Abstract
In Suncus murinus the ultrapotent capsaicin analogue resiniferatoxin (RTX) induced an emetic response in the dose range 1–1000 μg kg−1, s.c. The latency was inversely related to dose and ranged from 41.2±4.4 min. (1 μg kg−1, s.c.) to 2.7±0.6 min. (1000 μg kg−1, s.c.).
The emetic response to RTX (10 or 100 μg kg−1, s.c.) was blocked or markedly reduced by pre-treatment with RTX (100 μg kg−1, s.c.), 8-OH-DPAT (100 μg kg−1, s.c.), morphine (2 mg kg−1, s.c.), neonatal capsaicin (100 mg kg−1, s.c.) and the NK1 receptor antagonist CP-99,994 (10–20 mg kg−1, s.c.) but not by the 5-HT3 receptor antagonist tropisetron (200 μg kg−1, s.c.).
RTX (100 μg kg−1, s.c.) induced c-fos-like immunoreactivity in the area postrema and parts of the nucleus tractus solitarius. This pattern is consistent with the proposal that the emetic effect is mediated via one or both of these structures and an involvement of substance P is discussed.
RTX (10 and 100 μg kg−1, s.c.) had broad-spectrum antiemetic effects in Suncus as indicated by its ability to block or markedly reduce the emetic response to motion (1 Hz, 4 cm lateral, 10 min.), cisplatin (20 mg kg−1, i.p.), intragastric copper sulphate (40 mg kg−1, p.o.), nicotine (10 mg kg−1, s.c.) and RTX (100 μg kg−1, s.c.) itself.
It is proposed that the site of the anti-emetic effect is in the nucleus tractus solitarius and mechanisms involving the modulation of substance P release are discussed.
The general utility of Suncus for investigations of vanilloid receptors is reviewed in the light of the exquisite sensitivity of the emetic reflex in this species to resiniferatoxin.
Keywords: Emesis, vomiting, NK1 receptor, substance P, resiniferatoxin, capsaicin, motion, vanilloid receptors, anti-emetic
Introduction
Resiniferatoxin (RTX) is a naturally occurring ultrapotent analogue of capsaicin found in the latex of plants of the species Euphorbia and first isolated from a cactus-like plant found in Morocco–Euphorbia resinifera. Its general pharmacological properties have been reviewed extensively (Szallasi & Blumberg, 1989, 1990; Szallasi, 1994, 1995).
Prompted by the observation that RTX could be administered subcutaneously to rats and mice without apparent algesic effects and could block responses mediated by small diameter visceral afferents, Andrews & Bhandari (1993) investigated whether RTX had any effect against the emetic response to stimuli mediated by activation of abdominal vagal afferents, the vast majority of which are unmyelinated. Intragastric copper sulphate and whole body X-radiation were selected as two such stimuli and RTX (100 μg kg−1, s.c.) markedly reduced or blocked the emetic response to these agents in the ferret. Loperamide, an opioid receptor agonist known to induce emesis via a medulla oblongata site of action and not requiring the abdominal vagi for its emetic effects (Bhandari et al., 1992) was investigated as a ‘control' for the site of action of RTX. Surprisingly, the emetic effect of loperamide was also reduced by RTX. This led Andrews & Bhandari (1993) to conclude that RTX had broad spectrum anti-emetic effects as it affected emesis induced by both central and peripherally acting stimuli. They proposed that RTX acted by depletion of a neurotransmitter (substance P or CGRP) in the nucleus tractus solitarius. These conclusions are of particular interest in the light of the recognition of the wide-ranging anti-emetic effects of neurokinin1 receptor antagonists (see Discussion section and Watson et al., 1995a,1995b). Because of the cost of using RTX in a large animal and the absence of a characterized emetic response to motion in the ferret it was decided to undertake further studies of the anti-emetic effects of RTX in Suncus murinus, the house musk shrew. This animal belongs to the Insectivora considered to be the most primitive and earliest Eutherians and hence phylogenetically more closely related to primates than carnivores, rodents and lagomorphs (Colbert, 1958; Abe, 1967). The adults of this species weigh between 30–80 g and the emetic response to a wide range of stimuli including motion has been well characterized (Ueno et al., 1988; Torii et al., 1991a, 1993; Matsuki et al., 1992; Mutoh et al., 1992; Andrews et al., 1996). During the course of the studies it became apparent that Suncus vomited readily in response to RTX and therefore the scope of the study was extended to investigate the mechanism and site of this novel effect of RTX.
Methods
Animals
Studies were performed on adult (>3 months of age) Suncus murinus of either sex weighing between 30 and 80 g. They were housed in groups of 4–6 in temperature- (∼24°C) and humidity-controlled rooms with a 12 : 12 light-dark cycle. The animals were obtained from established breeding colonies either at the University of Tokyo or at St. George's Hospital Medical School. They were maintained on commercial food pellets and allowed free access to water. Studies were conducted according to the guiding principles for the care and use of laboratory animals approved by the Japanese Pharmacological Society or under the Animals (Scientific Procedures) Act 1986.
Induction and observation of emesis
The emetic response in Suncus usually consists of retching (rhythmic abdominal contractions) and vomiting (forceful oral expulsion of upper gastrointestinal contents). However, whilst the occurrence of retching can be observed, in contrast to species such as the ferret, cat and dog, the frequency of individual retches (∼4 Hz) is too high to be counted by direct observation (Andrews et al., 1996). For this reason the responses are quantified in terms of ‘emetic episodes' as has been the case in previous studies of this species (e.g. Ueno et al., 1988; Tattersall et al., 1995).
Drug-induced emesis
Animals were observed individually in transparent perspex cages (20 cm×10 cm×10 cm). Nicotine, resiniferatoxin or vehicle were administered by subcutaneous injection into the interscapular region. Following nicotine (10 mg kg−1, s.c.) animals were observed for 1 h, a period exceeding that previously demonstrated to be adequate to encompass the entire response to this agent (Tattersall et al., 1994). In the initial emetic studies with RTX (0.1–1000 μg kg−1) an observation period of 2 h was used but as episodes were not noted beyond 1 h, this was reduced to 1 h. A 2-h period was used in the anti-emetic studies to ensure that the agents studied had not merely modified the temporal pattern of the response.
