Abstract
To investigate the quantitative relationship between elevation in the intracellular Ca2+ concentration ([Ca2+]i) and nitric oxide (NO) production, the changes in [Ca2+]i and NO production were determined in parallel, using fluorimetry of fura-2 and 2,3-diaminonaphthalene, respectively, in endothelial cells ex vivo of pig aortic valves.
The extent of [Ca2+]i elevation was quantitatively assessed by two parameters: the level of peak [Ca2+]i elevation and the area under the [Ca2+]i curve during treatment (the integrated [Ca2+]i elevation). The amount of NO production was expressed as a percentage of that obtained with 10 μM ATP for 3 min.
ATP, bradykinin, thrombin, and ionomycin were used as stimulation to induce NO production, and all these caused [Ca2+]i increases and NO production in a concentration-dependent manner.
The relationships between the peak [Ca2+]i and NO production or between the integrated [Ca2+]i elevation and NO production were well described by a straight line. However, the slope value of the linear relationship in both cases varied with the type of stimulation, with thrombin giving the greatest value, followed by ATP, bradykinin and ionomycin.
These data suggest that in endothelial cells ex vivo: (1) [Ca2+]i elevation regulates NO production, but (2) the peak [Ca2+]i elevation- or the integrated [Ca2+]i elevation-NO production relationships varies depending on the type of agonists. Our results thus demonstrate the presence of the agonists-dependent modulation of the relationship between [Ca2+]i elevation and NO production in endothelial cells ex vivo.
Keywords: Cytosolic calcium concentration; nitrite; fura-2, 2,3-diaminonaphthalene; pig aortic valve
Introduction
Endothelial cells produce nitric oxide (NO) through the activation of endothelial constitutive NO synthase (ecNOS) and induce vasodilation (Moncada et al., 1991). It was reported that NO production by ecNOS was initiated by an elevation of the cytosolic Ca2+ concentration ([Ca2+]i) (Busse & Mülsch, 1990; Förestermann et al., 1991). In fact, ecNOS is a Ca2+-calmodulin-dependent enzyme, and the interaction of ecNOS with the Ca2+-calmodulin complex caused the activation of the enzyme (Nathan & Xie, 1994a). In endothelial cells, ecNOS was shown to be localized to the specialized invaginations of plasma membrane termed caveolae, and translocates from caveolae to the cytosol during the stimulation of endothelial cells by agonists (Feron et al., 1996; Michel & Feron, 1997). The caveolae contain a specific protein component, caveolin, which interacts with ecNOS. This interaction regulates not only the localization of ecNOS but also the activity of the enzyme (Michel & Feron, 1997). The Ca2+-calmodulin complex is also suggested to impair the interaction and cause translocation (Michel et al., 1997). On the other hand, ecNOS has been shown to be activated in a Ca2+-independent manner through the direct phosphorylation of ecNOS by Akt/protein kinase B (Dimmeler et al., 1999; Fulton et al., 1999). As a result, the relation between Ca2+ signal and NO production is suggested to vary with the type of stimulation. Therefore, it is important to determine the relationship between [Ca2+]i and NO production in parallel using different agonists in endothelial cells. However, little is known regarding the quantitative relationship between [Ca2+]i elevation and NO production.
Most studies of NO production and [Ca2+]i regulation have been performed on cultured endothelial cells (Wang et al., 1996). However, the culture procedure has been reported to alter the physiological properties of endothelial cells such as an expression of several proteins including membrane receptors and angiotensin-converting enzyme (De Nucci et al., 1988; Del Vecchio & Smith, 1981). The Ca2+-independent inducible NO synthase (iNOS) is one such protein. The expression of iNOS was shown to be extensively induced by growth factors and cytokines (Moncada et al., 1991), which are well-known major components of the culture medium. It is thus possible that cultured endothelial cells can consistently produce NO independently of Ca2+, through the activation of iNOS, but not necessarily of ecNOS (Moncada et al., 1991; Nathan & Xie, 1994b). Therefore, considerable caution should be taken when the in vivo characteristics of the Ca2+-dependent activation of ecNOS are inferred from results that are obtained in endothelial cells in culture.
In order to circumvent the possible problems and limitations in studies with cultured cells, we developed a technique to monitor the changes in [Ca2+]i in endothelial cells ex vivo on the surface of intact aortic valves (Aoki et al., 1991). In the present study, to clarify the quantitative relationship between [Ca2+]i elevation and NO production, we measured these two parameters in parallel in the same endothelial cells ex vivo on pig aortic valves using four different agonists. We herein report the existence of agonist-dependent modulation of the relationship between [Ca2+]i elevation and NO production in endothelial cells ex vivo.
