Abstract
The effect of IL-1ra on response to intraplantar (i.pl.) injection of LPS, carrageenin, bradykinin, TNFα, IL-1β, IL-8, PGE2 and dopamine was investigated in a model of mechanical hyperalgesia in rats.
IL-1ra inhibited hyperalgesic response to LPS, carrageenin, bradykinin, TNFα, and IL-1β, but not responses to IL-8, PGE2 and dopamine.
A sheep anti-rat IL-1ra serum potentiated response to LPS, carrageenin, bradykinin, TNFα and IL-1β but not IL-8.
Carrageenin and LPS stimulated and production of immunoreactive TNFα, IL-1β and IL-1ra in the skin of injected paws.
The inhibition by IL-1ra of the hyperalgesic response to carrageenin was not affected by antibodies neutralizing IL-4 and IL-10.
In mice, IL-1ra inhibited the nociceptive response to i.p. injection of acetic acid.
These data suggest that IL-1ra, released at sites of inflammation, limits inflammatory hyperalgesia. This effect is independent of (IL-1ra-induced) IL-4 and IL-10 and appears to be the result of antagonism by IL-1ra of IL-1β-stimulated eicosanoid production.
Keywords: Inflammatory hyperalgesia, interleukin-1 receptor antagonist, bradykinin, tumour necrosis factor alpha, interleukin-1β, interleukin-8, prostaglandin E2
Introduction
There is now a substantial body of evidence to show that the release of PGs and sympathomimetic amines occurs subsequent to the release of a cascade of cytokines and other mediators (Ferreira et al., 1988; 1993; Cunha et al., 1991; 1992; Safieh-Garabedian et al., 1995; Woolf et al., 1996; 1997; Banner et al., 1998; Poole et al., 1999). For example, in a model of mechanical hyperalgesia, carrageenin and LPS induced tissue formation of bradykinin, which stimulated the release of TNFα (Ferreira et al., 1993; Poole et al., 1999). The TNFα stimulated the release of IL-1β and IL-6, which stimulated the production of cyclo-oxygenase products, and IL-8, which stimulated the production of sympathomimetic mediators (Cunha et al., 1992).
A diverse group of compounds have been described which inhibit the production or action of pro-inflammatory cytokines (usually TNFα and IL-1β) and which have potential as anti-hyperalgesics with which to treat inflammatory pain. These include tripeptide analogues of IL-1β and α-melanocyte stimulating hormone (Ferreira et al., 1988; Poole et al., 1992; Safieh-Garabedian et al., 1997), and thalidomine, pentoxifylline and chlorpromazine (Sampaio et al., 1991; Weinberg et al., 1992; Gorizontova & Mironava, 1995; Dubost et al., 1997; Ohtsuka et al., 1997; Sommer et al., 1998). In addition, the ‘anti-inflammatory' cytokines IL-10 and IL-4 have also been shown to limit inflammatory hyperalgesia, by inhibiting the production of hyperalgesic cytokines and prostaglandins (Poole et al., 1995; Cunha et al., 1999).
Another ‘anti-inflammatory' or ‘inhibitory' cytokine that is released during the inflammatory process is IL-1ra (Dinarello & Thompson, 1991). IL-1ra is a specific receptor antagonist of IL-1 that competitively antagonizes binding of IL-1α and IL-1β to the IL-1 receptor, IL-1RI (Eisenberg et al., 1990; Granowitz et al., 1991a). It is the IL-1R1 that transduces the biological effects of IL-1(α and β), with the IL-1RII appearing to serve as a decoy receptor (Colotta et al., 1993). IL-1ra inhibits hypotension caused by both Gram-negative bacteria/LPS and also by Gram-positive organisms (Ohlsson et al., 1990; Aiura et al., 1993), production of PGs and IL-6, induced by IL-1 and LPS, respectively (Arend et al., 1990; Fischer et al., 1992), IL-1-induced fever (Opp & Krueger, 1991), adhesion of neutrophils and eosinophils to endothelial cells, and LPS-induced neutrophil accumulation (McIntyre et al., 1991). In view of the anti-hyperalgesic effects of other agents that inhibit the production or action of IL-1 (Ferreira et al., 1988; Poole et al., 1992; 1995; Watkins et al., 1994; 1995; Cunha et al., 1999), we have tested the possibility that IL-1ra limits inflammatory hyperalgesia by diminishing cytokine-mediated hyperalgesic response to carrageenin, bradykinin, and pro-inflammatory cytokines.
Methods
Test of mechanical hyperalgesia
All experiments were conducted in accordance with NIH guidelines on the welfare of experimental animals and with the approval of the USP (Ribeirão Preto) Ethics Committee.
A constant pressure of 20 mmHg was applied to the hind-paws of rats and discontinued when they presented a typical freezing reaction (reaction time). This reaction was characterized by a reduction in the movements that animals usually made to escape from the position imposed by the experimental situation. These were followed by alterations in respiratory frequency with the onset of a typical shivering reaction. The intensity of hyperalgesia was quantified as the variation in reaction time (delta reaction time) obtained by subtracting values measured 3 h after administration of hyperalgesic substances from (control) reaction times, measured before injection at time zero (Ferreira et al., 1978; 1988). Reaction times were typically 32–34 s (with s.e.mean of 0.5–1.0 s) before injection and 13–16 s after stimulation with hyperalgesic agents. Multiple paw treatments did not alter basal reaction times. Different individuals prepared the solutions to be injected, made the injections, and measured the reaction times.