The pharmacology of the emetic response to RTX (10 or 100 μg kg−1 s.c.) was investigated by administration of the following agents (or 154 mM NaCl vehicle) at doses and pretreatment times (30 min) previously demonstrated to have anti-emetic effects against other stimuli in Suncus: morphine sulphate (2 mg kg−1, s.c., Selve et al., 1994; Andrews et al., 1996), CP-99,994 (10 mg kg−1 s.c., Tattersall et al., 1995), tropisetron (200 μg kg−1, s.c., Torii et al., 1991b), 8-hydroxy-2-(di-n-propylamino) tetralin (8-OH-DPAT, 100 μg kg−1, s.c., Okada et al., 1994).
Motion-induced emesis
Animals were placed individually in a transparent observation cage (10×15×12 cm) on a reciprocal shaker (TAITEC R-30 mini, Taiyo Scientific Industrial Co. Ltd., Japan) 60 min. after treatment with RTX vehicle (Tween 80 : ethanol : saline in the ratio 1 : 1 : 8), RTX (1,10 or 100 μg kg−1, s.c.), or nicotine (10 mg kg−1, s.c.). They were allowed 5 min acclimatization as previously described before the shaker started (Okada et al., 1994). The motion stimulus used was a 4 cm horizontal oscillation at a frequency of 1 Hz applied for 10 min. This stimulus has previously been shown to induce emesis reliably in this species (Ueno et al., 1988). The vast majority of emetic episodes occurred during motion exposure with isolated episodes in the following minute after motion ceased. Individual animals were exposed to this stimulus on no more than two occasions separated by at least 1 week to avoid adaptation to motion exposure (Ueno et al., 1988).
The same protocol was used to test animals which had been treated with capsaicin or vehicle neonatally.
Surgery
Abdominal vagotomy
Under barbiturate anaesthesia (sodium pentobarbital, 50 mg kg−1, i.p.) the abdomen was opened via a midline incision. The dorsal and ventral abdominal vagal trunks were identified running over the oesophagus, mobilized, ligated and sectioned. The abdomen was closed in layers and the animal treated with antibiotic. Recovery was uneventful, with the animals gaining weight and appearing to eat and drink normally. At least 1 week was allowed between surgery and emetic testing.
Cerebral ventricular cannulation
For intracebroventricular (i.c.v.) administration of RTX or vehicle, a stainless steel guide cannula was implanted at least 1 week prior to testing. For surgery, animals were anaesthetized with sodium pentobarbital (50 mg kg−1., i.p.) and placed in a stereotaxic frame. Under aseptic conditions a sagittal incision about 1 cm long was made in the skin between the inter-ocular and inter-aural lines. Connective tissue was cleared from the skull to expose bone. The convergence point of skull sutures (lambda) was used as the reference point for implantation. A hole 1.0 mm in diameter was drilled through the skull, 9.3 mm rostral and 0.9 mm lateral, to lambda and the guide cannula implanted at a depth of 3.4 mm. The cannula was anchored to a small stainless steel screw inserted into the right side of the skull. The cannula and anchor screw were secured with dental cement. Recovery from surgery was uneventful. For drug administration, a fine needle attached to a microsyringe (Hamilton) was inserted through the guide cannula and after ensuring that its tip was located in the ventricle 3 μl of vehicle (Tween 80 : ethanol : saline in the ratio 1 : 1 : 8) or RTX (0.6–6.0 μg) was injected. Animals were observed as described above. At the end of the observation period 3–5 μl of dye (trypan blue or methylene blue) was injected via the guide cannula, the animal killed (anaesthetic overdose) and the brain removed. The brain was cut coronally at the site of the guide cannula. These studies showed that the tip of the cannula was located in the third ventricle and that injected dye stained the ventricles.
c-fos immunocytochemistry
The animals were injected with resiniferatoxin (100 μg kg−1, s.c.) or vehicle s.c. and then observed for 2 h. After 2 h they were killed by anaesthetic overdose (Euthatal) and perfused immediately with ice-cold saline (154 mM NaCl) via a catheter inserted directly into the left ventricle of the heart. The right atrium was opened to release the blood and saline. When the fluid coming out of the right atrium was free of blood perfusion continued with 4% paraformaldehyde in 0.1% phosphate-buffered saline (PBS). Once the body was completely rigid, the brain including the medulla oblongata was removed and placed in 4% paraformaldehyde for at least 48 h.
Medulla oblongata slices were cut 100 μm thick using a vibraslice (Campden Instruments 752 M) and the sections placed into the wells of a 24-well culture plate containing immunobuffer (Reynolds et al., 1991). Immunobuffer composition: NaCl (7 gl−1), KCl (0.37 gl−1), Na2HPO4 (1.21 gl−1), Na2HPO4 (1.80 gl−1), Tris Base (Trizma) (1.2 g−1l), Triton X-100 (3 mll−1), merthiolate (0.4 gl−1); pH 7.4. Sections were washed in this buffer before adding 1% H2O2 and then rewashed. Sections were then blocked in 2% normal rabbit serum (Vectastain ABC kits, Vector Laboratories, Peterborough, U.K.) overnight. The next day the sections were placed in fos antibody (OA11-824; Cambridge Research Biochemicals, Northwich, U.K.; 1 : 2000 in immunobuffer and rabbit serum) for 48 h. After this time sections were washed and placed in the secondary rabbit anti-sheep antibody (1 : 200 in immunobuffer; Vectastain ABC kit) and left for a further 24 h.
The excess of secondary antibody was removed, the sections again washed and then placed in avidin-biotin horseradish peroxidase for 3 h. After washing in 50 mM Tris, the sections were reacted with 3,3′-diaminobenzidine complex (DAB) in 0.1 M PBS. After 10 min. the colour change was developed using 0.1% H2O2. After washing with 50 mM Tris, sections were mounted on to slides, allowed to dry, dehydrated (100% ethanol for 10 min) and defatted (Histoclear National Diagnostics, Hull, U.K. for 10 min).
Drugs
Nicotine (Sigma) was dissolved in 154 mM NaCl as were tropisetron (RBI), 8-hydroxy-DPAT (Sigma), cisplatin (Sigma), copper sulphate (Sigma), CP-99,994 ((+)-(2S,3S)-3-3(2-methoxybenzyl-amino)-2-phenylpiperidine–a gift from Pfizer Inc.) morphine hydrochloride (Sigma). RTX (Sigma and RBI) and capsaicin were made up in Tween 80 : ethanol : 154 mM NaCl in the ratio 1 : 1 : 8.
