Abstract
Based on previous in vitro studies, inhibition of K+ recycling in thick ascending limb (TAL) is expected to lower Na+ reabsorption through (i) reducing the luminal availability of K+ to reload the Na+-2Cl−-K+ cotransporter and (ii) diminishing the lumen positive transepithelial potential difference which drives paracellular cation transport.
This issue was investigated in anaesthetized rats employing microperfusion of Henle's loop downstream from late proximal tubular site with K+-free artificial tubular fluid in nephrons with superficial glomeruli.
The unselective K+ channel blocker Cs+ (5–40 mM) dose-dependently increased early distal tubular delivery of fluid and Na+ with a maximum increase of ∼20 and 185%, respectively, indicating predominant effects on water-impermeable TAL.
The modest inhibition of Na+ reabsorption in response to the 15 mM of Cs+ but not the enhanced inhibition by 20 mM Cs+ was prevented by luminal K+ supplementation. Furthermore, pretreatment with 20 mM Cs+ did not attenuate the inhibitory effect of furosemide (100 μM) on Na+-2Cl−-K+ cotransport.
Neither inhibitors of large (charybdotoxin 1 μM) nor low (glibenclamide 250 μM; U37883A 100 μM) conductance K+ channels altered loop of Henle fluid or Na+ reabsorption.
The intermediate conductance K+ channel blockers verapamil and quinine (100 μM) modestly increased early distal tubular Na+ but not fluid delivery, indicating a role for this K+ channel in Na+ reabsorption in TAL. As observed for equieffective concentrations of Cs+ (15 mM), Na+ reabsorption was preserved by K+ supplementation.
The results indicate that modest inhibition of K+ channels lowers the luminal availability of K+ and thus transcellular Na+ reabsorption in TAL. More complete inhibition lowers paracellular Na+ transport probably by reducing or even abolishing the lumen positive transepithelial potential difference. Under the latter conditions, transcellular Na+ transport may be restored by paracellular K+ backleak.
Keywords: Cs+, Ba2+, potassium recycling, ATP sensitive potassium channels, micropuncture
Introduction
Reabsorption of Na+ in the thick ascending limb of Henle's loop (TAL) occurs through (1) transcellular pathways involving the Na+-2Cl−-K+ cotransporter in the apical membrane and (2) paracellular pathways driven by the lumen positive transepithelial potential difference. In vitro studies indicated that the recycling of K+ through K+ channels in the apical membrane of TAL may contribute to both transcellular and paracellular Na+ reabsorption in that tubule segment. First, because luminal K+ concentration is significantly lower than Na+ or Cl− concentration, K+ recycling via K+ channels in the apical membrane appears necessary to supply K+ for reloading the Na+-2Cl−-K+ cotransporter (Greger, 1985; Weinstein, 1992). Second, recycling of K+ to the lumen contributes to the lumen positive transepithelial potential difference which favours paracellular transport of cations (Greger, 1985; Guggino, 1991). Based on the above, pharmacological blockade of apical K+ channels in TAL is predicted to inhibit Na+ reabsorption. Indeed, in isolated TAL it was shown that addition of the unselective K+ channel blocker Ba2+ to the luminal perfusate decreased active transport as measured by the short circuit current (Greger & Schlatter, 1981).
The first aim of the present study was to evaluate the effect of unselective K+ channel blockade on Na+ reabsorption in TAL in vivo. Early distal tubular fluid collections were performed during orthograde microperfusion of Henle's loop from late proximal tubular site in anaesthetized Munich-Wistar-Frömter (MWF) rats. Nephrons with superficial glomeruli were studied in order to approach the early distal tubule relatively close to the macula densa. Effects on water-impermeable TAL were deduced by comparing effects on fluid and Na+ reabsorption during loop of Henle perfusion. In a first set of experiments, the effect of the unselective K+ channel blocker Cs+ (Van Driessche & Zeiske, 1985) was studied. In further studies, K+ or furosemide were added to the perfusate in the presence of Cs+ to test whether a reduction in the luminal availability of K+ or an interference with Na+-2Cl−-K+ cotransporter activity significantly contributed to the inhibitory effect of Cs+ on Na+ reabsorption. Because Cs+ was found to interfere with K+ measurements employing a micro-flame photometer and thus the effect of Cs+ on the concentration of K+ in early distal tubular fluid could not be assessed, additional studies were performed with the unselective K+ channel blocker Ba2+ which did not interfere with K+ measurements.
The second aim of the study was to determine which kind of K+ channel may contribute to Na+ reabsorption in TAL in vivo. In the apical membrane of TAL, three types of K+ channels have been identified by patch clamp investigations: low conductance (∼30pS), intermediate conductance (60–70pS), and large conductance (100–200pS) K+ channels (Bleich et al., 1990; Guggino et al., 1987a; Wang, 1994; Wang et al., 1997). Pharmacological inhibition of these channels has been reported in electrophysiological studies in vitro: in cell-attached and cell excised patch clamp studies in the apical membrane of TAL, quinine as well as the Ca2+ channel blocker verapamil were found to inhibit the intermediate-conductance K+ channels (Bleich et al., 1990). In comparison, glibenclamide and U37883A were reported to inhibit apical low-conductance K+ channels in rat TAL (Wang et al. 1995a,1995b). Finally, in cultured TAL cells, charybdotoxin (CTX) was shown to effectively block the large-conductance K+ channel (Guggino et al., 1987b). Based on these in vitro observations, we studied loop of Henle reabsorption during luminal application of the above mentioned K+ channel blockers to obtain further information on the potential type of K+ channel involved in Na+ reabsorption in TAL in vivo. Finally, for the respective drugs which inhibited Na+ reabsorption in TAL, we performed experiments with luminal K+ supplementation to assess whether the drugs acted through reducing the luminal availability of K+.
