Abstract
K+ has been proposed to be EDHF in small arteries. We compared ACh-stimulated, EDHF-mediated dilatation/relaxation with raised [K+]o in rat mesenteric arteries.
In pressurized arteries, ACh (10 μM) dilated all arteries. Raising [K+]o from 5.88 to 10.58 mM only dilated 30% of arteries. Ba2+ (30 μM) did not affect dilatation to ACh, but abolished 40% of dilatations to raised [K+]o.
If [K+]o was lowered to 1.18 mM, restoring [K+]o to 5.88 mM produced dilatation which was depressed by Ba2+ or ouabain (1 mM). Combined application of Ba2+ and ouabain abolished dilatation. In 1.18 mM K+, dilatation to ACh was depressed by ouabain, but not by Ba2+. Combined application of Ba2+ and ouabain depressed dilatation further. Gap junction inhibitors (Gap-27; 300 μM and 18-α-glycyrrhetinic acid; 100 μM) also depressed dilatation to ACh.
In arteries mounted isometrically, ACh (1 μM) relaxed endothelium intact (+E), but not endothelium denuded (−E) arteries. Raising [K+]o from 5.9–10.9 mM failed to relax all arteries. When [K+]o was lowered to 1 mM, raising [K+]o to 6 mM produced relaxation. In −E arteries, relaxation was unaffected by Ba2+ but abolished by ouabain. In +E arteries, Ba2+ depressed and ouabain abolished relaxation. In +E arteries, with 1 mM K+, ACh relaxation was depressed by ouabain but not Ba2+. The combined application of Ba2+ and ouabain further depressed relaxation.
In summary, both EDHF and raised [K+]o dilate/relax rat mesenteric arteries, though sensitivities to barium and ouabain differ. K+ may be a relaxing factor in this tissue, but its characteristics differ from EDHF. Gap junction inhibitors depress EDHF, implying an important role for myo-endothelial gap junctions.
Keywords: Potassium, EDHF, ouabain, barium, gap junction, mesenteric artery, rat
Introduction
In a variety of arteries, a component of endothelial-dependent relaxation to agents such as acetylcholine (ACh), bradykinin and A23187 persists after inhibition of nitric oxide synthase (NOS) and cyclo-oxygenase (COX). This NO- and prostacyclin-independent relaxation is accompanied by an endothelium-dependent hyperpolarization of the vascular smooth muscle and has been suggested to be mediated by an endothelium derived hyperpolarizing factor (EDHF) (Chen et al., 1988; Taylor & Weston, 1988; Feletou & Vanhoutte, 1988; Corriu et al., 1996; Ohlmann et al., 1997; Zygmunt et al., 1997). The identity of EDHF, and its mechanism of action has remained elusive. It has recently been reported that EDHF is potassium (Edwards et al., 1998), and that elevating extracellular potassium can mimic the effects of EDHF in rat hepatic and mesenteric arteries. In contrast it has also been reported that potassium does not mimic the effects of EDHF in rat mesenteric (Lacy et al., 2000), porcine coronary and guinea-pig carotid arteries (Quignard et al., 1999).
EDHF-mediated relaxation is depressed by either apamin or charybdotoxin (ChTx) alone in some arteries, but more usually complete block of EDHF-mediated relaxation is achieved only with a combination of these toxins (Corriu et al., 1996). Previously it has been assumed that the K+ current mediated by toxin sensitive channels located on the smooth muscle explained the hyperpolarization. However, it is now proposed that ChTx and apamin act on the endothelium, not the smooth muscle, in order to block EDHF-mediated relaxations (Edwards et al., 1998; Doughty et al., 1999). This has significant implications for the mechanism by which EDHF induces relaxation, as, for smooth muscle to hyperpolarize, there must be either effective electrical coupling of endothelial and smooth muscle cells, or, an additional toxin-sensitive, endothelium-dependent mechanism that induces hyperpolarization and relaxation in the smooth muscle. It has been postulated that K+ efflux from the endothelium through apamin- and ChTx-sensitive channels is sufficient to raise K+ in the myoendothelial space (Edwards et al., 1998), leading to hyperpolarization and relaxation of the smooth muscle by stimulation of inward rectifier K+ channels (Kir) (Edwards et al., 1988; Knot et al., 1996; McCarron & Halpern, 1990) and/or the electrogenic Na+/K+-ATPase (Prior et al., 1998; Edwards et al., 1999). Edwards et al. (1998) demonstrated that barium, which blocks Kir, and ouabain, which inhibits the Na+/K+-ATPase, abolished EDHF mediated hyperpolarization in rat hepatic and mesenteric arteries. Lacy et al. (2000) have also demonstrated that both barium and ouabain significantly depresses relaxations mediated by raised potassium, but only in the presence of an intact endothelium.
We have compared the ability of raised extracellular K+ ([K+]o) and ACh to mediate dilatation and relaxation of rat mesenteric arteries. Additionally, we have compared the effects of ouabain, barium and gap junction inhibitors on these responses. Some of this work has been published previously in abstract form (Doughty & Langton, 1999a,1999b).
Methods
200–300 g male Wistar rats were killed by stunning and cervical dislocation. Third order superior mesenteric arteries were dissected in physiological saline solution (PSS) containing (in mM): NaCl 119; KCl 4.7; NaHCO3 25; KH2PO4 1.18; CaCl2 1.8; MgSO4 1.2; glucose 11; EDTA 0.027; Nω-nitro-L-arginine methyl ester (L-NAME) 0.1; indomethacin 0.0028. The pH was 7.4 when gassed with 95% O2/5% CO2.
Pressure myography
Leak-free segments of artery of at least 1 mm in length were mounted between two glass cannulae in an arteriograph (Living Systems Instrumentation, Burlington, VT, U.S.A.) at room temperature (18–21°C) and pressurized to 80 mmHg, under conditions of no lumenal flow. The artery lumen was filled with the standard PSS. Constant pressure was maintained via a pressure servo control system (PS200, Living Systems Instrumentation). Pressure transducers at both ends of the artery allowed continual monitoring of intralumenal pressure. Arteries were viewed through a Nikon TMS inverted microscope and a measurement of the internal diameter was made from a video image using a video dimension analyser (V91, Living Systems Instrumentation). The arteriograph was continually superfused with the standard PSS at a rate of 25 ml min−1. The superfusing PSS was warmed to 37°C and no myogenic constriction of arteries was seen. Therefore, arteries were constricted with 0.3–1 μM PE applied in the superfusate. Pressure and diameter measurements were recorded to computer via a Digidata 1200B interface using Axoscope software version 7 (Axon Instruments, CA, U.S.A.). Intralumenal Perfusion: Intralumenal solution changes were made as previously described (Doughty et al., 1999). Intralumenal Solutions: During all experiments, the PSS contained 100 μM L-NAME and 2.8 μM indomethacin.