Statistics
Statistical comparisons were made using unpaired sample nonparametric (Mann-Whitney) tests or ANOVA as appropriate.
Results
The emetic effects of RTX and their pharmacology will be described before the anti-emetic effects of RTX itself against several stimuli.
The behavioural and emetic effect of RTX
General behavioural effects
RTX was given subcutaneously at doses of 0.1, 1, 10, 100, 500 or 1000 μg kg−1 to groups of male Suncus. It is important to emphasize that at none of the doses used did RTX appear to have any algesic effect as indicated by continuous vocalization, piloerection, urination, defaecation or scratching at the site of injection. This observation is consistent with the previous studies in the ferret, rat and mouse using similar dose ranges (Andrews & Bhandari, 1993; Woods et al., 1994; Szallasi & Blumberg, 1989; de Vries & Blumberg 1989). Two alterations in behaviour were observed and these are briefly described, although they were not quantified. Within 5 min of injection a slight tremor of the head was noted and at the same time mild twitching of the facial muscles around the eyes was apparent, although this was not necessarily synchronous in both eyes. Twitching of the peri-orbital muscles was seen in only one of six animals given 0.1 μg kg−1 RTX but it was not accompanied by head tremor. At higher doses the twitching continued throughout the observation period. There was some initial indication that RTX reduced the overall activity of the animals at doses >1 μg kg−1 but they became active again at the time of onset of emesis at which time some hindlimb motor weakness (e.g. splaying and incoordination) was noted. This was particularly noticeable at doses of >100 μg kg−1, although it apparently did not interfere with the ability of the animal to retch or vomit. With RTX doses of 100 μg kg−1 s.c., overt salivation was observed, although this was not observed at higher or lower doses. With the doses of 500 and 1000 μg kg−1 about 30 min after administration intense ano-genital grooming was observed, together with scratching of the flanks with the hindlimbs and occasional licking of the paws of the hindlimbs which lasted about 15 min. This appeared to be accompanied by cutaneous vasodilatation, although the pigmented nature of the skin made this difficult to assess.
The vehicle used for RTX (see Methods) did not induce any of the above effects in Suncus.
Emetic effects
Neither RTX vehicle nor a dose of 0.1 μg kg−1 RTX (n=6) induced emesis in any of the animals tested. Over the range 1–100 μg kg−1, s.c. RTX induced emesis in a dose-related manner in terms of the latency and intensity (emetic episodes) of the response (Figure 1). Three out of seven animals responded to 1 μg kg−1 with a few sporadic episodes at a latency of 41.2±4.4 min. In contrast, all animals in the group responded to 10 μg kg−1, s.c. (n=7) and 100 μg kg−1, s.c. (n=7). The latency of the response decreased with increasing dose and although the difference in latency between 1.0 and 10 μg kg−1, s.c. failed to reach significance (P=0.06), there was a significant difference in the latency between 10 and 100 μg kg−1, s.c. (P=0.007). There was a significant increase in the number of emetic episodes between 1 and 10 μg kg−1, s.c (P=0.007), although there was no change in the number of episodes as the dose increased to 100 μg kg−1, s.c. However, the response to 100 μg kg−1, s.c. was considered to be more intense as although the number of episodes was similar to the 10 μg kg−1 dose, they occurred over a shorter time (2.4±0.3 min vs 7.8±2.1 min, P=0.026).
Figure 1.
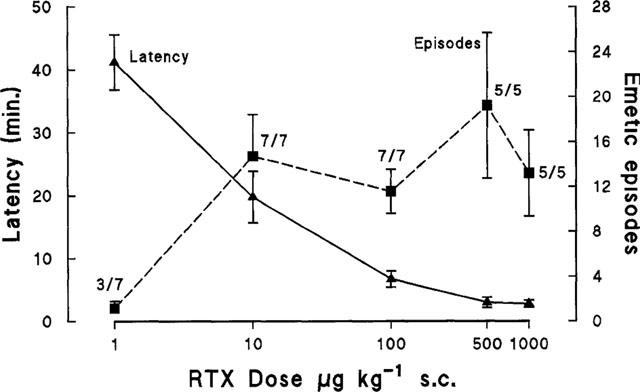
The relationship between the log-dose of resiniferatoxin (RTX) given subcutaneously to groups of Suncus and the mean±s.e.mean latency and number of emetic episodes induced over a 1-h observation period. The number of animals responding over the number tested is shown for each dose. For the dose of 1000 μg kg−1 the value for latency is calculated from the three animals with an early response (see text for details).
With doses of 500 μg kg−1, s.c. (Figure 2) and 1000 μg kg−1, s.c. the responses were more complex. The latency of the response to 500 μg kg−1, s.c. was significantly shorter than with 100 μg kg−1, s.c. (3.0±0.8 min, n=5 vs 6.7±1.3 min, P=0.048) and the total number of episodes increased, although not significantly (19.4±6.5 vs 11.6±2.0). A notable feature of the response to this dose (500 μg kg−1) was that emesis occurred in two distinct bursts in three of the five animals studied; the first beginning 2.3±0.2 min after injection and consisting of 5.7±2.3 episodes over 1.4±0.6 min and the second burst starting at 41.7±5.1 min after injection, with 22.7±4.4 episodes over 7.8±1.0 min (Figure 2).
Figure 2.
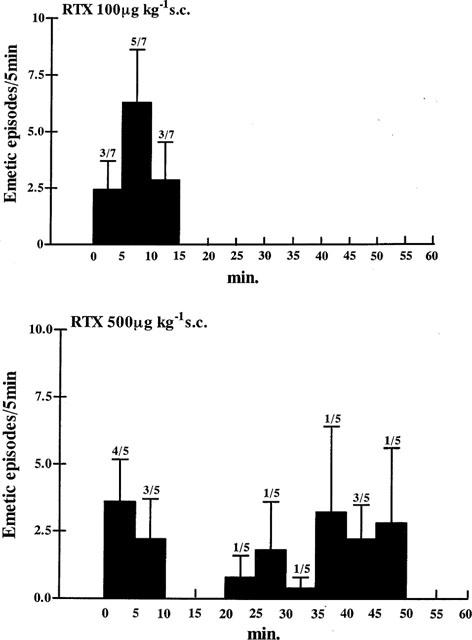
The profile of the emetic response to groups of Suncus given either 100 or 500 μg kg−1 s.c. The number (mean±s.e.mean) of episodes is plotted in 5 min time bins and the number of animals responding over those tested is shown for each time bin.