Hyperalgesia was measured 3 h after injections (in 100 μl, i.pl.) of hyperalgesic agents. These were: LPS (0.1 μg or 1 μg), carrageenin (10 or 100 μg), bradykinin (50 or 500 ng), TNFα (0.25 or 2.5 pg), IL-1β (0.5 or 0.05 pg), IL-8 (10 pg or 100 pg), PGE2 (100 ng) and dopamine (10 μg) into the hind-paws of rats. The effects of IL-1ra, anti-IL-1ra serum and other drugs were tested in Protocols 1–3. Protocol 1: IL-1ra (30–300 ng in 50 μl) or saline was injected in the (same) hind-paws, 30 min before hyperalgesic agents. Hyperalgesia was measured 3 h after injections (in 100 μl, i.pl.) of hyperalgesic agents. Protocol 2: Anti-IL-1ra serum or pre-immune serum (50 μl, i.pl) was injected to hind-paws to be injected with hyperalgesic agents, 30 min before hyperalgesic agents. Hyperalgesia was measured 3 h after injections (in 100 μl, i.pl.) of hyperalgesic agents. Protocol 3: Rats were injected i.pl. with 50 μg (in 50 μl) of antibody to IL-4 (BVDG), antibody to IL-10 (JEA-5), or with (control) antibody to ovalbumin. Fifteen min later, one of the following was injected (in 50 μl) into the same paws: saline (50 μl), IL-1ra (100 pg), IL-4 (10 ng) or IL-10 (100 ng). After a further 15 min, carrageenin (Cg, 100 μg) was injected and the hyperalgesia measured 3 h after this final injection.
The larger doses of hyperalgesic agents were the smallest that evoked maximum responses and the time interval of 3 h was a time at which responses to the chosen doses of hyperalgesic agents were all at or close to their peak (Ferreira et al., 1988; 1993; Cunha et al., 1991; 1992; Poole et al., 1995). The order of potency of the cytokines was IL-1β>TNFα>>IL-8 for these human sequence cytokines in rats (Cunha et al., 1992). Whether this order of potency reflects the order of potency of the endogenous cytokines of the rat is unknown. The rat sequence cytokines were not available for these studies and certain cytokines exhibit species preference or specificity (Lumpkin, 1987; Morstyn & Burgess, 1988; Stefferl et al., 1996) and the native cytokines remain to be tested.
Abdominal constriction test
The anti-hyperalgesic activity of IL-1ra in mice was assessed using the abdominal constrictions test (Koster et al., 1959; Collier et al., 1968). Mice were injected into the peritoneal cavities (i.p.) with acetic acid (0.8% w v−1, 0.1 ml 10 g−1) and the abdominal constrictions counted between 0 and 20 min after the injection of the acetic acid. IL-1ra (0.1–1.2 ng, 0.2 ml, i.p.) or saline was given 30 min before the acetic acid.
In vitro measurements
Saline (100 μl), carrageenin (100 μg 100 μl−1) and LPS (1 μg 100 μl−1) were injected into the hind-paws of rats and 0.5, 1, 2, 3 or 6 h later the animals were terminally anaesthetized. Skin paw samples were extracted for TNFα, IL-1β and IL-1ρa measurement as described previously (Safieh-Garabedian et al., 1995; Woolf et al., 1997). The ELISAs for rat IL-1β and rat TNFα were as described previously (Safieh-Garabedian et al., 1995; Rees et al., 1999). The protocol for the ELISA for rat IL-1ra was similar to the protocols for the ELISAs for rat IL-1β and rat TNFα. Briefly, sheep polyclonal anti-rat IL-1ra IgG, raised against rat recombinant IL-1ra (1 μg ml−1), in 100 μl PBS buffer, was used to coat microtiter plates (Nunc Maxisorb). After incubation (4°C overnight) and washing the plates in assay buffer (0.01 M phosphate, 0.05 M NaCl, 0.1% Tween 20, pH 7.2), 100 μl of standard (rat recombinant IL-1ra, or sample, was added to each well and incubated overnight at 4°C. After washing the plates, 100 μl of biotinylated, sheep polyclonal anti-rat IL-1ra IgG (diluted 1/1000 with assay buffer +1% normal sheep serum), was added to the plates and incubated for 1 h at room temperature. Subsequent steps were as for the ELISA of rat TNFα (Rees et al., 1999).
Results are presented as mean with s.e.mean of groups of five animals for the in vivo measurements and of groups of three animals for the in vitro cytokine measurements. Three independent in vitro experiments were performed, each giving similar results. The results of formal statistical tests are not reported: for the in vivo experiments (for which n=5), a mean value described as different from another mean value differed by more than three times (usually five to ten times) the larger s.e.mean, for in vitro experiments (for which n=3), a mean value described as different from another mean value differed by more than twice the larger s.e.mean.
