Abstract
Using fura-PE3 fluorimetry and α-toxin permeabilization, the characteristics of the contractile responses to neurokinin A (NKA) were determined in the pregnant rat myometrium.
NKA induced contractions in rat myometrium in a concentration-dependent manner. There were no significant differences in the maximum contractions and EC50 values between the pregnant and non-pregnant myometrium, however, the contraction of only the former was greatly enhanced in the presence of phosphoramidon (PPAD), an endopeptidase inhibitor.
In the pregnant myometrium, NKA induced sustained increases in [Ca2+]i and tension in normal physiological saline solution, while only small transient increases in [Ca2+]i and tension were observed in Ca2+-free solution.
Both diltiazem (10 μM) and SK-F 96365 (10 μM) significantly inhibited the NKA-induced elevations of [Ca2+]i and tension. The effects were additive when these drugs were used together.
NKA induced a significant leftward shift of the [Ca2+]i-tension curve obtained by changing the external Ca2+ (0–2.5 mM) during depolarization with high K+ solution. This Ca2+-sensitizing effect by NKA was also observed in the α-toxin permeabilized myometrium.
These results indicated that in the pregnant rat myometrium: (1) the responsiveness to NKA increased, although it was masked by the increase in the endopeptidase activity; (2) NKA induced contractions of the myometrium by increasing both [Ca2+]i and the myofilament Ca2+ sensitivity and (3) The NKA-induced [Ca2+]i elevation was partly due to the intracellular Ca2+ release and mainly due to the Ca2+ influx, which was thought to be through both voltage dependent calcium channels and non-specification channels.
Keywords: Pregnancy, neurokinin A, myometrium, endopeptidase
Introduction
Neurokinin A (NKA) belongs to a structurally related peptide family named tachykinins, which also includes substance P (SP) and neurokinin B. These tachykinins are mainly localized in the central nervous system in a large amount. However, these peptides are also distributed in the sensory nerves (mainly in the afferent C-fibres) and are widely distributed within the mammalian peripheral tissues (Gibbins et al., 1985; Otsuka & Yoshioka, 1993; Ottesen & Fahrenkrug, 1990; Regoli et al., 1994). The function of SP in the various peripheral tissues have been extensively investigated, however, the function of NKA, which is a product of the same gene as SP and usually co-localizes with SP (Otsuka & Yoshioka, 1993), in the peripheral tissues still remains unclear.
Since NKA-containing fibres innervate the uterus of various species (Gibbons et al., 1985; Shew et al., 1992; Traurig et al., 1988), it can be postulated that NKA might have a role in the regulation of the uterine function, especially in pregnancy. This speculation is supported by the following reports: (1) The pelvic neurectomies lead to uterine dystocia in the pregnant rat (Higuchi et al., 1987; Traurig et al., 1984; 1988), and afferent C-fibres may play a role in the neural regulation of parturition (Traurig et al., 1991); (2) NKA induced contractions in the rat myometrial strips (Fisher & Pennefather, 1997; Fisher et al., 1993; Pennefather et al., 1993) and (3) NK-2 receptor, which is preferentially activated by NKA, were predominant in the estrogen-primed rat myometrium (Fisher & Pennefather, 1998; 1999; Fisher et al., 1993; Magraner et al., 1998; Pennefather et al., 1993).
Regarding the mechanism underlying the NKA-induced contraction of myometrial smooth muscle, it has been reported to be largely dependent on the Ca2+ influx through voltage operated Ca2+ channel (VOC) and the Ca2+ release from intracellular Ca2+ store site in the estrogen-primed rat myometrium (Magraner et al., 1997). However, the direct measurements of [Ca2+]i and tension of the myometrial strips during activation by NKA have not been reported before. In the present study, using fura-PE3 fluorimetry and α-toxin permeabilization, we determined the mechanisms underlying the NKA-induced contraction of the pregnant rat myometrium.
Methods
The study protocol was approved by the Animal Care and Committee of Research Institute of Angiocardiology (Faculty of Medicine, Kyushu University). The pharmacological experiments were done according to the procedures described previously (Niiro et al., 1998). A brief description of them is shown below.
Tissue preparation
The virgin female Wister-Kyoto rats weighing 200–250 g were paired overnight with male rats and the next morning was considered to be day 1 of pregnancy when a vaginal plug was detected. The rats were sacrificed with ether on day 18 after mating. The myometrium of the pregnant rats was taken from the remote sites of implantation. The myometrium of the non-pregnant rats was also prepared from virgin rats without monitoring the oestrous cycle. The strips of myometrium (1 mm in width and 4 mm in length) were prepared using the middle part of the horn in a physiological saline solution (PSS) consisting of the following compositions (in mM): NaCl, 123; KCl, 4.7; CaCl2, 1.25; MgCl2, 1.2; KH2PO4, 1.2; NaHCO3, 15.5; D-glucose 11.5; gassed with 95% O2 and 5% CO2.
Tension measurement of intact myometrial strips
The myometrial strips were connected to a force transducer (TB-612T, Nihon Koden, Japan) and mounted vertically in a quartz organ bath. The strips were stimulated with 40 mM K+ PSS (an equimolar substitution of KCl for NaCl) every 15 min with a stepwise increase in the resting load until the maximal response was obtained. The contractile responses to NKA was quantitatively evaluated by the first peak level of contraction. These values obtained with 40 mM K+-depolarization were designated to be 100%.