With 1000 μg kg−1, s.c. only three out of five animals had an early emetic response with a latency of 2.7±0.6 min and lasting 5.1±3.3 min during which there were 10.0±0.6 episodes. A delayed onset response was present in four animals. This began at 31.5±1.8 min and lasted 4.9±1.2 min with 10.7±1.9 episodes. The number of emetic episodes over the entire observation period in this group was 13.2±3.8 (n=5).
The pharmacology of the emetic response to RTX: the effect of RTX, tropisetron, 8-OH-DPAT, CP-99,994 and morphine
A variety of pharmacological treatments were tested for their effects against emesis induced by RTX usually given at 100 μg kg−1, s.c. The control animals for each group have been combined into one larger control group of animals (n=16) treated with RTX and treatment vehicle alone.
RTX
In four animals, in which emesis had been induced by an injection of RTX 100 μg kg−1, s.c., administration of a second dose 60 min later failed to induce any response including decrease in activity and eye-twitching. To test whether the lack of emetic effect was due to a failure of the animals to respond specifically to RTX or to a refractory emetic reflex the effect of two administrations of another emetic agent, nicotine (10 mg kg−1, s.c.), given 60 min apart, was tested.
Nicotine (10 mg kg−1, s.c.) induced emesis in all animals tested with a latency of 2.6±0.1 min and 17.6±1.3 episodes over 3.8±0.3 min (n=14). A second challenge with nicotine (10 mg kg−1 s.c.) induced a response in all animals, although the latency was significantly increased (3.4±0.4 min, n=4, P=0.012). The magnitude (7.5±2.5 vs 17.6±1.3 episodes) and duration (1.42±0.36 vs 3.8±0.3 min) of the response were reduced, but not significantly. In a separate study, three of four animals which had received nicotine (10 mg kg−1, s.c.) 60 min prior to RTX administration responded to RTX (100 μg kg−1, s.c.) with an increased latency of 4.0±0.2 min (n=4, ns), although the number of episodes was significantly (P=0.03) reduced as compared to the control response.
Animals in which emesis had been induced by motion all responded to RTX (100 μg kg−1, s.c.) when tested 60 min later, although the latency was increased (8.5±1.6 min, n=6, after motion vs 5.7±0.5 min without prior motion) and the number of emetic episodes reduced (9.2±2.0 after motion vs 15.3±1.4 without prior motion), only the latter did so significantly (P=0.02).
Tropisetron
The magnitude of the emetic response to 10 μg kg−1, s.c. RTX was unaffected in three animals by the selective 5-HT3 receptor antagonist tropisetron (200 μg kg−1, s.c., control 14.7±3.7 episodes, n=6 with tropisetron 11.3±1.7 episodes, n=3) as was the latency of the response (control 19.8±4.1 min vs 16.5±1.8 min). As there appeared to be no effect on the response to this lower emetic dose of RTX, the higher dose was not studied.
8-OH-DPAT
The selective 5-HT1A receptor agonist 8-OH-DPAT (100 μg kg−1, s.c.) had a marked effect on the response to RTX (100 μg kg−1, s.c.) totally blocking emesis in three of four animals. The single responding animal had nine episodes beginning 8.18 min after RTX injection (RTX 100 μg kg−1 control 15.3±1.4 episodes, latency 5.7±0.5 min). In this study as in a previous one in Suncus (Okada et al., 1994) it was observed that 8-OH-DPAT itself stimulated locomotor activity. This increased locomotor activity was suppressed within 10 min of administration of RTX (100 μg kg−1 s.c.).
CP-99,994
The neurokinin1 receptor antagonist CP-99,994 was studied at two doses. The lower dose of 10 mg kg−1 s.c. blocked emesis in two out of five animals tested with 100 μg kg−1, s.c. RTX (3.2±1.4 episodes, 4.8±0.1 min latency, 4.6±1.7 min duration). The higher dose of 20 mg kg−1, s.c. blocked the response in all five animals tested. This higher dose of CP-99,994 appeared to increase spontaneous activity in the animals.
Morphine
Morphine (2 mg kg−1, s.c.) administration did not induce emesis confirming the previous observations of Selve et al. (1994) but did block the emetic response to RTX (100 μg kg−1, s.c.) in four animals tested.
Neonatal capsaicin
In eight animals which had been treated with capsaicin (100 mg kg−1, s.c.) neonatally the emetic response to RTX (100 μg kg−1, s.c.) was abolished but was present in two litter-mates treated neonatally with capsaicin vehicle (latency 6.3 and 5.3 min with 12 and 15 emetic episodes). The emetic response to motion was still present in capsaicin-treated animals and appeared unaffected by the capsaicin treatment when the response was compared to vehicle-treated animals (vehicle 14.2±1.5 episodes, latency 100±31 s n=5, neonatal capsaicin 17.0±3.8 episodes, latency 77±22 s n=5).
The effect of abdominal vagotomy on the emetic response to RTX
Abdominal vagotomy did not block the emetic response to RTX (100 μg kg−1, s.c.) or alter the latency of the response (vagotomy 2.0±0.5 min n=5, sham 2.2±0.4 n=5), although there was a marked reduction in the number of emetic episodes but not the number of responding animals when compared to the responses in sham-lesioned animals (vagotomy 5.4±1.3 episodes, n=5 vs sham 14.0±1.5 episodes, n=5, P=0.002).
Effect of i.c.v. administration of RTX
RTX given into the lateral ventricle induced a short latency (<3 min) emetic response at a dose of 0.6 μg 8.2±1.5 episodes were evoked with a latency of 2.6±0.50 min, n=5 and at 6 μg 18.4±2.0 episodes with a latency of 1.4±0.25 min, n=5). Behavioural responses similar to those seen in animals treated with 100 μg kg−1, s.c. RTX were observed in these animals. Injection of a similar volume of RTX vehicle (n=5) alone did not induce an emetic response.