Methods
The measurement of [Ca2+]i of the endothelial cells ex vivo
The changes in [Ca2+]i of the endothelial cells ex vivo were monitored using strips of the pig aortic valves as previously described (Aoki et al., 1991; Mizuno et al., 1998) except that a small chamber made with Sylgard (Dow Corning, U.S.A.) was used to reduce the solution volume to 250 μl. In brief, the aortic valves were obtained at a local abattoir and transferred to the laboratory. The valvular strips (5×8 mm) were dissected from the valve leaflets, and loaded with Ca2+ indicator dye, fura-2, by incubation in oxygenated (5% CO2 and 95% O2) Dulbecco's modified Eagle's medium containing 50 μM fura-2 acetoxymethyl ester (fura-2/AM), 5% foetal bovine serum, and 1 mM probenecid for 90 min at 37°C. It is possible that the exposure of the strips to 5% serum during the 90 min fura-2-loading period altered the physiological properties of endothelial cells in the same manner as the culture procedure. However, omitting the serum from the fura-2-loading mixture had little effect on the responsiveness to the agonists used in the present study, but it did cause a poor loading of fura-2 and also resulted in a poor recording of fluorescence. We therefore loaded the valvular strips in the presence of 5% serum. After loading with fura-2, valvular strips were equilibrated in normal physiological salt solution (PSS) at room temperature in order to remove the extracellular dye. Each valvular strip was then mounted horizontally with pins in a Sylgard chamber, and changes in the [Ca2+]i of the endothelial cells on the surface of the aortic side were monitored using a front-surface fluorometer CAM-OF-3 designed in collaboration with the Japan Spectroscopic Co. (Tokyo, Japan), as previously described (Kanaide, 1999; Mizuno et al., 1998). The fura-2 signals exclusively arose from the monolayered endothelial cells on the surface of the aortic side of the aortic valves (Aoki et al., 1994; Kuroiwa et al., 1995). The measurement was performed at 25°C to prevent any leakage of fluorescent dye (Kuroiwa et al., 1995). The fluorescence intensities at alternating (400 Hz) 340 nm (F340) and 380 nm (F380) excitation wave length and their ratios (R=F340/F380) were monitored at 500 nm emission. Before starting the experimental protocol, all strips were stimulated by 10 μM ATP for 3 min in order to obtain a reference response. The fluorescence ratio values were normalized (per cent fluorescence ratio) by assigning values in normal PSS and at the peak response to 10 μM ATP to be 0 and 100%, respectively.
The per cent fluorescence ratio was then converted to the absolute value of [Ca2+]i as follows. The fluorescence ratio at maximum and minimum fluorescence, and the β value in the original formula (Grynkiewicz et al., 1985) were first obtained in separate experiments as previously described (Kanaide, 1999). In brief, after recording the reference response to 10 μM ATP, valvular strips were treated with 10 μM ionomycin in Ca2+-free PSS, which caused a transient elevation in the fluorescence ratio. The maximum fluorescence was then obtained by the addition of extracellular Ca2+ to 2.5 mM. The subsequent incubation of strips in 2 mM EGTA-containing Ca2+-free PSS gave the minimum fluorescence. MnCl2 was then added to a final concentration of 3 mM to quench the fura-2 fluorescence and to obtain the level of autofluorescence. The values of per cent fluorescence ratio at the maximum and minimum fluorescence were normalized to 469.4±9.6 and −87.6±10.0% (n=5), respectively. The β value was calculated as the ratio of F380 at the minimum fluorescence to that at the maximum fluorescence after subtracting F380 of autofluorescence, and determined to be 2.32±0.36 (n=5). The fluorescence ratio (R) in the original formula was replaced by the per cent fluorescence ratio as described (Kanaide, 1999). Accordingly, the [Ca2+]i at 0 and 100% were 70.1±10 and 190±30 nM, respectively (n=5). All representative recordings and fluorimetry data were shown on a linear scale of absolute values of [Ca2+]i, after conversion from the per cent fluorescence ratio by computer-assisted planimetry. This conversion to the linear scale is essential when calculating the area under the [Ca2+]i curve.
To quantitatively evaluate the [Ca2+]i responses in endothelial cells, we measured two parameters: the peak [Ca2+]i and the area under the [Ca2+]i curve (the integrated [Ca2+]i elevation) (Figure 1). The area under the [Ca2+]i curve was determined by computer-assisted planimetry as shown in Figure 1, after converting fluorescence ratio to the linear scale of the absolute value of [Ca2+]i. The peak [Ca2+]i and the area under the [Ca2+]i curve were expressed in ‘%' and/or ‘nM' and ‘s×nM', respectively.
Figure 1.
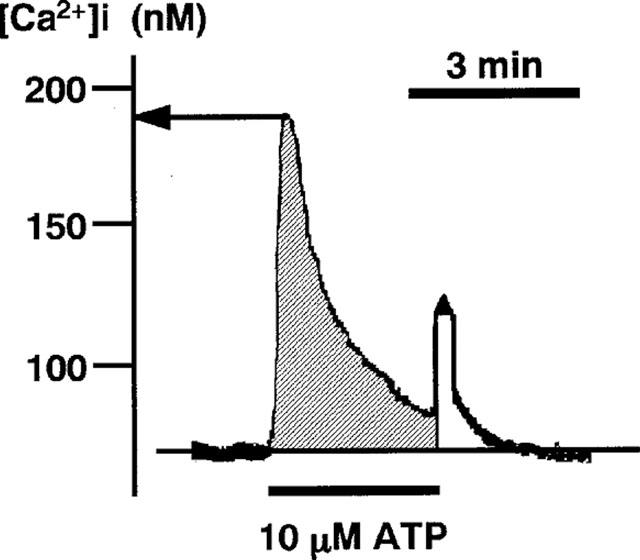
A recording of [Ca2+]i converted from the fura-2-fluorescence ratio (F340/F380). The trace of fluorescence ratio was converted onto the linear scale of the absolute value of [Ca2+]i as described in Methods. The [Ca2+]i (ordinate) is shown in nM. The peak [Ca2+]i is determined as indicated by an arrow. The shaded area indicates the area under the [Ca2+]i curve (integrated [Ca2+]i elevation).
