsule and bladder neck. Br. J. Urol. 1975;47:193–202. doi: 10.1111/j.1464-410x.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P. Muscarinic receptors–characterization, coupling and function. Pharmac. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- CAULFIELD M.P., BIRDSALL N.J.M. International Union of Pharmacology, XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- COHEN M., DREY K. Contractile responses in bladder body, bladder neck and prostate from rat, guinea-pig and cat. J. Pharmacol. Exp. Ther. 1989;248:1063–1068. [PubMed] [Google Scholar]
- DAIL W.G. Autonomic innervation of male reproductive genitalia The Autonomic Nervous System. Vol 3: Nervous Control of the Urogenital System 1993Switzerland: Harwood Academic Publishers; 69–101.ed. Maggi C.A. pp [Google Scholar]
- DOODS H.N., WILLIM K.D., BODDEKE H.W.G.M., ENTZEROTH M. Characterization of muscarinic receptors in guinea-pig uterus. Eur. J. Pharmacol. 1993;250:223–230. doi: 10.1016/0014-2999(93)90385-u. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., HEDGE S.S., WATSON N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- EGLEN R.M., MICHEL A.D., MONTGOMERY W.W., KUNYSZ E.A., MACHADO C.A., WHITING R.L. The interaction of parafluorohexahydosiladiphenidol at muscarinic receptors in vitro. Br. J. Pharmacol. 1990;99:637–642. doi: 10.1111/j.1476-5381.1990.tb12983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., WATSON N. Selective muscarinic receptor agonists and antagonists. Pharmacol. & Toxicol. 1996;78:59–68. doi: 10.1111/j.1600-0773.1996.tb00181.x. [DOI] [PubMed] [Google Scholar]
- ELTZE M. Muscarinic M1- and M2-receptors mediating opposite effects on neuromuscular transmission in rabbit vas deferens. Eur. J. Pharmacol. 1988;151:205–221. doi: 10.1016/0014-2999(88)90801-1. [DOI] [PubMed] [Google Scholar]
- ELTZE M., GMELIN G., WESS J., STROHMANN C., TACKE R., MUTSCHLER E., LAMBRECHT G. Prejunctional muscarinic receptors mediating inhibition of neurogenic contractions in the rabbit vas deferens are of the ganglionic M1-type. Eur. J. Pharmacol. 1988;158:233–242. doi: 10.1016/0014-2999(88)90072-6. [DOI] [PubMed] [Google Scholar]
- FARNSWORTH W.E., LAWRENCE M.H. Regulation of prostatic secretion in the rat. Proc. Soc. Exp. Biol. Med. 1965;119:373–376. doi: 10.3181/00379727-119-30185. [DOI] [PubMed] [Google Scholar]
- FARRELL J.I., LYMAN Y. A study of the secretory nerves of, and the action of drugs on the prostate gland. Am. J. Physiol. 1937;118:64–70. [Google Scholar]
- FERNÁNDEZ J.L.G., RIVERA L., LÓPEZ P.G., VELA-NAVARRETE R., GARCIA-SACRISTÁN A. Characterization of the muscarinic receptor mediating contraction of the dog prostate. J. Auton. Pharmacol. 1998;18:205–211. doi: 10.1046/j.1365-2680.1998.18486.x. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F. The classification of adrenoceptors (adrenergic receptors). An evaluation from the standpoint of receptor theory Handbook of Experimental Pharmacology Vol 33, Catecholamines 1972Berlin: Springer-Verlag; 283–335.ed. Blaschko H. & Muscholl E. pp [Google Scholar]
- HAYNES J.M., HILL S.J. Beta-adrenoceptor-mediated inhibition of alpha1-adrenoceptor-mediated and field stimulation-induced contractile responses in the prostate of the guinea-pig. Br. J. Pharmacol. 1997;122:1067–1074. doi: 10.1038/sj.bjp.0701494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEDLUND H., ANDERSSON K-E, LARSSON B. Alpha-adrenoceptors and muscarinic receptors in the isolated human prostate. J. Urol. 1985;134:1291–1298. doi: 10.1016/s0022-5347(17)47714-7. [DOI] [PubMed] [Google Scholar]
- HORSFALL D.J., MAYNE K., RICCIARDELLI C., RAO M., SKINNER J.M., HENDERSON D.W., MARSHALL V.R., TILLEY W.D. Age-related changes in guinea-pig prostatic stroma. Lab. Invest. 1994;70:753–763. [PubMed] [Google Scholar]