The anti-emetic effects of RTX
Motion-induced emesis
A group of 12 animals with a demonstrable response to motion exposure consisting of 5.8±1.0 episodes with a latency of 134±21 s was divided into three groups and at least 1 week later exposed to motion following three different doses of RTX given 60 min earlier. At 1 μg kg−1 s.c. RTX three out of four animals responded to motion, although the response was not significantly affected by RTX. At the higher doses of 10 μg kg−1, s.c. and 100 μg kg−1, s.c. the emetic response to motion was blocked in all animals (n=4 animals each dose).
Nicotine-induced emesis
Pretreatment with 100 μg kg−1, s.c. RTX blocked the emetic response to nicotine (10 mg kg−1, s.c.) given 60 min later in one of four animals tested but significantly reduced the overall emetic response in the remainder (control 17.6±1.3, n=14 vs 1.2±0.5, n=4, P=0.01), although there was no significant effect on the latency of the response (control 2.6±1.5 min vs 4.0±0.2 min).
Cisplatin-induced emesis
No emesis was observed in four animals given cisplatin (20 mg kg−1, i.p.) 60 min after RTX administration (100 μg kg−1, s.c.).
Copper sulphate-induced emesis
No emesis was observed in four animals given copper sulphate (40 mg kg−1, p.o.) 60 min after treatment with RTX (100 μg kg−1, s.c.).
Induction of c-fos expression in the medulla oblongata by RTX
No fos-like immunoreactivity (FLI) was seen in the medulla oblongata of vehicle controls throughout the rostro-caudal extent of the NTS and area postrema (Figure 3). In contrast, in the RTX-treated (100 μg kg−1, s.c.) animals FLI was noted throughout the rostro-caudal extent of the area postrema (AP) and nucleus tractus solitarius (NTS) (Figure 3), although there was relative sparing of the subnucleus gelatinosus region of the NTS in the more rostral sections. FLI was also observed in the dorsal motor vagal nucleus but was less dense than in the NTS (Figure 3).
Figure 3.
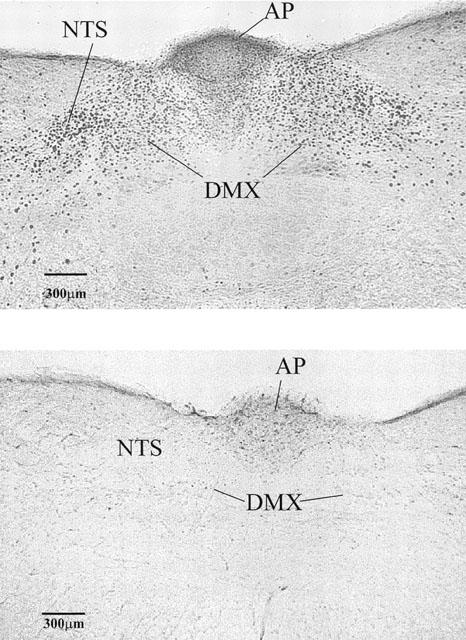
Photomicrographs of a representative transverse section of the caudal dorsal brainstem of Suncus showing fos-like immunoreactivity (dark stained cells) in one animal given RTX (100 μg kg−1 s.c. upper panel) and in another given its vehicle (lower panel). Note the intense fos-like immunoreactivity bilaterally in the nucleus tractus solitarius (NTS) and to a lesser extent in the body of area postrema (AP) in the RTX-treated animal. Fos-like immunoreactivity was noted in the dorsal motor vagal nucleus (DMX) but it was less dense than in the NTS.
Discussion
The results presented in this paper show that the capsaicin analogue RTX has two different but arguably related pharmacological effects in adult Suncus murinus: emesis and anti-emesis. These will be discussed separately.
Suncus murinus
The original premise of this study was to use Suncus to better characterize the anti-emetic effect of RTX originally described in the ferret by Andrews & Bhandari (1993). However, it became immediately apparent that RTX was emetic in Suncus and in fact it appears to be the most potent (expressed as dose in μg kg−1) emetic agent so far described in this species. Several features of the emetic response were dose-related over the range 1–500 μg kg−1, s.c. with perhaps the most notable features being the increase in the intensity (the number of emetic episodes compared to the time over which they occurred) and the reduction in latency with increasing dose of RTX even when a dose which caused emesis in all animals tested had been reached. This implies that the time taken to initiate an emetic response relies upon the intensity of activation of the pathway concerned. The latency of the emetic response to RTX (100 and 500 μg kg−1) is comparable to that of nicotine given via the same route (Tattersall et al., 1995) and which is also presumed to act centrally.
The pharmacological, micro-injection and vagotomy studies in Suncus indicate that the most likely explanation for the emesis is due to a release of substance P (or a closely related neurokinin) in the central nervous system and most probably in the medulla oblongata. Support for this hypothesis comes from several sources. Studies in the rat (MacLean et al., 1990) have demonstrated that capsaicin can induce the release of substance P from dissociated cultures of vagal sensory neurones and hence it is likely that RTX could have a similar action on the vagal terminals in the medulla oblongata. In Suncus RTX (1 μM) induces a release of immunoreactive substance P from the dorsal vagal complex in vitro (Matsuki, unpublished observations). Binding sites for [3H] RTX have been identified in the area postrema and nucleus tractus solitarius in the rat (Szallasi et al., 1995). In the dog application of capsaicin or RTX to the fourth ventricle induced firing of neurones in the medial solitary tract and transient fictive retching (Shiroshita et al., 1997). The lack of effect of the 5-HT3 receptor antagonist tropisetron at a dose which blocked the emetic response in Suncus to the cytotoxic drug cisplatin (Torii et al., 1991b) indicates that the mechanism of the emetic action of RTX differs from cytotoxic drugs and this is to some extent confirmed by the lack of effect of abdominal vagotomy. Further limited support for a release of substance P comes from the behavioural effects of RTX. Whilst the foot-tapping characteristic of central NK1 receptor activation in the Mongolian gerbil (Rupniak & Williams, 1994) was not observed in Suncus this behaviour is believed to be highly species-specific. However, the intense scratching of the flank and grooming of the genitals, licking of the hindlimbs and motor flaccidity, particularly of the hindlimbs, have all been reported with central administration of substance P receptor agonists (septide and senktide) in rats (Papir-Kricheli et al., 1987). These behavioural observations in Suncus require further investigation as Papir-Kricheli et al. (1987) suggested that different combinations of behaviours were due to activation of distinct substance P receptor types.