Drugs
The following materials were obtained from the sources indicated. Rat recombinant IL-1β, IL-1ra and TNFα, sheep anti-rat IL-1ra serum and pre-immune serum, human recombinant IL-1β (ampoule code: 86/680), IL-1ra (92/644), IL-8 (89/520), IL-10 (92/516), TNFα (87/650), murine IL-4 (91/656) (NIBSC, the specific activities of the ampouled materials are IL-1β: 100,000 international units (i.u.) 1 μg−1 ampoule−1, IL-1ra: 0.01 i.u. 10 μg−1 ampoule−1, IL-8: 1000 i.u. 1 μg−1 ampoule−1, IL-10: 5,000 i.u. 1 μg−1 ampoule−1, TNFα: 40,000 i.u. 1 μg−1 ampoule−1, murine IL-4: 10,000 i.u. 1 μg−1 ampoule−1). Carrageenin (FMC Corporation, Philadelphia, PA, U.S.A.). PGE2 and bradykinin (Sigma, St. Louis, MO, U.S.A.). Dopamine HCl (USP grade, Research Biochemicals International). Bacterial endotoxin from E. coli 055:B5 (referred to here as lipopolysaccharide, LPS, Difco Laboratories Ltd, West Molsey, Surrey, U.K.). Monoclonal IgG antibody to murine IL-4, BVDG (Professor F. Liew, University of Glasgow, U.K.). Monoclonal IgG antibody to murine IL-10, JEA-5 (Schering-Plough, U.S.A.). Control monoclonal antibody was a purified unrelated IgG raised against ovalbumin in our laboratory. The LPS content of the above materials, as measured in a Limulus Amoebocyte Lysate test, was of the order of 0.25 i.u. mg−1, which is equivalent to a little over 10−15 g of LPS in a hyperalgesic dose of IL-1β (0.5 pg), for example. The threshold hyperalgesic dose of LPS in the above model is some 100 ng, i.e. 10−7 g (based upon published data: Ferreira et al., 1993). Therefore, the doses of the hyperalgesic agents used contained amounts of LPS up to eight log10's less than the threshold hyperalgesic dose of LPS.
Animals
Male Wistar rats and Swiss mice, weighing 130–180 and 25–30 g, respectively, housed in temperature controlled rooms (22–25°C) with water and food ad libitum until use.
Results
Effect of IL-1ra on responses to hyperalgesic agents
Injection (in 100 μl, i.pl.) of IL-1β (0.5 pg), LPS (1 μg), carrageenin (100 μg), bradykinin (500 ng), TNFα (2.5 pg), IL-8 (100 pg), PGE2 (100 ng) and dopamine (10 μg) into the hind-paws of rats evoked hyperalgesic responses, measured 3 h after their injection (Figures 1 and 2). Treatment with IL-1ra (30–300 ng, 50 μl, i.pl.), 30 min before IL-1β, antagonized, in a dose-dependent manner, hyperalgesic responses to this cytokine (Figure 1). Similarly, hyperalgesic responses to LPS (1 μg), carrageenin (100 μg), bradykinin (500 ng) and TNFα (2.5 pg), but not to IL-8 (100 pg), PGE2 (100 ng) and dopamine (10 μg), were inhibited by treatment with IL-1ra (100 ng, 50 μl, i.pl.), 30 min before the hyperalgesic stimulus (Figure 2).
Figure 1.
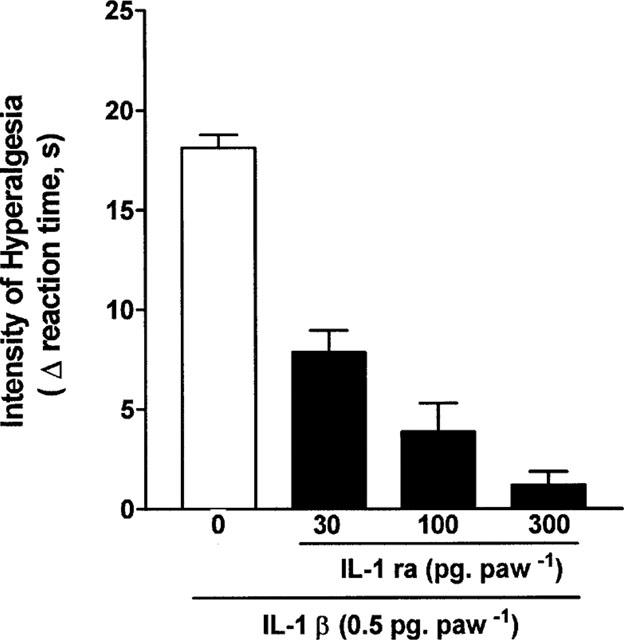
Antagonism by IL-1ra (30, 100 and 300 pg, 100 μl−1, i.pl.) of the hyperalgesic responses to IL-1β (IL-1β, 0.5 pg 100 μg−1, i.pl.). Saline (0) or IL-1ra was injected into paws to be injected with IL-1β, 30 min before the cytokine injection. The intensity of hyperalgesia was measured 3 h after injection of IL-1β. Results are expressed as means±s.e.mean in groups of five rats.
Figure 2.
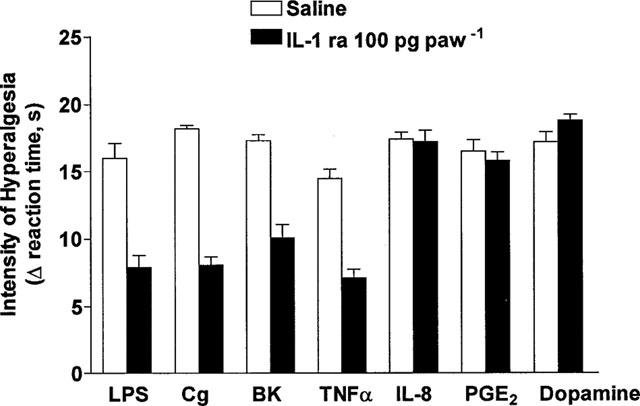
Effect of saline or IL-1ra (100 pg 100 μg−1, i.pl.) on hyperalgesic responses to injections (in 100 μl, i.pl.) or LPS (1 μg), carrageenin (Cg, 100 μg), bradykinin (BK, 500 ng), TNF-α (2.5 pg), IL-8 (100 pg), PGE2 (100 ng) and dopamine (10 μg). IL-1ra was injected into paws to be injected with one of the hyperalgesic agents, 30 min before the hyperalgesic agent. Hyperalgesia was measured 3 h after injection of hyperalgesic agents. Results are expressed as mean±s.e.mean in groups of five rats.