The measurement of the NO production of the endothelial cells ex vivo
The NO production in endothelial cells ex vivo was measured in parallel to the measurement of [Ca2+]i in the same aortic valve. The agonists were applied to the aortic valve pinned in the Sylgard chamber by exchanging the solutions (250 μl), and changes in [Ca2+]i were recorded for 3 min using front-surface fluorimetry. At the end of the 3 min treatment, a 200 μl aliquot of the bathing solution was sampled from the chamber and then subjected to a 2,3-diaminonaphthalene fluorimetry assay of NO production as previously described (Misko et al., 1993). To obtain the basal NO production, PSS without agonists (250 μl) was applied to the aortic valve for 3 min.
The free radical NO is very rapidly (≪10 s) oxidized to a stable derivative, nitrite, in a physiological solution (Robert, 1993). In the 2,3-diaminonaphthalene fluorimetry assay, the concentration of nitrite in PSS was directly measured, and thereby the production of NO by endothelial cells was assessed. Before adding fluorescence dye, EDTA was added to a final concentration of 5 mM to the sample solutions to avoid any possible interference with the fluorescence measurement by divalent cation (Damiani & Burini, 1986). Twenty μl of freshly prepared 2,3-diaminonaphthalene (0.05 mg ml−1 in 0.62 N HCl) were then added to 200 μl of the sample solution and mixed immediately. After a 15 min-incubation at 25°C, the reaction of nitrite with 2,3-diaminonaphthalene was then terminated by the addition of 10 μl of 2.8 N NaOH. The intensity of the fluorescent signal produced by the reaction product, 1-(H)-naphthotriazole, was measured at 365 nm excitation and at 450 nm emission with a fluorometer (Jobin Yvon, France). Standard sodium nitrite solutions (60 nM–3 μM) were made fresh before each measurement, and a standard curve for the relationship between fluorescence intensity and nitrite concentration in PSS was obtained (Figure 2). Using this standard curve, the level of NO production during the treatment with agonists for 3 min was calculated. The repetitive application of 10 μM ATP (3 min) at intervals of 20 min induced reproducible elevations of [Ca2+]i and NO production (Figure 3). Therefore, the response to 10 μM ATP was registered before starting each experiment, and the level of NO production was normalized to that obtained with 10 μM ATP, because aortic valvular strips were not exactly the same size. Data are thus expressed as a percentage, assigning the background level obtained with buffer only as 0% and the value obtained after 3 min stimulation by 10 μM ATP as 100%.
Figure 2.
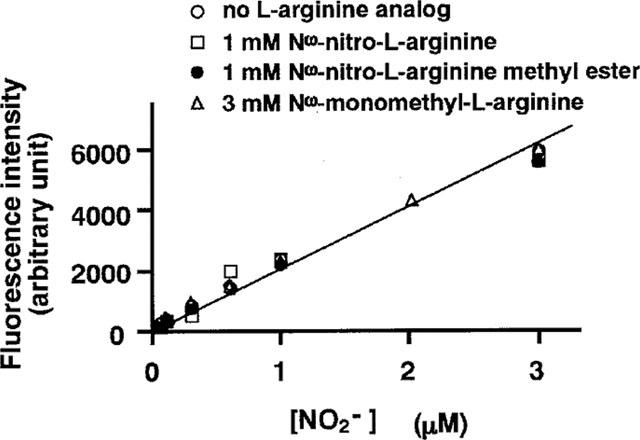
A standard curve for the relationship between fluorescence intensity and nitrite concentration, repeated in the presence of various analogues of L-arginine. The fluorescence intensities of 0.06, 0.1, 0.3, 0.6, 1, 2, and 3 μM nitrite solutions were obtained in the absence and presence of 1 mM NΩ-nitro-L-arginine (L-NOARG), 1 mM NΩ-nitro-L-arginine methyl ester (L-NAME), and 3 mM NΩ-monomethyl-L-arginine (L-NMMA). The fluorescence intensities (arbitrary unit) were obtained by subtracting the background fluorescence of physiological salt solution from the measured values.
Figure 3.
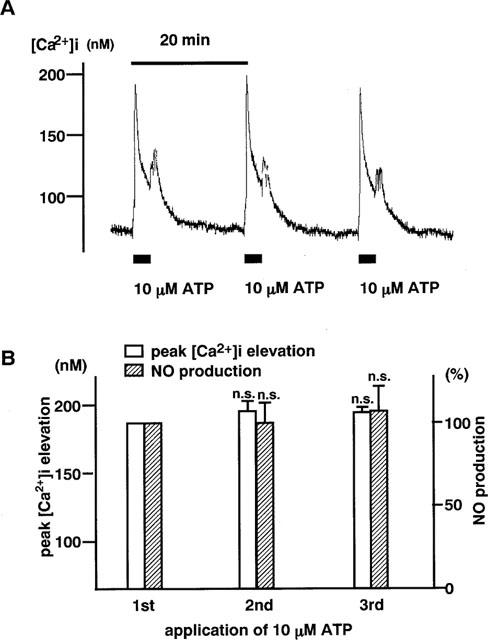
The [Ca2+]i elevation and NO production induced by repetitive stimulation with ATP in endothelial cells ex vivo. (A) A representative recording of transient elevation of [Ca2+]i in response to repetitive 3 min stimulations with 10 μM ATP at 20 min intervals. (B) A summary of the [Ca2+]i elevation and NO production induced by repetitive application of 10 μM ATP (n=9). The NO production was expressed as a percentage, assigning the result obtained with the first application of 10 μM ATP to be 100%.