The pattern of c-fos activation in the medulla oblongata supports the hypothesis that RTX either directly or indirectly activates two key regions involved in the emetic response, namely the area postrema and the nucleus tractus solitarius (but see also section below on anti-emetic effects). This pattern of activation is consistent with the hypothesis that RTX induces emesis by activation of the emetic pathway in the medulla oblongata with the area postrema and part of the nucleus tractus solitarius being the most likely sites. The observation that neonatal capsaicin treatment abolished the emetic effects of RTX when tested in adult Suncus would initially suggest that RTX does indeed exert its effect via vagal afferents. However, neurotoxic effects of capsaicin within the central nervous system either directly or indirectly (secondary to C-fibre afferent damage) have been reported (Ritter & Dinh, 1993, 1988).
The emetic response to RTX can be blocked by the neurokinin1 receptor antagonist CP-99,994, although higher doses are required in Suncus than other laboratory species. This has been related to species-dependent differences in the affinity of CP99,994 for the NK1 receptor (Tattersall et al., 1995; 1996) with IC50 values of 1.97 nM for ferret medulla oblongata, 0.45 nM for human recombinant NK1 receptors in IM9 cells and 12 nM for Suncus brain (Bountra et al., 1993; Tattersall et al., 1993; 1994; 1995; 1996; Watson et al., 1995a; Lucot et al., 1997). However, because of its broad-spectrum anti-emetic effect the blockade of the RTX response by CP-99,994 cannot be regarded as more than supportive of the proposal that RTX induces emesis via the central release of SP. Preliminary studies in Suncus using immunohistochemistry have demonstrated the presence of substance P-like immunoreactivity concentrated in the dorsal vagal complex (Leslie, Andrews & Matsuki, unpublished observations).
The blockade of the emetic response to RTX by morphine and 8-OH-DPAT is not surprising as both have potent broad-spectrum anti-emetic effects in Suncus and other species against stimuli including motion and cisplatin (Selve et al., 1994; Okada et al., 1994; Lucot, 1995; Rudd & Naylor, 1995). It is proposed that they suppress transmission at a critical locus in the emetic pathway, most likely the nucleus tractus solitarius (Lucot, 1995; Rudd & Naylor, 1995).
The blockade of the RTX response by RTX administered 1 h earlier could involve two mechanisms which cannot be distinguished by the present study. Firstly, it could be argued that the first dose of RTX released so much of the SP in the critical location that insufficient SP remained to trigger an emetic response with a second RTX challenge. Secondly, if the first dose of RTX is still bound to its receptors, it would act as an ‘antagonist' to a second challenge.
Anti-emetic effects
RTX at a dose of 100 μg kg−1, s.c. in the ferret reduced or blocked the emetic response to subsequent challenge with intragastric copper sulphate, total body X-radiation and subcutaneous loperamide (Andrews & Bhandari, 1993). It was concluded that RTX possessed broad-spectrum anti-emetic effects and that it acted by depleting substance P or CGRP at a central site in the emetic pathway, possibly the nucleus tractus solitarius. Such a mechanism is consistent with the broad-spectrum antiemetic effects of the neurokinin1 receptor antagonists (Bountra et al., 1993; Tattersall et al., 1993; 1994; 1995; 1996; Gardner et al., 1994; Watson et al., 1995a,1995b; Lucot et al., 1997). The present study extends the spectrum of anti-emetic action of RTX to another species and broadens it to include RTX itself, the cytotoxic drug cisplatin, motion and nicotine in addition to demonstrating the blockade of copper sulphate in a second species.
Because RTX induces emesis in Suncus at the same dose (10 and 100 μg kg−1) at which the anti-emetic effects have been demonstrated, control experiments were undertaken to examine the interdependence of consecutive emetic challenges. This was done to counter the argument that animals failed to respond because they had already had an emetic response to another stimulus.
We propose that RTX causes emesis by releasing SP, perhaps with a co-transmitter, such as glutamate (Saha et al., 1995), at a critical site in the emetic pathway argued to be the nucleus tractus solitarius, and the depletion of SP (perhaps with a co-transmitter) is responsible for the subsequent anti-emetic effects. In the decerebrate dog Shiroshita et al. (1997) reported that capsaicin and RTX after initially inducing firing in the medial nucleus tractus solitarius and fictive retching both abolished the neuronal and retching responses to vagal afferent stimulation.
However, other mechanisms may also be involved in the anti-emetic effects of RTX. For example, inhibition of transmitter release as capsaicin has been shown to produce inhibition of voltage-activated Ca++ channels by increasing intracellular Ca++ via vanilloid receptors (Bevan & Docherty, 1993; Szallasi, 1994; Liu et al., 1996) and this has also been demonstrated for RTX (Winter et al., 1990). Desensitization of NK1-receptors could also occur as indicated by a study showing that injection of capsaicin into the hindpaw of a rat induced internalization of NK1-receptors in the spinal cord (Mantyh et al., 1995).
In conclusion, the results from this study may serve two purposes. Firstly, the sensitivity of the emetic reflex to RTX in Suncus indicates that it may provide an in vivo model for investigating the structure-activity relationships of substances acting on the vanilloid receptors. Secondly, in view of the broad-spectrum anti-emetic effects of RTX studies of the mechanism and site of action, particularly the relationship to modulation of neurotransmitter release (e.g. SP) may give novel insights into the identification of compounds with anti-emetic activity against a wide range of central and peripheral emetic stimuli.
Acknowledgments
We wish to acknowledge the financial assistance of SmithKline Beecham and Merck Sharpe and Dohme, and Pfizer Inc for the gift of CP-99,994, and Dr W. Miner (Pfizer) for comments on this study, and Mr M. Lacey for technical assistance.