Wire myography
Segments of mesenteric artery were mounted in a Mulvany-Halpern wire myograph in HEPES-buffered PSS (HPSS) with the composition (in mM) NaCl, 118; NaHCO3, 25; KCl, 4.7; KH2PO4, 1.2; CaCl2, 1.8; MgSO4, 1.2; HEPES 5, glucose 11, pH 7.4, supplemented with indomethacin (10−5 M) and L-NAME (4×10−4 M) at 37°C for the recording of isometric tension. Artery segments were sequentially stretched until the wall tension was equivalent to a transmural pressure of 100 mmHg (Mulvany & Halpern, 1977), the diameter was calculated and set to 90% of this value and the tissue allowed to equilibrate for 45 min. Arterial segments were then constricted with phenylephrine (10−5 M) and relaxation responses to acetylcholine (10−6 M) and K+ (5 mM, added concentration) tested. The K+ concentration of the HPSS was then reduced to 1 mM by isotonic replacement of KCl with NaCl and a reduction in the concentration of KH2PO4. ACh and potassium were re-tested, firstly in the new low K+ HPSS and then in the low K+ HPSS in the presence of Ba2+ (30 μM), ouabain (1 mM) or a combination of these inhibitors. Where responses were required from endothelium-denuded vessels the endothelium was removed from the same vessels mounted in the myograph by rubbing a hair through the lumen. Relaxant responses to ACh and potassium were then tested again. Relaxation responses are expressed as percentage of the maximum relaxation available in each vessel.
Drugs
All drugs were made up as stock solutions in milli-Q water, unless otherwise stated, diluted in the experimental solution and applied in the superfusate. 18-α-glycyrrhetinic acid was dissolved in DMSO, such that the concentration of DMSO did not exceed 0.1% in the final solution. Gap-27 which was applied by intralumenal perfusion in pressurized arteries. Indomethacin was dissolved in 2% Na2CO3 or ethanol. All drugs were supplied by Sigma. The Protein and Nucleic Acid Chemistry Laboratory, at Leicester University, synthesized Gap-27. All data are expressed as mean values±s.e.mean for ‘n' experiments. Statistical significance was tested using a Student's t-test on paired data, unless stated otherwise. P<0.05 was regarded as significant.
Results
Ten μM ACh dilated all pressurized arteries tested to their passive diameter (passive: 283±6.9 μm; level of tone: 170±8.2 μm; ACh: 283±6.7 μm) (n=30), whereas raising [K+]o in the superfusing PSS from 5.88 mM (normal) to 10.58 mM (high), by doubling the concentrations of KCl in the standard PSS, fully dilated only nine out of 30 arteries to their passive diameter (an example is shown in Figure 2A). In the remaining 21 arteries raising [K+]o produced a weak dilatation (n=5), or a small constriction (n=16) (see Figure 1C). An example of failure of high [K+]o to produce dilatation is shown in Figure 1A. Even if raising [K+]o failed to dilate, 10 μM ACh in the presence of high [K+]o was still able to dilate to the passive diameter (n=7) (Figure 1A and B). In rat coronary artery Kir is present in small but not large rat coronary arteries (Quayle et al., 1996). However, the ability of raised [K+]o to produce dilatation in rat mesenteric arteries was not correlated with the artery diameter for the range tested (200–400 μm) (Figure 1C). Arteries that did not dilate on raising the extracellular concentration of K+ from 5.88–10.58 mM were subjected to further increases in [K+]o. Raising superfusing [K+]o to 15.28 mM produced no further effect when compared with 10.58 mM, whereas raising [K+]o to 19.98 mM caused constriction (n=3) (Figure 1D). Increasing [K+]o in the lumen was not more effective at eliciting dilatation. If high [K+]o in the superfusate failed to produce dilatation, then it also failed to produce dilatation when co-applied lumenally (n=3). These data are summarized in Figure 1E.
Figure 2.
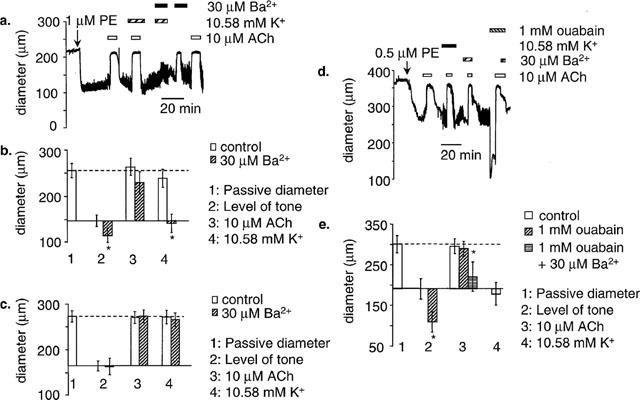
The effect of Ba2+ and ouabain on ACh- and K+-induced dilatation. (a) In 10 pressurized arteries where high (10.58 mM) produced dilatation, 30 μM Ba2+ did not affect ACh-induced dilatations, but four out of 10 K+-induced dilatations were blocked. This shows a representative example where 30 μM Ba2+ blocked K+-induced dilatation. (b) Mean data for K+-induced dilatations blocked by 30 μM Ba2+ (n=4). (c) Mean data for K+-induced dilatations unaffected by 30 μM Ba2+ (n=6). (d) In pressurized arteries where high (10.58 mM) K+ failed to produce dilatation, 1 mM ouabain increased the level of tone, but had no significant effect on ACh-induced dilatation. Combined application of 30 μM Ba2+ and 1 mM ouabain significantly depressed ACh-induced dilatation. (e) Mean data from five similar experiments. *Shows significance (P<0.05) compared to control.
Figure 1.
