Abstract
Because Prostaglandin E2 (PGE2) and dibutiryl cyclic AMP (dbcAMP) modulate the production and effects of haemopoietic cytokines in allergy, we examined their ability to modulate responses of myeloid progenitors to GM-CSF, and of eosinophil precursors to IL-5.
The ability of PGE2, dbcAMP, rolipram, forskolin, dbcGMP and PGD2, to modulate the responses to GM-CSF and IL-5 in colony formation (progenitor) and eosinophil differentiation (precursor) assays using bone-marrow from nonsensitized or from intranasally-challenged, ovalbumin-sensitized mice of five strains was studied.
PGE2 (10−7 M) inhibited GM-CSF-stimulated colony formation in bone-marrow from BP-2 mice. This effect was duplicated by dbcAMP (0.3–1×10−6 M), Rolipram (10−5 M) and forskolin (3×10−5 M), but not Prostaglandin D2 (10−6 M). Inhibition affected similarly all myeloid colony types. Progenitors from sensitized and challenged BP-2 mice were also inhibited by PGE2 and cyclic AMP. PGE2 inhibited progenitors from C57BL/10, CBA/J and A/J, but not BALB/c mice. However, BALB/c progenitors were sensitive to dbcAMP and Forskolin (10−4 M). In contrast, in precursor assays, PGE2 (10−7–10−9 M) blocked responses to IL-5 in bone-marrow from BP-2 and BALB/c mice, either naïve or sensitized and challenged, to a similar extent. PGD2 (10−6 M) was ineffective, as was PGE2 (10−7 M), if added after 48 h of culture.
In conclusion, PGE2 inhibits the responses of bone-marrow myeloid progenitors to GM-CSF and of eosinophil precursors to IL-5, in naïve or ovalbumin sensitized and challenged mice. These effects are duplicated by cyclic AMP-elevating agents. In the BALB/c strain, the resistance of progenitors, but not precursors, to PGE2 inhibition, indicates these developmental stages are separate targets for PGE2 modulation.
Keywords: Prostaglandin E2, haemopoiesis, GM-CSF, IL-5, bone-marrow
Introduction
Studies on asthma in patients and in animal models have shown upregulation of responses to the haemopoietic cytokines after allergen exposure in humans (Sehmi et al., 1997), mice (Gaspar Elsas et al., 1997) and dogs (Inman et al., 1996). Therefore, changes in the ability of haemopoietic cells to respond to cytokines is a potential target for therapeutic modulation. In a canine model, Prostaglandin E2 (PGE2) blocks the upregulation by allergen-induced serum factors of the responses of haemopoietic progenitors to Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF; Inman et al., 1997). Hence, it is of interest to evaluate whether PGE2 similarly affects the upregulation of bone-marrow responses to eosinopoietic cytokines in the murine model, which is more convenient for genetic and immunologic studies, and also involves allergen-induced, circulating factors (Gaspar Elsas et al., 1997).
Several early studies reported that different prostaglandins as well as cyclic AMP (cAMP)-elevating agents inhibited proliferation of murine (Kurland et al., 1977) and human (Taetle & Koessler, 1980) haemopoietic cells in vitro. Early in vivo studies also showed enhanced myelopoiesis associated with inhibition of prostaglandin biosynthesis in mice (Pelus, 1989), as well as induction of a myelopoiesis inhibitor in spleen and bone-marrow cultures from PGE2-injected mice (Gentile & Pelus, 1988). However, these studies are difficult to interpret, because: (a) the conditioned media used to stimulate haemopoietic cells in vitro contain several different, potentially interacting, growth factors; (b) the in vivo experiments do not address the direct effects of PGE2 on haemopoietic cells, since many different cell populations, as well as PGE2-induced mediators were involved (Pelus & Gentile, 1988, Gentile & Pelus, 1988). More recently, prostaglandins were shown to down-modulate murine haemopoiesis (O'Reilly & Gamelli, 1990, Kozubik et al., 1994), possibly acting through cyclic AMP-dependent mechanisms (Gamelli et al., 1994). PGE2 and dbcAMP directly inhibited murine GM colony formation (Gamelli et al., 1994). However, prostaglandin effects on murine and human bone-marrow may still involve inhibition of colony-stimulating factor production by stromal cells (Gamelli et al., 1998; Bug et al., 1998). As to the eosinophil lineage, which is relevant to asthma, selective inhibition of progenitors by PGE2 has not been reported, but cyclic AMP-elevating agents may either up- or down-modulate human GM-CSF-dependent mature eosinophil survival (Hallsworth et al., 1996).
We have reevaluated the direct effects of PGE2 and cyclic AMP-elevating agents on responses to exogenously added, recombinant, eosinopoietic cytokines in murine bone-marrow. We used assays that distinguish between their effects on progenitors (colony-forming cells) and precursors (noncolony-forming, lineage-committed cells derived from the former; Bagby, 1994), because these two classes of targets are differently affected by allergen challenge: progenitors do not increase in total number, but become increasingly committed to eosinophil colony formation, while precursors yield larger numbers of terminally differentiated eosinophils (Gaspar Elsas et al., 1997). Since the effects of PGE2 and dbcAMP, as those of the eosinopoietic cytokines themselves, may be modified by allergen exposure, we further examined whether the ability of these agents to modulate responses to GM-CSF and to IL-5 was modified by ovalbumin-sensitization and challenge.