Fura-PE3 loading and measurement of cytosolic Ca2+ concentration [Ca2+]i
The myometrial strips from the pregnant rats were incubated in Dulbecco's-modified Eagle medium gassed with 5% CO2 and 95% O2 containing 50 μM fura-PE3/AM and 5% foetal bovine serum for 6 h at 37°C. The strips were equilibrated in normal PSS for at least 1 h before the measurements. Front-surface fluorimetry (Niiro et al., 1998) was used to monitor the changes in the fluorescence intensity of the fura-PE3-Ca2+ complex. The fluorescence (500 nm) intensities at alternating (400 Hz) excitation (340 and 380 nm) and the ratio (F340/F380) were continuously measured. The data were stored in a Macintosh computer using a data acquisition system (MacLab). The fluorescence ratio (F340/F380), which indicates [Ca2+]i, was expressed as a percentage, assigning the values in normal (5.9 mM K+) and 40 mM K+ PSS to be 0 and 100%, respectively. The absolute values of [Ca2+]i were estimated in separate measurements as previously described (Kanaide, 1999). The [Ca2+]i levels of the pregnant myometrial smooth muscle cells at 0 and 100% were 190±46 nM and 485±123 nM, respectively (n=8). All simultaneous measurements of [Ca2+]i and force were performed at 37°C.
Tension measurement of α-toxin permeabilized myometrial strips
The permeabilization of the rat myometrium was carried out according to the methods described by Niiro et al. (1998) using α-toxin instead of β-escin. Small strips of the day 18 pregnant rat myometrium were mounted between two tungsten wires. The strips were permeabilized in a relaxing solution (mM): potassium methanesulphonate 100, Na2ATP 2.2, MgCl2 3.38, ethyleneglycol-bis (β-aminoethylether)-N′,N′,N′,N′-tetra acetic acid (EGTA) 10, creatine phosphate 10, Tris-maleate 20 (pH=6.8) containing 5000 units ml−1 Staphylococcus aureus α-toxin for 60 min. The activating solution containing the indicated concentration of free Ca2+ was made by adding an appropriate amount of CaCl2 to the relaxing solution, using the Ca2+-EGTA binding constant of 106/M (Saida & Nonomura, 1978). The tension measurements of the permeabilized tissue were all performed at room temperature. The tension in the relaxing solution and maximal tension induced by 10 μM Ca2+ were taken as 0 and 100%, respectively.
Drugs and solutions
The composition of the normal physiological salt solution (normal PSS) was described above. High-K+ PSS was made by an equimolar substitution of KCl for NaCl. The Ca2+-free solution (Ca2+-free PSS) containing 2 mM EGTA instead of 1.25 mM CaCl2 was produced by an exclusion of CaCl2 from the normal PSS. Each solution mentioned above was gassed with a mixture of 5% CO2 : 95% O2 (pH 7.4 at 37°C). The composition of the solution for the permeabilized preparations was described above. Neurokinin A was purchased from the Peptide Institute (Osaka, Japan). Diltiazem, phosphoramidon (N- (α-L-rhamnopyranosyl- oxyhydroxy-phosphinyl)-L-leucyl-L-tryptophan), and α-toxin were from Sigma (St Louis, MO, U.S.A.). SK&F 96365 (1-{β-[-3-(4-methoxyphenyl)propoxy]-4-methoxyphenyl}-1H-imidazole hydro-chloride) was purchased from Calbiochem (La Jolla, CA, U.S.A.). Guanosine-5′-triphosphate (GTP) and guanosine-5′-O-(β-thiodiphosphate) (GDPβS) were purchased from Boehringer Mannheim (Germany). EGTA was from Dojindo Laboratories (Kumamoto, Japan). All other chemicals were of the highest grade commercially available.
Statistical analysis
All data were expressed as the mean±s.e.mean. One strip obtained from one animal was used for each experiment, therefore the number of experiments (n value) also indicates the number of animals. Statistically significant differences were determined by an analysis of variance (ANOVA) followed by Scheffe's post-hoc test between more than three groups. P<0.05 were considered to be significant. An analysis of co-variance was used to determine the non-overlapping (or shift) of the [Ca2+]i-force relationship. The four-parameter logistic model was used to fit the sigmoidal curve to the concentration response of each drug (De Lean et al., 1978). All data were collected using a computerized data acquisition system (MacLab; Analog Digital Instruments, Australia, Macintosh; Apple Computer, U.S.A.).
Results
Effects of NKA on the contractility of the pregnant or non-pregnant rat myometrium
Application of NKA (100 nM) to the pregnant (Figure 1a) or non-pregnant (Figure 1c) rat myometrial strips in the normal PSS induced an initial rapid phasic contraction which reached a peak within 1 min and was followed by a tonic response of low amplitude with a superimposed rhythmic contraction. This oscillatory response usually continued for more than 20 min (Figure 1a,c), but disappeared in some strips (data not shown). When the developed tension was evaluated as an absolute value, 40 mM K+-depolarization produced 393±25 mg/mg wet weight (n=12) in the non-pregnant myometrium and 611±61 mg/mg wet weight (n=12) in the pregnant myometrium. Therefore, we evaluated the tension development induced by NKA relative to the 40 mM K+-depolarization-induced effect. There appeared to be no difference in the NKA-induced contractions between the pregnant and non-pregnant rat myometrium (Figure 1a,c). However, when the strips were treated with phosphoramidon (PPAD), an endopeptidase inhibitor, the NKA-induced contraction was greatly enhanced in the pregnant myometrium (Figure 1b). On the other hand, the enhancing effect of PPAD, if any, in the non-pregnant myometrium was not remarkable (Figure 1d).
Figure 1.