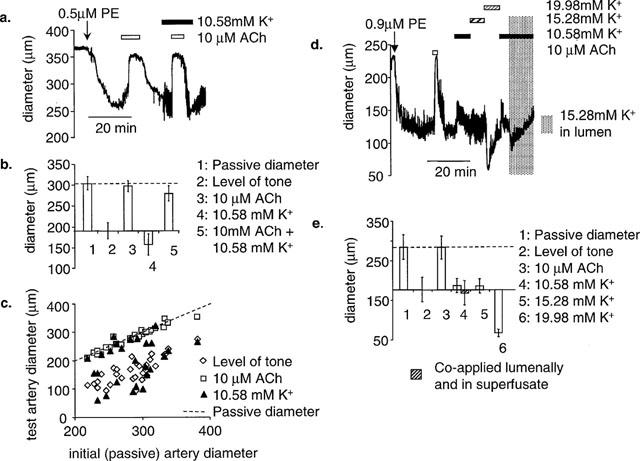
The ability of 10 μM ACh and 10.58 mM K+ to dilate rat mesenteric arteries. (a) A representative example of a pressurized artery that failed to dilate to on stepping from normal (5.88 mM) to high (10.58 mM) K+. 10 μM ACh was able to dilate this vessel close to its passive diameter both under control conditions (5.8 mM K+) and in high K+. (b) Mean data from seven similar experiments. (c) Scatter plot showing the relationship between the initial (passive) artery diameter (x-axis) and the level of tone of the arteries after PE constriction, in the presence of 10 μM ACh or high [K]o. In all arteries tested (n=30) ACh dilated to the passive diameter, whereas high [K+] only dilated to the passive diameter in nine arteries. The failure of high [K+] do dilate was not related to the diameter of the artery. (d) In three pressurized arteries where high (10.58 mM) K+ failed to produce dilatation, K+ was raised further in 4.7 mM increments by adding KCl from a 1 M stock. K+ failed to produce dilatation and at higher concentrations (19.98 mM) started to constrict. 10.58 mM K+ also failed to dilate when applied in the lumen. (e) Mean data (n=3).
Ba2+ is an effective blocker of Kir channels in resistance arteries (IC50: 8 μM at −40 mV) (Quayle et al., 1993). Thirty μM Ba2+ blocked four of the 10 dilatations to high [K+]o. An example is shown in Figure 2A, and the data from these four arteries is summarized in Figure 2B. In these arteries 30 μM Ba2+ also significantly increased the level of tone. In the remaining six arteries, where 30 μM Ba2+ was ineffective against dilatations to high [K+]o, there was also no effect on the level of tone, and this is summarized separately in Figure 2C. ACh dilatations were not significantly depressed by 30 μM Ba2+, even in arteries where the level of tone was increased and dilatation to high [K+]o was abolished (n=10) (Figure 2B and C).
We tested the effect of ouabain, which blocks the Na+/K+-ATPase, on ACh-induced dilatations. In 20 experiments we failed to observe dilatation to raised [K+]o, and therefore were unable to test the effect of ouabain on a K+-induced dilatation. One mM ouabain caused pronounced constriction but had no significant effect on ACh dilatations when applied alone (n=5) (Figure 2E). However, applied in combination, 30 μM Ba2+ and 1 mM ouabain significantly depressed the ACh dilatation (n=5). These data are summarized in Figure 2D, E.
In arteries where high [K+]o failed to produce dilatation, [K+] in the PSS was lowered to 1.18 mM by omitting KCl. Subsequent restoration of [K+] to 5.88 mM (restoring KCl) resulted in a dilatation of all the arteries tested (n=8). This dilatation on returning to 5.88 mM K+ was sustained as long as the K+ was elevated (>30 min) (Figure 3C). Thirty μM ACh dilated towards passive diameter in all arteries tested, both in PSS containing normal [K+]o and PSS containing low [K+]o (n=8). These data are summarized in Figure 3.
Figure 3.
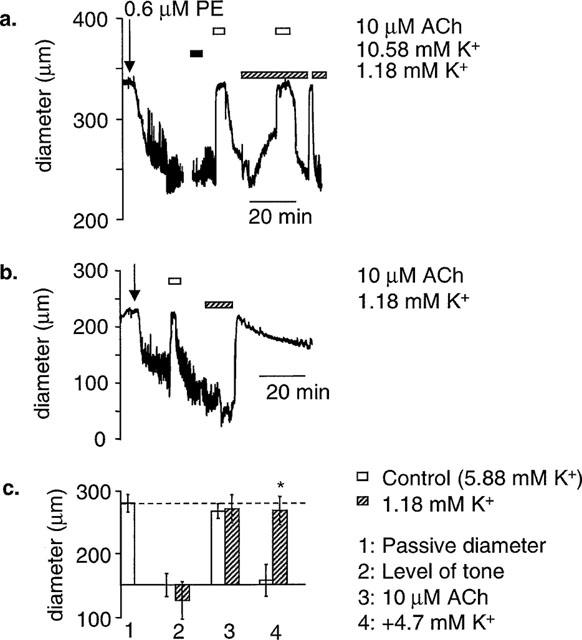
Stepping [K+] from 1.18–5.88 mM. In pressurized arteries where stepping from 5.88 mM (normal) to 10.58 mM K+ failed to produce dilatation, dilatation could be induced by lowering K+ to 1.18 mM K+ and stepping back to 5.88 mM (normal) K+. (a) A representative trace. (b) An alternative example showing that dilatation to raised [K]o is sustained for >30 min. (c) Mean data from seven similar experiments. *Shows significance (P<0.05) compared to control.
The effects of ouabain and barium on ACh- and K+-induced dilatations were re-tested in 1.18 mM K+. Thirty μM Ba2+ did not significantly depress either the dilatation to 10 μM ACh (n=4), or the dilatation elicited by stepping [K+] back to 5.88 mM (n=4). In contrast, 1 mM ouabain significantly depressed the dilatation to both 10 μM ACh (n=5) and 5.88 mM [K+]o (n=5). Combined application of 30 μM Ba2+ and 1 mM ouabain depressed the dilatation to 10 μM ACh to a greater extent than ouabain alone (n=4), but abolished dilatation to 5.88 mM [K+]o (n=4) (Figure 4). It should be noted that at normal [K+]o, the ACh-induced dilatation was insensitive to ouabain and Ba2+ when applied separately, but when [K+]o was lowered to 1.18 mM the ACh-induced dilatation became sensitive to ouabain alone in some arteries.