- HULME E.C., BIRDSALL N.J.M., BUCKLEY N.J. Muscarinic receptor subtypes. Ann. Rev. Pharmacol. Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- JAMES S., CHAPPLE C.R., PHILLIPS M.I., GREENGRASS P.M., DAVEY M.J., TURNER-WARWICK R.T., MILROY E.J.G., BURNSTOCK G. Autoradiographic analysis of alpha-adrenoceptors and muscarinic cholinergic receptors in the hyperplastic human prostate. J. Urol. 1989;142:438–444. doi: 10.1016/s0022-5347(17)38780-3. [DOI] [PubMed] [Google Scholar]
- KERR P.M., HILLIER K., WALLIS R.M., GARLAND C.J. Characterization of muscarinic receptors mediating contractions of circular and longitudinal muscle of human isolated colon. Br. J. Pharmacol. 1995;115:1518–1524. doi: 10.1111/j.1476-5381.1995.tb16645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMANO CARVALHO T.L., HODSON N.P., BLANK M.A., WATSON P.F., MULDERRY P.K., BISHOP A.E., GU J., BLOOM S.R, POLAK J.M. Occurrence, distribution and origin of peptide-containing nerves of guinea-pig and rat male genitalia and the effects of denervation on sperm characteristics. J. Anat. 1986;149:121–141. [PMC free article] [PubMed] [Google Scholar]
- LAMBRECHT G., FEIFEL R., MOSER U., WAGNER-RODER M., CHOO L.K., CAMUS J., TASTENOY M., WAELBROECK M., STROHMANN C., TACKE R., RODRIGUES DE MIRANDA J.F., CHRISTOPHE J., MUTSCHLER E. Pharmacology of hexahydo-difenidol, hexahydro-sila-difenidol and related selective muscarinic antagonists. Trends Pharmacol. Sci. 1989;10 Suppl:60–64. [PubMed] [Google Scholar]
- LATIFPOUR J., GOUSSE A., YOSHIDA M., WEISS R.M. Muscarinic receptors in diabetic rat prostate. Biochem. Pharmacol. 1991;42:S113–S119. doi: 10.1016/0006-2952(91)90400-y. [DOI] [PubMed] [Google Scholar]
- LAU W.A.K. Neurotransmission to the prostate: role of acetylcholine 1998. Masters of Science thesis. Monash University, Australia
- LAU W.A.K., PENNEFATHER J.N. Neurotransmission to the smooth muscle of the prostate gland from the guinea-pig. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 1995;2:89. [Google Scholar]
- LAU W.A.K., PENNEFATHER J.N. Muscarinic receptor subtypes in the rat prostate glands. Eur. J. Pharmacol. 1998;343:151–156. doi: 10.1016/s0014-2999(97)01535-5. [DOI] [PubMed] [Google Scholar]
- LAU W.A.K., PENNEFATHER J.N., MITCHELSON F.J. Cholinergic facilitation of neurotransmission in the guinea-pig prostate gland. J. Auton. Nerv. Syst. 1997;65:147. doi: 10.1038/sj.bjp.0703409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAU W.A.K., VENTURA S., PENNEFATHER J.N. Pharmacology of neurotransmission to the smooth muscle of the rat and the guinea-pig prostate glands. J. Auton. Pharmacol. 1998;18:349–356. doi: 10.1046/j.1365-2680.1998.1860349.x. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., FARRIES T., BIRDSALLN J. Pharmacological characterization of guanine nucleotide exchange reactions in membranes from CHO cells stably transfected with human muscarinic receptors m1-m4. Life Sci. 1993;52:449–456. doi: 10.1016/0024-3205(93)90301-i. [DOI] [PubMed] [Google Scholar]
- LEPOR H., KUHAR M.J. Characterization and localization of the muscarinic cholinergic receptor in human prostate tissue. J. Urol. 1984;132:397–402. doi: 10.1016/s0022-5347(17)49636-4. [DOI] [PubMed] [Google Scholar]
- LUTHIN G.R., WANG P., ZHOU H., DHANASEKARAN D., RUGGIERI M.R. Role of m1 receptor-G protein coupling in cell proliferation in the prostate. Life Sci. 1997;60:963–968. doi: 10.1016/s0024-3205(97)00035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAINI A., ARCHER C., WANG C.Y., HASS G.P. Comparative pathology of benign prostatic hyperplasia and prostate cancer. In vivo. 1997;11:293–300. [PubMed] [Google Scholar]
- MCVARY K.T., MCKENNA K.E., LEE C. Prostate Innervation. The Prostate. 1998;8:2–13. [PubMed] [Google Scholar]
- NAJBAR-KASZKIEL A.T., DI IULIO J.L., LI C.G., RAND M.J. Characterisation of excitatory and inhibitory transmitter systems in prostate glands of rats, guinea-pigs, rabbits and pigs. Eur. J. Pharmacol. 1997;337:251–258. doi: 10.1016/s0014-2999(97)01270-3. [DOI] [PubMed] [Google Scholar]
- OHKAWA H. Sympathetic neuromuscular transmission in the smooth muscle of guinea-pig prostate gland. Int. J. Fertil. 1983;28:68–77. [PubMed] [Google Scholar]