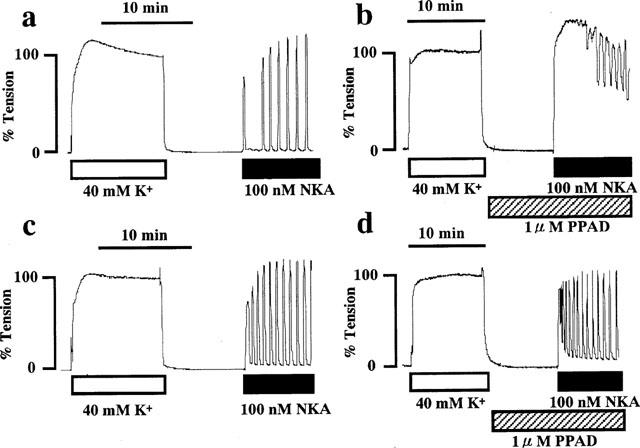
Representative recordings of tension developed by neurokinin A (NKA) in day 18 pregnant and non-pregnant rat myometrium in the absence and presence of phosphoramidon (PPAD). NKA (100 nM) was applied to day 18 pregnant (a,b) and non-pregnant (c,d) rat myometrium in the absence (a,c) or presence (b,d) of 1 μM PPAD. PPAD was applied 10 min before and during the application of NKA. The developed tension was expressed as a percentage, assigning the values in normal (5.9 mM K+) and 40 mM K+ PSS to be 0 and 100%, respectively. The traces shown are representative of six similar independent experiments.
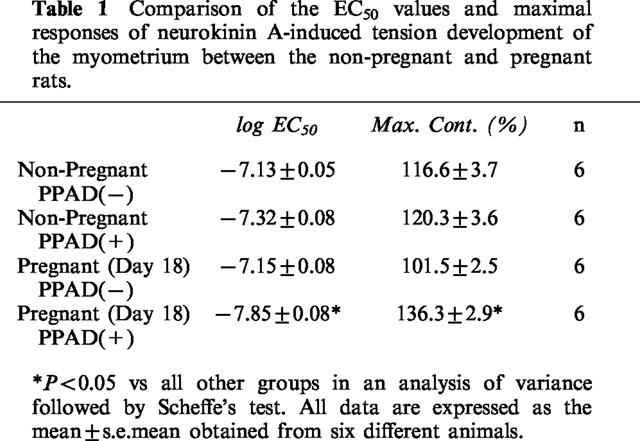
For a quantitative analysis of the NKA-induced contraction of the pregnant or non-pregnant myometrium, the concentration-response relationships of NKA were determined in the presence or absence of PPAD. Since an initial peak value of the first contraction was reproducible for each NKA concentration, we measured this value to construct the concentration-response curves for the NKA-induced myometrial contraction (Niiro et al., 1998). Figure 2 shows the concentration-response relationships of the contractions induced by the various concentrations of NKA (0.3 nM–10 μM) in the four different groups, namely, non-pregnant without PPAD, non-pregnant with PPAD, pregnant without PPAD and pregnant with PPAD. The log EC50 value and maximal contraction level of each curve are shown in Table 1. Without PPAD, there was no significant difference in the log EC50 values and maximal contraction levels between non-pregnant and pregnant myometrium. However, when the strips were treated with PPAD, a significant difference was observed in the log EC50 values and the maximal contraction levels between the non-pregnant and the pregnant myometrium. The PPAD-induced leftward shift was significant in the pregnant myometrium, but it was not significant in the non-pregnant myometrium.
Figure 2.
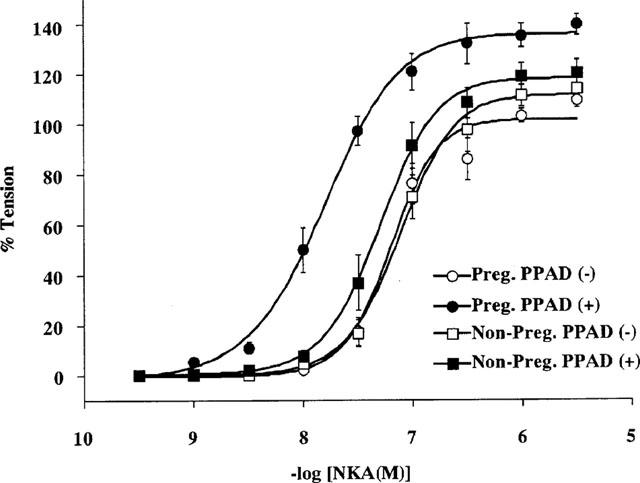
Concentration-response relationships of NKA-induced rat myometrial contraction. Various concentrations of NKA (0.3 nM–10 μM) were applied to the day 18 pregnant and non-pregnant rat myometrium in the presence or absence of PPAD and the peak level of each tension development was plotted. Each tension experiment was performed under conditions similar to those shown in Figure 1. Each symbol shows the mean value of the NKA-induced developed tension from six different rats. Vertical bars represent the s.e.mean.
Table 1.
Comparison of the EC50 values and maximal responses of neurokinin A-induced tension development of the myometrium between the non-pregnant and pregnant rats.
Mechanisms underlying the NKA-induced myometrial contraction in the pregnant myometrium
Because the response to NKA in the pregnant myometrium was significantly greater than that in the non-pregnant myometrium, the subsequent studies on the mechanism of contraction were performed using the myometrial strips from pregnant rat on day 18 of gestation. Figure 3a shows the representative recordings of the changes in [Ca2+]i and tension induced by 100 nM NKA in normal PSS in the presence of 1 μM PPAD. The application of NKA induced rapid rises in both [Ca2+]i and tension, which reached a peak level within 1 min, followed by a gradual decline with superimposed oscillations. The initial peak levels of [Ca2+]i and tension were 120.5±5.4 and 129.2±3.4%, respectively (n=8). In a Ca2+-free PSS containing 0.1 mM EGTA, NKA induced a small [Ca2+]i transient accompanied by a small transient contraction (Figure 3b). A summary of the six similar experiments is shown in Figure 3d, which demonstrated that NKA induced significant increases in [Ca2+]i and tension in the Ca2+-free PSS. Since the NKA-induced transient increase in [Ca2+]i in the EGTA-containing Ca2+-free PSS was unexpectedly small probably due to the Ca2+-chelating effect by EGTA, we tried to detect the intracellular Ca2+ release in normal PSS in the presence of 3 mM Ni2+. As shown in Figure 3c,d, NKA induced a significant increase in [Ca2+]i in the presence of Ni2+. As a result, the increase in [Ca2+]i in PSS containing Ni2+ was much bigger than that in a Ca2+-free PSS containing EGTA.