NΩ-nitro-L-arginine (L-NOARG) and NΩ-nitro-L-arginine methyl ester (L-NAME) have been reported to interfere with the assay of nitrite/nitrate with the modified Griess reaction (Greenberg et al., 1995). Therefore, we examined the effects of L-NOARG, L-NAME, and NΩ-monomethyl-L-arginine (L-NMMA) on the standard curve for the 2,3-diaminonaphthalene assay. As shown in Figure 2, these L-arginine analogues had no effect on the standard curve.
Chemicals and solutions
Sodium salt of ATP was obtained from Boehringer Mannheim (Mannheim, Germany). Fura-2/AM and 2,3-diaminonaphthalene were purchased from DOJINDO (Kumamoto, Japan). Thrombin, probenecid, fendiline and L-NOARG were obtained from Sigma-Adrich (St Louis, MO, U.S.A.). Bradykinin was purchased from the Peptide Institute (Osaka, Japan). L-arginine was obtained from the Ishizu Pharma. Co. (Tokyo, Japan). L-NAME and L-NMMA were purchased from Wako Pure Chemical (Tokyo, Japan). The composition of the normal PSS was (in mM): L-arginine 1, NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25 and D-glucose 11.5. All solutions were gassed with a mixture of 5% CO2 and 95% O2 (pH adjusted to 7.4 at 25°C).
Data analysis
Each value is the mean±s.e.mean of the number of experiments as indicated. The unpaired Student's t-test was used to determine statistical significance. An analysis of variance was used to determine the concentration-dependency of the drug effects. An analysis of covariance was used to determine the significance of difference in the relationships between [Ca2+]i elevation and NO production. A P-value of less than 0.05 was considered to be statistically significant. All data was collected at the sampling rate of 17 Hz using a computerized data acquisition system (MacLab, Analog Digital Instruments, Australia; Macintosh, Apple Computer, U.S.A.).
Results
In order to investigate the relationship between [Ca2+]i and NO production in endothelial cells ex vivo, we used ATP, bradykinin and thrombin as three [Ca2+]i-elevating agonists which also increase the production of NO. In addition to these agonists, we used ionomycin, a Ca2+ ionophore, which directly elevates [Ca2+]i without activation of intracellular signalling systems in endothelial cells (Parsaee et al., 1993). As shown in Figures 4 and 5, all of these drugs (ATP, n=4–9; bradykinin, n=4–11, thrombin, n=4–11 and ionomycin, n=4–11) elevated [Ca2+]i of endothelial cells ex vivo, in a concentration-dependent manner (P<0.05 by an analysis of variance). The [Ca2+]i elevations consist of a transient component and a subsequent sustained elevation with a decline, except for thrombin which caused only a transient [Ca2+]i elevation. The initial peak elevations of [Ca2+]i were resistant to the removal of extracellular Ca2+, and therefore considered to be mainly mediated by the release of intracellular Ca2+ (data not shown). Since the time course of [Ca2+]i elevation differed with type of stimulation, we evaluated [Ca2+]i elevation by two parameters; the peak of [Ca2+]i elevation and the integrated [Ca2+]i elevation (the area under the [Ca2+]i curve) (Figure 1). As shown in Figures 4B and 5, regardless of the parameter, a similar concentration-dependency (P<0.05 by an analysis of variance) of the [Ca2+]i elevation was obtained for each agonist. It was also noted that washing out the agonists using normal PSS induced a transient increase in [Ca2+]i (Figures 1, 3 and 4). A similar transient [Ca2+]i increase was also observed even when the normal PSS was changed to the normal PSS (data not shown). The net increase in [Ca2+]i induced by changing the buffer was similar regardless of the presence or absence of agonists. Therefore, this transient [Ca2+]i elevation was considered to be mainly due to the mechanical shear stress to endothelial cells.
Figure 4.
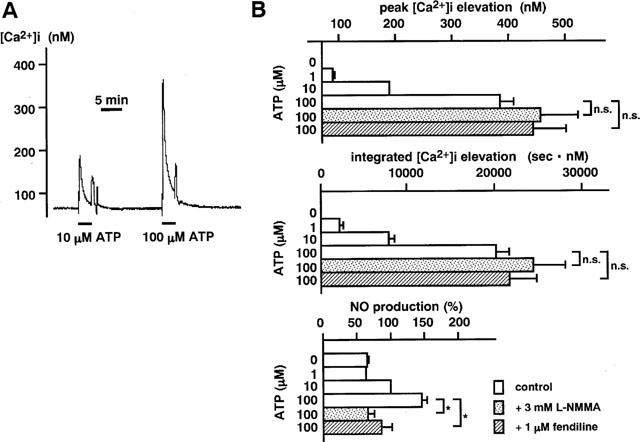
The concentration-dependent [Ca2+]i elevation and NO production induced by ATP in endothelial cells. (A) A representative recording of the changes in [Ca2+]i induced by 3 min stimulation with 10 μM (reference) and 100 μM ATP. The values obtained with the 2nd stimulation with ATP were normalized by reference to those obtained with the first stimulation with 10 μM ATP treatment. (B) Summary of the peak [Ca2+]i elevation, the integrated [Ca2+]i elevation and the NO production induced by ATP in the absence (control) and presence of 3 mM NΩ-monomethyl-L-arginine (L-NMMA) and 1 μM fendiline. L-NMMA and fendiline were applied 10 min before the stimulation with ATP. Data are the mean±s.e.mean (n=4–9). *Significantly different (P<0.05); n.s., not significant (P>0.05).