Methods
Animals and animal procedures
For most of the study, male and female BP-2 and BALB/c mice bred at Elevage Janvier (Le Genest Saint-Isle, France), were used at 6–8 weeks of age. BALB/c mice bred at Fiocruz, Rio de Janeiro (Brazil), yielded comparable results. A/J, CBA/J and C57BL/10 mice bred at Fiocruz were also used. BALB/c and BP-2 mice were immunized with two s.c. 0.4 ml injections of 100 μg ovalbumin mixed with 4 mg ml−1 Al(OH)3 in 9% NaCl, at 7 day intervals. BALB/c mice were intranasally challenged with 10 μg ovalbumin in 50 μl 0.9% NaCl, 1 week after the second injection, a procedure that increases responses to eosinopoietic cytokines but does not induce hyperreactivity (Gaspar Elsas et al., 1997). In selected experiments, BP-2 mice were submitted to repeated challenges (twice a day, for 2 days), which induce intense bronchopulmonary hyperreactivity in this strain (Eum et al., 1995; Haile et al., 1999). All groups were sacrificed 24 h after completing the challenge procedure. Animal handling followed the standard procedures adopted at the Institut Pasteur, Paris (France).
Reagents
Heat-inactivated Foetal Calf Serum (FCS), L-glutamate and culture media were from Gibco (Life Technologies SARL, Cergy Pontoise, France), Diff-Quick from DADE Diagnostika GmbH (Unterschleisshei, Germany) and Harris' Hematoxylin from Réactifs RAL (Paris, France). Recombinant rmIL-5 was from Pharmingen (San Diego, CA, U.S.A.). Dibutiryl cyclic AMP (dbcAMP), dibutiryl cyclic GMP (dbcGMP) were from Biolmol Research Laboratories Inc., Plymouth, U.S.A. Forskolin was from Sigma France. Rolipram was from Sanofi Labs., Montpellier, France. Prostaglandins E2 (PGE2) and D2 (PGD2) were from Cayman Chemical Co., Ann Arbor, U.S.A. PGE2 was prepared as a 10−3 M stock solution in pure ethanol and diluted in medium before use. Control cultures with ethanol at the same final concentration found in PGE2-treated cultures were included. Isoproterenol was from Sigma and prepared as a 10−5 M stock solution in medium. Ovalbumin (5× crystallized) was from ICN Biomedicals, Inc. (Costa Mesa, CA, U.S.A.), Al(OH)3 from Merck (Darmstadt, Germany).
Bone-marrow cell studies
Bone-marrow cell harvest, identification and separation
Bone-marrow cells, collected by flushing the two femurs of 5–8 mice with RPMI 1640 medium containing 10% FCS, were washed and counted. The frequency of cells stained for eosinophil peroxidase (EPO) following the protocol of Ten et al. (1989) was determined in cytocentrifuge smears (Gaspar Elsas et al., 1997). The cytochemical pattern of EPO+ cells in stained bone-marrow preparations was identified to that described by Horton et al. (1996).
Progenitor assays
1 ml semi-solid cultures were established in 35 mm culture dishes, from 2×105 cells, at least in triplicate, in a mixture of Iscove's modified Dulbecco's medium, with 20% FCS and agar to 0.3% final concentration (Kurland et al., 1977; Gaspar Elsas et al., 1997), with GM-CSF at 2 ng ml−1, alone or in association with any of the following: PGE2, PGD2, Rolipram, forskolin, isoproterenol, dbcAMP, or dbcGMP, at the various concentrations detailed in Results. A colony was defined as progenitor-derived ensemble larger than 50 cells (Bagby, 1994). Colonies were scored at day 7 under the inverted microscope at low magnification, and the frequency of eosinophil colonies was determined on agar layers dried (50°C), mounted on microscope slides, stained for EPO, and scored under high magnification (Gaspar Elsas et al., 1997). We have previously confirmed that these conditions were adequate for counting total myeloid colonies and for accurate differential counts of myeloid colony types on dried agar layers (Gaspar Elsas et al., 2000).
Precursor assays
Liquid bone-marrow cultures (106 cells in a 1 ml volume, in a 24-well cluster, Gaspar Elsas et al., 1997) were seeded in RPMI 1640 medium, with 10% FCS, 2 mM L-glutamine, and penicillin-streptomycin, at 37°C, 5% CO2, 95% air, for 7 days, at least in triplicate, with rmIL-5 (Pharmingen, 0.01–1 ng ml−1), alone or in association with PGE2, PGD2, forskolin, dbcAMP, or dbcGMP, at the various concentrations detailed in Results. The frequency of EPO+ cells was determined after 7 days of culture in cytocentrifuge smears. We have previously confirmed that these conditions were adequate for evaluating eosinophil differentiation in both naïve and sensitized and challenged murine bone-marrow, and that the increase in per cent EPO+ cells, as directly determined on cytocentrifuge smears, was paralleled by actual increases in the total numbers of EPO+ cells in these cultures, as indirectly determined from total and differential cell counts (Gaspar Elsas et al., 2000).
Statistical analysis
The data were analysed with the help of the Systat for Windows version 4 software, using factorial analysis of variance, and with the Tukey correction for multiple comparisons between different treatments.