Figure 3.
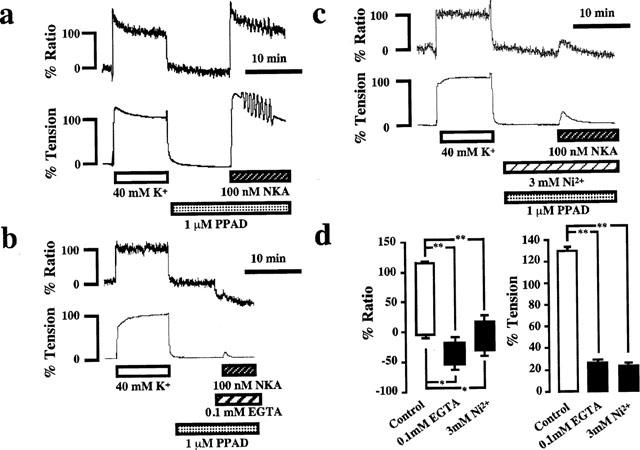
NKA-induced intracellular Ca2+ release. (a–c) Representative recordings showing the effect of NKA (100 nM) on the [Ca2+]i (upper trace) and tension (lower trace) of the pregnant rat myometrium in normal PSS (a), in Ca2+-free PSS containing 0.1 mM EGTA (b) and in normal PSS containing 3 mM Ni2+ (c). PPAD and Ni2+ was added 10 min before and during NKA application. (b) Ca2+-free PSS with 0.1 mM EGTA was applied 1 min before and during the addition of NKA. (d) Summary of the inhibitory effect of Ca2+-free PSS with 0.1 mM EGTA and Ni2+ on the NKA-induced increases in [Ca2+]i and tension. The bottom and top of each column indicate the levels of [Ca2+]i and tension just before the NKA application and at the peak elevation induced by NKA, respectively, under the indicated conditions. All data are expressed as the mean±s.e.mean. (The vertical bars) obtained from experiments similar to those shown in a–c using 4–6 different animals. *P<0.05, **P<0.01.
In an attempt to clarify the Ca2+ influx pathway, the various concentrations of diltiazem (an L-type Ca2+ channel blocker) or SK-F 96365 (a non-selective Ca2+ channel blocker or receptor-operated Ca2+ channel blocker (Merritt et al., 1990)) were applied during the activation by 100 nM NKA. As shown in Figure 4a, the pre-treatment with 10 μM diltiazem significantly inhibited the NKA-induced increases in [Ca2+]i and tension. Figure 4b demonstrates that the pre-treatment by 10 μM SK-F 96365 also significantly inhibited the NKA-induced increases in [Ca2+]i and tension. Figure 4c summarizes the results obtained from the experiments performed in a manner similar to those shown in Figure 4a,b (n=6). Diltiazem induced the maximum effect to the NKA-induced increase in [Ca2+]i and tension at 10 μM. Even if the concentration of diltiazem was raised to 30 μM, there was no additional effect. On the other hand, the application of 10, 30 and 100 μM SK-F 96365 inhibited the NKA-induced increases in [Ca2+]i and tension in a dose-dependent manner. However, the application of 10 μM diltiazem plus 10 μM SK-F 96365 more effectively inhibited the NKA-induced increase in [Ca2+]i compared with the effects by 10 μM diltiazem or 10 μM SK-F 96365 alone. This combined use of diltiazem and SK-F 96365 more effectively inhibited the NKA-induced increase in [Ca2+]i compared with 30 μM diltiazem alone, which is 1.5 times higher than the former in terms of the molar concentration. Although no statistical significance could be obtained, the inhibitory effect by 10 μM diltiazem plus 10 μM SK-F 96365 tends to be greater than that by 30 μM SK-F 96365 alone, which is also 1.5 times higher than the former in terms of the molar concentration. In addition, the treatment with diltiazem or SK-F 96365 completely inhibited the occurrence of the oscillations in [Ca2+]i and tension (n=6). In contrast, when the Ca2+ concentration of the normal PSS was raised to 2.5 mM, NKA-induced oscillations could be observed even in the strips which did not cause oscillations in the normal PSS containing 1.25 mM Ca2+ (n=5, data not shown).
Figure 4.
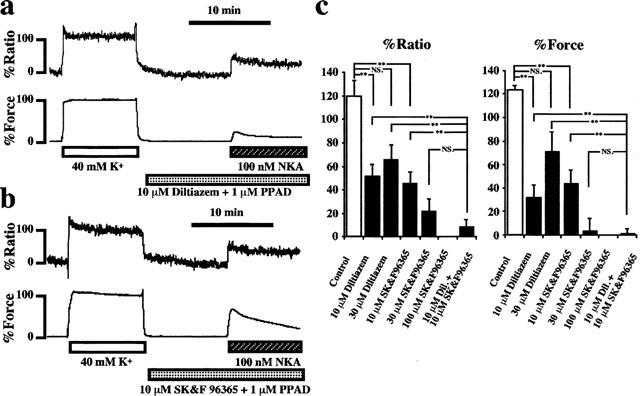
Effects of diltiazem and SK-F 96365 on the NKA-induced changes in [Ca2+]i and tension in the pregnant rat myometrium. (a and b) Representative recordings showing the effect of 10 μM diltiazem (a) and 10 μM SK-F 96365 (b) on the NKA-induced increases in [Ca2+]i (upper trace) and tension (lower trace) of the pregnant rat myometrium in normal PSS. Diltiazem or SK-F 96365 was applied 10 min before and during NKA application in conjunction with 1 mM PPAD. (c) Summary of the inhibitory effect of diltiazem and SK-F 96365 on the NKA-induced increases in [Ca2+]i and tension. The data were obtained from 5–7 independent experiments done in a manner similar to those shown in a and b. Various concentrations of diltiazem (10–30 μM) and/or SK-F 96365 (10–100 μM) were applied as illustrated under each column. *P<0.01, **P<0.001, NS.; not significant.