Figure 4.
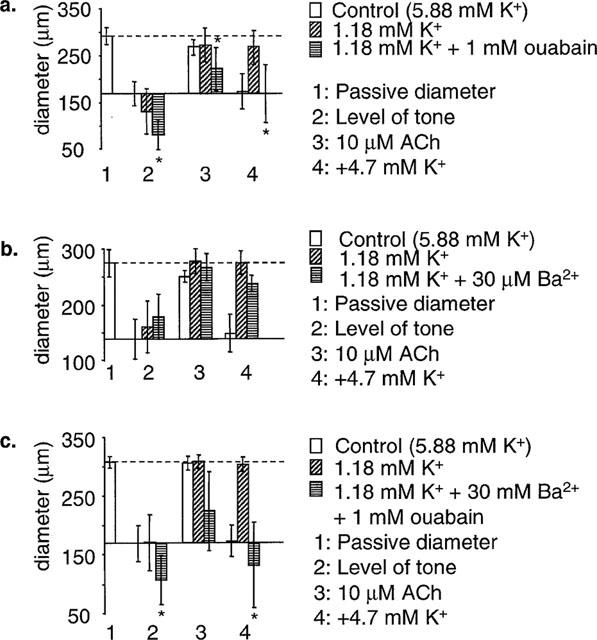
The effects of ouabain and barium in 1.18 mM K+ in pressurized arteries. Dilatation to K+ was induced in endothelium intact arteries by lowering [K+] to 1.18 mM K+, and stepping back to 5.88 mM. (a) Dilatation to 10 μM ACh and K+ was significantly depressed by 1 mM ouabain (n=5). (b) Dilatation to 10 μM ACh was unaffected by 30 μM Ba2+, and K+ dilatation was depressed, though not significantly (n=4). (c) Dilatation to 10 μM ACh was depressed by 1 mM ouabain and 30 μM Ba2+ in some arteries, but the mean reduction was not significant. K+ dilatation was abolished by 1 mM ouabain and 30 μM Ba2+ (n=4). *Shows significance (P<0.05) compared to control.
The effects of ouabain and barium were compared in both endothelium-intact and endothelium-denuded arteries. Arteries mounted in a Mulvany–Halpern myograph for isometric measurement of force were used for these experiments, to allow easier removal of the endothelium. In endothelium intact arteries, 1 μM ACh relaxed PE-induced tone, whereas in arteries with the endothelium removed failed to relax to ACh (n=8) (Figure 5A). No relaxation to K+ on raising the concentration from 5.9–10.9 mM was seen in this series of experiments, either in endothelium-intact or endothelium-denuded arteries (n=8). In endothelium-denuded arteries, relaxation to K+ was induced by first lowering [K+]o to 1 mM K+, and then raising the concentration back to 6 mM. This relaxation was unaffected by 30 μM Ba2+ but abolished by 1 mM ouabain (n=8). In a similar experiment in endothelium-intact arteries, 1 μM ACh still relaxed in 1 mM [K+]o. Dilatation to ACh was unaffected by 30 μM Ba2+ but significantly depressed by 1 mM ouabain and abolished by the combination of ouabain and Ba2+. K+-induced relaxation was significantly depressed by 30 μM Ba2+ (in contrast to when the endothelium was denuded), and abolished by 1 mM ouabain and a combination of 1 mM ouabain and 30 μM Ba2+ (n=8) (Figure 5B). These observations were consistent with the effects of barium and ouabain seen in low [K+]o in pressurized arteries.
Figure 5.
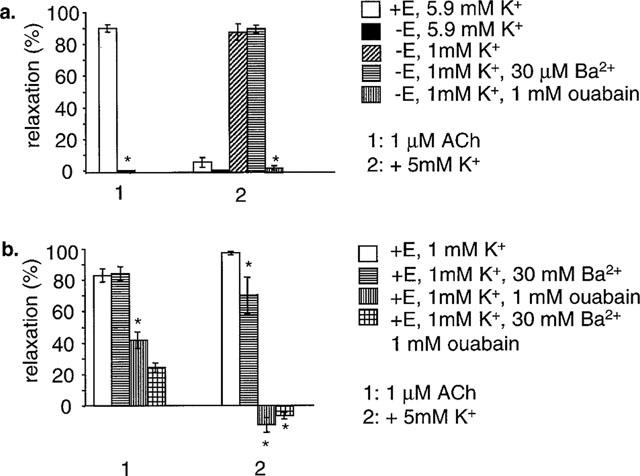
The effects of ouabain and barium in 1.18 mM K+ in isometric arteries. In isometric arteries (wire myography), relaxation to ACh was dependent on the presence of an intact endothelium. K+ failed to induce relaxation (5.9–10.9 mM step) both in the presence and absence of endothelium. (a) Relaxation to K+ was induced in endothelial denuded arteries by lowering [K+] to 1 mM K+, and stepping back to 6 mM. This relaxation was unaffected by 30 μM Ba2+ and abolished by 1 mM ouabain. Mean data for eight arteries are shown. Numbers next to columns are P values compared to the relevant control dilatation in the absence of barium and ouabain. (b) A similar experiment in endothelium-intact arteries. ACh still relaxed in 1 mM K+. This was unaffected by 30 μM Ba2+, and significantly depressed by both 1 mM ouabain, and a combination of 1 mM ouabain and 30 μM Ba2+. K+-induced relaxation was significantly depressed by 30 μM Ba2+, and abolished by 1 mM ouabain and a combination of 1 mM ouabain and 30 μM Ba2+. Mean data for eight arteries are shown. *Shows significance (P<0.05) compared to control.