Results
Effect of PGE2 and cAMP-elevating agents on myeloid progenitors in bone-marrow cultures from naïve BP-2 mice
To define the effect of PGE2 on colony formation by progenitors from naïve mice, semi-solid cultures were established from bone-marrow of BP-2 mice, in the presence of GM-CSF, alone or with PGE2 (10−7–10−9 M). As shown in Figure 1, a significant decrease in the total number of myeloid colonies formed in the presence of GM-CSF and PGE2 (10−7 M) was observed, relative to the control cultures with GM-CSF alone (P=0.03). At 10−8–10−9 M, the effect of PGE2 was not significant. As also shown in Figure 1, a significant decrease in myeloid colony formation was observed when cultures were established in the presence of GM-CSF and dbcAMP (3×10−7–10−6 M), relative to the GM-CSF controls (P=0.007 and P=0.005, respectively). dbcAMP at 10−7 M had no effect.
Figure 1.
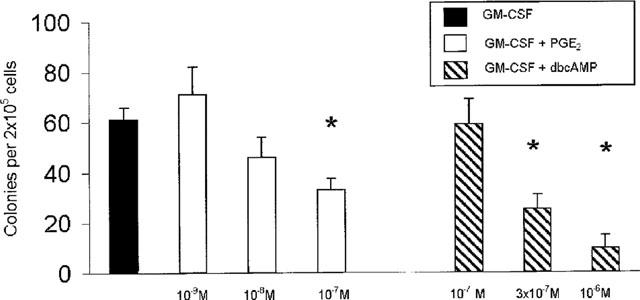
Effect of PGE2 and dbcAMP on GM-CSF-induced colony formation in naïve bone-marrow. The data are mean±s.e.mean of the number of myeloid colonies formed by bone-marrow from naïve BP-2 mice. GM-CSF was used at 2 ng ml−1. Asterisks indicate significant differences relative to the GM-CSF controls. Data are derived from 4–19 experiments.
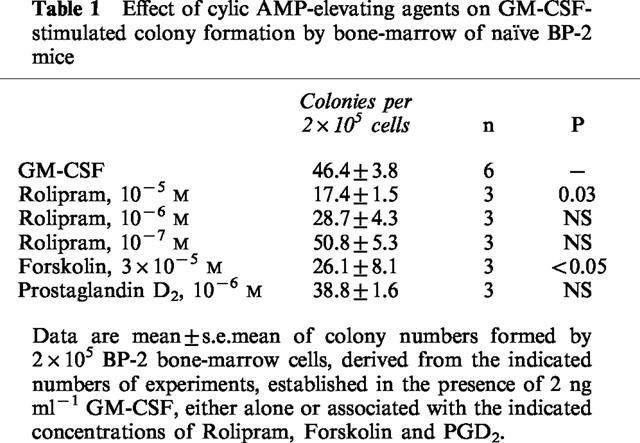
As shown in Table 1, a significant decrease in the total number of myeloid colonies formed by naïve BP-2 bone-marrow cells in the presence of GM-CSF and Rolipram (10−5 M) was observed, relative to the control cultures with GM-CSF alone (P=0.03). Rolipram, at 10−6–10−7 M, was ineffective. On the other hand, forskolin, at 3×10−5 M, also inhibited significantly colony formation (P<0.05). In contrast, Prostaglandin D2, at 10−6 M, had no effect.
Table 1.
Effect of cylic AMP-elevating agents on GM-CSF-stimulated colony formation by bone-marrow of naïve BP-2 mice
Effect of PGE2 and cylic AMP-elevating agents on myeloid progenitors in bone-marrow cultures from sensitized and challenged BP-2 mice
To define whether the ability of PGE2 and dbcAMP to down-modulate GM-CSF-stimulated colony formation was modified by allergen sensitization and challenge, we established bone-marrow cultures from BP-2 mice that had been sensitized to ovalbumin and either challenged repeatedly four times over 2 days or challenged once. In BP-2 mice challenged four times, GM-CSF-stimulated colony formation was decreased from 66±17 (mean±s.e.mean) in control cultures to 20±3.9 in cultures established in the presence of PGE2 at 10−7 M (P<0.001, n=8), and to 6±1.8 in cultures established with dbcAMP at 10−6 M (P<0.001, n=6). In contrast, PGE2 at 10−8–10−9 M, or dibutyryl cyclic GMP (dbcGMP), at 10−6 M, had no effect. Similar effects were observed in BP-2 mice challenged only once (P<0.001).
Effect of PGE2 and cyclic AMP-elevating agents on myeloid progenitors in naïve mice from different strains
To define whether the sensitivity to inhibition of colony formation by PGE2 was dependent on the genetic background of the bone-marrow donors, we established semi-solid bone-marrow cultures from mice of the A/J, CBA/J, C57BL/10 and BALB/c strains, in the presence of GM-CSF, alone or associated with PGE2 (10−7–10−9 M). As shown in Figure 2, GM-CSF-stimulated colony formation, which varied from one strain to the other, was significantly inhibited in bone-marrow from the A/J, CBA/J and C57BL/10 strains (P<0.01 in all cases) by addition of PGE2 at one or more of the concentrations tested. The effective dose range varied from one strain to the other, and A/J bone-marrow progenitors were significantly inhibited at a concentration (10−9 M) that was no longer effective for the other strains. In contrast, BALB/c progenitors were not inhibited at any of the PGE2 concentrations tested, and were unaffected even by PGE2 at 10−6 M.
Figure 2.