To examine the effect of NKA on the Ca2+-tension relationship, changes in [Ca2+]i and tension were monitored with a stepwise increment of the extracellular Ca2+ concentration ([Ca2+]o), during the depolarization with 40 mM K+ solution. Figure 5 shows the representative time courses of the changes in [Ca2+]i and tension induces by the cumulative application of CaCl2 during depolarization with 40 mM K+ solution in the absence (Figure 5a) or presence (Figure 5b) of 100 nM NKA plus 1 μM PPAD. When the bathing solution was changed from normal PSS to Ca2+-free PSS containing 0.1 mM EGTA, [Ca2+]i decreased to reach new steady levels even though the tension remained at the resting levels. The application of NKA induced only a transient elevation of [Ca2+]i and tension. In both cases (Figure 5a,b), the stepwise increase in [Ca2+]o (0.0125–5 mM) induced stepwise increases in [Ca2+]i and tension in a concentration-dependent manner. Figure 5c shows the [Ca2+]i-tension relationships plotted using the data points obtained from independent experiments done in a manner similar to those shown in Figure 5a,b. The presence of 100 nM NKA induced a leftward shift of the [Ca2+]i-tension curve (P<0.01 by an analysis of covariance, n=6), and moreover the curve was sifted leftward to the same extent in the presence of 1 μM NKA (data not shown).
Figure 5.
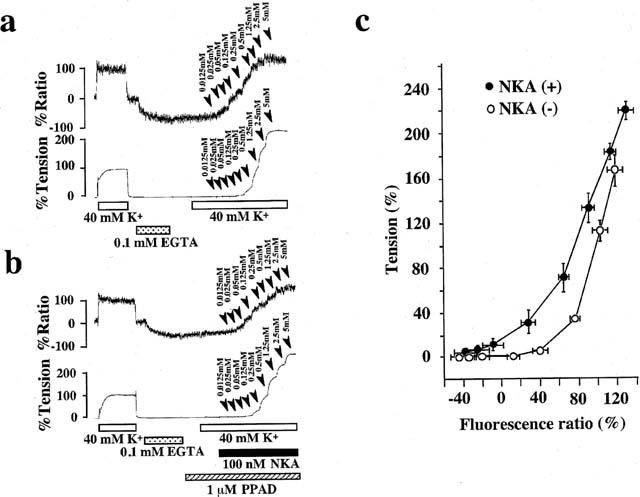
The effects of 100 nM NKA on the changes in [Ca2+]i and tension induced by the cumulative application of external Ca2+ to Ca2+-free 40K+ solution in the pregnant rat myometrium. (a and b) Representative recordings of changes in [Ca2+]i and tension induced by the cumulative application of external Ca2+ in Ca2+-free 40 mM K+ solution in the absence (a) or presence (b) of NKA in the pregnant rat myometrium. External Ca2+ (0.0125–5 mM) were applied cumulatively to Ca2+-free, 40 mM K+ solution in the absence (a : control) or presence (b) of 100 nM NKA. (b) NKA was applied 2 min before and during the cumulative application of extracellular Ca2+, and PPAD was applied 10 min before and during the application of NKA. The numbers with arrow heads on the traces represent the final extracellular Ca2+ concentrations. (c) [Ca2+]i-tension relationship in the presence or absence of 100 nM NKA. The data points were obtained from the measurements using the protocol shown in a and b. The data are expressed as the mean±s.e.mean. (n=6).
To elucidate the effect of NKA on the Ca2+ sensitivity of the contractile apparatus in the pregnant rat myometrium, we measured the tension development induced by NKA using the α-toxin permeabilized pregnant rat myometrium. As shown in Figure 6a, an application of 100 nM NKA, after a steady state contraction had been obtained by a mixture of 0.5 μM Ca2+, 1 μM GTP, and 1 μM PPAD, caused an additional force development at a constant [Ca2+]i. Subsequent application of 10 μM GTPγS, a non-hydrolyzable GTP analogue, induced further Ca2+ sensitization. Treatment with 1 mM GDPβS, a nonhydrolyzable GDP analogue for 10 min before and during the application of 100 nM NKA almost abolished both the NKA-induced and GTPγS-induced additional force development (Figure 6b).
Figure 6.