Potentiation by a sheep anti-IL-1ra serum of responses to hyperalgesic agents
A sheep anti-IL-1ra serum (50 μl, i.pl.), but not a pre-immune (control) serum (50 μl, i.pl.), injected 30 min before injections (i.pl.) of LPS (0.1 μg), carrageenin (10 μg), bradykinin (50 ng), TNFα (0.25 pg) and IL-1β (0.05 pg) potentiated responses, measured 3 h after injection of these hyperalgesic agents (LPS=+91%, carrageenin=+77%, bradykinin=+72%, TNFα=+114%, IL-1β=+134%). In contrast, hyperalgesic responses to IL-8 (10 pg, i.pl.) were little affected by the sheep anti-IL-1ra serum (Figure 3).
Figure 3.
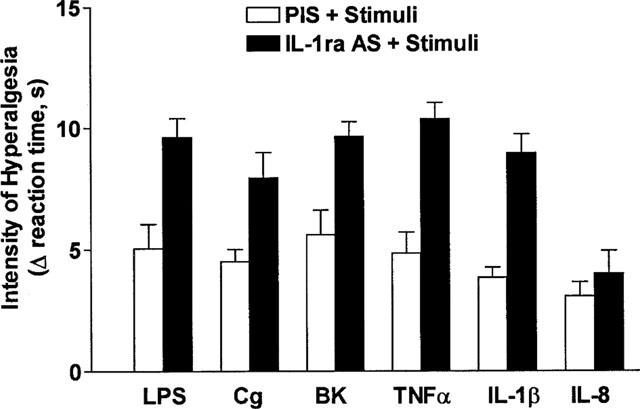
Effect of anti-rat IL-1ra serum (50 μl, i.pl.), and pre-immune serum on hyperalgesic responses to LPS (0.1 μg), carrageenin (Cg, 10 μg), BK (50 ng), TNFα (0.25 pg), IL-1β (0.05 pg), IL-8 (10 pg). The sera were injected into paws to be injected with one of the hyperalgesic agents, 30 min before the hyperalgesic agent. The intensity of hyperalgesia was measured 1 h after injection of the hyperalgesic agents. Results are expressed as means±s.e.mean in groups of five rats.
Stimulation by LPS and carrageenin of the production of TNF-α, IL-1β and IL-1ra in paw skin
Carrageenin (100 μg 100 μl−1, i.pl.) and LPS (1 μg 100 μl−1, i.pl.) stimulated the production of TNFα, IL-1β and IL-1ra in rat paw skin. The pattern of cytokine production was broadly similar for the two stimuli although there were some differences in the time courses for production of TNFα and IL-1β (Figure 4). TNFα: with carrageenin, concentrations of TNF-α were increased at 30 min (to 0.11±0.02 ng hind paw−1, +110%), peaked at 3 h (at 0.14±0.01 ng hind paw−1, +174%), and remained elevated at 6 h (0.12±0.02 ng hind paw−1, +135%); with LPS, concentrations of TNFα were not increased at 30 min but peaked earlier, at 1 h (at 0.33±0.09 ng hind paw−1, +547%), and had essentially returned to pre-injection concentrations at 6 h (0.07±0.01 ng hind paw−1, +37%). IL-1β: with carrageenin, concentrations of IL-1β were not increased at 30 min and 1 h but peaked at 2 h (at 1.90±0.22 ng hind paw−1, +164%), and remained elevated at 6 h (1.47±0.45 ng hind paw−1, +105%); with LPS, concentrations of IL-β similarly were not increased at 30 min and 1 h, but were increased at 2 h (at 1.26±0.83 ng hind paw−1, +75%), peaked later, at 3 h (at 2.25±0.14 ng hind paw−1, +213%), and remained elevated at 6 h (1.44±0.19 ng hind paw−1, +100%). IL-1ra: with carrageenin and LPS, IL-1ra concentrations were increased at 30 min (5.02±0.21 ng hind paw−1, +252%, and 3.25±0.23 ng hind paw−1, +128%, respectively), peaked at 2 h (5.9±0.12 ng hind paw−1, +313%, and 3.61±0.33 ng hind paw−1, +153%, respectively) and remained elevated at 6 h (5.02±0.12 ng hind paw−1, +252%, and 3.78±0.12 ng hind paw−1, +165%, respectively).
Figure 4.
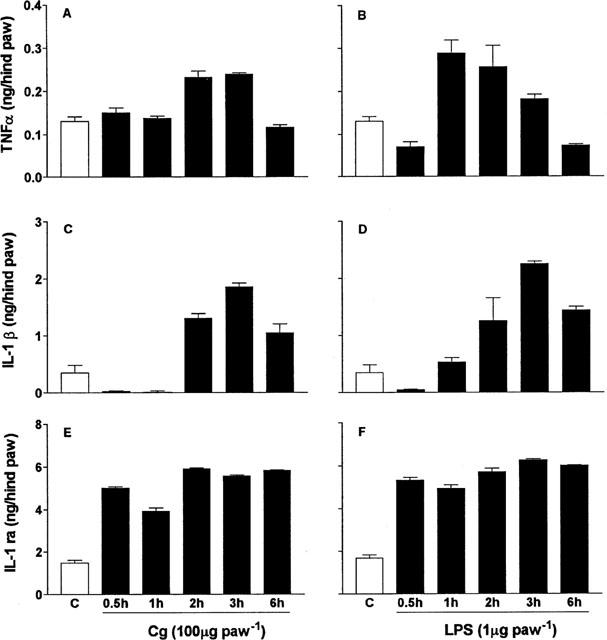
Concentrations of TNFα (A, B), IL-1β (C, D) and IL-1ra (E, F) in rat paws injected with carrageenin (Cg, filled columns, A, C, E) or LPS (B, D, F). Paws were injected (100 μl) with saline, carrageenin (Cg, 100 μg), or LPS (1 μg). At times after injection of 30 min, 1, 2, 3 and 6 h rats were killed and paw skin samples were extracted for cytokines, which were measured by ELISA. The results are reported as means±s.e.mean of three rats.