There is good evidence in mesenteric artery that smooth muscle and endothelium are electrically coupled by gap junctions (Dora et al., 1999). If EDHF-induced relaxation is dependent upon hyperpolarization of the endothelium by toxin-sensitive channels, then smooth muscle hyperpolarization/relaxation may occur by electrotonic coupling, or by transfer of a factor through the gap junction. Application of the gap junction inhibitor Gap 27 (300 μM) into the lumen of pressurized arteries depressed dilatations to 10 μM ACh (n=4), such that only a small, transient dilatation remained (Figure 6A). Similarly, dilatations to 10 μM ACh could also be depressed by another gap junction inhibitor, 18-α-glycyrrhetinic acid, applied in the superfusate (100 μM) (n=3) (Figure 6B). A small dilatation to raised [K+]o was observed under control conditions, which was unaffected by 18-α-glycyrrhetinic acid. It is interesting to note that the remaining dilatation to ACh was of similar amplitude and time course to the dilatation to raised K+ (see Discussion).
Figure 6.
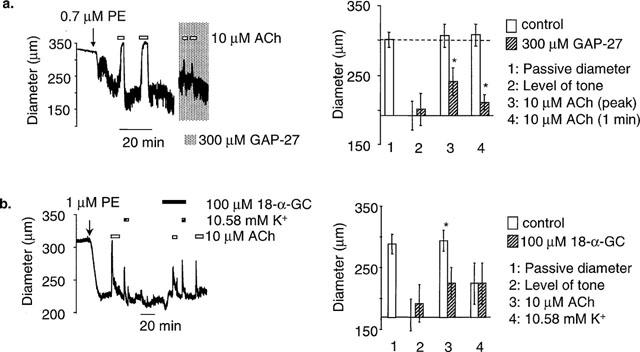
The effects of GAP-27 and 18-α-glycyrrhetinic acid in pressurized arteries. (a) Application of the gap junction inhibitor Gap-27 (300 μM) into lumen of the pressurized artery depressed ACh dilatations, such that only a small, transient dilatation remained. The gap in the data trace shows where Gap-27 was being loaded into the lumen. The bar graph shows mean data (n=4). *Shows significance (P<0.05) compared to control. (C) The gap junction inhibitor, 18-α-glycyrrhetinic acid (100 μM) in the superfusate depressed ACh dilatations, but not dilatations to high [K+]o. Mean data (n=3). *Shows significance compared to control.
Discussion
Pressure versus isometric myography
In contrast to previous studies on EDHF (Edwards et al., 1998; Quignard et al., 1999; Lacy et al., 2000), the effects of ACh and K+ were tested in both pressurized (isobaric) arteries, and arteries mounted for measurement of isometric force. Because the resting membrane potential of pressurized arteries is depolarized, compared to that of isometric arteries, it is more representative of the resting membrane potential of small arteries in vivo (Harder et al., 1984; 1987). This difference in membrane potential may influence the response to raised extracellular K+ or EDHF, and therefore was an important factor for consideration of the physiological relevance of EDHF. This study revealed no obvious difference between the response of pressurized and isometric arteries to EDHF or K+, and therefore both techniques were used interchangeably. Isometric arteries were preferred for experiments requiring denudation of the endothelium, because it is technically easier to strip the endothelium of these arteries without compromising the viability of the artery. In contrast, pressurized arteries were preferred for application of GAP-27, as intralumenal perfusion allowed very small quantities of the drug to be applied.
Involvement of Kir and Na+/K+ ATPase in EDHF
In our experiments, inhibition of Kir and Na+/K+-ATPase, with Ba2+ and ouabain respectively, was ineffective in blocking EDHF-induced relaxation/dilatation. In contrast, K+-induced dilatations could be blocked by low concentrations of Ba2+ alone in some arteries, suggesting that hyperpolarization by members of the Kir family can be sufficient to account for K+-induced dilatation (Nelson & Quayle, 1995). A similar mechanism has been suggested to underlie the relaxation of mesenteric arteries in response to hypercapnia (Okazaki et al., 1998). In other arteries, K+-induced dilatation was insensitive to Ba2+ alone, but was abolished by the combination of Ba2+ and ouabain. The variability in the effects of Ba2+ on dilatations to elevated [K+]o may reflect different densities of Kir throughout the mesenteric bed. It has previously been shown in the coronary circulation, that the density of Kir channels is inversely related to arterial size. The consequence of this is that mechanisms dependent upon the activation of Kir become increasingly important in smaller arteries (Quayle et al., 1993, 1996). This would be consistent with the hypothesis that EDHF becomes progressively more important as arterial diameter decreases (Shimokawa et al., 1996). However, our data show no correlation between artery diameter and the ability of K+ to produce Ba2+-sensitive dilatation. Interestingly, in arteries where Ba2+ depressed dilatations to raised [K+]o, Ba2+ also affected the level of tone, suggesting a role for Kir in setting resting membrane potential in this subset of mesenteric arteries. The reason for the failure of some mesenteric arteries to constrict to Ba2+ or dilate to raised [K+]o is unclear, and will require further experiments. However, the failure of arteries to dilate to raised [K+]o has also been reported by Lacy et al. (2000), who saw only transient relaxation in 30–40% of rat mesenteric arteries mounted for measurement of isometric force. This is unlikely to reflect differences in expression of Kir between animals because, in our experiments, adjacent sections of pressurized artery from the same animal could be seen to respond differently to raised [K]o, such that one section dilated and one section did not.
Differences in the Ba2+ and ouabain-sensitivity of EDHF-induced relaxation may reflect differences in the mechanism by which EDHF relaxes different arteries. There may also be differences between species or even different stains of the same species. We have used mesenteric artery from Wistar strain rats, whereas Edwards et al. (1998) studied mesenteric and hepatic arteries from Sprague-Dawley rats. Lacy et al. (2000) recently reported, using Sprague-Dawley rat mesenteric artery, that responses to EDHF and raised extracellular K+ differed, conflicting with the observations of Edwards et al. (1998), but agreeing very substantially with our study (JP Boyle, personal communications: The Lacy et al. 2000 study used both Wistar and Sprague-Dawley rats and no difference was observed between these strains). For this reason we do not feel that this species difference is sufficient to account for the differences between our study and the report of Edwards et al. (1998).