Abbreviations
- 5-HT3
5-hydroxytryptamine3 receptor
- NK1
tachykinin neurokinin1 receptor
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino) tetralin
- RTX
resiniferatoxin
References
- ABE H. Classification and biology of Japanese Insectivore (Mammalia). I. Studies on variation and classification. J. Fac. Agric. Hokkaido Univ. 1967;55:191–265. [Google Scholar]
- ANDREWS P.L.R., BHANDARI P. Resiniferatoxin, an ultrapotent capsaicin analogue, has anti-emetic properties in the ferret. Neuropharmacology. 1993;32:799–806. doi: 10.1016/0028-3908(93)90189-a. [DOI] [PubMed] [Google Scholar]
- ANDREWS P.L.R., TORII Y., SAITO H., MATSUKI N. The pharmacology of the emetic response to upper gastrointestinal tract stimulation in Suncus murinus. Eur. J. Pharmacol. 1996;307:305–313. doi: 10.1016/0014-2999(96)00275-0. [DOI] [PubMed] [Google Scholar]
- BEVAN S.J., DOCHERTY R.J.Cellular mechanisms of the action of capsaicin Capsaicin in the Study of Pain 1993London, U.K: Academic Press; 27–44.ed. Wood, J.N., pp [Google Scholar]
- BHANDARI P., BINGHAM S., ANDREWS P.L.R. The neuropharmacology of loperamide-induced emesis in the ferret: The role of the area postrema, vagus, opiate and 5-HT3 receptors. Neuropharmacology. 1992;31:735–742. doi: 10.1016/0028-3908(92)90034-m. [DOI] [PubMed] [Google Scholar]
- BOUNTRA C., BUNCE K., DALE T., GARDNER C., JORDAN C., TWISSELL D., WARD P. Anti-emetic profile of a non-peptide neurokinin NK1 receptor antagonist, CP-99,994, in ferrets. Eur. J. Pharmacol. 1993;249:R3–R4. doi: 10.1016/0014-2999(93)90673-6. [DOI] [PubMed] [Google Scholar]
- COLBERT E.H. New York, U.S.A: John Willey & Sons; 1958. Evolution of the Vertebrates. [Google Scholar]
- DE VRIES D.J., BLUMBERG P.M. Thermoregulatory effects of resiniferatoxin in the mouse: comparison with capsaicin. Life Sci. 1989;44:711–715. doi: 10.1016/0024-3205(89)90382-2. [DOI] [PubMed] [Google Scholar]
- GARDNER C.J., BOUNTRA C., BUNCE K.T., DALE T.J., JORDAN C.C., TWISSELL D.J., WARD P. Anti-emetic activity of neurokinin NK1 receptor antagonist is mediated centrally in the ferret. Br. J. Pharmacol. 1994;112:516P. [Google Scholar]
- LIU L., WANG Y., SIMON S.A. Capsaicin activated currents in rat dorsal root ganglion cells. Pain. 1996;64:191–195. doi: 10.1016/0304-3959(94)00097-2. [DOI] [PubMed] [Google Scholar]
- LUCOT J.B.5-HT1A receptor antagonists as anti-emetics Serotonin and the scientific basis of anti-emetic therapy 1995Oxford, U.K: Oxford Clin. Comm; 222–227.ed. Reynolds, D.J.M., Andrews, P.L.R. & Davis C.J. pp [Google Scholar]
- LUCOT J.B., OBACH R.S., MCLEAN S., WATSON J.W. The effect of CP-99,994 on the responses to provocative motion in the cat. Br. J. Pharmacol. 1997;120:116–120. doi: 10.1038/sj.bjp.0700888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN D.B., WHEELER F., HAYES L. Basal and stimulated release of substance P from dissociated cultures of vagal sensory neurons. Brain Res. 1990;519:308–314. doi: 10.1016/0006-8993(90)90093-q. [DOI] [PubMed] [Google Scholar]
- MANTYH P.W., DEMASTER E., MALHOTRA A., GHILHARDI J.R., ROGERS S.D., MANTYH C.R., LIU H., BASBAUM A.I., VINGA S.R., MAGGIO J.E., SIMONE D.A. Receptor endocytosis and dendrite reshaping in spinal neurons after somatosensory stimulation. Science. 1995;268:1629–1632. doi: 10.1126/science.7539937. [DOI] [PubMed] [Google Scholar]
- MATSUKI N., TORII Y., UENO S., SAITO H.Suncus murinus as an experimental animal model for emesis and motion sickness Mechanisms and Control of Emesis 1992Montrouge, France: INSERM/John Libbey Eurotext, Paris; 323–329.ed. Bianchi, A.L., Grelot, L., Miller, A.D. & King, G.I. pp [Google Scholar]
- MUTOH M., IMANISHI H., TORII Y., TAMURA M., SAITO H., MATSUKI N. Cisplatin-Induced Emesis in Suncus murinus. Japan. J. Pharmacol. 1992;58:321–324. doi: 10.1254/jjp.58.321. [DOI] [PubMed] [Google Scholar]
- OKADA F., TORII Y., SAITO H., MATSUKI N. Antiemetic Effects of Serotonergic 5-HT1A-Receptor Agonists in Suncus murinus. Japan. J. Pharmacol. 1994;64:109–114. doi: 10.1254/jjp.64.109. [DOI] [PubMed] [Google Scholar]
- PAPIR-KRICHELI D., FREY J., LAUFER R., GILON C, , CHOREV M., SELINGER Z., DEVOR M. Behavioural effects of receptor-specific substance P agonists. Pain. 1987;31:263–276. doi: 10.1016/0304-3959(87)90041-8. [DOI] [PubMed] [Google Scholar]
- REYNOLDS D.J.M, , BARBER N.A., GRAHAME-SMITH D.G., LESLIE R.A. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43964) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- RITTER S., DINH T.T. Capsaicin-induced neuronal degeneration: Silver impregnation of cell bodies, axons and terminals in the central nervous system of the adult rat. J. Comp. Neurol. 1988;271:79–90. doi: 10.1002/cne.902710109. [DOI] [PubMed] [Google Scholar]
- RITTER S., DINH T.T.Capsaicin-induced degeneration in rat brain and retina Capsaicin in the study of pain 1993New York: Academic Press; 105–138.ed. Wood, J.N. pp [Google Scholar]
- RUDD J.A., NAYLOR R.J.Opioid receptor involvement in emesis and anti-emesis Serotonin and the scientific basis of anti-emetic therapy 1995Oxford: Oxford Clin. Comm; 208–221.ed. Reynolds, D.J.M., Andrews, P.L.R. & Davis C.J. pp [Google Scholar]