Failure of anti-IL-4 antibodies and anti-IL-10 antibodies to reverse the effect of IL-1ra
The inhibition (−39%) by IL-1ra (100 ng 50 μg−1, i.pl.) of the hyperalgesic response to carrageenin (100 μg 100 μg−1, i.pl.) was unaffected by the presence of antibodies neutralizing rat IL-4 (BVDG, −41%) and rat IL-10 (JEA-5, −38%, Figure 5). In contrast, BVDG essentially reversed the inhibitory effect of IL-4 (from −47 to −10%) and JEA-5 reversed the inhibitory effects of IL-10 (from −55 to −5%, Figure 5).
Figure 5.
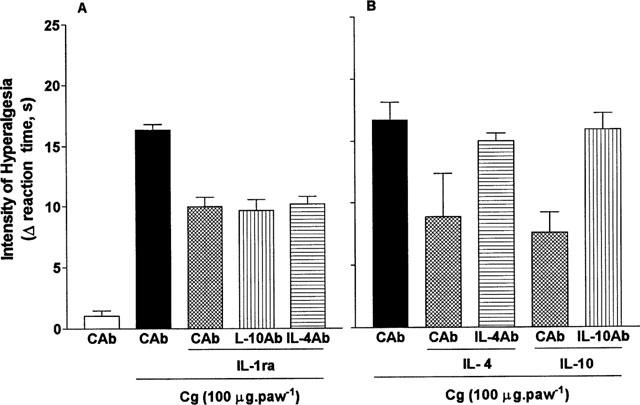
Effect of antibodies neutralizing IL-4 and IL-10 on the anti-hyperalgesic effect of IL-1ra. Rats were injected i.pl. with 50 μg (in 50 μl) of antibody to IL-4 (IL-4Ab, BVDG), IL-10 (IL-10Ab, JEA-5), or with (control) antibody to ovalbumin. Fifteen min later, one of the following was injected (in 50 μl) into the same paws: saline (50 μl), IL-1ra (100 pg, A), IL-4 (10 ng, B) or IL-10 (100 ng, B). After a further 15 min, carrageenin (Cg, 100 μg (in 100 μl)) was injected and the hyperalgesia measured 3 h after this final injection. Results are expressed as means±s.e.mean in groups of five rats.
Inhibition by IL-1ra of acetic acid-induced abdominal constrictions
In mice, acetic acid (0.8%, w v−1, 0.1 ml 10 g−1) caused abdominal constrictions. The cumulative total number of constrictions was 64±5 (mean±s.e.mean) during the 20 min period following injection (Figure 6). IL-1ra (0.1–1.2 ng mouse−1, 0.2 ml, i.p.), given 30 min before the acetic acid, inhibited, in a dose-dependent manner, this response (Figure 6). IL-1ra (1.2 ng mouse−1) reduced the number of constrictions from 64±5 to 21±7 (−67%).
Figure 6.
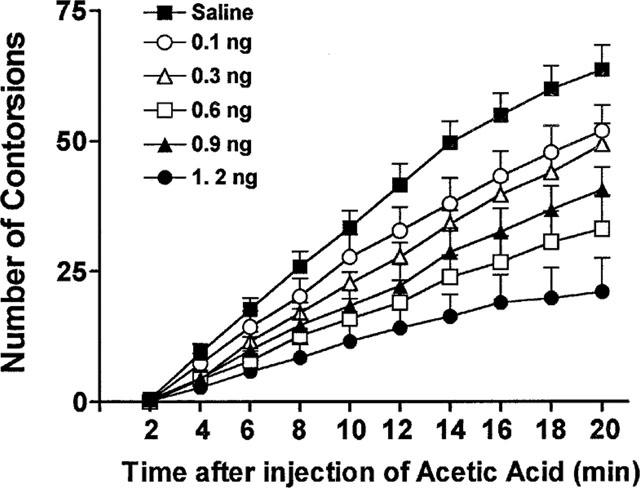
The anti-hyperalgesic effect of IL-1ra. Mice were injected into the peritoneal cavities (i.p.) with acetic acid (0.8% w v−1, 0.1 ml 10 g−1) and the cumulative abdominal constrictions counted between 0 and 20 min after the injection of the acetic acid. IL-1ra (0.1 ng, open circles; 0.3 ng, open triangles; 0.6 ng, filled triangles; 0.9 ng, open squares, 1.2 ng, filled circles; 0.2 ml, i.p.) or saline was given 30 min before the acetic acid. Results are expressed as means±s.e.mean in groups of at last six mice.