Figure 5.
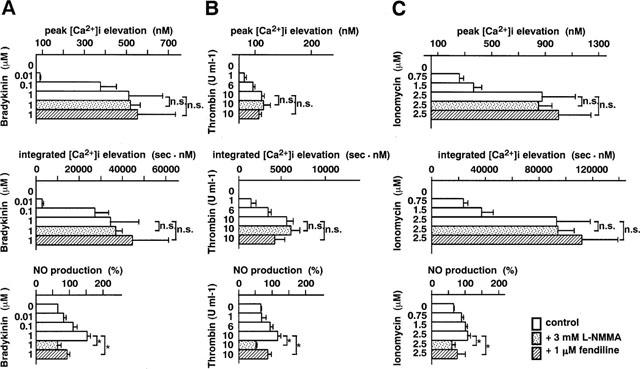
The concentration-dependent [Ca2+]i elevation and the NO production induced by bradykinin (A), thrombin (B) and ionomycin (C). The peak [Ca2+]i elevation, the integrated [Ca2+]i elevation and the NO production in the absence (control) and presence of 3 mM NΩ-monomethyl-L-arginine (L-NMMA) and 1 μM fendiline. L-NMMA and fendiline were applied 10 min before the stimulation. The data are the mean±s.e.mean (n=4–11). *Significantly different (P<0.05); n.s., not significant (P>0.05).
Agonist-induced concentration-dependent elevations of [Ca2+]i were associated with concentration-dependent elevations of NO production (Figures 4B and 5). The application of 3 mM L-NMMA or 1 μM fendiline, a calmodulin inhibitor, blocked the elevation of NO production induced by a maximal concentration of ATP, thrombin, bradykinin or ionomycin, while they had no significant effect on the [Ca2+]i elevations as assessed either by the peak [Ca2+]i or the integrated [Ca2+]i elevation (Figures 4B and 5). In the presence of L-NMMA (ATP, n=4; bradykinin, n=4; thrombin, n=4; ionomycin, n=5), the amount of NO production during treatment with these agonists did not significantly (P>0.05) differ from the basal production of NO in normal PSS without drugs. The amount of NO production during treatment with the agonists was significantly (P<0.05) but not completely inhibited by fendiline (ATP, n=4; bradykinin, n=4; thrombin, n=4; ionomycin, n=4). L-NMMA or fendiline had no significant effect (P>0.05) on the [Ca2+]i elevations induced by ATP, bradykinin, thrombin or ionomycin (Figures 4 and 5).
We next examined the relationship between the elevations of [Ca2+]i and NO production (Figure 6). In the case of each agonist, the relationships between the amount of NO production and either the peak [Ca2+]i level (r=0.734–0.547) or the integrated [Ca2+]i elevation (r=0.754–0.561) were well described by a linear regression (Figure 6). Namely, the relationships between NO production and either the peak [Ca2+]i or the integrated [Ca2+]i elevation were closely fitted to the following equation:
where X is the peak [Ca2+]i or the integrated [Ca2+]i elevation, Y is the magnitude of NO production, a is a slope for the peak or the integrated [Ca2+]i elevation-NO production relation, and b represents the NO production at rest. These results are compatible with previous observations in which NO production was found to be regulated by [Ca2+]i elevation in endothelial cells (Busse & Mülsch, 1990a). However, the slope value for the relation between NO production and the peak [Ca2+]i level and the relation between NO production and the integrated [Ca2+]i elevation varied depending on the types of stimuli, with thrombin giving the greatest value (0.92), followed by ATP (0.19), bradykinin (0.09) and ionomycin (0.03) in the relation between NO production and the peak [Ca2+]i level; and with thrombin giving the greatest value (0.0073), followed by ATP (0.0031), bradykinin (0.0011) and ionomycin (0.00033) in the relation between NO production and the integrated [Ca2+]i elevation. The slope values for the first three agonists are much larger (about 3–30 fold) than that for ionomycin. As a result, thrombin caused the greatest production of NO for a given change in [Ca2+]i among the agonists used in the present study.
Figure 6.