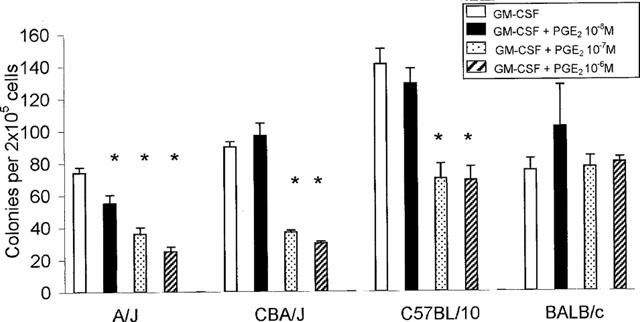
Effect of PGE2 on GM-CSF-induced colony formation in bone-marrow from different mouse strains. The data are mean±s.e.mean of the number of myeloid colonies formed by bone-marrow from naïve mice of the indicated strains. GM-CSF was used at 2 ng ml−1. Asterisks indicate significant differences relative to the respective GM-CSF controls (P<0.01 in all cases). Data are derived from 3–19 experiments.
To define whether unresponsiveness to PGE2 in BALB/c mice originated in a defect in cyclic AMP-dependent signalling mechanisms, we established semi-solid cultures in the presence of GM-CSF, alone or associated with PGE2, dbcAMP, forskolin or isoproterenol. Colony numbers per 2×105 bone-marrow cells plated were significantly decreased, from 77±8.3 (mean±s.e.mean; data are derived from 12 experiments) in the presence of GM-CSF alone, to 16±5.2 (P=0.029) when dbcAMP (10−6 M) was added, while PGE2 at either 10−7 or 10−6 M had no significant effect (92±10 and 95±5.4, respectively). In another series of experiments, colony formation by 2×105 cells was significantly decreased, from 54±2.6 in control cultures (mean±s.e.mean, data are derived from six experiments) to 25±4 in cultures established in the presence of forskolin, at 10−4 M (P<0.001). In contrast, forskolin at 3×10−5 M was ineffective. In a further series of experiments, isoproterenol at 10−6–10−11 M did not significantly inhibit colony formation by BALB/c bone-marrow cells. In contrast, isoproterenol at 10−6–10−7 M strongly inhibited colony formation by bone-marrow cells from C57BL/10 mice (not shown).
Lack of lineage-selectivity in progenitor inhibition by PGE2 and dbcAMP
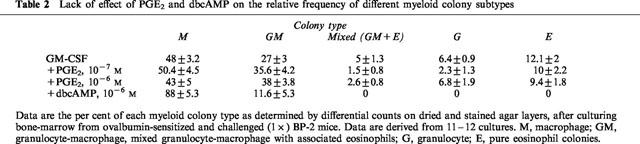
To define whether inhibition of colony formation in BP-2 bone marrow correlated with selective down-regulation of the eosinophil lineage, we performed differential colony counts on dried and stained semisolid cultures. As shown in Table 2, no significant difference in eosinophil colony frequency, either pure or mixed, was found between cultures established in the presence of GM-CSF alone and those to which PGE2 had been added, at 10−7–10−8 M, even though total myeloid colony number was significantly reduced in the latter (see above). No selective inhibition of GM colonies was observed. In contrast, cultures established in the presence of dbcAMP (10−6 M) contained no eosinophil colonies, either pure or mixed, nor granulocyte colonies.
Table 2.
Lack of effect of PGE2 and dbcAMP on the relative frequency of different myeloid colony subtypes
Effect of PGE2 and cyclic AMP-elevating agents on eosinophil precursors
To define whether PGE2 inhibited responses of eosinophil precursors to rmIL-5, liquid bone-marrow cultures were established from bone marrow of ovalbumin-sensitized and challenged (1×) BP-2 mice, which responds vigorously in a 7 day-eosinophil differentiation assay (Gaspar Elsas et al., 1997), in the presence of various concentrations of rmIL-5 and of PGE2. As shown in Figure 3, PGE2 (10−7–10−9) inhibited IL-5 driven eosinophil differentiation for all rmIL-5 concentrations. However, the effect was most marked when 1 ng ml−1 rmIL-5, which yields plateau stimulation (see Methods) was used. For further analysis, the effect of PGE2 was studied at 10−7 M, in the presence of 1 ng ml−1 rmIL-5, unless otherwise indicated. To define whether the effect of PGE2 on IL-5-driven eosinophil differentiation was immediate, bone-marrow cultures were established from BP-2 bone-marrow with rmIL-5 alone or associated with PGE2 at 10−7 M, and the per cent EPO+ cells was determined at various times after initiation of the culture. In parallel control cultures, bone-marrow was cultured for 7 days in the presence of rmIL-5, and PGE2 was added 30 min before cells were harvested. As shown in Figure 4, EPO+ cell numbers steadily increased from day 1 to day 7 of culture, reflecting eosinophil differentiation from EPO−precursors (per cent EPO+ cells in the initial BP-2 bone-marrow inoculum was 1.5±0.6, mean±s.e.mean, n=4). At 7 days of culture, plateau levels were reached in the control cultures. At day 7, EPO+ cell numbers were significantly reduced in cultures containing PGE2. Cultures containing rmIL-5 associated with effective concentrations of PGE2 presented, by day 7, large numbers of viable bone-marrow derived macrophages (which do not require haemopoietic factors to survive), but had fewer morphologically intact EPO+ cells than control cultures. However, EPO+ granules could be found inside macrophages, suggesting the latter had phagocytosed dead EPO+ cells. Cultures exposed to PGE2 for only 30 min showed no decrease in EPO+numbers relative to rmIL-5-treated controls.