In our hands the effects of Ba2+ and ouabain are complex. Whereas neither ouabain, nor Ba2+, or a combination of ouabain and Ba2+, are effective in abolishing EDHF-induced dilatation with normal levels of superfusing [K+]o, in low [K+]o, the dilatation to EDHF become more ouabain and Ba2+ sensitive. It is clear that elevation of external [K+] cannot fully explain EDHF and additional mechanisms must be involved. Ouabain has a pronounced constrictor effect on pressurized mesenteric arteries, which suggests that Na+/K+-ATPase is also involved in setting the resting membrane potential. This is consistent with reports showing that ouabain produces a large depolarization in porcine coronary artery (Quignard et al., 1999). Restoring [K+]o to normal (5.88 mM) after a period of low [K+]o (1.18 mM), resulted in a ouabain-sensitive relaxation consistent with elevated Na+/K+ ATPase activity (Prior et al., 1998; Lacey et al., 2000). However the relaxation might be expected to be transient, as the activity of the pump would cease as Na+ and K+ reach their new equilibrium levels. This was seen by Quignard et al. (1999). In our experiments relaxation elicited by restoring normal [K]+o (5.8 mM) was not transient, but was sustained, for up to 2 h in some experiments. In endothelium-denuded arteries mounted for isometric measurement of force, restoring [K+]o from a low K+ concentration produces a relaxation which is unaffected by Ba2+ but abolished by ouabain. Lacy et al. (2000) observed a similar endothelium-dependence of the effects of barium, such that in the absence of endothelium, relaxation to raised K+ was abolished by ouabain alone. This suggests that restoring [K+]o to 5.8 mM after a period low [K+]o produces relaxation entirely via effects on the Na+/K+ ATPase. However in a similar experiment, either in pressurized arteries or in arteries mounted for measurement of isometric force, with the endothelium intact, Ba2+ is partially effective in blocking relaxation. Our interpretation of this result is that the Kir channels in rat mesenteric artery are to be found on the endothelial cells rather than the smooth muscle cells. A similar conclusion was reached in a previous study of hypercapnia in these arteries (Okazaki et al., 1998).
It is important to note that evidence for Kir channels in mesenteric artery myocytes is lacking, despite several attempts to demonstrate their presence (Nelson & Quayle, 1995). Using RT–PCR, (Bradley et al., 1999) demonstrated the presence of messenger RNA encoding an inwardly rectifying K+ channel of the Kir channel family (Kir2.1) in rat mesenteric cells. However, no recordings of the native conductance in isolated mesenteric smooth muscle cells were shown or described. Indeed, Kir currents have only been identified in one voltage-clamp study of intact mesenteric arterioles of the guinea-pig (Edwards & Hirst, 1988), and it is possible that the recorded currents were generated by the endothelium. Evidence for Kir is also limited in other arterial smooth muscle where EDHF is present (Quignard et al., 1999; Quayle et al., 1997). Our data suggest that a major source of Ba2+-sensitive hyperpolarizing current/factor is the endothelium, and this is transmitted to smooth muscle by gap junction coupling. Increases in [K+]o generated by K+ flux through toxin-sensitive K+ channels could play a supporting role in this mechanism, by causing endothelial hyperpolarization via activation of the inward rectifier and by actions on the smooth muscle Na+/K+-ATPase. Although Kir has not been measured in mesenteric endothelial cells to our knowledge, evidence from endothelial cells obtained from other blood vessels shows that Kir is a major determinant of endothelial membrane potential (Nilius et al., 1997; Voets et al., 1996).
The lack of effectiveness of Ba2+ and ouabain in blocking EDHF is not consistent with the hypothesis that K+ is the primary signal for smooth muscle relaxation. If K+ was EDHF it would need to hyperpolarize the smooth muscle cells through a Ba2+- and ouabain-insensitive mechanism, and there is no evidence for this, as dilatation to elevated [K+]o, unlike EDHF, was effectively abolished by the combination of Ba2+ and ouabain.
Gap junctions and EDHF
The effectiveness of the gap junction inhibitors, Gap 27 and 18α-glycyrrhetinic, in blocking EDHF may suggest that gap junction coupling is important in EDHF-induced relaxation. Gap 27 is a small peptide targeted to a conserved sequence in part of the second extracellular loop of connexin 43. Six connexin subunits form the connexon – half of a functional gap junction, which is formed when connexons from two cells dock. Gap 27 is thought to prevent docking between the two connexons. This peptide has been shown to be an effective inhibitor of gap junction mediated dye transfer (Dora et al., 1999) and also attenuates endothelial-dependent relaxations in rabbit conduit arteries (Chaytor et al., 1998). The structurally unrelated gap junction inhibitor, 18α-glycyrrhetinic acid, has also been shown to block EDHF-induced relaxation in rabbit iliac arteries (Taylor et al., 1998). The mechanism of block is not well understood. Gap junction inhibitors do not appear to depress dilatation to high [K+]o. Indeed, in the presence of gap junction inhibitors, the amplitude and time course of the residual dilatation to EDHF closely resembles the dilatation to high [K+]o. This is consistent with the idea that K+ may contribute to, but is not the major mechanism of, EDHF.
Myo-endothelial gap junctions are unlikely to be the only target for Gap 27 and 18α-glycyrrhetinic acid. Gap junctions between myocytes are likely also to be disrupted. Gap 27 and 18α-glycyrrhetinic acid do not significantly effect PE-stimulated tone, implying that the ability of the muscle to contract is not impaired. In addition gap junction inhibitors also have little or no effect on relaxation to vasodilators such as sodium nitroprusside and levcromakalim. These data tend to suggest that even if gap junctions between myocytes are being broken down that this does not, in itself, prevent contraction or relaxation of arterial smooth muscle. We cannot exclude the possibility that inhibition of gap junction communication would have a more dramatic effect on hyperpolarization-mediated relaxation, but if EDHF is a transferable factor, such as K+, then it might be expected to act on each smooth muscle cell, irrespective of whether they were electrically coupled. We interpret these data using gap junction blockers to reflect an important role for myo-endothelial gap junctions in the mechanism of EDHF-mediated relaxation.
K+-induced dilatation can fail where an EDHF response is present
ACh-mediated EDHF vasorelaxation is a robust response, eliciting relaxation in all arteries tested in our experiments. In contrast K+-induced dilatation was observed in less than half of pressurized arteries tested in one series of experiments, with dilatations occurring at a substantially lower frequency overall. None of the arteries tested mounted for isometric measurement of force showed significant relaxation to raised [K+]o. This is at variance with some recent reports, in which a transient relaxation to raised [K+]o is observed in a proportion of arteries mounted for isometric measurement (Edwards et al., 1999; Lacy et al., 2000). There is no obvious explanation for this discrepancy at this stage.