Methods
All animal experimentation was conducted in accord with the NIH Guide for the Care and Use of Laboratory Animals and the German Law on the Protection of Animals. Experiments were performed in male Munich-Wistar-Frömter rats receiving normal rat chow and weighing between 200 and 400 g.
Surgical preparation
Surgical preparation was performed as described before (Huang et al., 1998; 1999; Vallon et al., 1999). Briefly, rats were anaesthetized with thiobutabarbital (Inactin® 120 mg kg−1 i.p.). Animals were then placed on a temperature-controlled operating table to keep rectal temperature at 37°C. After tracheostomy (PE-200), a catheter (PE-50) was inserted into the right jugular vein for infusion of Ringer saline (mM: NaCl 111, KCl 4.7, NaHCO3 30) at a rate of 1.3% of body weight h−1. Arterial blood pressure was recorded via a catheter (PE-50) which was inserted in the left femoral artery and connected to a pressure transducer (P23dB, Gould-Statham, Oxnard, CA, U.S.A.). The left kidney was exposed by flank incision, carefully freed of perirenal fat and immobilized in a lucite cup. The kidney was covered with prewarmed paraffin oil. Both the urinary bladder and the left ureter were cannulated (PE-50) to maintain free urine drain. After completion of the surgical preparation, the animals were allowed to stabilize for 90 min before starting micropuncture experiments.
Loop of Henle microperfusion
Tubular reabsorption in the loop of Henle was studied during orthograde microperfusion from late proximal tubular site as previously described (Huang et al., 1998; 1999). Proximal and distal nephron configuration of nephrons with superficial glomeruli were identified by intratubular application of small amounts of stained artificial tubular fluid. A perfusion pipette directly attached to a calibrated microperfusion pump (pump 1, Hampel, Neu-Isenburg, Germany) and filled with K+-free artificial tubular fluid (ATF in mM: NaCl 130, NaHCO3 10, CaCl2 2, urea 7.5, stained with 0.1% FD&C fast green, pH=7.4) was inserted into the last surface loop of the proximal tubule. A wax block was introduced immediately upstream to the perfusion pipette and venting the tubular system upstream to the wax block allowed the filtrate to escape freely onto the surface. Three to five minutes elapsed at an initial perfusion before a collecting pipette (7–9 μm outer diameter) was inserted into the first accessible distal tubular loop and a timed collection of tubular fluid (at least 2.5 min in duration) was begun, utilizing a short mineral oil block. Following this collection, the perfusate escaped from the collecting hole or escaped downstream after pushing away the short mineral oil block. Subsequently, another perfusion pipette connected to a second microperfusion pump (pump 2) and filled with ATF containing drug or vehicle was inserted in close proximity to the first pump. Pump 1 was switched off and pump 2 was started. After a time period of 5 min, a second tubular collection was performed. In experiments applying BaCl2 or CsCl, the concentration of NaCl in the perfusate in the experimental periods was adjusted to maintain the same concentration of Cl− in the perfusion fluid as in the respective control periods.
Analytical methods
Early distal tubular flow rate was measured by transferring the collected fluid sample to a constant bore capillary. Na+ and K+ concentration in the early distal tubular fluid were determined as previously described (Huang et al., 1998; 1999) with a micro flame photometer which was developed and built by Rolf Englert and Klaus Stieler (Department of Pharmacology, University of Tübingen) based on the original conception by Wolfgang Hampel (Wissenschaftlicher Gerätebau, Frankfurt/Main). Concentrations of K+ in tubular fluid of experiments with Cs+ could not be determined because Cs+ interfered with K+ measurements. In part of the experiments, the Cl− concentration in the early distal tubular fluid was measured as previously described (Vallon et al., 1999) with the electrometric titration method (Ramsey et al., 1955). The equipment for the latter (Microtitre ET-1, World Precision Instruments, Sarasota, FL, U.S.A.) was kindly placed at our disposal by Prof D. Häberle (Department of Physiology, University of Munich, Germany).
Materials and statistics
CsCl, BaCl2, furosemide, quinine, verapamil, glibenclamide, and charybdotoxin were purchased from Sigma (St. Louis, MO, U.S.A.). U37883A was kindly provided by Pharmacia & Upjohn (Kalamazoo, MI, U.S.A.). Quinine, U37883A and glibenclamide were first dissolved in DMSO. These stock solutions were diluted in ATF to give the highest concentration of the respective drug tested with a final DMSO concentration of 0.03%. These solutions were further diluted in ATF containing 0.03% DMSO to give perfusates with lower drug concentrations. Data are presented as mean±s.e.mean. Data from control and experimental period were compared using paired or unpaired Student's t-test. P values <0.05 were considered statistically significant.