Figure 3.
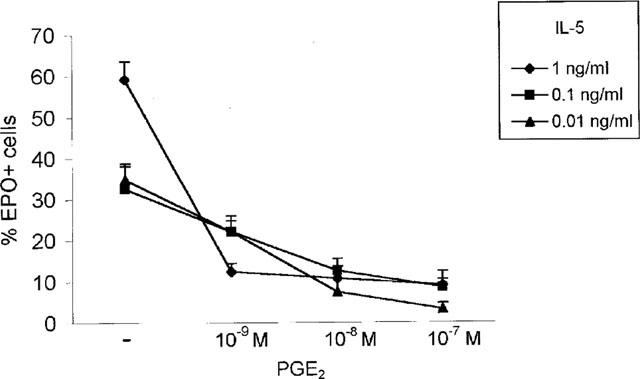
Effect of PGE2 on rmIL-5-driven eosinophil differentiation in bone-marrow from BP-2 mice. Data are mean±s.e.mean of the per cent EPO+ cells in liquid cultures established from ovalbumin-sensitized and challenged BP-2 mice, with IL-5 at the indicated concentrations, without PGE2 (−) or with addition of PGE2, (10−7–10−9 M) for 7 days. Inhibition was significant for all PGE2 concentrations, relative to the respective control cultures. Data are derived from three experiments.
Figure 4.
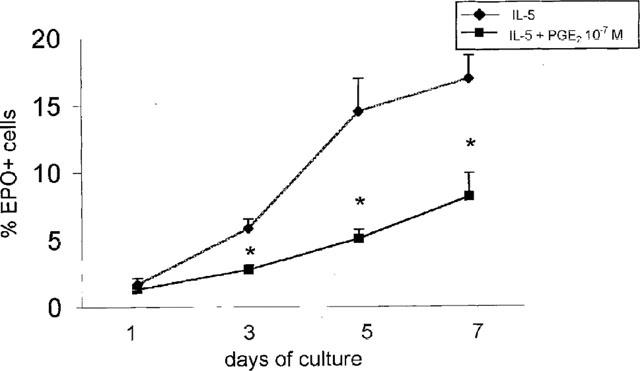
Effect of PGE2 on IL-5-driven eosinophil differentiation in bone-marrow from BP-2 mice. Data are mean±s.e.mean of the per cent EPO+ cells in liquid cultures established from naïve BP-2 mice, in the presence of 1 ng ml−1 rmIL-5, without PGE2 (IL-5) or with addition of PGE2, (10−7 M) for the indicated number of days. Asterisks indicate significant differences relative to the respective rmIL-5 controls. Data are derived from 4–10 experiments.
To define whether unresponsiveness to PGE2 in bone-marrow progenitors was associated with similar defect in eosinophil precursors, liquid cultures were established from bone-marrow of naïve or of ovalbumin-sensitized and challenged BALB/c mice, for 7 days, in the presence of rmIL-5, alone or associated with PGE2. As shown in Figure 5, eosinophil differentiation occurred in the presence of rmIL-5 in bone-marrow from both naïve and immune mice. Confirming previous observations (Gaspar Elsas et al., 1997), higher plateau levels were attained in cultures of sensitized and challenged bone-marrow. Addition of PGE2, at 10−6–10−8 M, inhibited significantly the responses to rmIL-5 in naïve and bone-marrow (P<0.003 for all comparisons). In contrast, no inhibition was observed with PGE2 at 10−9 M, nor with PGD2, at 10−6–10−7 M. On the other hand, bone-marrow from sensitized and challenged mice was similarly inhibited by PGE2 at 10−7–10−8 M. This shows that, in contrast to BALB/c progenitors, eosinophil precursors in this strain are as sensitive to inhibition by PGE2 as those from BP-2 mice. Hence, the study of the BALB/c strain makes it possible to clearly distinguish between PGE2 effects on progenitors and on precursors.
Figure 5.
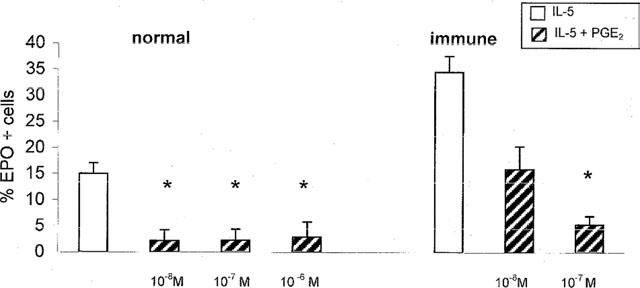
Effect of PGE2 on IL-5-driven eosinophil differentiation in bone-marrow from BALB/c mice. Data are mean±s.e.mean of the per cent EPO+ cells in liquid cultures established from naïve (normal) or ovalbumin-sensitized and challenged (immune) BALB/c mice in the presence of IL-5 (1 ng ml−1) alone or with PGE2 at the indicated concentrations. Asterisks indicate significant differences relative to the respective rmIL-5 controls (P<0.003 in all cases). Data are derived from 9–10 experiments.