- PENNEFATHER J.N., LAU W.A.K., CHIN C., STORY M.E., VENTURA S. α1L-Adrenoceptors mediate noradrenaline-induced contractions of the guinea-pig prostate stroma. Eur. J. Pharmacol. 1999;384:25–30. doi: 10.1016/s0014-2999(99)00667-6. [DOI] [PubMed] [Google Scholar]
- PONTARI M.A., LUTHIN G.R., BRAVERMAN A.S., RUGGIERI M.R. Characterization of muscarinic cholinergic receptor subtypes in rat prostate. J. Receptor & Signal Transduction Research. 1998;18:151–166. doi: 10.3109/10799899809047742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYFORD W., NOBLE M.J., AUSTENFELD M.A., WEIGEL J., MEBUST W.K., SHAH G.V. Muscarinic cholinergic receptors promote growth of human prostate cancer cells. The Prostate. 1997;30:160–166. doi: 10.1002/(sici)1097-0045(19970215)30:3<160::aid-pros3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- RICCIARDELLI C., HORSFALL D.J., SKINNER J.M., HENDERSON D.W., MARSHALL V.R., TILLEY W.D. Development and characterization of primary cultures of smooth muscle cells from the fibromuscular stroma of the guinea-pig prostate. In Vitro Cell Dev. Biol. 1989;25:1016–1024. doi: 10.1007/BF02624135. [DOI] [PubMed] [Google Scholar]
- ROFFEL A.F., ELZINGA C.R.S., ZAAGSMA J. Cholinergic contraction of the guinea-pig lung strip is mediated by muscarinic M2-like receptors. Eur. J. Pharmacol. 1993;250:267–279. doi: 10.1016/0014-2999(93)90391-t. [DOI] [PubMed] [Google Scholar]
- RUDOLF K., EBERLEIN W., ENGEL W., WIELAND H.A., WILLIM K.D., ENTZEROTH M., WIENEN W, , BECK-SICKINGER A.G., DOODS H.N. The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur. J. Pharmacol. 1994;271:R11–R13. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- RUGGIERI M.R., COLTON M.D., WANG P., WANG J., SMYTH R.J., PONTARI M.A., LUTHIN G.R. Human prostate muscarinic receptor subtypes. J. Pharmacol. Exp. Ther. 1995;274:976–982. [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND N.O. Effects of acetylcholine and some other smooth muscle stimulants on the electrical and mechanical responses of the guinea-pig vas deferens to nerve stimulation. Acta Physiol. Scand. 1973;89:1–9. doi: 10.1111/j.1748-1716.1973.tb05491.x. [DOI] [PubMed] [Google Scholar]
- SJOSTRAND N.O., SWEDIN G. On the mechanism of the enhancement by smooth muscle stimulants of the motor responses of the guinea-pig vas deferens to nerve stimulation. Acta Physiol. Scand. 1974;90:513–521. doi: 10.1111/j.1748-1716.1974.tb05616.x. [DOI] [PubMed] [Google Scholar]
- SOMOGYI G.T., DE GROAT W.C. Modulation of the release of [3H]norepinephrine from the base and body of the rat urinary bladder by endogenous adrenergic and cholinergic mechanisms. J. Pharmacol. Expt. Ther. 1990;255:204–210. [PubMed] [Google Scholar]
- SOMOGYI G.T., TANOWITZ M., DE GROAT W.C. M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. J. Physiol. 1994;480:81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMOGYI G.T., TANOWITZ M., ZERNOVA G., DE GROAT W.C. M1 muscarinic receptor-induced facilitation of ACh and noradrenaline release in the rat urinary bladder is mediated by protein kinase C. J. Physiol. 1996;496:245–254. doi: 10.1113/jphysiol.1996.sp021681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMOGYI G.T., ZERNOVA G., TANOWITZ M., DE GROAT W.C. Role of L- and N-type Ca2+ channels in muscarinic receptor-mediated facilitation of ACh and noradrenaline release in the rat urinary bladder. J. Physiol. 1997;499:645–654. doi: 10.1113/jphysiol.1997.sp021957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K., PENNEFATHER J.N., SMITH K. Characterization of the muscarinic receptor subtypes present in the vas deferens. Proc. Aust. Soc. Clin. Exp. Pharmacol. Toxicol. 1995;2:90. [Google Scholar]
- YAZAWA H., HONDA K. The M3-muscarinic cholinoceptor subtype in rat prostate and its down regulation by aging. Jap. J. Pharmacol. 1993;61:319–324. doi: 10.1254/jjp.61.319. [DOI] [PubMed] [Google Scholar]