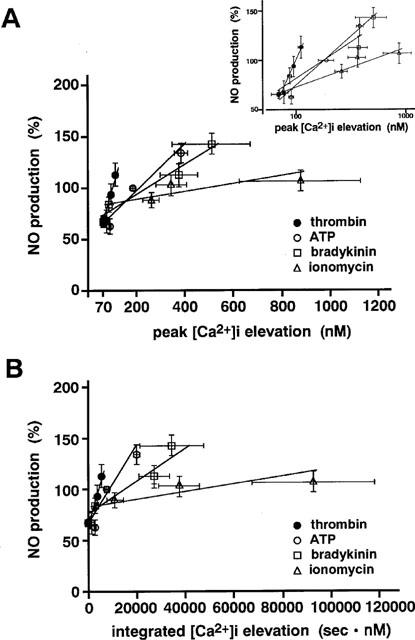
Stimulus-specific alteration of the relationships between NO production and the [Ca2+]i elevation. The relationship between NO production and either the peak [Ca2+]i elevation (A) or the integrated [Ca2+]i elevation (B) for ATP, bradykinin, thrombin and ionomycin were constructed from the data obtained in the absence of L-NMMA and fendiline as shown in Figures 4 and 5. Inset, a semilog plot of the same data shown in (A), showing the relationship at low [Ca2+]i. All data are the mean±s.e.mean (n=4–11).
Discussion
In the present study, we demonstrated, for the first time, in endothelial cells ex vivo, that: (1) for a given agonist, [Ca2+]i elevation quantitatively correlated with the NO production, however; and (2) the NO production for a given elevation of [Ca2+]i differed with type of agonists, with thrombin causing the greatest production, followed by ATP, bradykinin and ionomycin. Among these agonists, thrombin caused the most potent activation of NO production for a given [Ca2+]i change. Thus the Ca2+-sensitivity of the NO production process varied with the type of stimulation. The correlation between [Ca2+]i elevation and NO production was also supported by the finding that fendiline, a calmodulin inhibitor partially blocked the NO production induced by agonists. Since ATP, bradykinin, and thrombin, but not ionomycin, are known to activate receptor-coupled G-proteins and their related signal transduction systems, our results suggested that the Ca2+-sensitivity of the NO production process might be influenced by a G-protein-coupled intracellular signal. Ionomycin causes [Ca2+]i elevation mainly by activating Ca2+ influx, while the agonists cause both Ca2+ influx and Ca2+ release from the intracellular store sites. It is thus also possible that the [Ca2+]i elevation due to the Ca2+ release activates ecNOS more efficiently than the [Ca2+]i elevation due to the Ca2+ influx. However, the precise mechanism of the modulation of the Ca2+ sensitivity remains to be elucidated.
The phosphorylation of ecNOS or alteration of the intracellular environment such as pH or another ion could be a mechanism which alters the relationship between the Ca2+ signal and NO production. Some pathways activated by agonists in endothelial cells, including protein kinase A and C, have been reported to be related to the reduction of NOS activity (Hirata et al., 1995; Ohara et al., 1995). Recently, Akt/protein kinase B has been shown to directly phosphorylate ecNOS and thereby activate the production of NO independently of [Ca2+]i (Dimmeler et al., 1999; Fulton et al., 1999). The Akt/protein kinase B was suggested to be activated through phosphatidylinositol 3-kinase-mediated signal transduction (Dimmeler et al., 1999; Fulton et al., 1999). Since bradykinin was shown to activate phosphatidylinositol 3-kinase, it is possible that bradykinin activated ecNOS in a Ca2+-independent manner, thereby causing an apparent potentiation of the Ca2+-sensitivity of the NO production process. However, there is no published report showing the activation of Akt/protein kinase B by any of the agonists used in the present study including bradykinin. Thrombin has been shown to couple with a novel family of trimeric G-proteins, G12 (Aragay et al., 1995; Post et al., 1996), which activates such small GTP-binding proteins as Rho and Rac (Buhl et al., 1995; Collins et al., 1996). There is a possibility that these small GTP-binding proteins are involved in the potentiation of the Ca2+-sensitivity of the NO production process. However, this possibility remains to be elucidated. The activity of ecNOS was shown to be dependent on the pH level and to increase with alkalinization within the range of pH between 5.5–7.5 (Fleming et al., 1994). Thrombin also induced activation of the Na+-H+ exchanger and intracellular alkalinization in various cells including endothelial cells (Gerritsen et al., 1989; Yasutake et al., 1996). However, the difference in the Ca2+-sensitivity of the NO production process was not only seen between the receptor agonists and ionomycin, but also noted among the receptor agonists. Therefore, the relative contribution of these possible mechanisms as discussed above may vary with the type of agonist, and such variations may cause differences in the Ca2+-sensitivity of the NO production process.
In the present study, it was shown that the [Ca2+]i elevation, as assessed by both peak [Ca2+]i and integrated [Ca2+]i elevation, closely correlated to the NO production (Figure 6). However, it was reported in cultured endothelial cells that the amount of NO production stimulated with agonists hyperbolically correlated to the area under the [Ca2+]i curve, but not to the peak [Ca2+]i (Wang et al., 1996). The cause for such a discrepancy is unknown, however, it might be due to the difference in the preparations, namely endothelial cells ex vivo versus cultured endothelial cells. To investigate the physiological role of [Ca2+]i elevation in NO production in endothelial cells, the use of endothelial cells ex vivo, instead of cultured cells, may be important. In the present study, we used endothelial cells ex vivo on aortic valve. We previously demonstrated that agonist-induced elevations of [Ca2+]i in endothelial cells ex vivo on aortic valves are associated with the relaxation of arterial strips without endothelium which were placed in close proximity to the valvular strips (Miyagi et al., 1996), and that monolayered endothelial cells ex vivo on the surface of the aortic valves are also active in the uptake of acetylated low-density lipoprotein (Kuroiwa et al., 1995; Miyagi et al., 1996). These characteristics thus indicate that functional endothelial cells are located on the surface of the aortic valves. Using fluorimetry of 2,3-diaminonaphthalene, the present studies directly show that the valvular endothelial cells ex vivo produce NO through an L-arginine-NOS pathway. Thus, the endothelial cells ex vivo from the aortic valves are useful for the study of the physiological properties of endothelial cells, including [Ca2+]i regulation and NO production.