To define whether PGE2 could act after bone-marrow cells had been exposed to rmIL-5, we established cultures in the presence of rmIL-5, and added PGE2 as a single dose (to a final 10−7 M concentration) to the cultures at various intervals. As shown in Table 3, while PGE2 added at day 1 was as effective as PGE2 present from the beginning of the culture in inhibiting responses to rmIL-5 from both BALB/c and BP-2 bone-marrow, addition of PGE2 after culturing cells for 48 h in rmIL-5 alone was ineffective, even though these cultures were exposed to PGE2 for 5 more days. These findings indicate that the target for PGE2 is a relatively immature precursor, that probably becomes more resistant at later stages.
Table 3.
Effect of late addition of PGE2 on IL-5-driven eosinophil differentiation in liquid culture

Inhibition of responses to rmIL-5 could be duplicated by cyclic AMP-elevating agents: in experiments with BP-2 bone-marrow, the per cent EPO+ was significantly decreased from 27±5.6 (mean±s.e.mean; data derived from six cultures) in the presence of rmIL-5 alone, to 3.1±1.2 (P<0.001) when PGE2 (10−7 M) was added, and to 10.8±2.8 (P<0.01) with addition of forskolin (3×10−5 M). Addition of dbcAMP (10−6 M) to cultures from both BP-2 and BALB/c mice totally blocked responses to rmIL-5, and no intact EPO+ cells were present by day 7.
Discussion
Several studies suggest that prostaglandins and cyclic AMP-elevating agents exert regulatory effects on haemopoiesis, both by modulating production of haemopoietic factors (Quill et al., 1989; O'Reilly & Gamelli, 1990; Gamelli et al., 1994; Bug et al., 1998; Borger et al., 1998; Kaminuma et al., 1997), and by affecting the response to these factors (Gamelli et al., 1998; Kozubik et al., 1994; Piacibello et al., 1991). Our findings provide further evidence that PGE2 directly down-regulates the progenitor response to GM-CSF in murine bone-marrow. Experiments using several cyclic AMP-elevating agents yielded similar results, suggesting that these effects of PGE2 involve cyclic AMP-dependent pathways. We have further examined whether the inhibitory effects of PGE2 and dbcAMP, which are known to be modified by the state of maturation of the target cells (Piacibello et al., 1991), by the presence of concurrent stimulatory signals (Visnjic et al., 1995) and by target cell priming (Hallsworth et al., 1996), would also be modified by allergen sensitization and challenge. Since similar effects were observed in bone-marrow cultures from ovalbumin-sensitized and challenged mice, and since PGE2 (and other putative inhibitory prostaglandins) are generated both in vivo and in bone-marrow cultures (Gamelli et al., 1994), the possible contribution of endogenous prostaglandin production to modulation of haemopoietic progenitors in murine bone-marrow must be taken into account when the effects of allergen sensitization and challenge on production of eosinopoietic cytokines (Minshall et al., 1998), or on response to these cytokines (Gaspar Elsas et al., 1997) are studied.
Our findings also allow for a comparison of the effects of PGE2 and dbcAMP. Although both stimuli inhibited myeloid progenitors and eosinophil precursors, some differences were observed. As a rule, dbcAMP inhibited both targets more profoundly than PGE2. Furthermore, dbcAMP completely inhibited formation of eosinophil and granulocyte colonies, an effect that was not detectable in PGE2-treated cultures, even at very high prostaglandin concentrations. On the other hand, our findings differ from those of previous reports (Kurland et al, 1977; Taetle & Koessler, 1980) in that: (a) we found no significant potentiation of colony formation by dbcGMP and (b) GM progenitors were not selectively inhibited by PGE2 in our conditions. The reason for these discrepancies may be linked to the different experimental conditions, since these early studies used conditioned media, the effects of which are known to differ in important ways from those of pure, recombinant cytokines, due to the presence of a mixture of active factors in the former (Bagby, 1994).
The inhibitory effects of PGE2 varied among different inbred strains, and bone-marrow progenitors from BALB/c mice were totally resistant to PGE2 over a large series of experiments. This argues against the effects of PGE2 being due to nonspecific toxicity for bone-marrow progenitors. Furthermore, progenitors from BALB/c bone-marrow were inhibited by dbcAMP and by forskolin. This suggests that BALB/c mice have a defect in the progenitor response to PGE2 that can be circumvented by cyclic AMP-elevating agents. Since isoproterenol did not inhibit colony formation in this strain, while it strongly suppressed progenitors from the C57BL/10 strain, which also responded to PGE2, it is possible that the defect in BALB/c progenitors affects receptor-cyclase coupling, although a decreased sensitivity of the cyclase to activating stimuli (such as forskolin) may also be present.
We have also analysed whether PGE2 blocked lineage-specific responses to rmIL-5 in eosinophil precursors, an issue which has not been addressed in previous studies (Inman et al., 1997; Sehmi et al., 1997; Gaspar Elsas et al., 1997). Our findings indicate that PGE2 strongly inhibits IL-5-driven eosinophil differentiation. The availability of BALB/c mice, which resist inhibition of colony formation, made it possible to show that blockade of eosinophil precursor responses to rmIL-5 by PGE2 can occur in the absence of any demonstrable effect on myeloid progenitors. Also, in the BP-2 strain, eosinophil progenitors were not inhibited by PGE2 at 10−8 M, but this prostaglandin concentration was inhibitory for eosinophil precursor responses to rmIL-5. Finally, the examination of the frequency of the different types of myeloid colonies provided no evidence of a significant effect on the eosinophil lineage at the progenitor level. In contrast, the analysis of the lineage-specific responses of eosinophil precursors to rmIL-5 revealed a significant effect of PGE2 at concentrations lower than those that inhibited the responses of progenitors of most mouse strains to GM-CSF. Again, inhibition of precursor responses did not seem to involve nonspecific toxicity, because: (a) addition of PGE2 after 48 h of the beginning of the culture did not inhibit responses to rmIL-5, and (b) cultures established in the presence of rmIL-5 and PGE2 did show a progressive increase in EPO+ cell numbers, showing that the conditions in the culture were permissive for the survival and differentiation of these cells, even though the magnitude of the plateau response was strongly reduced.