It was shown by Edwards et al. (1998), using a K+-sensitive microelectrode, that, during stimulation of EDHF, K+ concentrations in the myoendothelial space of hepatic arteries rose to around 11 mM. It was proposed that this elevation in K+ is due to efflux of K+ from the endothelium through Ca2+-activated K+ channels. Toxins which block Ca2+-activated K+ channels, ChTx and apamin, are known to block EDHF (Waldron & Garland, 1994) and their sites of action are have been proposed to be on the endothelium (Doughty et al., 1999). Raising [K+]o in pressurized arteries from 5.88–10.58 mM should have been sufficient to mimic this elevation of K+ in the media, as these arteries are only 3–4 smooth muscle cell layers thick (Aalkjaer & Mulvany, 1983). Arteries mounted for isometric recording of force also failed to relax to elevated [K+]o suggesting that the restricted access of K+ to either the outside or inside of the arteries in the pressure myograph does not explain the absence of dilatation. Indeed, in arteries where 10.58 mM [K+]o failed to relax, raising [K+]o to higher concentrations (19.98 mM) resulted in a constriction. Raising [K+]o in the lumen of the vessels was no more effective at dilating arteries than high [K+]o superfusion. In contrast, Edwards et al. (1998) observed relaxations to K+ even at concentrations of 20–25 mM. This is likely to be due to the fact that K+ was applied as a bolus of concentrated KCl to a constantly perfused chamber. Using this method, the concentration of K+ at the tissue cannot be known. It should be noted that arteries that failed to dilate to raised [K+]o could be completely dilated by the opener of ATP-sensitive K+ channels, BRL38227 (data not shown), suggesting the membrane potential of the smooth muscle is significantly positive to EK.
Gap junctions and other factors are involved in EDHF-mediated vasodilatation
There is general agreement that EDHF-mediated hyperpolarization relies on an increased K+ conductance. Increased [K+]o in a diffusionally restricted space (myoendothelial space) may be the consequence and not the cause of EDHF. The failure of raised [K+]o to dilate over 50% of pressurized mesenteric arteries, and all isometrically mounted arteries suggests that this is not the primary mechanism of EDHF. If Kir channels are present on the endothelium, an increase in [K+]o would tend to support a toxin-sensitive hyperpolarization of the endothelium, which is coupled to smooth muscle hyperpolarization/relaxation via gap junctions. Likewise, Na+/K+-ATPase on the smooth muscle would support smooth muscle cell hyperpolarization, but this is unlikely to be the principal mechanism of relaxation given the apparent importance of gap junctions in our experiments. Further evidence for this is illustrated in Edwards et al. (1998) (Figure 4) which shows that block of Kir and Na+/K+-ATPase, using Ba2+ and ouabain, prevented hyperpolarization but not relaxation to EDHF in mesenteric arteries. Vanhoutte (1998) also questioned if a single layer of endothelial cells, which average less than one micron thick, could contain sufficient K+ to significantly elevate the concentration of K+ in the myoendothelial space. It is likely that the endothelium would be required to recycle or replenish intracellular K+ in order to sustain a K+ efflux.
Even if EDHF is not K+, EDHF-mediated relaxation may be fundamentally dependent on hyperpolarization of the endothelium by toxin-sensitive K+ conductances. Endothelial hyperpolarization may lead to hyperpolarization of smooth muscle if functional myoendothelial gap junctions coupling exists. Gap junction inhibitors have recently been demonstrated to be very effective inhibitors of EDHF (Chaytor et al., 1998; Dora et al., 1999; Taylor et al., 1998). This observation is supported by our own data.
The possibility remains that a diffusable factor, independent of K+ efflux from the endothelium, may contribute a component to EDHF, but this would also need to be directly or indirectly toxin-sensitive. Compelling evidence that EDHFs is likely to include a humoral factor that is not explained by K+ come from the reports of experiments using sandwich (or donor) preparations (Chen et al., 1991; Mombouli et al., 1996). Possible candidates for this factor are briefly reviewed in (Vanhoutte, 1998) and include epoxyeicosatrienoic acids (EETs), cannabinoids, such as anandamide, and residual NOS inhibitor-insensitive NO production.
Conclusions
EDHF cannot be mimicked by raised K+ in rat mesenteric arteries since raising K+ from concentrations that are close to physiological values induces relaxation in less than 50% of arteries tested, and then only in endothelium-intact preparations. In addition, EDHF- and K+-induced dilatations show different sensitivities to Ba2+ and ouabain. K+-dilatations are abolished either by Ba2+ or a combination of Ba2+ and ouabain, whereas EDHF is only partially depressed by a combination of Ba2+ and ouabain. Gap junction inhibitors depress EDHF, but not dilatation to K+, suggesting that there is an important role for gap junctions in EDHF-mediated relaxation of mesenteric arterial smooth muscle.
Acknowledgments
The authors wish to thank Paul Kendrick for technical support. This work was supported by British Heart Foundation grant number PG/97182 (J.M. Doughty and P.D. Langton) and Medical Research Council grant number G9609076 (J.P. Boyle).