In summary, our data suggest that in endothelial cells ex vivo: (1) transient elevation of [Ca2+]i is necessary for NO production; and (2) agonists can modulate the [Ca2+]i-NO production relation, the extent of which varies depending on the agonist (thrombin>ATP>bradykinin>ionomycin). We therefore propose the presence of cross-talk between the Ca2+ signalling system and other signal transduction systems, which in turn results in modulation of the Ca2+-sensitivity of ecNOS in endothelial cells ex vivo, and that this modulation is stimulus-specific.
Acknowledgments
We thank Mr B. Quinn for comments and help with the manuscript. This study was supported in part by Grants-in-Aid for Scientific Research (No. 10557072, 11838013, 11670687), for the Encouragement of Young Scientists (No. 10770308) from the Ministry of Education, Science, Sports and Culture, Japan, by the Research Grant for Cardiovascular Diseases (11C-1) from the Ministry of Health and Welfare, Japan, and by grants from the Vehicle Racing Commemorative Foundation, the Foundation for the Promotion of Clinical Medicine and Suzuken Memorial Foundation.
Abbreviations
- [Ca2+]i
cytosolic Ca2+ concentration
- ecNOS
endothelial constitutive NO synthase
- fura-2/AM
fura-2 acetoxymethyl ester
- iNOS
inducible NO synthase
- L-NAME
Nω-nitro-L-arginine
- L-NMMA
Nω-monomethyl-L-arginine
- L-NOARG
Nω-nitro-L-arginine
- NO
nitric oxide
References
- AOKI H., KOBAYASHI S., NISHIMURA J., KANAIDE H. Sensitivity of G-protein involved in endothelin-1-induced Ca2+ influx to pertussis toxin in porcine endothelial cells in situ. Br. J. Pharmacol. 1994;111:989–996. doi: 10.1111/j.1476-5381.1994.tb14841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOKI H., KOBAYASHI S., NISHIMURA J., YAMAMOTO H., KANAIDE H. Endothelin induces the Ca2+-transient in endothelial cells in situ. Biochem. Biophys. Res. Commun. 1991;181:1352–1357. doi: 10.1016/0006-291x(91)92087-z. [DOI] [PubMed] [Google Scholar]
- ARAGAY A.M., COLLINS L.R., POST G.R., WATSON A.J., FERAMISCO J.R., BROWN J.H., SIMON M.I. G12 requirement for thrombin-stimulated gene expression and DNA synthesis in 1321N1 astrocytoma cells. J. Biol. Chem. 1995;270:20073–20077. doi: 10.1074/jbc.270.34.20073. [DOI] [PubMed] [Google Scholar]
- BUHL A.M., JOHNSON N.L., DHANASEKARAN N., JOHNSON G.L. Gα12 and Gα13 stimulate Rho-dependent stress fiber formation and focal adhesion assembly. J. Biol. Chem. 1995;270:24631–24634. doi: 10.1074/jbc.270.42.24631. [DOI] [PubMed] [Google Scholar]
- BUSSE R., MÜLSCH A. Calcium-dependent nitric oxide synthesis in endothelial cytosol is mediated by calmodulin. FEBS Lett. 1990;265:133–136. doi: 10.1016/0014-5793(90)80902-u. [DOI] [PubMed] [Google Scholar]
- COLLINS L.R., MINDEN A., KARIN M., BROWN J.H. Gα12 stimulates c-Jun NH2-terminal kinase through the small G proteins Ras and Rac. J. Biol. Chem. 1996;271:17349–17353. doi: 10.1074/jbc.271.29.17349. [DOI] [PubMed] [Google Scholar]
- DAMIANI P., BURINI G. Fluorometric determination of nitrite. Talanta. 1986;33:649–652. doi: 10.1016/0039-9140(86)80151-5. [DOI] [PubMed] [Google Scholar]
- DEL VECCHIO P.J., SMITH J.R. Expression of angiotensin-converting enzyme activity in cultured pulmonary artery endothelial cells. J. Cell. Physiol. 1981;108:337–345. doi: 10.1002/jcp.1041080307. [DOI] [PubMed] [Google Scholar]
- DE NUCCI G., GRYGLEWSKI R.J., WARNER T.D., VANE J.R. Receptor-mediated release of endothelium-derived relaxing factor and prostacyclin from bovine aortic endothelial cells is coupled. Proc. Natl. Acad. Sci. U.S.A. 1988;85:2334–2338. doi: 10.1073/pnas.85.7.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIMMELER S., FLEMING I., FISSLTHALER B., HERMANN C., BUSSE R., ZEIHER A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- FERON O., BELHASSEN L., KOBZIK L., SMITH T.W., KELLY R.A., MICHEL T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J. Biol. Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- FLEMING I., HECKER M., BUSSE R. Intracellular alkalinization induced by bradykinin sustains activation of the constitutive nitric oxide synthase in endothelial cells. Circ. Res. 1994;74:1220–1226. doi: 10.1161/01.res.74.6.1220. [DOI] [PubMed] [Google Scholar]