Discussion
Interleukin-1ra is a cytokine with anti-inflammatory activity because it is a receptor antagonist of the pro-inflammatory cytokine IL-1β. In the present study, a model of inflammatory hyperalgesia (a rat paw pressure test) was used to assess whether or not IL-1ra was able to exert an anti-hyperalgesic effect. In this test, carrageenin and LPS cause hyperalgesia by stimulating the release of bradykinin, which initiates two pathways, each of which involves the release of cytokines and other mediators. The two pathways are: (i) carrageenin/LPS→bradykinin→IL-6→IL-1β→PGs and (ii) carrageenin/LPS→bradykinin→TNFα→IL-8→sympathomimetics (Ferreira et al., 1978; 1988; 1993; Cunha et al., 1991; 1992; Poole et al., 1999).
Local injection of IL-1ra inhibited hyperalgesic responses to the inflammatory stimuli carrageenin and LPS and responses to the endogenous hyperalgesic mediators bradykinin, TNFα and IL-1β, which are induced by carrageenin and LPS. That the inhibition by IL-1ra of the hyperalgesic effects of mediators other than IL-1β was only partial is consistent with the contribution of the IL-1β-independent pathway (carrageenin/LPS→bradykinin→TNFα→IL-8→sympathomimetics) to the hyperalgesic responses to carrageenin, LPS, bradykinin and TNFα. Similarly, the absence of an effect of IL-1ra on hyperalgesic responses to IL-8, PGE2 and dopamine was predicted because the mode of action of these mediators is not IL-1β-dependent. IL-8 evokes local production of sympathetic amines, and sympathetic amines and PGE2 sensitize nociceptors, as shown in the pathways shown above.
The enhancement of hyperalgesic responses to carrageenin, LPS, bradykinin TNF-α and IL-1β by treatment with an anti-IL-1ra serum suggest that endogenous IL-1ra serves to limit inflammatory hyperalgesia. The absence of an effect of the anti-IL-1ra serum on hyperalgesic responses to IL-8 was predicted because, as noted above, the mode of action of IL-8 is not IL-1β-dependent. IL-8 evokes local production of sympathetic amines which sensitize nociceptors (Ferreira et al., 1978). The restriction of an anti-hyperalgesic effect of IL-1ra to mediators involved in the IL-1β-dependent pathway (carrageenin/LPS→bradykinin→IL-6→IL-1β→PGs) suggests that the mechanism of action of IL-1ra was (competitive) antagonism of IL-1 receptors.
The cytokines IL-10 and IL-4 also have a role in limiting the development of inflammatory hyperalgesia triggered by carrageenin, bradykinin and TNF-α (Poole et al., 1995; Cunha et al., 1999). Since IL-1ra can induce the production of IL-10 and IL-4, the possibility that the anti-hyperalgesic effect of IL-1ra was secondary to the production of IL-10 and IL-4 was tested in experiments in which antibodies neutralizing rat IL-10 and IL-4 failed to reverse the inhibition by IL-1ra of carrageenin-evoked hyperalgesia (while reversing inhibition by IL-10 and IL-4, respectively). So, although IL-1ra joins IL-10 and IL-4 in the class of anti-hyperalgesic cytokines, its mode of action is different. It is a competitive receptor-antagonist of a pro-inflammatory cytokine (IL-1), rather than a functional antagonist, like IL-10 and IL-4, which inhibit production of pro-inflammatory cytokines.
Consistent with the anti-hyperalgesic effect of exogenous IL-1ra and the hyperalgesic effect of an anti-IL-1ra serum, endogenous concentrations of immunoreactive IL-1ra were found to be elevated in rat paw skin at all time intervals between 30 min and 6 h subsequent to the injection (i.pl.) of carrageenin and LPS. Interestingly the increase in skin concentrations of immunoreactive IL-1ra preceded increases in skin immunoreactive IL-1β, which is a prime stimulus for the production of IL-1ra (Arend et al., 1998). The time-course of the production of IL-1β and IL-1ra accords with the results of Granowitz et al. (1991b), who reported that, following i.v. injection of LPS in human volunteers, plasma concentrations of IL-1ra remained elevated for 24 h whereas plasma concentrations of IL-1β fell to pre-injection values between 6 and 12 h. Consistent with the role of TNFα and IL-1β in inflammatory hyperalgesia, paw skin concentrations of these cytokines increased markedly following injection of carrageenin and LPS, with increases in TNFα preceding increases in IL-1β, in agreement with the literature (Safieh-Garabedian et al., 1995; Woolf et al., 1997; Miller et al., 1997).
Recently, IL-1β-induced eiosanoid production was shown to have a major role in acetic acid-induced induced abdominal constrictions in mice, with an anti-IL-1β antiserum reducing by more than 50% the response to acetic acid (Ribeiro et al., 2000). We report here that IL-1ra effected a similar reduction in the response to acetic acid, providing further evidence for the anti-hyperalgesic properties of IL-1ra. The explanation for these potent effects of anti-IL-1β serum and very modest doses of IL-1ra may be that by injecting them i.p. they had access to vagal paraganglia on the abdominal vagus which can bind biotinylated IL-1ra (Goehler et al., 1997).
In summary, IL-1ra, produced at the site of inflammation, limits inflammatory hyperalgesia in two different models, one in rats and one in mice. The mode of action of IL-1ra appears to result from its capacity to antagonise the stimulation by IL-1β of the production of eicosanoid products, and not from the capacity of IL-1ra to stimulate the production of anti-inflammatory cytokines such as IL-10 and IL-4. Notwithstanding the fact that IL-1ra has to be administered parenterally, its potent anti-hyperalgesic properties suggest its evaluation as a treatment for inflammatory pain that is refractory to existing analgesic drugs.