Results
Effects of Cs+
Adding Cs+ to the perfusate caused a dose-dependent reduction of fluid, Na+ and Cl− reabsorption in the loop of Henle as illustrated by a rise in early distal tubular fluid and ion delivery (Figure 1). Cs+ elicited a maximum rise in early distal tubular fluid delivery of about 20% indicating a modest inhibition of fluid and electrolyte reabsorption in water-permeable segments of Henle's loop. Furthermore, early distal tubular delivery of Na+ and Cl− increased by a maximum of about 186 and 255%, respectively. The lower increase in Na+ as compared to Cs− delivery probably reflected the supplementation of Cs+ for Na+ in the perfusate, whereas the Cl− concentration in the perfusate was kept constant. The about 10 fold greater increase in early distal tubular delivery of Na+ or Cl− as compared to fluid proposed a significant inhibition of Na+ and Cl− reabsorption in water-impermeable TAL in addition to effects on water-permeable segments of Henle's loop. Further supporting this notion, it was observed that whereas Cs+ at 10 mM already elicited a near maximum rise in early distal fluid delivery, the delivery to the early distal tubule of Na+ and Cl− especially increased with higher Cs+ concentrations, i.e., in the absence of further effects on water-permeable segments.
Figure 1.
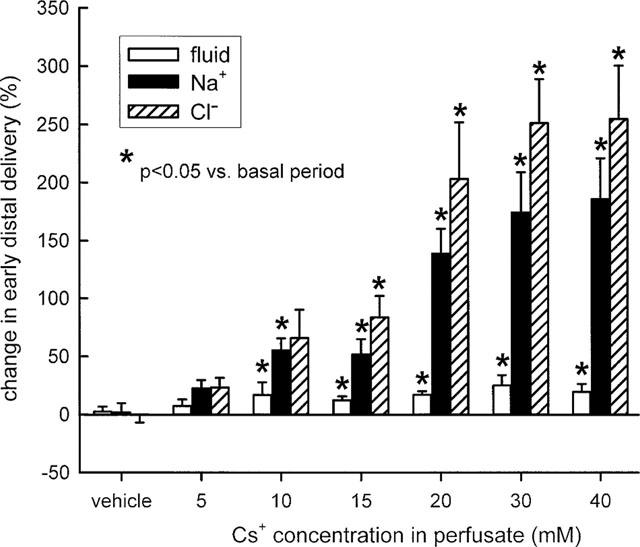
Effect of Cs+ on loop of Henle reabsorption of fluid, Na+, and Cl−. Depicted are the changes in fractional early distal tubular delivery during perfusion of Henle's loop with vehicle or Cs+ as compared to basal measurements. n=5–9 nephrons each group.
Further experiments revealed that supplementation of 40 mM K+ to the perfusate eliminated the inhibition of fluid or Na+ reabsorption in the loop of Henle in response to 15 mM of Cs+ (Figure 2) indicating that a reduction in the luminal K+ availability contributed to the modest inhibition of Na+ reabsorption observed with this concentration of Cs+. In comparison, the more pronounced inhibitory effect of 20 mM Cs+ on Na+ reabsorption was not significantly reduced by luminal supplementation of 20 or 40 mM K+ to the perfusate (Figure 3). As expected, adding furosemide (100 μM) to the perfusate did not alter loop of Henle fluid reabsorption but significantly increased early distal tubular Na+ concentration and thus Na+ delivery (Figure 4). Further supporting the notion that high concentrations of Cs+ (20 mM) did not reduce the luminal K+ availability and thus inhibit Na+ reabsorption in TAL by preventing the K+ reloading of the Na+-2Cl−-K+ cotransporter, it was observed that pretreatment of Henle's loop with Cs+ (20 mM) did not reduce the effect of furosemide on loop of Henle Na+ reabsorption (Figure 4). As shown in Figure 4, the sum of the changes in early distal Na+ delivery observed (a) in response to furosemide with no pretreatment and (b) in Cs+-time control experiments equalled about 0.80 (0.54+0.25) nmol min−1, which was even lower than the rise of 1.17 nmol min−1 observed in response to furosemide with Cs+ pretreatment. The more pronounced effect of furosemide on Na+ delivery during Cs+ pretreatment could be accounted for by the increased Na+ delivery to furosemide-sensitive TAL through actions of Cs+ on upstream water-permeable segments.
Figure 2.
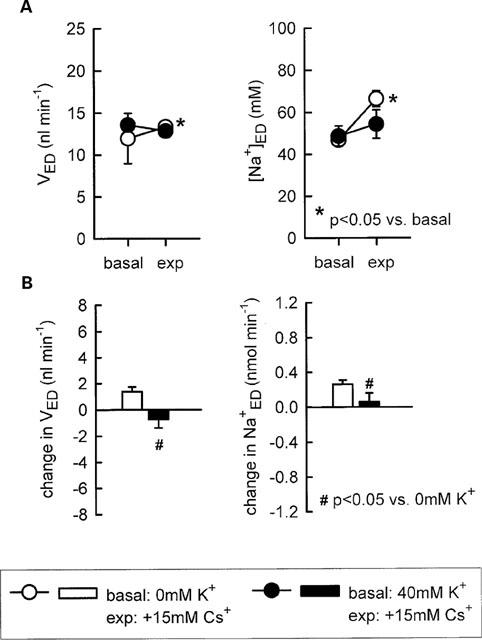
Influence of K+ supplementation on the effect of 15 mM Cs+ on loop of Henle reabsorption of fluid and Na+. Depicted are values for early distal tubular flow rate and Na+ concentration (VED, [Na+]ED-A) as well as the changes in early distal tubular flow rate and Na+ delivery as compared to basal measurements (B). n=7–8.
Figure 3.