- FÖRESTERMANN U., POLLOCK J.S., SCHMIDT H.H.H., HELLER M., MURAD F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fraction of bovine aortic endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1991;88:1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULTON D., GRATTON J.P., MCCABE T.J., FONTANA J., FUJIO Y., WALSH K., FRANKE T.F., PAPAPETROPOULOS A., SESSA W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERRITSEN M.E., PERRY C.A., MOATTER T., CRAGOE E.J.J., MEDOW M.S. Agonist-specific role for Na+-H+ antiport in prostaglandin release from microvessel endothelium. Am. J. Physiol. 1989;256:C831–C839. doi: 10.1152/ajpcell.1989.256.4.C831. [DOI] [PubMed] [Google Scholar]
- GREENBERG S.S., XIE J., SPITZER J.J., WANG J.F., LANCASTER J., GRISHAM M.B., POWERS D.R., GILES T.D. Nitro containing L-arginine analogs interfere with assays for nitrate and nitrite. Life Sci. 1995;57:1949–1961. doi: 10.1016/0024-3205(95)02181-h. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- HIRATA K., KURODA R., SAKODA T., KATAYAMA M., INOUE N., SUEMATSU M., KAWASHIMA S., YOKOYAMA M. Inhibition of endothelial nitric oxide synthase activity by protein kinase C. Hypertension. 1995;25:180–185. doi: 10.1161/01.hyp.25.2.180. [DOI] [PubMed] [Google Scholar]
- KANAIDE H. Measurement of [Ca2+]i in smooth muscle strips using front-surface fluorimetry Methods in Molecular Biology 1999Totowa, NJ: Humana Press Inc; 269–277.ed. Lambert, D.G. pp [DOI] [PubMed] [Google Scholar]
- KUROIWA M., AOKI H., KOBAYASHI S., NISHIMURA J., KANAIDE H. Mechanism of endothelium-dependent relaxation induced by substance P in the coronary artery of the pig. Br. J. Pharmacol. 1995;116:2040–2047. doi: 10.1111/j.1476-5381.1995.tb16409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL T., FERON O. Nitric oxide synthases: which, where, how, and why. J. Clin. Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL J.B., FERON O., SACKS D., MICHEL T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997;272:15583–15536. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- MISKO T.P., SCHILLING R.J., SALVEMINI D., MOORE W.M., CURRIE M.G. A fluorometric assay for the measurement of nitrite in biological samples. Anal. Biochem. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- MIYAGI Y., KOBAYASHI S., NISHIMURA J., FUKUI M., KANAIDE H. P2U receptor is linked to cytosolic Ca2+ transient and release of vasorelaxing factor in bovine endothelial cells in situ. J. Physiol. 1996;492:751–761. doi: 10.1113/jphysiol.1996.sp021343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIZUNO O., HIRANO K., NISHIMURA J., KUBO C., KANAIDE H. Mechanism of relaxation induced by thrombin in pig coronary artery strips with endothelium. Eur. J. Pharmacol. 1998;351:67–77. doi: 10.1016/s0014-2999(98)00292-1. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M.J., HIGGS E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NATHAN C., XIE Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994a;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- NATHAN C., XIE Q.W. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 1994b;269:13725–13728. [PubMed] [Google Scholar]
- OHARA T., SAYEGH H.S., YAMIN J.J., HARRISON D.G. Regulation of endothelial constitutive nitric oxide synthase by protein kinase C. Hypertension. 1995;25:415–420. doi: 10.1161/01.hyp.25.3.415. [DOI] [PubMed] [Google Scholar]
- PARSAEE H., MCEWAN J.R., MACDERMOT J. Bradykinin-induced release of PGI2 from aortic endothelial cell lines: responses mediated selectively by Ca2+ ions or a staurosporine-sensitive kinase. Br. J. Pharmacol. 1993;110:411–415. doi: 10.1111/j.1476-5381.1993.tb13825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POST G.R., COLLINS L.R., KENNEDY E.D., MOSKOWITZ S.A., ARAGAY A.M., GOLDSTEIN D., BROWN J.H. Coupling of the thrombin receptor to G12 may account for selective effects of thrombin on gene expression and DNA synthesis in 1321N1 astrocytoma cells. Mol. Biol. Cell. 1996;7:1679–1690. doi: 10.1091/mbc.7.11.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERT A.S. Southwestern internal medicine conference: Nitric oxide. Am. J. Med. Sci. 1993;306:348–358. [Google Scholar]
- WANG Y.W., SHIN W.S., KAWAGUCHI H., INUKAI M., KATO M., SAKAMOTO A., UEHARA Y., MIYAMOTO M., SHIMAMOTO N., KORENAGA R., ANDO J., TOYO-OKA T. Contribution of sustained Ca2+ elevation for nitric oxide production in endothelial cells and subsequent modulation of Ca2+ transient in vascular smooth muscle cells in coculture. J. Biol. Chem. 1996;271:5647–5655. doi: 10.1074/jbc.271.10.5647. [DOI] [PubMed] [Google Scholar]
- YASUTAKE M., HAWORTH R.S., KING A., AVKIRAN M. Thrombin activates the sarcolemmal Na+-H+ exchanger. Evidence for a receptor-mediated mechanism involving protein kinase C. Circ. Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]