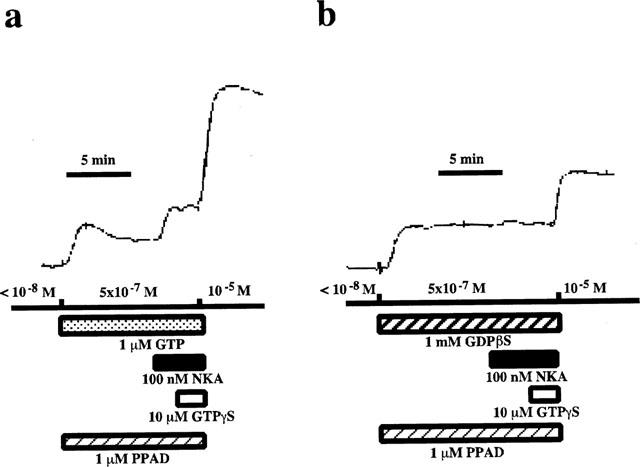
Effect of NKA on the Ca2+-induced contraction of the α-toxin permeabilized pregnant rat myometrium. After the permeabilization with 5000 units ml−1 α-toxin, the pregnant rat myometrial strip was contracted by 0.5 μM Ca2+ in the presence of either 1 μM GTP (a) or 1 mM GDPβS (b). When the tension reached the steady state, 100 nM NKA was applied. After the application of NKA, GTPγS (10 μM) was added. Maximal tension was induced by 10 μM Ca2+. The developed tension was expressed as a percentage, assigning the values in the relaxing solution ([Ca2+]i <10−8 M) and in the activating solution ([Ca2+]i=10−5 M) to be 0 and 100% respectively.
Discussion
In the present study, we attempted to address the following two questions: (1) Is there any change in the responsiveness of the pregnant rat myometrium to NKA during pregnancy? (2) What mechanisms are involved in the NKA-induced contraction of the rat myometrium? Concerning the first question, we found that the responsiveness to NKA was increased during pregnancy, however, the increased endopeptidase activity of the pregnant myometrium masked this increased responsiveness. As for the second question, our results indicated that NKA induces contraction of the myometrium not only by increasing [Ca2+]i but also by increasing the Ca2+ sensitivity of the contractile apparatus. The NKA-induced increase in the [Ca2+]i was mainly due to the Ca2+ influx from the extracellular space and partly due to the intracellular Ca2+ release. The Ca2+ influx was thought to be through both the voltage dependent calcium channels and non-specific cation channels. Moreover, NKA induced the G protein-dependent additional tension development in the α-toxin permeabilized pregnant rat myometrium.
The change in the responsiveness of the pregnant myometrium to NKA was assessed in the experiments shown in Figures 1, 2 and Table 1. As shown in Figures 1a,c and 2, the NKA-induced contractions of the pregnant and non-pregnant myometrium were substantially the same. However, the treatment by PPAD, which inhibits the endopeptidase and prevents the degradation of NKA, revealed that the NKA-induced contraction of the pregnant myometrium was much greater than that of the non-pregnant myometrium (Figure 1b,d). As shown in Figure 2 and Table 1, the EC50 value of the concentration-response curve for NKA in the pregnant myometrium was significantly lower than that in the non-pregnant myometrium in the presence of PPAD. In contrast, the maximal response of the pregnant myometrium was significantly greater than that of the non-pregnant myometrium in the presence of PPAD. Thus, the quantitative analysis shown in Figure 2 and Table 1 clearly supported the conclusion that the NKA-induced contraction of the pregnant myometrium was much greater than that of the non-pregnant myometrium, when the endopeptidase activity was inhibited by PPAD.
The increased responsiveness of the pregnant rat myometrium in the presence of PPAD could be explained by the increase in the NKA receptors in this tissue. Although the direct evidence for this in terms of the binding study or mRNA expression study is apparently still not available, it has been reported that NK-2 receptor, which is preferentially activated by NKA, may be the major tachykinin receptor subtype expressed in the oestrogen-primed rat uterus (Fisher & Pennefather, 1998; 1999; Fisher et al., 1993; Magraner et al., 1998; Pennefather et al., 1993). It is thus possible to speculate that the NK-2 receptors in the pregnant rat myometrium might also increase. The reason why the increased responsiveness of the pregnant myometrium was not visible in the absence of PPAD, may be explained by the increased endopeptidase activity of the pregnant myometrium. For example, the previous studies indicated that inhibition of endopeptidase markedly enhanced the effects of NKA on the oestrogen-primed rat uterus (Fisher & Pennefather, 1997; Fisher et al., 1993; Magraner et al., 1998; Pennefather et al., 1993). Furthermore, Ottlecz and colleagues (1991) reported that the endopeptidase activity increases and reaches a peak level at late pregnancy and thereafter slowly decreases until term-pregnancy. These authors indicated that the uterine endopeptidase may play an important role in regulating uterine smooth muscle contraction during the later stages of pregnancy through its action on oxytocin and perhaps other biologically active peptides (Ottlecz et al., 1991). Among the several peptide hormones involved in parturition, oxytocin has been proposed to be a key regulator of parturition (Soloff et al., 1979), because uterine sensitivity to oxytocin increases markedly at term. It was thus speculated that NKA might also contribute to the onset and/or maintenance of labour at term, because the responsiveness of the pregnant myometrium to NKA also increased.
In the present study, we found this contraction to be accompanied by an increase in [Ca2+]i (Figure 3a). These NKA-induced increases in [Ca2+]i were thought to be mainly due to the Ca2+ influx from the extracellular space and partly due to the intracellular Ca2+ release, because NKA induced a small transient increase in [Ca2+]i in the absence of extracellular Ca2+ or in the presence of Ni2+ in normal PSS (Figure 3b,c,d). These observations were consistent with the idea that NKA may activate the receptors which are coupled to a phospholipase C (PLC) mediated by a G protein, because oxytocin, one of the typical PLC-coupled receptor agonists, causes a similar response (Wray, 1993).