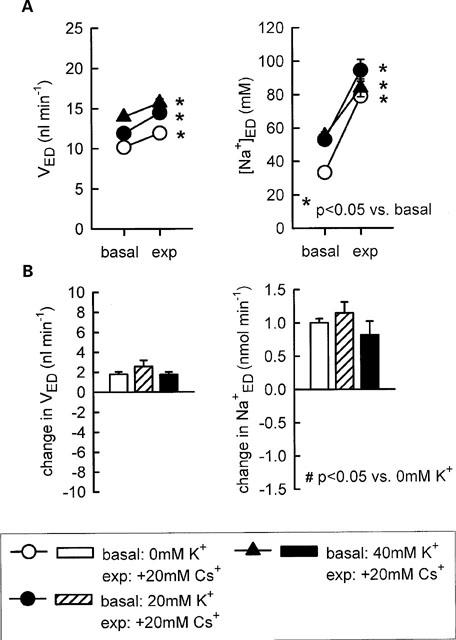
Influence of K+ supplementation on the effect of 20 mM Cs+ on loop of Henle reabsorption of fluid and Na+. Depicted are values for early distal tubular flow rate and Na+ concentration (VED, [Na+]ED-A) as well as the changes in early distal tubular flow rate and Na+ delivery as compared to basal measurements (B). n=5–7.
Figure 4.
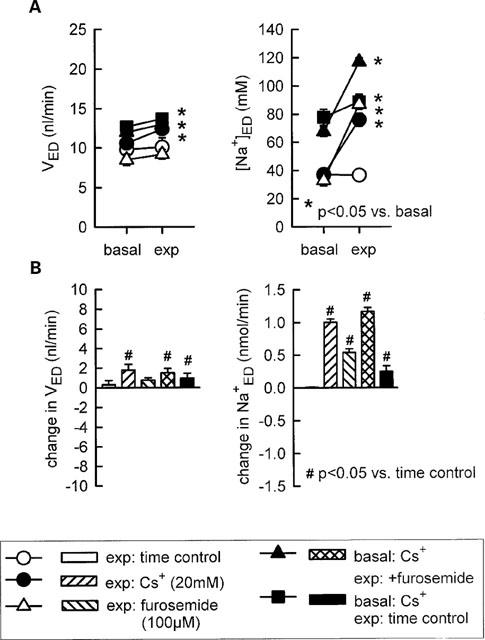
Influence of Cs+ pretreatment on the effect of furosemide on loop of Henle reabsorption of fluid and Na+. Depicted are values for early distal tubular flow rate and Na+ concentration (VED, [Na+]ED-A) as well as changes in early distal tubular flow rate and Na+ delivery as compared to basal measurements (B). Basal: perfusion with artificial tubular fluid (if not otherwise indicated); exp, experimental period. n=5–8.
Effects of Ba2+
Adding Ba2+ to the perfusate elicited a dose-dependent inhibition of loop of Henle fluid and Na+ reabsorption. Similar to the response to Cs+, the fractional increase in early distal tubular delivery of Na+ was significantly more pronounced than the rise in fluid delivery indicating effects on TAL in addition to effects on water-permeable segments (Table 1). Supporting the concept that high concentrations of Ba2+ did not reduce the luminal availability of K+ it was observed that (i) inhibition of loop of Henle Na+ reabsorption by Ba2+ was associated with a significant rise in early distal tubular K+ concentration and K+ delivery and (ii) the inhibitory effect on Na+ reabsorption could not be eliminated by adding 40 mM K+ to the perfusate (Table 1).
Table 1.
Effect of Ba2+ on loop of Henle fluid and electrolyte reabsorption

Effects of verapamil or quinine
At concentrations of 10 or 100 μM, both verapamil and quinine were found to increase early distal tubular Na+ delivery without changing flow rate, indicating effects on water-impermeable TAL. In comparison, application of verapamil at either 1 μM or 1 mM did not significantly alter Na+ reabsorption. Similarly, quinine at 1 mM appeared to exhibit a smaller effect than at 100 μM. Thus, bell-shaped dose response curves were observed for both drugs with regard to Na+ reabsorption in Henle's loop (Figures 5 and 6). Supplementation of 40 mM K+ to the perfusate prevented the modest inhibitory effects of 100 μM verapamil or 100 μM quinine on Na+ reabsorption in the loop of Henle (Figure 7).
Figure 5.
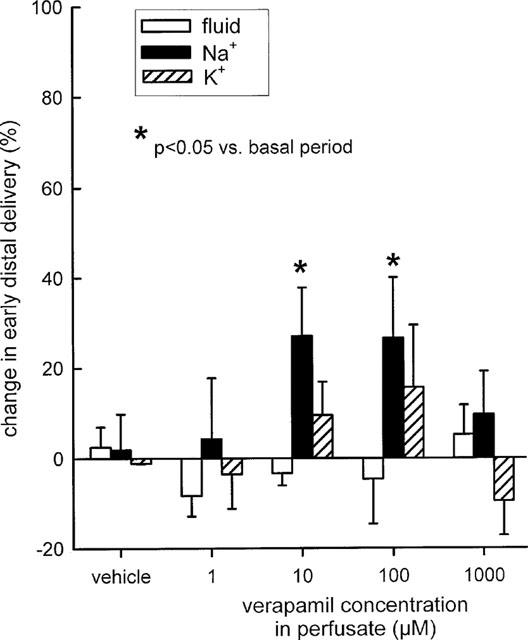
Effect of verapamil on loop of Henle reabsorption of fluid, Na+, and K+. Depicted are changes in early distal tubular delivery of fluid, Na+, and K+ as compared to basal measurements. n=5–7.