Abbreviations
- ACh
acetylcholine
- ChTx
charybdotoxin
- COX
cyclo-oxygenase
- −E
endothelium denuded
- +E
endothelium intact
- EDHF
endothelium derived hyperpolarizing factor
- HPSS
HEPES-buffered physiological saline solution
- [K+]o
extracellular K+
- L-NAME
Nω-nitro-L-arginine methyl ester
- NOS
nitric oxide synthase
- PSS
physiological saline solution
References
- AALKJAER C., MULVANY M.J. Human and rat resistance vessels: A comparison of their morphological and pharmacological characteristics. Gen. Pharmacol. 1983;14:85–87. doi: 10.1016/0306-3623(83)90070-8. [DOI] [PubMed] [Google Scholar]
- BRADLEY K.K., JAGGAR J.H., BONEV A.D., HEPPNER T.J., FLYNN E.R.M., NELSON M.T., HOROWITZ B. K(ir)2.1 encodes the inward rectifier potassium channel in rat arterial smooth muscle cells. J. Physiol. 1999;515:639–651. doi: 10.1111/j.1469-7793.1999.639ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J. Physiol. 1998;508:561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H., WESTON A.H. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br. J. Pharmacol. 1988;95:1165–1174. doi: 10.1111/j.1476-5381.1988.tb11752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., YAMAMOTO Y., MIWA K., SUZUKI H. Hyperpolarization of arterial smooth muscle induced by endothelial humoral substances. Am. J. Physiol. 1991;260:H1888–H1892. doi: 10.1152/ajpheart.1991.260.6.H1888. [DOI] [PubMed] [Google Scholar]
- CORRIU C., FELETOU M., CANET E., VANHOUTTE P.M. Endothelium-derived factors and hyperpolarization of the carotid artery of the guinea-pig. Br. J. Pharmacol. 1996;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORA K.A., MARTIN P.E.M., CHAYTOR A.T., EVANS W.H., GARLAND C.J., GRIFFITH T.M. Role of heterocellular gap junctional communication in endothelial-dependent smooth muscle hyperpolarization: Inhibition by a connexin-mimetic peptide. Biochem. Biophy. Res. Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- DOUGHTY J.M., LANGTON P.D. The effects of ouabain and barium of EDHF- and 10mM K-induced dilatations of pressurized rat mesenteric artery. J. Physiol. 1999a;518P:34P. [Google Scholar]
- DOUGHTY J.M., LANGTON P.D. Gap junction inhibitors depress EDHF in rat mesenteric arteries. J. Physiol. 1999b;521P:61P. [Google Scholar]
- DOUGHTY J.M., PLANE F., LANGTON P.D. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am. J. Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- EDWARDS F.R., HIRST G.D.S. Inward rectification in submucosal arterioles of guinea-pig ileum. J. Physiol. 1988;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS F.R., HIRST G.D.S., SILVERBERG G.D. Inward rectification in rat cerebral arterioles; Involvement of potassium ions in autoregulation. J. Physiol. 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FELETOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigations of endothelium-derived hyperpolarizing factor (EDHF) in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELETOU M., VANHOUTTE P.M. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br. J. Pharmacol. 1988;93:515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARDER D.R. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circulation Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., GILBERT R., LOMBARD J.H. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am. J. Physiol. 1987;253 4:F778–F781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- KNOT H.J., ZIMMERMANN P.A., NELSON M.T. Extracellular K+-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K+ channels. J. Physiol. 1996;492:419–430. doi: 10.1113/jphysiol.1996.sp021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACY P.S., PILKINGTON G., HANVESAKUL R., FISH H.J., BOYLE J.P., THURSTON H. Evidence against potassiumas an endothelium-derived hyperpolarizing factor in rat mesenteric small arteries. Br. J. Pharmacol. 2000;129:605–611. doi: 10.1038/sj.bjp.0703076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARRON J.G., HALPERN W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am. J. Physiol. – Heart and Circul. Physiol. 1990;259:H902–H908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- MOMBOULI J.-V., BISSIRIOU I., AGBOTON V.D., VANHOUTTE P.M. Bioassay of endothelium-derived hyperpolarizing factor. Biochem. Biophys. Res. Commun. 1996;221:484–488. doi: 10.1006/bbrc.1996.0621. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. – Cell Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- NILIUS B., VIANA F., DROOGMANS G. Ion channels in vascular endothelium. Ann. Rev. Physiol. 1997;59 doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- OHLMANN P., MARTINEZ M.C., SCHNEIDER F., STOCLET J.C., ANDRIANTSITOHAINA R. Characterization of endothelium-derived relaxing factors released by bradykinin in human resistance arteries. Br. J. Pharmacol. 1997;121:657–664. doi: 10.1038/sj.bjp.0701169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI K., ENDOU M., OKUMURA F. Involvement of barium-sensitive K+ channels in endothelium-dependent vasodilation produced by hypercapnia in rat mesenteric vascular beds. Br. J. Pharmacol. 1998;125:168–174. doi: 10.1038/sj.bjp.0702048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR H.M., WEBSTER N., QUINN K., BEECH D.J., YATES M.S. K+ induced dilation of a small renal artery: no role for inward rectifier K+ channels. Cardiovasc. Res. 1998;37:780–790. doi: 10.1016/s0008-6363(97)00237-x. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., DART C., STANDEN N.B. The properties and distribution of inward rectifier potassium currents in pig coronary arterial smooth-muscle. J. Physiol. 1996;494:715–726. doi: 10.1113/jphysiol.1996.sp021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUAYLE J.M., MCCARRON J.G., BRAYDEN J.E., NELSON M.T. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am. J. Physiol. 1993;265:C1363–C1370. doi: 10.1152/ajpcell.1993.265.5.C1363. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiolog. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- QUIGNARD J.-F., FELETOU M., THALLON C., VILAINE J.-P., DUHAULT J., VANHOUTTE P.M. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br. J. Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIMOKAWA H., YASUTAKE H., FUJII K., OWADA M.K., NAKAIKE R., FUKUMOTO Y., TAKAYANAGI T., NAGAO T., EGASHIRA K., FUJISHIMA M., TAKESHITA A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J. Cardiovas. Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- TAYLOR H.J., CHAYTOR A.T., EVANS W.H., GRIFFITH T.M. Inhibition of the gap junctional component of endothelium-dependent relaxations in rabbit iliac artery by 18-alpha glycyrrhetinic acid. Br. J. Pharmacol. 1998;125:1–3. doi: 10.1038/sj.bjp.0702078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR S.G., WESTON A.H. Endothelium-derived hyperpolarizing factor: A new endogenous inhibitor from the vascular endothelium. Trends Pharmacol. Sci. 1988;9:272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Old-timer makes a comeback. Nature. 1998;396:213–216. doi: 10.1038/24261. [DOI] [PubMed] [Google Scholar]
- VOETS T., DROOGMANS G., NILIUS B. Membrane currents and the resting membrane potential in cultured bovine pulmonary artery endothelial cells. J. Physiol. 1996;497:95–107. doi: 10.1113/jphysiol.1996.sp021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Effect of potassium channel blockers on L-NAME insensitive relaxations in rat small mesenteric artery Canadian J. Physiol. Pharmacol. 199472115(Abstract) [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., LARSSON B., HOGESTATT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]