Since the major pathway for increasing [Ca2+]i during activation by NKA was thought to be due to the Ca2+ influx from the extracellular space, we further characterized this pathway using two different types of Ca2+ channel blockers, diltiazem or SK-F 96365. The Ca2+ influx pathway during activation by NKA was thought to be through both the L-type Ca2+ channels and the non-selective Ca2+ channels, because the combined use of 10 μM diltiazem plus 10 μM SK-F 96365 more effectively inhibited the NKA-induced increase in [Ca2+]i compared with the effects by 10 μM diltiazem alone, 30 μM diltiazem alone, 10 μM SK-F 96365 alone or 30 μM SK-F 96365 alone (Figure 4a–c). These observations could not be explained by the involvement of a single type of calcium channel in the NKA-induced Ca2+ influx. In addition, the NKA-induced oscillations in [Ca2+]i and tension were thought to involve, at least in part, the Ca2+ influx from the extracellular space, because the NKA-induced oscillations could not be observed in the presence of EGTA, Ni2+, diltiazem or SK-F 96365 (Figures 3 and 4). This conclusion was further supported by the observation that NKA-induced oscillations could be observed when the Ca2+ concentration of the normal PSS was raised to 2.5 mM even in the strips which did not cause oscillations in the normal PSS containing 1.25 mM Ca2+ (n=5, data not shown).
As mentioned above, the present results indicated that NKA may be a PLC-coupled receptor agonist. We next investigated the effect of NKA on the Ca2+ sensitivity of the contractile apparatus, because recent research on smooth muscle contraction have shown the increase in Ca2+ sensitivity to be one of the mechanisms for smooth muscle contraction (Nishimura et al., 1988; Somlyo & Somlyo, 1994). In the rat myometrium, the Ca2+-sensitizing mechanism has also been reported to be involved in the contraction induced by such contractile agents as oxytocin, carbachol, prostaglandin F2α, prostaglandin E2 (Izumi et al., 1995, 1996) and galanin (Niiro et al., 1998). As shown in Figure 5, NKA induced a significant leftward shift of the Ca2+-tension relationship obtained by the cumulative application of CaCl2 during depolarization with 40 mM K+ solution in the absence or presence of NKA plus PPAD. The NKA-induced Ca2+-sensitization was further confirmed using the permeabilized preparations. As shown in Figure 6, NKA induced an enhancement of tension development caused by Ca2+ in the α-toxin permeabilized myometrial strips in the presence of GTP and this NKA-induced Ca2+-sensitization could be blocked by GDPβS (Figure 6b). These results clearly indicated that NKA activates G-proteins and consequently induces an increase in the Ca2+ sensitivity of the contractile apparatus. This finding is consistent with previous reports which showed that NKA induced Ca2+ sensitization of the contractile element of canine colonic smooth muscle (Sato et al., 1994). It is thus concluded that NKA induces the contraction of the rat myometrium not only by increasing [Ca2+]i but also by increasing the Ca2+ sensitivity of the contractile apparatus.
In conclusion, our present study indicated that NKA induced the contraction of the pregnant rat myometrium by increasing both [Ca2+]i and the Ca2+ sensitivity of the myofilament. The NKA-induced increase in the [Ca2+]i was mainly due to the Ca2+ influx from the extracellular space and was also partly due to the intracellular Ca2+ release. The Ca2+ influx was thought to be through both voltage dependent Ca2+ channels and non-specific cation channels. The increase in the Ca2+-sensitivity is mediated by G-protein. Furthermore, the responsiveness of the rat myometrium to NKA was increased during pregnancy, although the increased endopeptidase activity of the pregnant myometrium masked this increased responsiveness. We therefore are tempted to speculate that NKA might contribute to the onset and/or maintenance of labour at term, since the activity of the endopeptidase has been reported to decrease at term (Ottlecz et al., 1991), however, further studies are called for before any definitive conclusions can be made.
Acknowledgments
We thank Mr Brian Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (Nos. 10557072, 11838013, 11670687), for Encouragement of Young Scientists (No. 10770308), and for Creative Basic Research Studies of Intracellular Signaling Network from the Ministry of Education, Science, Sports and Culture, Japan, and by the Yokoyama Rinshoyakuri, Mochida Memorial Foundation for Medical and Pharmaceutical Research and Foundation for the Promotion of Clinical Medicine.
Abbreviations
- [Ca2+]i
the intracellular Ca2+ concentration
- EGTA
ethyleneglycol-bis(β-aminoethylether)-N′,N′,N′,N′-tetra acetic acid
- GDPβS
guanosine-5′-O-(β-thiodiphosphate)
- GTP
guanosine-5′-triphosphate
- NKA
neurokinin A
- PLC
phospholipase C
- PPAD
phosphoramidon
- PSS
physiological salt solution
- SP
substance P
- VOC
voltage operated Ca2+ channel
References
- DE LEAN A., MUNSON P.J., RODBARD D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am. J. Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Potencies of agonists acting at tachykinin receptors in the oestrogen-primed rat uterus: effects of peptidase inhibitors. Eur. J. Pharmacol. 1997;335:221–226. doi: 10.1016/s0014-2999(97)01229-6. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Structure-activity studies of analogues of neurokinin A mediating contraction of rat uterus. Neuropeptides. 1998;32:405–410. doi: 10.1016/s0143-4179(98)90063-4. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N. Tachykinin receptors mediating contractions of oestrogen-primed rat uterus: classification using non-peptide agonists. Clin. Exp. Pharmacol. Physiol. 1999;26:729–735. doi: 10.1046/j.1440-1681.1999.03119.x. [DOI] [PubMed] [Google Scholar]