Taken together, our findings indicate that GM-CSF-responsive myeloid progenitors and IL-5-stimulated eosinophil precursors, which are known to differ in proliferative potential and colony-forming ability (Bagby, 1994) constitute separate targets for down-modulation by PGE2.
Acknowledgments
Supported by grants from Papes-Fiocruz, FINEP, CNPq-RHAE, INSERM-Fiocruz, CAPES-COFECUB and by Institute Pasteur-INSERM. M. Bodstein is a fellow from PiBiC-FioCRUZ. We thank Drs Mohamed Hatmi and Daniel Vial (Institut Pasteur) for the generous gift of reagents and helpful advice.
Abbreviations
- dbcAMP
dibutiryl cyclic adenosine monophosphate
- GM-CSF
granulocyte-macrophage colony stimulating factor
- IL-3
interleukin-3
- IL-5
interleukin-5
- PGD2
prostaglandin D2
- PGE2
prostaglandin E2
References
- BAGBY G.C.Hematopoiesis The Molecular Basis of Blood Diseases 1994Philadelphia: W.B. Saunders; 71–106.(2nd Edn.) eds. Stamatoyannopoulos, G., Nienhuis, A.W., Majerus, P.W., Varmus, H. pp [Google Scholar]
- BORGER P., VELLENGA E., GRINGHUI S.I., TIMMERMAN J.A.B., LUMMNEN C., POSTMA D.S., KAUFFMAN H.K. Prostaglandin E2 differentially modulates IL-5 gene expression in activated human T lymphocytes depending on the costimulatory signal. J. Allergy Clin Immunol. 1998;101:231–240. doi: 10.1016/s0091-6749(98)70388-4. [DOI] [PubMed] [Google Scholar]
- BUG G., AMAN J., HUBER C., PESCHEL C., DERIGS H.G. cAMP analogues downregulate the expression of granulocyte macrophage colony-stimulating factor (GM-CSF) in human bone marrow stromal cells in vitro. Mediators Inflamm. 1998;7:195–199. doi: 10.1080/09629359891135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUM S.Y., HAILÉ S., LEFORT J., HUERRE M., VARGAFTIG B.B. Eosinophil recruitment into the respiratory epithelium following antigenic challenge in hyper-IgE mice is accompanied by interleukin-5-dependent bronchial hyperresponsiveness. Proc. Natl. Acad. Sci. U.S.A. 1995;92:12290–12294. doi: 10.1073/pnas.92.26.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMELLI R.L., HE L.K., LIU H. Marrow granulocyte-macrophage progenitor cell response to burn injury as modified by endotoxin and indomethacin. J. Trauma. 1998;44:469–474. doi: 10.1097/00005373-199409000-00002. [DOI] [PubMed] [Google Scholar]
- GAMELLI R.L., HE L.K., LIU H., RICKEN J.D. Burn wound infection-induced myeloid suppression: the role of prostaglandin E2, elevated adenylate cyclase, and cyclic adenosine monophosphate. J. Trauma. 1994;37:339–346. doi: 10.1097/00005373-199803000-00008. [DOI] [PubMed] [Google Scholar]
- GASPAR ELSAS M.I., JOSEPH D., XAVIER, ELSAS P., VARGAFTIG B.B. Rapid increase in bone marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am. J. Resp. Cell. Mol. Biol. 1997;17:404–413. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- GASPAR ELSAS M.I., MAXIMIANO E.S., JOSEPH D., ALVES L., TOPILKO A., VARGAFTIG B.B., ELSAS P.X. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br. J. Pharmacol. 2000;129:1543–1552. doi: 10.1038/sj.bjp.0703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENTILE P.S., PELUS L.M. In vivo modulation of myelopoiesis by prostaglandin E2. IV. Prostaglandin E2 induction of myelopoietic inhibitory activity. J. Immunol. 1988;141:2714–2720. [PubMed] [Google Scholar]
- HAILE S., LEFORT J., JOSEPH D., GOUNON P., HUERRE M., VARGAFTIG B.B. Mucous-cell metaplasia and inflammatory-cell recruitment are dissociated in allergic mice after antibody- and drug-dependent cell depletion in a murine model of asthma. Am. J. Resp. Cell. Mol. Biol. 1999;20:891–902. doi: 10.1165/ajrcmb.20.5.3446. [DOI] [PubMed] [Google Scholar]
- HALLSWORTH M.P., GIEMBYCZ M.A., BARNES P.J., LEE T.H. Cyclic AMP-elevating agents prolong or inhibit eosinophil survival depending on prior exposure to GM-CSF. Br. J. Pharmacol. 1996;117:79–86. doi: 10.1111/j.1476-5381.1996.tb15157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTON M.A., LARSON K.A., LEE J.J., LEE N.A. Cloning of the murine eosinophil peroxidase gene (mEPO): characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J. Leukocyte Biol. 1996;60:285–294. doi: 10.1002/jlb.60.2.285. [DOI] [PubMed] [Google Scholar]