Figure 6.
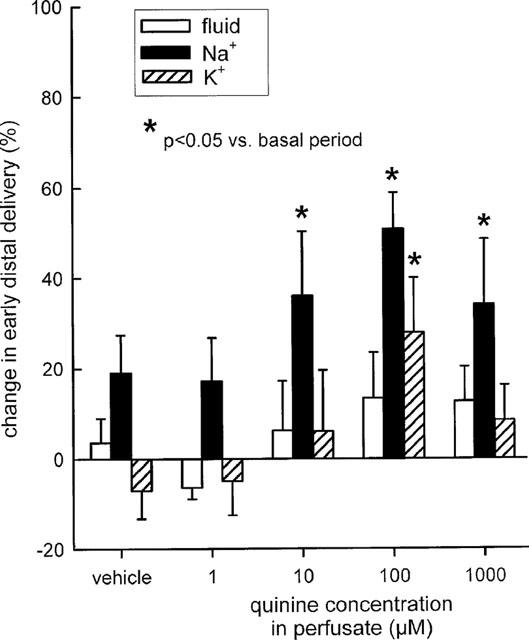
Effect of quinine on loop of Henle reabsorption of fluid, Na+, and K+. Depicted are changes in early distal tubular delivery of fluid, Na+, and K+ as compared to basal measurements. n=5–7.
Figure 7.
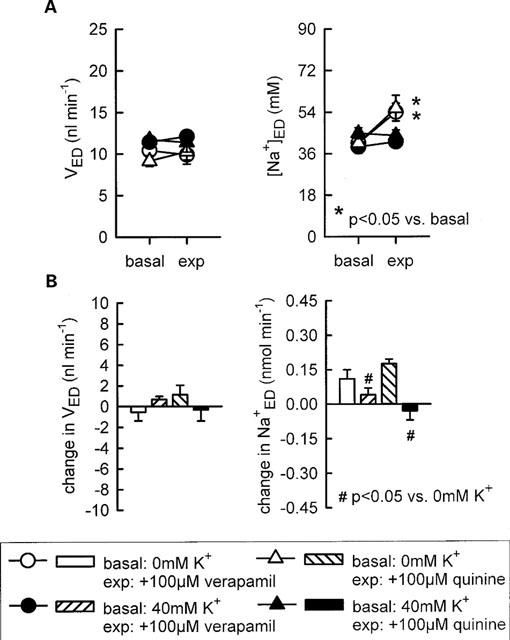
Influence of K+ supplementation on the effect of verapamil or quinine on loop of Henle reabsorption of fluid and Na+. Depicted are values for early distal tubular flow rate and Na+ concentration (VED, [Na+]ED-A) as well as the changes in early distal tubular flow rate and Na+ delivery as compared to basal measurements (B). n=5–6.
Effects of glibenclamide, U37883A, or charybdotoxin
No significant changes in early distal tubular flow rate or concentrations of Na+ or K+ were found in response to U37883A (50 or 100 μM) or glibenclamide (250 μM) (Table 2). Similarly, charybdotoxin (1 μM) did not significantly alter loop of Henle reabsorption (Table 2).
Table 2.
Effect of U37883A, glibenclamide, or charybdotoxin (CTX) on early distal tubular flow rate and Na+ and K+ concentration (VED, [Na+]ED, [K+]ED) during loop of Henle perfusion

Discussion
Studies in isolated perfused tubule have established the concept that recycling of K+ through K+ channels in the apical membrane of TAL contributes to both transcellular and paracellular Na+ reabsorption in that segment. To elicit the role of K+ channels in Na+ reabsorption in TAL in vivo, effects of various K+ channel blockers were studied in microperfusion experiments of Henle's loop.
Cs+ is an unselective K+ channel blocker (Van Driessche & Zeiske, 1985) which was reported to inhibit apical K+ conductance in colon and cortical collecting tubule of the rabbit (O'Neil, 1983; Wills et al., 1982). The present results indicated that application of Cs+ elicited a minor inhibitory effect on water-permeable segments in Henle's loop as well as a prominent inhibition of Na+ reabsorption in water-impermeable TAL, compatible with a significant role of Cs+-sensitive K+ channels in Na+ reabsorption in TAL in vivo. It was further found that high concentrations of Cs+, i.e., 20 mM, did not apparently lower to a significant extent the luminal availability of K+ necessary to reload the Na+-2Cl−-K+ cotransporter inasmuch as application of Cs+ did not reduce the inhibitory effect of furosemide on Na+ reabsorption in TAL. The latter suggestion was further supported by the finding that supplementation of 20 or 40 mM of K+ to the perfusate did not restore Na+ reabsorption in the loop of Henle during application of high concentrations of Cs+. By exclusion, these studies indicate that high concentrations of Cs+ predominantly inhibited the paracellular pathway of Na+ reabsorption in TAL, which most likely resulted from lowering the lumen positive transepithelial potential difference.