Acknowledgments
The authors gratefully acknowledge the technical assistance of Ieda R. dos Santos, Giuliana Bertozi Franciso and Sérgio Roberto Rosa. This work was supported by grants from FAPESP and CNPq (Brazil). The rat cytokine reagents were prepared with funding from the European Community Concerted Action Programme Biomed I: ‘Cytokines in the Brain' (PL931450) and Biomed II: ‘Anti-inflammatory Cytokines In Neurological Disease' (PL97-2492).
Abbreviations
- BK
bradykinin
- Cg
carrageenin
- IgG
immunoglobulin G
- IL
interleukin
- IL-1ra
interleukin-1 receptor antagonist
- LPS
lipopolysaccharide
- MAb
monoclonal antibody
- PGE2
prostaglandin E2
- TNFα
tumour necrosis factor
References
- AIURA K., GELFAND J.A., BURKE J.F., THOMPSON R.C., DINARELLO C.A. Interleukin-1 (IL-1) receptor antagonist prevents Staphylococcus epidermis-induced hypotension and reduces circulating levels of tumour necrosis factor and IL-1 beta in rabbits. Infect. Immun. 1993;61:3342–3350. doi: 10.1128/iai.61.8.3342-3350.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AREND W.P., MALYAK M., GUTHRIDGE C.J., GABAY C. Interleukin-1 receptor antagonist: role in biology. Ann. Rev. Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- AREND W.P., WELGUS H.G., THOMPSON R.C., EISENBERG S.P. Biological properties of recombinant human myocyte-derived interleukin 1 receptor antagonist. J. Clin. Invest. 1990;85:1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANNER L.R., PATTERSON P., ALLCHORNE A., POOLE S., WOOLF C.J. Leukaemia inhibitory factor is an anti-inflammatory and analgesics cytokine. J. Neurosci. 1998;18:5456–4262. doi: 10.1523/JNEUROSCI.18-14-05456.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIER H.O.J., DINNEEN J.C., JOHNSON C.A., SCHNEIDER C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOTTA F., RE F., MUZIO M., BERTINI R., POLENTARUTTI N., SIRONI M,. , GIRI J., DOWLER S.K., SIMS J.E., MANTOVANI A. Interleukin-1 type II receptors: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. Interleukin-8 as a mediator of sympathetic pain. Br. J. Pharmacol. 1991;104:765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., FERREIRA S.H. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LORENZETTI B.B., VEIGA F.H., FERREIRA S.H. Cytokine mediated inflammatory hyperalgesia limited by interleukin-4. Br. J. Pharmacol. 1999;126:45–50. doi: 10.1038/sj.bjp.0702266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINARELLO C.A., THOMPSON R.C. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunol. Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- DUBOST J.J., SOUBRIER M., RISTORI J.M., BEAUJON G., OUALID T., BUSSIÈRE J.L., SAUVEZIE B. An open study of the anti-TNF alpha agent pentoxifylline in the treatment of rheumatoid arthritis. Rev. Rhum. Engl. Ed. 1997;64:789–793. [PubMed] [Google Scholar]
- EISENBERG S.P., EVANS R.J., AREND W.P., VERDERBER E., BREWER M.T., HANNUM C.H., THOMPSON R.C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990;343:341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., BRISTOW A.F., POOLE S. Interleukin-1β as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., CORREA F.M.A. Central and peripheral antialgesic action of aspirin like drug. Eur. J. Pharmacol. 1978;53:39–48. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., POOLE S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER E., MARANO M.A., VAN ZEE K.J., ROCK C.S., HAWES A.S., THOMPSON W.A., DEFORGE L., KENNEDY J.S., REMICK D.G., BLOEDOW D.C., THOMPSON R.C., LOWRY S.F., MOLDAWER L.L. Interleukin-1 receptor blockade improves survival and hemodynamic performance in Escherichia coli septic shock, but fails to alter host response to sublethal endotoxemia. J. Clin. Invest. 1992;89:1551–1557. doi: 10.1172/JCI115748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOEHLER L.E., RELTON J.K., DRIPPS D., KIECHLE R., TARTAGLIA N., MAIER S.F., WATKINS L.R. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain. Res. Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- GORIZONTOVA M.P., MIRONOVA I.V. The effect of prophylactic administration of pentoxifyllin (trental) on development of a neuropathic pain syndrome and microcirculatory disorders caused by it. Biull. Eksp. Biol. Med. 1995;119:485–487. [PubMed] [Google Scholar]
- GRANOWITZ E.V., CLARK B.D., MANCILLA J., DINARELLO C.A. Interleukin-1 receptor antagonist competitively inhibits the binding of interleukin-1 to the type II interleukin-1 receptor. J. Biol. Chem. 1991a;266:14147–14150. [PubMed] [Google Scholar]
- GRANOWITZ E.V., SANTOS A.A., POUTSIAKA D.D., CANNON J.G., WILMORE D.W., WOLFF S.M., DINARELLO C.A. Production of interleukin-1 receptor antagonist during experimental endotoxaemia. Lancet. 1991b;338:1423–1424. doi: 10.1016/0140-6736(91)92725-h. [DOI] [PubMed] [Google Scholar]
- KOSTER R., ANDERSON M., DE BEER E.J. Acetic acid for analgesic screening. Federation Proc. 1959;18:412. [Google Scholar]
- LUMPKIN M.D. The regulation of ACTH secretion by IL-1. Science. 1987;238:452–545. doi: 10.1126/science.2821618. [DOI] [PubMed] [Google Scholar]