- INMAN M.D., DENBURG J.A., ELLIS R., DAHLBACK M., O'BYRNE P.M. Allergen-induced increase in bone marrow progenitors in airway hyperresponsive dogs: regulation by a serum hemopoietic factor. Am. J. Respir. Cell Mol. Biol. 1996;15:305–311. doi: 10.1165/ajrcmb.15.3.8924277. [DOI] [PubMed] [Google Scholar]
- INMAN M.D., DENBURG J.A., ELLIS R., DAHLBÄCK M., O'BYRNE P.M. The effect of treatment with budesonide or PGE2 in vitro on allergen-induced increases in canine bone marrow progenitors. Am. J. Respir. Cell. Mol. Biol. 1997;17:634–661. doi: 10.1165/ajrcmb.17.5.2746. [DOI] [PubMed] [Google Scholar]
- KAMINUMA O., MORI A., OGAWA K., WADA K., KIKKAWA H., NAITO K., SUKO M., OKUDAIRA H. Two differential effects of cyclic adenosine 3′,5-monophosphate on IL-5 production by antigen-specific human T cell line. J. Pharm. Exp. Therap. 1997;283 Iss 1:345–349. [PubMed] [Google Scholar]
- KOZUBIK A., HOFMANOVA J., POSPISIL M., NETIKOVA J., HOLA J., LOJEK A. Effects of drugs inhibiting prostaglandin or leukotriene biosynthesis on postirradiation haematopoiesis in mouse. Int. J. Radiat. Biol. 1994;65:369–377. doi: 10.1080/09553009414550431. [DOI] [PubMed] [Google Scholar]
- KURLAND J.I., HADDEN J.W., MOORE M.A.S. Role of cyclic nucleotides in the proliferation of committed granulocyte-macrophage progenitor cells. Cancer Res. 1977;37:4534–4538. [PubMed] [Google Scholar]
- MINSHALL E.M., SCHLEIMER R., CAMERON L., MINNICOZZI M., EGAN R.W., GUTIERREZ-RAMOS J.C., EIDELMAN D.H., HAMID Q. Interleukin-5 expression in the bone marrow of sensitized Balb/c mice after allergen challenge. Am. J. Respir. Crit. Care Med. 1998;158:951–957. doi: 10.1164/ajrccm.158.3.9709114. [DOI] [PubMed] [Google Scholar]
- O'REILLY M., GAMELLI R.L. Indomethacin augments granulocyte-macrophage colony-stimulating factor-induced hematopoiesis following 5-FU treatment. Exp. Hematol. 1990;18:974–978. [PubMed] [Google Scholar]
- PELUS L.M. Blockade of prostaglandin biosynthesis in intact mice dramatically augments the expansion of committed myeloid progenitor cells (colony-forming units, granulocyte, macrophage) after acute administration of recombinant human IL-1 alpha. J. Immunol. 1989;143:4171–4179. [PubMed] [Google Scholar]
- PELUS L.M., GENTILE P.S. In vivo modulation of myelopoiesis by prostaglandin E2. III. Induction of suppressor cells in marrow and spleen capable of mediating inhibition of CFU-GM proliferation. Blood. 1988;71:1633–1640. [PubMed] [Google Scholar]
- PIACIBELLO W., FERRERO D., SANAVIO F., BADONI R., STACCHINI A., SEVERINO A., AGLIETTA M. Responsiveness of highly enriched CFU-GM subpopulations from bone marrow, peripheral blood, and cord blood to hemopoietic growth inhibitors. Exp. Hematol. 1991;19:1084–1089. [PubMed] [Google Scholar]
- QUILL H., GAUR A., PHIPPS R.P. Prostaglandin E2-dependent induction of granulocyte-macrophage colony-stimulating factor secretion by cloned murine helper T cells. J. Immunol. 1989;142:813–818. [PubMed] [Google Scholar]
- SEHMI R., WOOD L.J., WATSON R., FOLEY R., HAMID Q., O'BYRNE P.M., DENBURG J.A. Allergen-induced increases in IL-5 Receptor α-subunit expression on bone marrow-derived CD34+ cells from asthmatic subjects. A novel marker of progenitor cell commitment towards eosinophilic differentiation. J. Clin. Invest. 1997;100:1–10. doi: 10.1172/JCI119789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAETLE R., KOESSLER A. Effects of cyclic nucleotides and prostaglandins on normal and abnormal human myeloid progenitor proliferation. Cancer Res. 1980;40:1223–1229. [PubMed] [Google Scholar]
- TEN R.M., PEASE L.R., MCKEAN D.J., BELL M.P., GLEICH G.J. Molecular cloning of human Eosinophil Peroxidase. Evidence for the existence of a Peroxidase multigene family. J. Exp. Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VISNJIC D., BATINIC D., LASIC Z., KNOTEK M., MARUSIC M., BANFIC H. Phorbol 12-myristate 13-acetate-mediated signalling in murine bone marrow cells. Biochem. J. 1995;310:163–170. doi: 10.1042/bj3100163. [DOI] [PMC free article] [PubMed] [Google Scholar]