If high concentrations of Cs+ lowered the luminal availability of K+, one would expect Cs+ to lower the K+ concentration in early distal tubular fluid, especially in nephrons with superficial glomeruli as employed in the present study. Because Cs+ interferes with measurements of K+ in the employed micro flame photometry, this issue could not be answered. However, additional studies were performed with the unselective K+ channel blocker Ba2+ which unlike Cs+ does not interfere with K+ measurement. In isolated rat TAL, Ba2+ was previously shown to inhibit low-conductance (Wang, 1994), intermediate-conductance (Bleich et al., 1990) and large-conductance (Guggino et al., 1987b) K+ channels. In isolated-perfused mouse TAL Ba2+ was found to reduce transcellular conductance (Hebert & Andreoli, 1986; Hebert et al., 1984) and in isolated rabbit TAL, active NaCl transport was shown to be inhibited by luminal Ba2+ (Greger, 1985). In accordance with the above and previous microperfusion studies (Wang et al., 1995a), the present study indicated that luminal application of Ba2+ significantly inhibited Na+ reabsorption in TAL. Furthermore, the inhibition of Na+ reabsorption in Henle's loop by high concentrations of Ba2+ was associated with a significant rise in early distal tubular K+ concentration and similar to effects of high concentrations of Cs+, the inhibitory effect on Na+ reabsorption could not be eliminated by adding 40 mM K+ to the perfusate. Thus, similar to high concentrations of Cs+, the present findings with high concentrations of Ba2+ did not support the notion that unselective K+ channel blockade inhibited Na+ reabsorption in TAL by limiting the luminal availability of K+.
Assuming that high concentrations of Cs+ or Ba2+ effectively inhibited apical K+ channels in TAL, but at the same time did not limit luminal K+ availability to reload the Na+-2Cl−-K+ cotransporter, K+ must have entered the lumen through alternate pathways. Although the present studies cannot answer this issue, it may be speculated that high concentrations of both drugs lowered the lumen positive transepithelial potential difference to an extent which allowed paracellular back leak of sufficient K+ to satisfy the affinity of the Na+-2Cl−-K+ cotransporter which for K+ is only about 1–2 mM (Greger, 1985).
In the luminal membrane of rat TAL, an intermediate-conductance K+ channel (∼70pS) and a low conductance K+ channel (∼30pS) have been identified by cell-attached or cell-excised patch clamp experiments (Bleich et al., 1990; Wang, 1994; Wang et al. 1997). In cultured medullary TAL cells, a large-conductance K+ channel which was efficiently inactivated by charybdotoxin was described (Guggino et al., 1987b). The latter K+ channel appeared to be silent under physiological conditions and was proposed to become activated during changes of cell volume or external stretch (Guggino, 1991). In the present study, Na+ reabsorption in the loop of Henle was not altered by luminal application of charybdotoxin, supporting the notion that the large conductance K+ channel does not contribute to the K+ recycling involved in Na+ reabsorption in TAL. It was claimed from in vitro studies that the intermediate-conductance K+ channel contributes by about 80% to the apical K+ conductance in TAL during control conditions (Wang et al., 1997; Wang & Lu, 1995). To further assess the contribution of this type of K+ channel in Na+ reabsorption in TAL in vivo, two drugs, namely verapamil and quinine, which have been previously shown to lower either the open probability or the current amplitude of the intermediate-conductance K+ channel in TAL in vitro (Bleich et al., 1990), were used. Although neither of the two drugs appeared as effective as Ba2+ or Cs+, evidence is provided that both drugs inhibited Na+ reabsorption in TAL. Verapamil, which was shown to be the most potent inhibitor for the intermediate-conductance K+ channel in TAL in vitro (Bleich et al., 1990), exhibited a modest inhibitory effect on Na+ reabsorption in TAL at concentrations of 10–100 μM as indicated by an increase in early distal tubular Na+ delivery of about 30% without affecting fluid delivery during the loop of Henle perfusion. A qualitatively and quantitatively similar effect on Na+ reabsorption in TAL was observed in response to quinine. Furthermore, higher concentrations of both verapamil or quinine were less effective in inhibiting Na+ reabsorption in TAL indicating that other effects of the drugs may have offset the effects on K+ channel. For example, both verapamil and quinine can reduce intracellular Ca2+ concentration (Arruda, 1983; Montrose-Rafizadeh & Guggino, 1991). The latter is expected to enhance reabsorption in TAL through lowering PKC activation (Bailly, 1998). In any event, the present studies with both verapamil and quinine are in accordance with a significant role of intermediate-conductance K+ channels in Na+ reabsorption in TAL in vivo. It was further observed that the modest inhibitory effects of verapamil and quinine on Na+ reabsorption in TAL could be prevented by luminal supplementation of K+. Thus, in contrast to high concentrations of Cs+ or Ba2+, these findings support the notion that verapamil and quinine acted through inhibiting the luminal availability of K+. Interestingly, the similarly modest effect on Na+ reabsorption in TAL in response to lower concentrations of Cs+, i.e., 15 mM, could also be prevented by luminal K+ supplementation. Thus, it appears that the mechanism of Na+ transport inhibition in TAL by K+ channel blockade depends on the extent of K+ channel blockade (see Figure 8).
Figure 8.