- MCINTYRE K.M., STEPAN G.J., KOLINSKY K.D., BENJAMIN W.R., PLOCINSKI J.M., KAFFKA K.L., CAMPEN C.A., CHIZZONITE R.A., KILIAN P.L. Inhibition of interleukin 1 (IL-1) binding and bioactivity in vitro and modulation of acute inflammation in vivo by IL-1 receptor antagonist and anti-IL-1 receptor monoclonal antibody. J. Exp. Med. 1991;173:931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER A.J., LUHESI G.N., ROTHWELL N.J., HOPKINS S.J. Local cytokine induction by LPS in the rat air pouch and its relationship to the febrile response. Am. J. Physiol. 1997;272:R857–861. doi: 10.1152/ajpregu.1997.272.3.R857. [DOI] [PubMed] [Google Scholar]
- MORSTYN G., BURGESS A.W. Hemopoietic growth factors: a review. Cancer Res. 1988;48:5624–5637. [PubMed] [Google Scholar]
- OHLSSON K., BJORK P., BERGENFELDT M., HAGEMAN R., THOMPSON R.C. Interleukin-1 receptor antagonist reduces mortality from endotoxin shock. Nature. 1990;348:550–552. doi: 10.1038/348550a0. [DOI] [PubMed] [Google Scholar]
- OHTSUKA H., HIGUCHI T., MATSUZAWA H., SATO H., TAKAHASHI K., TAKAHASHI J., YOSHINO T. Inhibitory effect on LPS-induced tumour necrosis factor, in calves treated with chlorpromazine or pentoxifylline. J. Vet. Med. Sci. 1997;59:1075–1077. doi: 10.1292/jvms.59.1075. [DOI] [PubMed] [Google Scholar]
- OPP M.R., KRUEGER J.M. Interleukin-1 receptor antagonist blocks interleukin-1 induced sleep and fever. Am. J. Physiol. 1991;260:R453–457. doi: 10.1152/ajpregu.1991.260.2.R453. [DOI] [PubMed] [Google Scholar]
- POOLE S., BRISTOW A.F., LORENZETTI B.B,. , DAS R.E., SMITH T.W., FERREIRA S.H. Peripheral analgesic activities of peptides related to alpha-melanocyte stimulating hormone and interleukin-1 beta 193–195. Br. J. Pharmacol. 1992;106:489–492. doi: 10.1111/j.1476-5381.1992.tb14361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., CUNHA F.Q., SELKIRK S., LORENZETTI B.B., FERREIRA S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1. Br. J. Pharmacol. 1995;115:684–688. doi: 10.1111/j.1476-5381.1995.tb14987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POOLE S., LORENZETTI B.B., CUNHA J.M., CUNHA F.Q., FERREIRA S.H. Bradykinin B1 and B2 receptors, tumor necrosis factor a and inflammatory hyperalgesia. Br. J. Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REES G., GEE C.K., WARD H.L., BALL C., TARRANT G.M., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-α: expression in recombinant picia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999;10:383–392. [PubMed] [Google Scholar]
- RIBEIRO R.A., VALE M.L., THOMAZZI S.M., PASCHOALATO A.B.P., POOLE S., FERREIRA S.H., CUNHA F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., KANAAN S.A., JALAKHIAN R.H., JABBUR S.J., SAADE N.E. Involvement of interleukin-1 beta, nerve growth factor, and prostaglandin-E2 in the hyperalgesia induced by intraplantar injections of low doses of thymulin. Brain Behav. Immun. 1997;11:185–200. doi: 10.1006/brbi.1997.0491. [DOI] [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., POOLE S., ALLCHORNE A., WINTER J., WOOLF C.J. Interleukin-1 beta contributes to the inflammation-induced increase in nerve-growth factor levels and inflammatory hyperalgesia. Br. J. Pharmacol. 1995;115:1265–1275. doi: 10.1111/j.1476-5381.1995.tb15035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPAIO E.P., SARNO E.N., GALILLY R., KOHN Z.A., KAPLAN Z. Thalidomide selectively inhibits tumour necrosis factor-∞ production by stimulated human monocytes. J. Exp. Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMER C., MARZINIAK M., MYERS M.M. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998;74:83–91. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- STEFFERL A., HOPKINS S.J., ROTHWELL N.J., LUHESHI G.N. The role of TNF-alpha in fever: opposing actions of human and murine TNF-alpha and interactions with IL-beta in the rat. Br. J. Pharmacol. 1996;118:1919–1924. doi: 10.1111/j.1476-5381.1996.tb15625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS L.R., GOEHLER L.E., RELTON J., BREWER M.T., MAIER S.F. Mechanism of tumour necrosis factor-alpha (TNF-alpha) hyperalgesia. Brain-Res. 1995;692:244–250. doi: 10.1016/0006-8993(95)00715-3. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., WIERTELAK E.P., GOEHLER L.E., SMITH K.P., MARTIN D., MAIER S.F. Characterization of cytokine induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- WEINBERG J.B., MASON S.N., WORTHAM T.S. Inhibition of tumour necrosis factor-alpha (TNF-alpha) and interleukin-1 beta (IL-1 beta) messenger RNA (mRNA) expression in HL-60 leukemia cells by pentoxifylline and dexamethasone: dissociation of acivicin-induced TNF-alpha and IL-1 beta mRNA expression from acivicin-induced monocytoid differentiation. Blood. 1992;79:3337–3343. [PubMed] [Google Scholar]
- WOOLF C.J., ALLCHORNE A., SAFIEH-GARABEDIAN B., POOLE S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLF C.J., QING-PING M.A., ALLCHORNE A., POOLE S. Peripheral cell types contributing to the hyperalgesic action of the nerve growth factor in inflammation. J. Neurosci. 1996;16:2716–2723. doi: 10.1523/JNEUROSCI.16-08-02716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]