- RUPNIAK N.M.J., WILLIAMS A.R. Differential inhibition of foot tapping and chromodacryorrhoea in gerbils by CNS penetrant and non-penetrant tachykinin NK1 receptor antagonists. Eur. J. Pharmacol. 1994;265:179–183. doi: 10.1016/0014-2999(94)90430-8. [DOI] [PubMed] [Google Scholar]
- SAHA S., BATTEN T.F.C., MCWILLIAM P.N. Glutamate, γ-aminobutyric acid and tachykinin-immunoreactive synapses in the cat nucleus tractus solitarii. J. Neurocytol. 1995;24:55–60. doi: 10.1007/BF01370160. [DOI] [PubMed] [Google Scholar]
- SELVE N., FRIDERICHS E., REIMANN W., REINARTZ S. Absence of emetic effects of morphine and loperamide in Suncus murinus. Eur. J. Pharmacol. 1994;256:287–293. doi: 10.1016/0014-2999(94)90554-1. [DOI] [PubMed] [Google Scholar]
- SHIROSHITA Y., KOGA T., FUKUDA H. Capsaicin in the 4th ventricle abolishes retching and transmission of emetic vagal afferents to solitary nucleus neurons. Eur. J. Pharmacol. 1997;339:183–192. doi: 10.1016/s0014-2999(97)01370-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. The vanilloid (capsaicin) receptor: receptor types and species differences. Gen. Pharmacol. 1994;25:223–243. doi: 10.1016/0306-3623(94)90049-3. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. Autoradiographic visualisation and pharmacological characterization of vanilloid (capsaicin) receptors in several species, including man. Acta. Physiol. Scand. 1995;155 Suppl 629:1–68. [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;20:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sciences. 1990;47:1399–1408. doi: 10.1016/0024-3205(90)90518-v. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., NILSSON S., FARKAS-SZALLASI T., BLUMBERG P.M., HÖKFELT T., LUNDBERG J.M. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–221. doi: 10.1016/0006-8993(95)01094-7. [DOI] [PubMed] [Google Scholar]
- TATTERSALL F.D., RYCROFT W., FRANCIS B., PEARCE D., MERCHANT K., MACLEOD A.M., LADDUWAHETTY T., KEOWN L., SWAIN C., BAKER R., CASCIERI M., BER E., METZGER J., MACINTYRE D.E., HILL R.G., HARGREAVES R.J. Tachykinin NK1 receptor antagonists act centrally to inhibit emesis induced by the chemotherapeutic agent cisplatin in ferrets. Neuropharmacology. 1996;35:1121–1129. doi: 10.1016/s0028-3908(96)00020-2. [DOI] [PubMed] [Google Scholar]
- TATTERSALL F.D., RYCROFT W., HARGREAVES R.J., HILL R.G. The tachykinin NK1 receptor antagonist CP-99,994 attenuates cisplatin-induced emesis in the ferret. Eur. J. Pharmacol. 1993;250:R5–R6. doi: 10.1016/0014-2999(93)90649-3. [DOI] [PubMed] [Google Scholar]
- TATTERSALL F.D., RYCROFT W., HILL R.G., HARGREAVES R.J. Enantioselective inhibition of apomorphine-induced emesis in the ferret by the neurokinin1 receptor antagonist CP-99,994. Neuropharmacology. 1994;33:259–260. doi: 10.1016/0028-3908(94)90018-3. [DOI] [PubMed] [Google Scholar]
- TATTERSALL F.D., RYCROFT, MARMONT N., CASCIERI M., HILL R.G., HARGREAVES R.J. Enantiospecific inhibition of emesis induced by nicotine in the house musk shrew (Suncus murinus) by the neurokinin1 (NK1) receptor antagonist CP-99,994. Neuropharmacology. 1995;34:1697–1699. doi: 10.1016/0028-3908(95)00164-6. [DOI] [PubMed] [Google Scholar]
- TORII Y., SAITO H., MATSUKI N. 5-hydroxytryptamine is emetogenic in the house musk shrew, Suncus murinus. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991a;344:564–567. doi: 10.1007/BF00170653. [DOI] [PubMed] [Google Scholar]
- TORII Y., SAITO H., MATSUKI N. Selective blockade of cytotoxic drug-induced emesis by 5-HT3 receptor antagonists in Suncus murinus. Japan. J. Pharmacol. 1991b;55:107–113. doi: 10.1254/jjp.55.107. [DOI] [PubMed] [Google Scholar]
- TORII Y., SHIKITA M., SAITO H., MATSUKI N. X-Irradiation-Induced Emesis in Suncus murinus. J. Radiat. Res. 1993;34:164–170. doi: 10.1269/jrr.34.164. [DOI] [PubMed] [Google Scholar]
- UENO S., MATSUKI N., SAITO H. Suncus murinus as a new experimental model for motion sickness. Life Sciences. 1988;43:413–420. doi: 10.1016/0024-3205(88)90520-6. [DOI] [PubMed] [Google Scholar]
- WATSON J.W., GONSALVES S.F., FOSSA A.A., MCLEAN S., SEEGER T., OBACH S., ANDREWS P.L.R. The anti-emetic effects of CP-99,994 in the ferret and the dog: role of the NK1 receptor. Br. J. Pharmacol. 1995a;115:84–94. doi: 10.1111/j.1476-5381.1995.tb16324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON J.W., NAGAHISA A., LUCOT J.B., ANDREWS P.L.R.The tachykinins and emesis: towards complete control Serotonin and the scientific basis of anti-emetic therapy 1995bOxford, UK: Oxford Clin. Comm; 233–238.ed. Reynolds, D.J.M., Andrews, P.L.R. & Davis, C.J. pp [Google Scholar]
- WINTER J., DRAY A., WOOD J.N., YEATS J.C., BEVAN S. Cellular mechanism of action of resiniferatoxin: a potent sensory neuron excitotoxin. Brain Res. 1990;520:131–140. doi: 10.1016/0006-8993(90)91698-g. [DOI] [PubMed] [Google Scholar]
- WOODS A.J., STOCK M.J., GUPTA A.N., WONG T.T.L., ANDREWS P.L.R. Thermoregulatory effects of resiniferatoxin in the rat. Eur. J. Pharmacol. 1994;264:125–133. doi: 10.1016/0014-2999(94)00445-5. [DOI] [PubMed] [Google Scholar]