- FISHER L., PENNEFATHER J.N., HALL S. Tachykinin receptors in the rat isolated uterus. Regul. Pept. 1993;46:396–398. doi: 10.1016/0167-0115(93)90098-s. [DOI] [PubMed] [Google Scholar]
- GIBBINS I.L., FURNESS J.B., COSTA M., MACINTYRE I., HILLYARD C.J., GIRGIS S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pigs. Neurosci. Lett. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- HIGUCHI T., UCHIDE K., HONDA K., NEGORO H. Pelvic neurectomy abolishes the fetus-expulsion reflex and induces dystocia in the rat. Exp. Neurol. 1987;96:443–455. doi: 10.1016/0014-4886(87)90061-6. [DOI] [PubMed] [Google Scholar]
- IZUMI H., BIAN K., BUKOSKI R.D., GARFIELD R.E. Agonists increase the sensitivity of contractile elements for Ca2+ in pregnant rat myometrium. Am. J. Obstet. Gynecol. 1996;175:199–206. doi: 10.1016/s0002-9378(96)70275-2. [DOI] [PubMed] [Google Scholar]
- IZUMI H., BYAM-SMITH M., GARFIELD R.E. Gestational changes in oxytocin- and endothelin-1-induced contractility of pregnant rat myometrium. Eur. J. Pharmacol. 1995;278:187–194. doi: 10.1016/0014-2999(95)00089-4. [DOI] [PubMed] [Google Scholar]
- KANAIDE H. Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry Methods in Molecular Biology, Vol. 114: Calcium Signaling Protocols 1999Humana Press Inc.: Totowa, NJ; (eds) D.G. Lambert [DOI] [PubMed] [Google Scholar]
- MAGRANER J., MORCILLO E., AUSINA P., PINTO F.M., MARTIN J.D., MOREAU J., ANSELMI E., BARRACHINA M.D., CORTIJO J., ADVENIER C., CANDENAS M.L. Effects of Mn2+ on the responses induced by different spasmogens in the oestrogen-primed rat uterus. Eur. J. Pharmacol. 1997;326:211–222. doi: 10.1016/s0014-2999(97)85416-7. [DOI] [PubMed] [Google Scholar]
- MAGRANER J., PINTO F.M., ANSELMI E., HERNANDEZ M., PEREZ-AFONSO R., MARTIN J.D., ADVENIER C., CANDENAS M.L. Characterization of tachykinin receptors in the uterus of the oestrogen-primed rat. Br. J. Pharmacol. 1998;123:259–268. doi: 10.1038/sj.bjp.0701613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.A., MOORES K.E., RINK T.J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIIRO N., NISHIMURA J., HIRANO K., NAKANO H., KANAIDE H. Mechanisms of galanin-induced contraction in the rat myometrium. Br. J. Pharmacol. 1998;124:1623–1632. doi: 10.1038/sj.bjp.0702004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMURA J., KOLBER M., VAN BREEMEN C. Norepinephrine and GTP-gamma-S increase myofilament Ca2+ sensitivity in alpha-toxin permeabilized arterial smooth muscle. Biochem. Biophys. Res. Commun. 1988;157:677–683. doi: 10.1016/s0006-291x(88)80303-6. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., YOSHIOKA K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- OTTESEN B., FAHRENKRUG J. Regulatory peptides and uterine function Uterine Function. Molecular and Cellular Aspects 1990New York: Plenum Press; 393–422.(eds) Carsten, M.E. & Miller, J.D. pp [Google Scholar]
- OTTLECZ A., WALKER S., CONRAD M., STARCHER B. Neutral metalloendopeptidase associated with the smooth muscle cells of pregnant rat uterus. J. Cell. Biochem. 1991;45:401–411. doi: 10.1002/jcb.240450414. [DOI] [PubMed] [Google Scholar]
- PENNEFATHER J.N., ZENG X.P., GOULD D., HALL S., BURCHER E. Mammalian tachykinins stimulate rat uterus by activating NK-2 receptors. Peptides. 1993;14:169–174. doi: 10.1016/0196-9781(93)90025-c. [DOI] [PubMed] [Google Scholar]
- REGOLI D., BOUDON A., FAUCHERE J.L. Receptors and antagonists for substance P and related peptides. Pharmacol. Rev. 1994;46:551–599. [PubMed] [Google Scholar]
- SAIDA K., NONOMURA Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J. Gen. Physiol. 1978;72:1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO K., SANDERS K.M., GERTHOFFER W.T. Sensitization of the contractile system of canine colonic smooth muscle by agonists and phorbol ester. J. Physiol. 1994;481:677–688. doi: 10.1113/jphysiol.1994.sp020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEW R.L., PAPKA R.E., MCNEILL D.L. Galanin and calcitonin gene-related peptide immunoreactivity in nerves of the rat uterus: localization, colocalization, and effects on uterine contractility. Peptides. 1992;13:273–279. doi: 10.1016/0196-9781(92)90108-f. [DOI] [PubMed] [Google Scholar]
- SOLOFF M.S., ALEXANDROVA M., FERNSTROM M.J. Oxytocin receptors: triggers for parturition and lactation. Science. 1979;204:1313–1315. doi: 10.1126/science.221972. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- TRAURIG H.H., PAPKA R.E., RUSH M.E. Effects of capsaicin on reproductive function in the female rat: role of peptide-containing primary afferent nerves innervating the uterine cervix in the neuroendocrine copulatory response. Cell Tissue Res. 1988;253:573–581. doi: 10.1007/BF00219748. [DOI] [PubMed] [Google Scholar]
- TRAURIG H.H., PAPKA R.E., SHEW R.L. Substance P and related peptides associated with the afferent and autonomic innervation of the uterus. Ann. N.Y. Acad. Sci. 1991;632:304–313. doi: 10.1111/j.1749-6632.1991.tb33118.x. [DOI] [PubMed] [Google Scholar]
- TRAURIG H.H., SARIA A., LEMBECK F. Substance P in primary afferent neurons of the female rat reproductive system. Naunyn-Schmiedeberg's Arch. Pharmacol. 1984;327:254–259. doi: 10.1007/BF00501440. [DOI] [PubMed] [Google Scholar]
- WRAY S. Uterine contraction and physiological mechanisms of modulation. Am. J. Physiol. 1993;264:C1–C18. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]