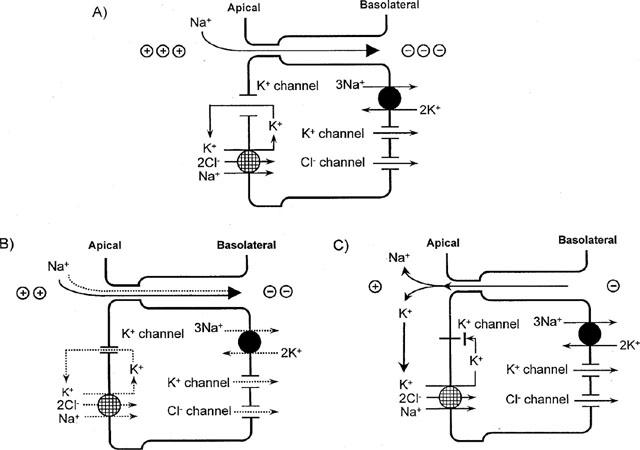
Proposed model for a dependency of the mechanism of Na+ transport inhibition in TAL on the extent of K+ channel blockade. (A) Under basal conditions, luminal K+ channels conduct K+ recycling back to the lumen to (i) reload the Na+-2Cl−-K+ cotransporter in the apical membrane and (ii) set up the lumen positive transepithelial potential difference which drives paracellular Na+ transport. (B) Partial blockade of luminal K+ channels (e.g., by 15 mM Cs+ or 100 μM verapamil or quinine) attenuates K+ recycling back to the lumen and reduces the luminal availability of K+ for reloading the Na+-2Cl−-K+ cotransporter and thus to a certain extent the transcellular Na+ transport. The partial blockade of K+ channels is insufficient, however, to reduce the transepithelial lumen positive potential difference to an extent which would significantly inhibit paracellular Na+ transport. (C) More complete blockade of luminal K+ channels (e.g., by 20 mM of Cs+ or Ba2+) lowers the lumen positive transepithelial potential difference to an extent which inhibits paracellular reabsorption or even allows for paracellular back leak of Na+. Paracellular back leak of K+ reloads the Na+-2Cl−-K+ cotransporter and maintains transcellular Na+ reabsorption.
In the present study we could confirm our previous observation that the two low-conductance K+ channel blockers, glibenclamide or U37883A, did not apparently affect Na+ reabsorption in TAL (Huang et al., 1999). These two substances at the same concentrations have been reported previously to inhibit luminal low-conductance ATP-sensitive K+ channels in vitro and were found to reduce fluid, Na+ and K+ reabsorption in the loop of Henle during in vivo perfusion in Sprague-Dawley (SD) rats (Wang et al., 1995a,1995b). The different rat strains used in the two studies cannot account for the different results because we could confirm the negative response of U37883A on the loop of Henle reabsorption in SD rats (Huang et al., 1999). The different results of these studies may indicate that depending on the respective conditions, the contribution of low-conductance K+ channels to Na+ reabsorption in TAL and/or the responsiveness of these channels to blockade by glibenclamide or U37883A may vary. Such a possibility would be supported by the findings that the activity of the low-conductance K+ channel can be modulated by various factors such as intracellular pH, exogenous ATP, [Ca2+]i, or PKA (Wang, 1994; Wang et al., 1997). Furthermore, the sensitivity to glibenclamide of a cloned inward rectifying K+ channel from rat outer medulla (ROMK channel), which exibits characteristics of low-conductance K+ channels in apical TAL (Hebert, 1995), was shown to vary in dependence of coexpression and potential coupling with cystic fibrosis transmembrane regulator (CFTR) (McNicholas et al., 1996). Clearly, further studies are needed to assess a potential variation in the contribution of low-conductance K+ channels to Na+ reabsorption in TAL.
In summary, evidence was provided that unselective K+ channel blockade can significantly lower Na+ reabsorption in TAL in vivo. Additional studies indicated a role for intermediate conductance K+ channels in this response. Finally, the mechanism of Na+ transport inhibition in TAL by K+ channel blockade appeared to depend on the extent of K+ channel blockade.
Acknowledgments
This work was supported by grants provided by the Deutsche Forschungsgemeinschaft (DFG Os 42/9-1) and the Federal Ministry of Education and Research (BMBF 01 EC 9405 and 01 KS 9602).
Abbreviations
- EXP
experimental period
- K+ED
early distal K+ delivery
- [K+]ED
early distal tubular K+ concentration
- MWF
Munich-Wistar-Frömter
- Na+ED
early distal Na+ delivery
- [Na+]ED
early distal Na+ concentration
- SD
Sprague-Dawley
- TAL
thick ascending limb of Henle's loop
- VED
early distal tubular flow rate
References
- ARRUDA J.A.L. Characterization of the effect of quinine on Na+ transport by the toad and turtle bladders. J. Pharmacol. Exp. Ther. 1983;224:297–301. [PubMed] [Google Scholar]
- BAILLY C. Transducing pathways involved in the control of NaCl reabsorption in the thick ascending limb of Henle's loop. Kidney. Int. 1998;65 Suppl:S29–S35. [PubMed] [Google Scholar]
- BLEICH M., SCHLATTER E., GREGER R. The luminal K+ channel of the thick ascending limb of Henle's loop. Pflügers. Arch. 1990;415:449–460. doi: 10.1007/BF00373623. [DOI] [PubMed] [Google Scholar]
- GREGER R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol. Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- GREGER R., SCHLATTER E. Presence of luminal K+, a prerequisite for active NaCl transport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflügers. Arch. 1981;392:92–94. doi: 10.1007/BF00584588. [DOI] [PubMed] [Google Scholar]
- GUGGINO S.E., GUGGINO W.B., GREEN N., SACKTOR B. Ca2+-activated K+ channels in the cultured medullary thick ascending limb cells. Am. J. Physiol. 1987a;252:C121–C127. doi: 10.1152/ajpcell.1987.252.2.C121. [DOI] [PubMed] [Google Scholar]
- GUGGINO S.E., GUGGINO W.B., GREEN N., SACKTOR B. Blocking agents of Ca2+-activated K+ channels in cultured medullary thick ascending limb cells. Am. J. Physiol. 1987b;252:C128–C137. doi: 10.1152/ajpcell.1987.252.2.C128. [DOI] [PubMed] [Google Scholar]
- GUGGINO W.B. Regulation of K+ channels in the thick ascending limb Nephrology 1991Tokyo: Springer-verlag; 1685–1697.(edi) Michinobu Hatano (Volume II) pp [Google Scholar]
- HEBERT S.C. An ATP-regulated, inwardly rectifying potassium channel from rat kidney (ROMK) Kidney. Intern. 1995;48:1010–1016. doi: 10.1038/ki.1995.383. [DOI] [PubMed] [Google Scholar]
- HEBERT S.C., ANDREOLI T.E. Ionic conductance pathways in the mouse medullary thick ascending limb of Henle. The paracellular pathway and electrogenic Cl− absorption. J. Gen. Physiol. 1986;87:567–590. doi: 10.1085/jgp.87.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBERT S.C., FRIEDMAN P.A., ANDREOLI T.E. Effects of antidiurectic hormone on cellular conductive pathways in mouse medullary thick ascending limb of Henle. I. ADH increases transcellular conductive pathways. J. Membr. Biol. 1984;80:201–219. doi: 10.1007/BF01868439. [DOI] [PubMed] [Google Scholar]
- HUANG D.Y., OSSWALD H., VALLON V. Intratubular application of sodium azide inhibits loop of Henle reabsorption and tubuloglomerular feedback response in anesthetized rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:367–373. doi: 10.1007/pl00005266. [DOI] [PubMed] [Google Scholar]
- HUANG D.Y., OSSWALD H., VALLON V. Eukaliuric natriuresis and diuresis in response to the KATP channel blocker U37883A: micropuncture studies on the tubular site of action. Br. J. Pharmacol. 1999;127:1811–1818. doi: 10.1038/sj.bjp.0702742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNICHOLAS C.M., GUGGINO W.B., SCHWIEBER E.M., HEBERT S.C., GIEBISCH G.H., EGAN M.E. Sensitivity of a renal K+ channels (ROMK2) to the inhibitory sulfonylurea compound, glibenclamide, is enhanced by co-expression with the ATP-binding cassette transporter CFTR. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8083–8088. doi: 10.1073/pnas.93.15.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTROSE-RAFIZADEH C., GUGGINO W.B. Role of intracellular calcium in volume regulation by rabbit medullary thick ascending limb cells. Am. J. Physiol. 1991;260:F402–F409. doi: 10.1152/ajprenal.1991.260.3.F402. [DOI] [PubMed] [Google Scholar]
- O'NEIL R.G. Voltage-dependent interaction of barium and cesium with the potassium conductance of the cortical collecting duct apical cell membrane. J. Membr. Biol. 1983;74:165–173. doi: 10.1007/BF01870505. [DOI] [PubMed] [Google Scholar]
- RAMSEY J.A., BROWN R.H.J., CROGHAN P.C. Electrometric titration of chloride in small volume. J. Exp. Biol. 1955;32:822–829. [Google Scholar]
- VALLON V., RICHTER K., BLANTZ R.C., THOMSON S., OSSWALD H. Glomerular hyperfiltration in experimental diabetes mellitus: potential role of tubular reabsorption. J. Am. Soc. Nephrol. 1999;10:2569–2576. doi: 10.1681/ASN.V10122569. [DOI] [PubMed] [Google Scholar]
- VAN DRIESSCHE W., ZEISKE W. Ionic channels in epithelial cell membrane. Physiol. Rev. 1985;65:833–903. doi: 10.1152/physrev.1985.65.4.833. [DOI] [PubMed] [Google Scholar]
- WANG T., WANG W.H., KLEIN-ROBBENHAAR G., GIEBISCH G. Effects of glyburide on renal tubule transport and potassium-channel activity. Renal. Physiol. Biochem. 1995a;18:169–182. doi: 10.1159/000173914. [DOI] [PubMed] [Google Scholar]
- WANG T., WANG W., KLEIN-ROBBENHAAR G., GIEBISCH G. Effects of a novel KATP channel blocker on renal tubule function and K+ channel activity. J. Pharmacol. Exp. Ther. 1995b;273:1382–1389. [PubMed] [Google Scholar]
- WANG W. Two types of K+ channels in thick ascending limb of rat kidney. Am. J. Physiol. 1994;267:F599–F605. doi: 10.1152/ajprenal.1994.267.4.F599. [DOI] [PubMed] [Google Scholar]
- WANG W., HEBERT S.C., GIEBISCH G. Renal K+ Channels: Structure and Function. Annu. Rev. Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- WANG W.H., LU M. Effect of arachidonic acid on activity of the apical K channel in the thick ascending limb of the rat kidney. J. Gen. Physiol. 1995;106:727–743. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINSTEINSodium chloride transport in the loop of Henle The kidney: physiology and pathophysiology 1992IIUSA: Raven Press; 1977–1983.Seldin & Giebisch (edi) [Google Scholar]
- WILLS N.K., ZEISKE W., VAN DRIESSCHE W. Noise analysis reveals K+ channel conductance in the apical membrane of rabbit colon. J. Membr. Biol. 1982;69:187–197. doi: 10.1007/BF01870398. [DOI] [PubMed] [Google Scholar]


